Abstract
The free radical theory of aging proposes the accumulation of altered, less active and toxic molecules of DNA, RNA, proteins and lipids caused by reactive oxygen species and reactive nitrogen species. Neurodegenerative disorders are characterized by an abnormal accumulation of oxidatively damaged macromolecules inside cells and in the extracellular space. Proteins involved in the formation of aggregates are β-amyloid, tau, α-synuclein, parkin, prion proteins and proteins containing polyglutamine. These abnormal aggregated proteins influence normal cellular metabolism. Additionally, deposition of abnormal proteins induces oxidative stress and proteasomal as well as mitochondrial dysfunction that ultimately lead to neuronal cell death.
In this review we focus on the impact of oxidative and nitrative stress in the aging brain and, consequently, on the generation of modified proteins, as these post-translational modifications are assumed to play an important role in the development of neurodegenerative diseases.
Keywords: oxidative stress, nitrative stress, neurodegenerative diseases, protein degradation, aging
Introduction
Cellular exposure to irradiation, oxidant pollutants such as ozone and nitrogen species, xenobiotics, drugs, or heavy metals are known to generate reactive oxygen species, reactive nitrogen species and electrophiles that lead to oxidative stress (1,2). Reactive oxygen species are also produced endogenously by a variety of enzymes and as by-products of cellular respiration (3). Additionally, physiological responses such as the activation of neutrophils during inflammation and infection are accompanied by the production of reactive oxygen and reactive nitrogen species (4). There are several mechanisms for protection against overwhelming reactive oxygen and reactive nitrogen species production; however, high levels of reactive oxygen species production could shift the balance between oxidant production and defense mechanisms towards pro-oxidative conditions, a situation referred to as oxidative stress. This leads to an enhanced macromolecular damage, mutations, apoptosis and degeneration of tissues, premature aging, cellular transformation and cancer (5–7).
It has been reported that markers of oxidative and nitrative stress, like modified lipids, DNA and proteins, are increased with aging (8). Especially the broad spectrum of diverse protein modifications leads to abnormal and damaged proteins and protein aggregation. Abnormal proteins can be produced by a multitude of events, including faulty post-translational modifications, oxidation and/or nitration of amino acid residues by reactive oxygen or reactive nitrogen species (9–11), halogenations of amino acid residues (12), modification of proteins by lipid peroxidation products (e.g. HNE and isoketals), glycation/glycoxidation (13) and chemical modifications like deamidation and racemisation (14,15). Abnormal proteins can also be produced by non-post-translational mechanisms, for example, gene mutations, aberrant mRNA splicing, or overexpression due to triplication of genes (16).
Studies on oxidatively, modified proteins have revealed an age-related increase in the content of protein carbonyls (17), glycated proteins (13), oxidized methionine (11) and an accumulation of enzymes that are impaired in their catalytic activity (18). However, although a broad spectrum of protein modifications are known, the best studied marker for age-related protein oxidation is currently protein carbonylation (17).
Beside the modifications made by oxidative/nitrative stress, it is also assumed that post-translational modifications like ubiquitination and phosphorylation may also contribute to the aggregation or fibrillization of proteins as this could be found in aggregates in diverse neurodegenerative diseases (19,20).
The term neurodegeneration refers to a continuous neuronal damage often triggered by protein aggregates formed of abnormally modified proteins (excessive misfolding). The accumulation of these misfolded proteins leads to a progressive loss of neurons in an age-dependent manner (21). Alzheimer, Parkinson, Prion, Huntington and motor neuron disorders (amyotrophic lateral sclerosis) are all considered to emerge due to accumulation of post-translationally modified, especially misfolded, proteins (22). The exact mechanisms of abnormal folding are not fully understood, however, speculations lead to the presumption that genetic and environmental factors (especially oxidative stress) are involved (22).
Neurodegenerative diseases have different symptoms and a multitude of causes. Frequently, mitochondrial function is impaired and there is an increased oxidative damage accompanied by defects in the ubiquitin-proteasome system and accumulation of abnormal, aggregated proteins. However, some hereditary forms are caused by genetic mutations and many sporadic cases are of unknown origin, perhaps due to environmental factors (23). This is confirmed by growing evidence which supports the hypothesis that oxidative stress triggers a cascade of events leading to cell death in multiple neurodegenerative diseases, such as Parkinson disease, Alzheimer disease, Huntington disease and amyotrophic lateral sclerosis (ALS).
There is considerable debate regarding the toxicity versus protective effects of protein aggregates. However, there is consensus that under some circumstances, the toxicity predominates. It is frequently reviewed that protein misfolding appears to be a common feature in progressive neuronal dysfunction (24,25).
In this review we discuss the role of protein post-translational modifications in the development of neurodegenerative diseases, mentioned above. In doing so, major emphasis was placed on the aging process itself and two most prevalent diseases: Alzheimer and Parkinson disease.
Protein oxidation during aging
Aging is generally described as an increased risk of disease and death with time (26). Many factors contribute to aging, including genetics and environment, leading to deleterious changes in the organism over time. Aging is a highly complex progress and one of the many problems in studying aging is the difficulty of separating the aging process itself from abnormal processes involved in age-related diseases, since aging is often accompanied by subclinical parameters involved in age-related diseases (e.g. plaque formation, local inflammation, etc.).
One hypothesis of aging is often mentioned: the oxidative stress or free radical theory (27). Oxidative stress or free radicals can damage molecules and if removal systems fail, these damaged molecules accumulate and interfere with cellular and systemic systems (28). The different hypotheses might interact with each other, for example, oxidative stress might be involved in the mutation theory (27). Some investigations describe that mutations in single genes can extend life span (29,30), however, most of the commonly accepted theories of aging propose that aging involves some unrepaired molecular damage that affects homeostasis in organisms (26). Mutations have the potential to affect essential functions in cells and facilitate the formation of destabilizing macromolecules, metabolites and reactive intermediates. Additionally, they can be involved in loss of function in protective enzymes or they are responsible for destabilization of regulatory pathways (28). However, the discussion of mutation accumulation in aging is a subject of debate and beyond the scope of this review (for further details see (28,31,32).
One of the hallmarks of aging is the accumulation of oxidized and modified proteins, resulting at least in part from a failure of protein maintenance (33,34). There is increasing evidence that biological aging is accompanied by post-translational modifications of proteins, like protein tyrosine nitration (reviewed in (35)). Some time ago it was proposed that in older individuals one-third of proteins bear carbonyl groups (36). Amino acid side chains and peptide backbones get oxidized in reactions with reactive oxygen and nitrogen species. Oxidized protein degradation is accomplished by the proteasomal system, however, it is now well established and described that proteasomal function is commonly impaired with age (37–39). This age-related impairment in proteasomal function is believed to contribute to the accumulation of oxidized proteins during aging (37,40). Presumably this is due to decreased proteasomal subunit expression or alteration of proteasomal subunits and therefore inactivation. Additionally highly oxidized and cross-linked proteins may act as endogenous inhibitors of proteasomal activity (37,40). An accumulation of oxidized proteins has been investigated in replicative and nonreplicative senescence of human fibroblasts (41–44), a valid model for studying aging, and the proteasomal activity and oxidized protein-repair enzymes like methionine sulfoxide reductases, that are also impaired in these cell systems during senescence (45,46).
To gain further insight into the mechanisms that might be implicated in the reduced proteasomal activity in replicative senescent cells, senescent WI-38 fibroblasts have been investigated for their content of modified proteins. Indeed, oxidized proteins and proteins modified by the lipid peroxidation product 4-hydroxy-2-nonenal were found to accumulate with serial passaging of human fibroblasts and keratinocytes (39,47).
Other endogenously generated products, probably correlating with aging, are the so called “age pigments”. These typically brown pigments are a highly heterogenous mixture of substances, however, they can be distinguished in two types: lipofuscin and advanced glycation end products (AGE) (13,48). AGEs are formed by reaction of amino acid side chains with reactive intermediates from carbohydrates (reviewed in (13)). Lipofuscin is generated by an extensive adduct formation and protein cross-linking, sometimes mediated by lipid peroxidation products, e.g. 4-hydroxy-2-nonenal, malondialdehyde and others (49). Many age pigments are fluorescent products; therefore, they can be conveniently measured and regarded as markers of aging. However, the question remains unsolved whether age pigments are a consequence or a cause of aging. In the case of lipofuscin, it has been traditionally assumed that this pigment is a by-product of the aging process (reviewed in (48)), conversely the “lysosomal-mitochondrial axis” theory of aging postulates its contribution to aging (50,51). It is known that lipofuscin accumulates in lysosomes leading to their dysfunction and probably prevent efficient autophagy (52). Autophagy is described as an important longevity assurance mechanism, therefore, lipofuscin may be linked to aging (53).
Aging is still a complex barely understood phenomenon and more investigations are needed to obtain detailed information. An important goal of research should be the identification und characterization of post-translationally modified proteins in vivo to discover specific biomarkers of aging for diagnostic purposes and for the development of therapies.
Of special importance is the accumulation of abnormal and modified proteins in so called post-mitotic cells. Post-mitotic cells are not capable of cell division and therefore the amount of post-translationally modified proteins cannot be divided and passed to daughter cells in lower concentrations. Post-mitotic cells, like neurons or skeletal muscles, are therefore especially prone to the build of protein aggregates during aging.
Evidence of oxidative and nitrative stress in the aging brain
In mammalian brain cells, reactive oxygen species and reactive nitrogen species are continuously generated as a consequence of normal oxygen cellular metabolism. It is assumed that some of the consumed oxygen is converted into superoxide. Responsible for this fact are reactions catalyzed by NAD(P)H oxidase, xanthine oxidase, phospholipase A2 and neuronal nitric oxide synthase as well as redox-reactive compounds of the mitochondrial electron transport chain leading to oxidative byproducts. Microglia, the macrophagial/inflammatory cells of the brain, produce high amounts of nitric oxide (NO) and peroxynitrite (ONOO−) if activated (54). To eliminate the damage induced by these highly reactive species a multilevel antioxidative defense system composed of low-molecular weight antioxidants and enzymes is present in cells. In the aging brain, it is suggested that the antioxidant defense is somewhat weak, and a decline in the defense plays a role in increased oxidative stress (55,56). Therefore, the products of protein oxidation can be readily detected in the brain, especially, oxidized sensitive amino acids as histidine, methionine and cysteine (57) and protein carbonyls were detected in the aging brain (58). Oxidation of proteins in the presence of monosaccharides such as glucose and aldehydes, such as methylglyoxal, can further result in the formation of irreversible advanced glycation endproducts (AGEs) via the Maillard reaction (59). Advanced glycation endproducts (AGEs) and the deposition of AGEs-cross-linked proteins occur in many degenerative diseases in an early state (60–62).
Lipid peroxidation occurs when hydrogen atoms are abstracted from polyunsaturated fatty acids. The brain seems to have an excess of polyunsaturated fatty acids that are susceptible to lipid peroxidation, therefore the brain is prone to oxidative stress. Due to this and the fact that neurons, which are damaged cannot be renewed, as they are post-mitotic cells, it was proposed that oxidative damage, including protein damage plays a dominant role in the pathogenesis of several neurodegenerative diseases (63). Investigations of neurodegenerative tissues revealed oxidative stress markers (e.g. protein oxidation and nitration, lipid peroxidation and nucleic acid oxidation) in higher concentrations compared to healthy tissues (23,64,65).
Beside the modification in macromolecules, oxidative stress is also involved in several cellular functions, such as apoptosis activation, excitotoxicity (glutameric over-stimulation), ion transport and calcium mobilization (66). Excitotoxicity and apoptosis are the two main causes of cell death in brain (67,68).
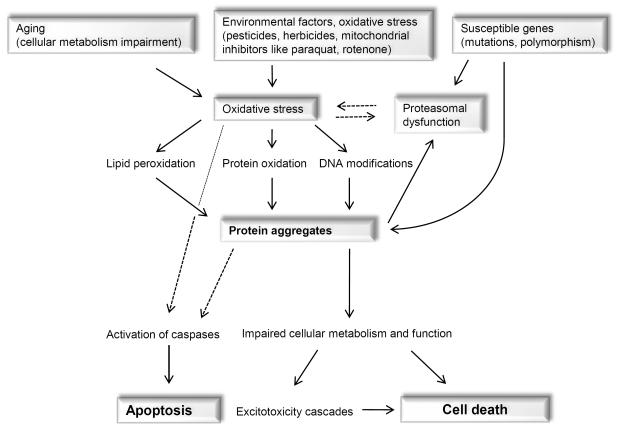
Oxidized or nitrated proteins are usually removed by the proteasome. However, inhibition of the proteasome or decreasing proteasomal activity leads to an accumulation of abnormal proteins (69). In a vicious cycle these abnormal proteins could overload the proteasome and block it, or they may stimulate reactive species formation, e.g. via the activation of microglia cells. It seems likely that all these events could contribute to the dramatic vicious cycle: increased oxidative stress leads to modified proteins; these may inhibit proteasomal activity which contributes to an accumulation of damaged proteins and these may increase oxidative stress. A hypothetical pathway leading to oxidative stress and associated neurodegeneration is shown in Fig. 1.
Fig. 1.
A hypothetical pathway leading to neuronal degeneration
Damaged protein accumulation appears due to aging, mutations in genes and environmental factors. These accumulated materials have an influence on proteasomal function and induce oxidative stress in cells, which influences mitochondrial function leading to a cascade of intracellular events and finally to cell death. The dotted lines represent hypothetical conclusions.
In summary, reactive oxygen or reactive nitrogen species may initiate neurodegeneration due to damage to mitochondria, inhibition of the proteasome, rise in Ca2+ and activation of a series of enzymes, initiation of apoptosis and protein accumulation. Especially common pathways in the pathogeneses of neurodegenerative diseases are mitochondrial dysfunction and oxidative stress. In fact, genetic and non-genetic studies revealed the central role of these pathways, accompanied by impairment in the protein degradation pathway, the ubiquitin-proteasomal system.
Specific oxidative and nitrative post-translationally modified proteins in the brain
In order to emphasize the role of oxidative and nitrative protein damage we have summarized those which are most commonly monitored in human brains (Table 1). Proteins modified by oxidative and/or nitrative reactive species tend to form aggregates that frequently contain nitrated proteins, bear carbonyl groups, have attached aldehydes such as HNE or carry AGE-products (70,71). Oxidized and nitrated proteins are usually removed by the proteasome.
Table 1.
Protein aggregates in selected neurodegenerative diseases
| Characteristic protein aggregates; Inclusions | Genes | References | |
|---|---|---|---|
|
| |||
| Alzheimer disease | Amyloid plaques (senile plaques, extracellular) | Presenilin 1 and 2, Apo E, APP | (90,93,94,98) |
| Diffuse amyloid deposits, neurofibrillary tangels (intracellular) | |||
| Parkinson disease | Lewy bodies consisting of α-synuclein, ubiquitin, parkin, synphilin-1 | α- synuclein, parkin, UCHL1 | (93,139,147,151,153) |
| Amyotrophic lateral sclerosis | Inclusion bodies consisting of superoxide dismutase, ubiquitin, neurofilaments | Superoxide dismutase | (170,171) |
| Huntington disease | Inclusion bodies consisting of huntingtin, ubiquitin, proteasome, heat-shock proteins | Huntingtin | (93,175) |
| Friedreich's ataxia | Frataxin aggregates | Frataxin | (93,178) |
| Prion disease | Prion proteins | Prion | (180) |
| Ceroid lipofuscinoses | Lysosomal, fluorescent material | Lysosomal proteases | (182) |
The degradation of many intracellular proteins, like cyclins, transcription factors and other short lived regulatory proteins and some damaged proteins require an initial ubiquitination as a recognition signal for the 26S proteasome (69,72,73). Ubiquitin, a 76-amino acid protein, is generally attached to substrate proteins through the formation of a covalent peptide bound by sequential enzymatic reactions, composed of ubiquitin-activating enzyme (E1), ubiquitinconjugating enzyme (E2) and ubiquitin ligase (E3) (69).
It was shown that ubiquitin is not required for the degradation of oxidatively modified proteins (74,75). This ubiquitin-independent degradation is carried out by the 20S proteasome, as observations described that the 26S proteasome is decreased during oxidative stress whereas the 20S proteasome is unaffected (74,75). Evidence exists that proteins can be degraded by the 20S proteasome without the requirement of ubiquitin conjugation (reviewed in Jariel-Encontre (76)).
Recently it was again suggested that as well ubiquitin as the 26S proteasome are involved in the degradation of misfolded or damaged proteins due to oxidative stress (77). Whether this is a contradiction or simply a special pathway of the degradation of oxidized protein should be clarified in the future.
Regardless this debate, it is a fact that inhibition of the proteasome leads to the accumulation of oxidized, nitrated and ubiquitinated proteins that causes cellular damage. Additionally, if mitochondria are damaged, this will influences ATP production which in turn interferes with the degradation of proteins by the ubiquitin-proteasome system as the ubiquitin-degradation process is ATP dependent.
Protein aggregates, listed in Table 1, are assumed to accumulate due to limited degradation, which might be due to a failure of the proteasomal system, lack of ubiquitinylation, extensive oxidation/modification or a mutation of the substrate protein. In any case it should be considered that non-degraded accumulated protein aggregates undergo further oxidation. It is still debated whether these protein aggregates are toxic to neurons. In general, it is assumed that the early stage of aggregate formation is more toxic than the final insoluble complexes. This can be based on the fact that toxic oligomers are sequestered into insoluble molecules (78) on the other hand large aggregates may interfere with cellular metabolism, including proteasome inhibition and oxidant formation (79,80).
Posttranslational modifications of proteins in neurodegenerative diseases
All diseases listed in Table 1 have in common that, in specific regions of the brain, proteins or their fragments undergo changes in conformation and/or function and form aggregates.
Frequently, there are simultaneous appearances of different pathological lesions, suggesting that there are similar mechanisms of cellular injury that may contribute to neurodegenerative diseases. To recapitulate the main facts from the former sections it is important to remember that the various neurodegenerative diseases have in common an impaired mitochondrial function, increased oxidative damage, defects in ubiquitin-proteasome system, accumulation of abnormal proteins, changes in metal metabolism, excitotoxicity and inflammation (25,81–88).
Alzheimer disease
Alzheimer disease is the most common neurodegenerative disease in elderly people. It is associated with progressive memory deficits, cognitive impairment and personality changes including language communication, emotional reactions and other social functions. Pathological features in Alzheimer disease are loss of neurons and synapses in the neocortex, hippocampus and other subcortical regions of the brain (89). The main histological features are extracellular protein deposits called senile β-amyloid plaques and intraneuronal neurofibrillary tangles (89). Neurofibrillary tangles, one hallmark of Alzheimer disease, consist of pairs of filaments, twisted around each other (`paired helical filaments') and are built up from abnormal hyperphosphorylated microtubule associated protein (MAP) tau (90). While normal tau stabilizes microtubules and promotes their constitution, the abnormal phosphorylated tau does not stabilize microtubules. Hyperphosphorylation of tau decreases its turnover and abets the self-assembly into tangles of paired helical or straight filaments, neurophil threads and dystrophic plaque neuritis (20,91). Finally, these modifications impair the axoplasmic flow and lead to a slow progressive degeneration of the affected neurons. Phosphorylation of tau is regulated by phosphatases, however, the activity of the major enzyme, protein phoshatase-2A (PP2A) is down-regulated in Alzheimer diseased brains whereas its inhibitors, I1PP2A and I2PP2A, are overexpressed (90). Abnormal hyperphosphorylation does not only occur in Alzheimer diseased brains, it is also found in other related neurodegeneration diseases, called tauopathies (90). Mutation studies showed that there are some missense mutations in the tau protein (R406W, G272V, P301L and V337M), which make tau a preferable substrate for hyperphosphorylation (91). Hyperphosphorylated tau makes tau resistant to calcium activated proteases, calpains and the ubiquitin-proteasome pathway (92), however, accumulated hyperphosphorylated tau becomes polyubiquitinated with time (19).
Senile plaques localized extracellular, consist of degenerating and frequently swollen axons, neurites and glia cells. Although the main protein is the β-amyloid peptide, senile plaques contain many different proteins, like hyperphosphorylated tau, ubiquitin, acetyl cholinesterase, α1-antichymotrypsin, proteoglycans, caspases, presenilin 1/2 and apolipoprotein E (93). Apolipoprotein E plays a key role in brain lipid metabolism and it is essential for brain development and maintenance. The primary cells involved in the synthesis of apolipoprotein E are astrocytes, and after traumatic injury and in several neurodegenerative diseases, the synthesis is increased (94). The gene encoding apoE is located on chromosome 19 and possesses three alleles: ApoE2, ApoE3 and ApoE4. They differ from each other by a single amino-acid substitution at position 112 and 158. Cysteine residues on both positions in apoE2 are associated with impaired binding to apoE receptors. ApoE4 has a cysteine at 158 and arginine at 112, whereas due to arginine at 112 apoE4 cannot form disulphide bonds with itself or with other proteins. This leads to the fact, that apoE4 protein facilitates plaque formation and this allele is a risk factor for the development of Alzheimer disease (94).
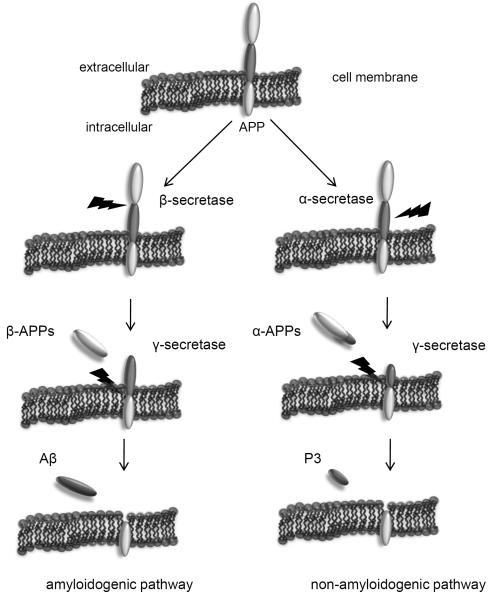
Senile plaques include a core of aggregated β-amyloid peptides (Aβ), and they are most common in the amygdala, hippocampus, and cerebral cortex. Deposits of Aβ that are not accompanied by neurites and glia are often called diffuse or preamyloid plaques (95). These protein aggregates, neurofibrillary tangles and plaques are detected in the brain of young adults with Down syndrome and to a lesser extend in the normally aging brain (95). However, in Alzheimer diseased brains, the concentration of soluble and insoluble, precipitated Aβ is significantly higher and the formation of plaques is considered to be an earlier event in the pathogenesis of Alzheimer disease then the formation of neurofibrillary tangles (95,96). However, correlation studies between cognitive impairment and histopathological changes have demonstrated that neurofibrillary tangles correlate better with the degree of dementia compared to plaques (97). Aβ are peptides, produced by the cleavage of larger proteins called amyloid precursor proteins (APPs). These APPs are located in the membrane in most cells, consisting of a short intracellular C terminal region and a larger N terminal region in the extracellular space (98). The exact function of APP is still unknown, but it is assumed that in neurons, it is necessary for survival and growth during brain development. APP exhibit a copper binding domain and it can be cleaved from the cells surface by several secretases (98,99). The metabolism of APP is shown in Fig. 2. Alpha-Secretase, a proteolytic enzyme, is involved in the normal turnover of APP. It releases the N-terminal region of APP (called α-APPs) in the extracellular space, whereas the fragment in the membrane left as a residue and can be cleared by γ-secretase to yield the soluble small fragment P3. In contrast to α-secretase, the release of Aβ involves β- and γ-secretases (87). The brain is rich in non-esterified cholesterol and this is involved in the cleavage of APP. In fact, statins which inhibit cholesterol synthesis, seems to decrease the risk of Alzheimer disease by decreasing β- and γ-secretase action and therefore by lowering Aβ formation (87).
Fig. 2.
Amyloid precursor protein (APP) processing
The “amyloidogenic pathway”: APP is cleaved sequentially by β-secretase and γ-secretase to produce Aβ. In the first step, the transmembrane protein APP is cleaved by β-secretase to yield β-APPs (“s” for soluble). The remaining protein is then cleaved by γ-secretase, releasing the amyloid beta fragment (Aβ) with 40 or 42 amino acids whereas Aβ42 shows stronger aggregation capacity (69).
The “non-amyloidogenic pathway”: Alternatively, APP is cleaved by α-secretase to yield the α-APPs (“s” for soluble)-fragment. The remaining protein is cleaved by γ-secretase to produce the P3 fragment (69).
A closer look at the Aβ peptide shows, that it can be 39 to 43 amino acids long, depending on where APP is cleaved. The most common structure of Aβ is Aβ1-40 or Aβ1-42. Aβ is cytotoxic and there are further modifications that may increase its neurotoxicity (100) but the exact mechanism has still not been clearly defined. Additionally to direct neurotoxicity, plaques can also activate an inflammatory response of microglia (101). Aβ was described to inhibit mitochondrial function and generate reactive oxygen species (81). The methionine residue at position 35 can react with bound copper to generate a methionine radical. Copper can further react with tyrosine residues to yield tyrosine radicals that cross-link Aβ via bi-tyrosine formation. This pathological capacity of copper and Aβ is regulated by APP. APP may bind copper and influences neuronal copper metabolism, as APP knockout-mice show increased copper concentration in brain (86). Thus metal chelating agents such as the drug clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) may have beneficial therapeutic effects; however, in larger doses it is found to be neurotoxic (102,103). Aβ binds further Zn2+ and Fe2+ and this metal binding capacity leads to peptide aggregation that can be avoided by treatment with chelators such as EDTA (104). Aβ metal complex may drive the Fenton-reaction and can contribute to the generation of reactive oxygen species in Alzheimer diseased brains (105,106). Another study showed that neurofibrillary tangles and senile plaques in Alzheimer disease are major sites for redox reactions indicating the essential role of metals for the generation of reactive oxygen species in these aggregates (107).
In addition to the direct generation of reactive oxygen species, Aβ can further stimulate intracellular pathways involved in the reactive oxygen species production as side-reactions. Aβ can activate NADPH oxidase in astrocytes and it is associated with the mitochondrial enzyme Aβ-binding alcohol dehydrogenase that catalyses oxidation (108). Coexistence of Aβ and Aβ-binding alcohol dehydrogenase causes mitochondrial reactive oxygen species production, lipid peroxidation and cytotoxicity (108). Additionally, Aβ can activate microglia cells resulting in the induction of inflammatory response. Plaques can increase cytokine production and activated microglia secrete proteases. Furthermore, markers for inflammation, like iNOS, COX-2 and 12/15-lipoxygenase are increased in Alzheimer disease (109,110). The role of mitochondrial degeneration should not be neglected, as this is one of the earliest signs in Alzheimer's diseases, arising before neurofibrillary tangles are evident (111).
Oxidative stress plays a major role in Alzheimer disease, believed to be stronger than in other neurodegenerative diseases (112). Oxidative damage in Alzheimer disease is proved as increased levels of DNA oxidation products like 8-hydroxydeoxyguanosine (8OHdG) in mitochondria and nucleus, protein carbonyls, methionine sulfoxide, nitrotyrosine and 4-HNE in brain tissues have been reported (84,113). Elevated levels of oxidized, nitrated and glycated proteins are found in plaques, helical filaments and in the Liquor cerebrospinalis from Alzheimer disease patients (114–116). This oxidative damage may result from mitochondrial dysfunction (117,118), from Aβ (119,120) and from glial activation (121,122).
AGEs are found to accumulate in Aβ (59) and NFTs and it could be shown that AGEs induce the release of various potentially neurotoxic inflammatory mediators such as NO, Il-1 and TNF-α (123). Oxidative stress was shown to reduce the repair activity of methionine sulfoxide reductase, an enzyme activity essential for repair of oxidized methionine residues (124). Interestingly the activity of the proteasome is also impaired, as hyperphosphorylated tau that is heavily ubiquitinated, forms cross-linked aggregates and inhibit the proteasome (125,126). A summary of protein modifications found in Alzheimer disease is shown in Tab. 2.
Table 2.
Diverse molecular modifications observed in Alzheimer diseased brains
| Modifications in Alzheimer disease | Observation | References |
|---|---|---|
|
| ||
| Oxidative protein modifications | Increases in protein carbonyls and other protein oxidation products (glutamic and aminoadipic semialdehydes), increased levels of methionine sulfoxide | (84,112,115) |
| oxidized SOD, creatine kinase and glutamine synthetase | ||
| UCHL1 is heavily oxidized | ||
| Nitrative protein modifications | Increased levels of nitrotyrosine neurofibrillary tangles | (115) |
| other nitrated proteins are β-actin, α-enolase and triosephosphate isomerase | ||
| Lipid peroxidation | Increased levels of HNE | (84,112,113) |
| Oxidative DNA damage | Increases in 8OHdG and other base oxidation products in mitochondria and nucleus | (84,112) |
| Mitochondrial dysfunction | Decreased activities of complex IV, alpha-ketoglutarate dehydrogenase, pyruvate dehydrogenase | (114) |
| Proteasome dysfunction | Decreased proteasomal activity, abnormal accumulation of proteins containing polyubiquitin | (116,163), |
It is necessary to mention that Aβ deposits are also found in several other diseases, like macular degeneration and myositis (127,128). Additionally, `amyloid' is also deposited in tuberculosis, rheumatoid arthritis and multiple myeloma (129–131). Therefore, the term `amyloid' is rather unspecific. Amyloid means a starch-like structure, but this is rather mistakable as it describes here a deposit protein-containing material and not carbohydrates. The term `β' refers to the β-sheet conformation that the Aβ is able to form.
Parkinson disease
Parkinson disease is associated with progressive loss of dopaminergic neurons in the substantia nigra pars compacta, and with deterioration of neurons in the locus coeruleus (132,133). It is the most common movement disorder in elderly people and the second most common neurodegenerative disease after Alzheimer disease. In general, Parkinson disease is characterized by tremor, bradykinesia, rigidity and finally postural instability (134) as well as decreased activity of mitochondrial complex I and increased lipid, protein and DNA oxidation are found in Parkinson disease (82,135,136).
Parkinson disease is associated with the appearance of round, intracytoplasmic proteinaceous inclusions termed Lewy bodies and dystrophic neuritis (Lewy neuritis). Lewy bodies have a characteristic electron-dense core from which filaments radiate and they are usually located in the cytoplasm. These aggregates are not specific in Parkinson disease, they have also been observed in other dementia disorders like dementia with Lewy bodies, in Alzheimer disease (about 15%) and in healthy people above the age of 60 (approximately 10%) (137,138). Lewy bodies consist of amino acids, but a precise biochemical composition has yet not been identified. Several cellular components have been found in Lewy bodies including structural elements, neurofilaments, synphilin-1, and constituents from the ubiquitin-proteasome system, phosphorylated proteins, α-synuclein, parkin and others (139).
Synphilin-1 is a α-synuclein binding protein whose function is unknown, however it is localized close to synaptic vesicles suggesting a role in synaptic vesicle transmission (140,141). Parkin, another protein found in Lewy bodies, is an ubiquitin ligase (E3) enzyme. It may be involved in the normal turnover of α-synuclein (142). The major cause of juvenile Parkinson disease results from mutations in this parkin gene (143) and this autosomal recessive hereditary disorder disrupts this specific E3 activity (110,144). The major constituent of Lewy bodies is α-synuclein, a 140-amino-acid protein of unknown function. It is highly expressed in the mammalian brain and associates with membrane and vesicular structures in presynaptic nerve termi (145). Limited studies with α-synuclein knockout mice display increased dopamine release after stimulation (146). Moreover, α-synuclein may inhibit dopamine synthesis, therefore α-synuclein may be a negative regulator of dopamine neurotransmission (147). Interestingly, in cultured cells, the toxic effects of α-synuclein appear to be selective for dopamine neurons as the accumulation of α-synuclein selectively degenerates dopaminergic neurons, but not non-dopaminergic neurons. Under this aspect, a selective role of α-synuclein in the metabolism of dopamine is suggested (148).
Mutations in α-synuclein have been demonstrated to lead to a greater aggregation-tendency compared to wild-type synuclein and to the formation of fibrils (149). Three rare mutations in the α-synuclein gene (A53T, A30P, E46K) are found to cause autosomal dominant familial Parkinson disease (150,151).
Early onset of Parkinson disease cases is caused by mutations in parkin. These mutations are therefore common in familial Parkinson disease. Familial Parkinson disease is furthermore induced by a genomic triplication of α-synuclein. This is found to double the expression of the protein and increase the propensity to self-aggregate (149,150). Overexpression of the mutant synuclein in the nigrostriatal areas of monkeys is associated with some cases of familial Parkinson disease (152). An overexpression of the wild-type gene had the same effect; however, chromosome abnormality of the gene encoding α-synuclein is a rare cause of familial Parkinson disease (149). Consequently, too much normal, as well as mutant α-synuclein has a negative effect. It is reported that cells transfected with mutant α-synuclein are more sensitive to apoptosis triggered by toxins, like hydrogen peroxide and iron. These cells show increased levels of oxidative damage and protein aggregates (153). α-Synuclein overexpression, mutant or normal form, has been linked to mitochondrial deficits (153) and apoptosis (154). However, data on the effect of overexpressed normal α-synuclein are not consistent. It protects in some studies the cells against toxins, in others not (153–155). A dependence on the cell lines used in different studies can be assumed, also there is a difference between the levels of overexpression, as a very high level of any protein may disturb neuronal metabolism.
The α-synuclein protein is natively unfolded (interestingly, as is the tau protein). In the aggregation process it forms oligomers that comprise heterogeneous structures and that are the precursors of higher-ordered aggregates. Some investigators propose that these oligomeric structures, also termed protofibrils, are rather pathogenic compared to the later formed fibrils (156). It was hypothesized that these annular protofibrils resemble pore-forming bacterial toxins and permeabilize cellular membranes (157). The fibrils are stable, amyloid-like structures and eventually form Lewy bodies in vivo. Aβ (described under “Alzheimer disease”) can also enhance the fibrillization of α-synuclein and the interaction of tau and α-synuclein promotes the fibrillization of both proteins which is supported by the co-occurrence of them in diverse neurodegenerative diseases (85,158).
Significant evidence exists to show enhanced oxidative stress in Parkinson disease (23,65,116,135,159), including markers of oxidative damage to biological structures (Table 3). For instance, proteins in Lewy bodies are generally oxidized, nitrated and contain products from lipid peroxidation that all promote aggregation (23). Especially selective tyrosine nitration of α-synuclein may play a role in fibril formation of unfolded native synuclein and decrease the rate of degradation by the proteasome (160). Brain tissue from patients died from Parkinson disease revealed increase oxidative damage and defective mitochondrial function like an impaired complex I activity. Investigations demonstrated that inhibitors of mitochondrial complex-1 lead to aggregation of α-synuclein in vitro as well as in animal models (161,162).
Table 3.
Diverse molecular modifications observed in Parkinson diseased brains
| Modifications in Parkinson disease | Observation | References |
|---|---|---|
|
| ||
| Oxidative protein modifications | Increased levels of protein carbonyls in substantia nigra, oxidized SOD1 | (82) |
| Increased levels of oxidized dopamine (cysteinyl-DOPA and cysteinyl-dopamine) | ||
| Nitrative protein modifications | Increased levels of nitrotyrosine in α-synuclein and Lewy bodies | (135,159) |
| Lipid peroxidation | HNE-products in Lewy bodies and increased peroxides | (82) |
| Oxidative DNA damage | Increased levels of DNA oxidation product 8OHdG in mitochondrial and total DNA | (82,135) |
| Glutathione | Decreased levels of GSH | (159) |
| Mitochondrial dysfunction | Decreased activities of complex I and alpha-ketoglutarate dehydrogenase | (159) |
| Proteasome dysfunction | Genetic defects in inherited Parkinson disease, decreased activities in sporadic Parkinson disease, decrease UCHL1 activity | (159) |
| oxidized Dopamine | Auto-oxidation of free non-vesicle dopamine to quinone | (159) |
Alpha-Synuclein has been also shown to bind copper and iron, therefore, a redox catalyzed reaction as described under Alzheimer disease and the binding of metals by Aβ can take place (86) with the result of α-synuclein aggregation. Interestingly, a decrease in ubiquitin-carboxy-terminal hydrolase L1 (UCHL1) activity is found in sporadic Parkinson disease (163). Mice with a knockout in the UCHL1 gene show widespread neurodegeneration, increase oxidative damage and protein aggregates (164). Due to all these facts, apoptosis and caspases chain activation are also described to be involved in cellular death in Parkinson disease (165).
To summarize, it seems that oxidative and nitrative stress promotes α-synuclein aggregation and accumulation (23).
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is the most common chronic progressive degeneration of motor neurons in the spinal cord, brainstem and motor cortex. The etiology of most ALS cases remains unknown, however approximately 10 % are inherited and thus have an early age of onset. Pathological features in ALS are progressive muscle weakness, atrophy and spasticity, followed by problems with speech and swallowing. The exact mechanisms underlying the development of ALS are unknown, however mutations in the Cu/Zn-superoxide dismutase 1 (CuZnSOD) contribute to the onset of this disease (166). SOD1 is an important antioxidant enzyme which converts the superoxide anion (O2−.) to hydrogen peroxide (H2O2). Amino acid residues in the mutant SOD1 are less affected in the active site, instead, amino-acid residues which are necessary for protein stabilization, access to the active site and dimer interaction are often altered in the disease (167). Most of the mutants exhibit decreased enzyme activity, but the activity is rarely below 30 %, perhaps a more drastic loss of SOD1 activity is a lethal mutation. More important is that mutant CuZnSOD molecules are generally less stable and form aggregated fibrils that can be toxic (but this has until yet not been proven). This less stable and misfolded protein releases Zn2+ and probably Cu2+. Because Zn2+ is held less tightly than Cu2+ the mutations favor the generation of zinc-deficient CuZnSODs (168). It is described that the copper in zinc-deficient CuZnSODs is more accessible, transferring electrons to oxygen to produce superoxide, therefore zinc-deficient CuZnSODs rapidly oxidizes many intracellular components. On the other hand, knock-out of the gene for the copper chaperone of CuZnSOD shows no effect in the disease development in transgenic mice models, overexpressing mutant CuZnSODs (169). Although the zinc-deficient CuZnSODs hypothesis is controversial, it shows how oxidative and nitrative stress can contribute to neurodegenerative diseases. In this disease, motor neurons are strongly affected, as they possess a lot of CuZnSOD (166). CuZnSOD is not only present in the cytosol but also in the mitochondrial intermembrane space. Mutant CuZnSOD can therefore influence mitochondrial function and indeed, this is observed in ALS (170). Oxidative biomarkers, like 8OHdG, protein carbonyls, glycated proteins, HNE and 3-nitrotyrosin are elevated in the spinal cord of ALS patients (171).
Motor neuron dysfunction is accompanied by neurofilament abnormalities and ubiquitin-positive inclusion bodies. The term `amyotrophic' indicates the muscle wasting, associated with the disease. There are several nomenclatures for this disease: Lou Gehrig's disease (named after the New York Yankees baseball player who got ALS in 1938), Charcot's disease (after the French neurologist who first described this disease in 1874) and motor neuron disease, but ALS refers only to one form of motor neuron disease, as others are also known. In all motor neuron diseases, patients usually die within a few years, due to pneumonia or respiratory failure and muscle paralysis (170).
Huntington disease
Huntington disease is an autosomal dominant neurodegenerative disorder that emerges as a consequence from a polyglutamine repeat expansion in the huntingtin gene. It is also known as Huntington's chorea. Chorea relates to the Latin choreus or Greek choros and means dancing. Accordingly, it describes the abnormal movement, observed in this disease. The pathological features characterizing this disease are cognitive impairment, neuropsychiatric symptoms and premature death (64). Usually these symptoms are accompanied by uncontrollable twitching, spasms and other motor skill impairments. Often this disease is related to degeneration of striatal neurons and astrocytosis (64). The exact mechanism of the pathogeneses remains unknown, but it has been suggested to be caused by the effect of mutant huntingtin on mitochondrial function (172,173).
The gene for huntingtin is located on chromosome 4 and is expressed in many tissues, especially in neurons and lungs. This gene is characterized for its repeated trinucleotide CAG, which encodes glutamine at the N-terminus of the protein. In the normal gene, this trinucleotide is repeated 9–39 times (on average: 19 times), however, in mutant genes, the trinucleotide repeat frequency is higher (36–121 times, on average: 43 times) (174). Therefore, in mutant genes, the polyglutamine sequence is longer. The abnormal huntingtin with very high repeat lengths gains a toxic function as it makes the protein more susceptible for aggregation and formation of inclusion bodies. These inclusion bodies are the typical histological markers of Huntington disease and it could be shown that they interact with proteins of the ubiquitin-proteasomal system like 20S core proteasome, the 19S proteasome regulator and ubiquitin (175). As demonstrated in transgenic mice, this abnormal protein is cleaved in fragments and these fragments are conjugated with ubiquitin. However, they are not efficiently degraded by the proteasome, therefore, leading to accumulation of aggregates forming neuronal inclusion bodies (175,176).
Increased oxidative stress markers are found in a transgenic mouse model of Huntington disease (R6/2), for example 8OHdG, lipid peroxidation, nitrotyrosine and mitochondrial dysfunction (83,173,177). Defects in energy metabolism due to mitochondrial dysfunction are described in Huntington diseased patients, possibly because mutant huntingtin has an influence on mitochondria (172,173).
Friedreich's ataxia
Friedreich's ataxia is the most common hereditary (autosomal recessive) ataxia, which means an impaired movement due to loss of motor co-ordination. It affects usually neurons with long neuritis receiving a death signal from the periphery. The pathological event is a mutation in a gene on chromosome 9, encoding the 210-amino acid protein frataxin which is involved in mitochondrial iron metabolism. It is known that an expansion of the trinucleotide (GAA) in the first intron leads to a decreased transcription of the gene. Therefore, the formation of Fe/S clusters in complexes I, II and III and in aconitase is not sufficient (178). It is assumed that less incorporation of Fe in Fe/S clusters leads to a raise of `free' iron in mitochondria, thus free radical reactions may be initiated. Frataxin expression and accumulation is elevated in several tissues, like cerebellum, spinal cord and heart. It is known, that premature death of Friedreich's ataxia patients are often associated with cardiac problems (178). The tissue specific increase in expression may explain the pathology.
Prion diseases
These diseases are all thought to be caused by prions. Prions are proteinaceous infectious particles, glycoproteins, with approximately 230 amino acids. They are found in all mammals in several tissues, especially in high concentrations in the brain. This `normal' prion protein is a glycoprotein located outside the cell by a glycosylphosphatidylinositol anchor. The N-terminal domain is associated with the binding of copper (179).
The `normal' prion protein is thought to be innocuous; however, it becomes `abnormal' when changing its conformation. This `abnormal' protein is a poor substrate for degradation and therefore accumulates in tissues. Accumulated proteins are known to influence cellular metabolism, induce neuronal dysfunction and cell death (180).
Prion diseases include Creutzfeldt-Jakob disease (CJD, a human neurodegenerative disease), bovine spongiform encephalopathy (BSE) and scrapie, a neurodegenerative disease of goats, sheep and others. It is assumed that `abnormal' prion proteins can trigger the disease when it gets access to neuronal tissue. It may alter the conformation of `normal' prion protein to generate more `abnormal' prion proteins. Studies with prion protein knockout mice show that they cannot develop the disease when infected with `abnormal' prion proteins; however, they show various abnormalities indicating an important role for `normal' prion protein (180). One possibility is that `normal' prion protein binds copper and therefore prevents oxidative damage in cells (181). In fact, prion protein knockout mice showed increase oxidative stress markers, like protein carbonyls and MDA (181).
Neuronal ceroid lipofuscinoses
Neuronal ceroid lipofuscinoses are inherited, autosomal recessive disorders emerging throughout lifetime. Some patients develop symptoms during infancy, early childhood and late childhood or after adolescence. Battan's disease is developed during childhood and Kuf's disease is called for the adult form of Neuronal ceroid lipofuscinoses. The pathological features include impaired vision and speech, muscular and mental degeneration. Neuronal ceroid lipofuscinoses can occur by inherited defects in lysosomal proteases, e.g. by an impaired palmitoyl protein thioesterase, that removes fatty acid from lipoproteins or by a defect in tripeptidyl peptidase (182–184).
Neuronal ceroid lipofuscinoses is accompanied by an accumulation of fluorescent pigments called ceroids in the cortex and cerebellum. These pigments are usually located in lysosomes, as lysosomes normally engulf cytoplasmic components in a process called autophagy and degrade them. In neuronal ceroid lipofuscinoses, however, engulfment continues but degradation of the material is impaired and, therefore, the material accumulates in lysosomes (184).
Conclusion
Despite numerous studies that have investigated the role of diverse aggregates, the formation of aggregates and their effects are still under debate. However, it does seem to clear that aggregates which are not degraded will extensively accumulate within cells. Indeed, such accumulation may be so extensive that they detract from the functional volume of the cell by gradually filling the whole cytosol. Post-mitotic cells, like neurons, cannot divide and therefore cannot dilute protein aggregates by passing aggregated material on to their daughter cells. Therefore, cell death is a likely outcome of the accumulation of oxidatively damaged and cross-linked protein aggregates in post-mitotic cells.
In most diseases it is still unsure, which pathogenic effect occurs first. Impaired proteasomal and/or mitochondrial function are regarded to have major roles in the pathogenesis of neurodegenerative diseases. Impaired proteasomal function leads to accumulation of aggregated materials. Aggregated proteins can bind directly to proteasomal subunits and impair the overall proteolytic activity of the proteasome complex. Moreover, aggregated proteins may overwhelm the degradation capacity of the proteasome, leading to further impairment. Currently, there seems to be sufficient data to put oxidative and nitrative stress in the center of mechanisms leading to neuronal loss and neurodegeneration. Additionally, a number of pathways and substances have been identified as potential contributors, including NAPDH oxidase, mitochondrial electron-transport chain, dopamine, iron and other metals, diverse protein aggregates, etc.
To summarize, a great deal of data exist to support the concept that the accumulation of protein aggregates contributes to neurodegenerative diseases. Accumulation apparently occurs in the aged brain due to both impaired clearance and increased formation. Protein aggregate formations seem to result from mitochondrial dysfunction, oxidative stress, defects in lysosomal system and impairment of the ubiquitin-proteasomal system. Protein studies on aggregates associated with neurodegenerative diseases have shown many similarities between aggregates in different diseases. They all seem to be oxidized and cross-linked, and to have the capacity to bind metals and to influence the redox state in brain tissues. Currently, no prevention or treatments for neurodegenerative diseases are known; we only have a limited arsenal of drugs to ease some disease symptoms and (sometimes) pain. However, animal models for ALS (165,185), Alzheimer disease (186), Parkinson disease (187) and aging brain (188) now allow the testing of potential therapeutic compounds and targets to prevent the occurrence of these diseases. Among them, therapeutic interventions targeted against oxidative stress may be a promising step in diminishing the burden of neurodegeneration.
Nonstandard abbreviations used
- Aβ
β-amyloid
- AGE
advanced glycation endproducts
- ALS
amyotrophic lateral sclerosis
- APP
amyloid precursor protein
- ATP
adenosine trisphosphate
- 8OdG
8-hydoxydeoxyguanosine
- 4-HNE
4-hydroxy-nonenal
- MDA
malondialdehyde
- SOD
superoxide dismutase
- UCHL1
ubiquitin carboxyl-terminal hydrolase L1
Reference List
- [1].Breimer LH. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3:188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- [2].Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic Biol Med. 1997;23:783–792. doi: 10.1016/s0891-5849(97)00016-6. [DOI] [PubMed] [Google Scholar]
- [3].McLennan HR, Degli EM. The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J Bioenerg Biomembr. 2000;32:153–162. doi: 10.1023/a:1005507913372. [DOI] [PubMed] [Google Scholar]
- [4].Thelen M, Dewald B, Baggiolini M. Neutrophil signal transduction and activation of the respiratory burst. Physiol Rev. 1993;73:797–821. doi: 10.1152/physrev.1993.73.4.797. [DOI] [PubMed] [Google Scholar]
- [5].Ward JF. The complexity of DNA damage: relevance to biological consequences. Int J Radiat Biol. 1994;66:427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- [6].Breen AP, Murphy JA. Reactions of oxyl radicals with DNA. Free Radic Biol Med. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- [7].Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- [8].Mendoza-Nunez VM, Ruiz-Ramos M, Sanchez-Rodriguez MA, Retana-Ugalde R, Munoz-Sanchez JL. Aging-related oxidative stress in healthy humans. Tohoku J Exp Med. 2007;213:261–268. doi: 10.1620/tjem.213.261. [DOI] [PubMed] [Google Scholar]
- [9].Stadtman ER. Role of oxidant species in aging. Curr Med Chem. 2004;11:1105–1112. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- [10].Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- [11].Stadtman ER, Van RH, Richardson A, Wehr NB, Levine RL. Methionine oxidation and aging. Biochim Biophys Acta. 2005;1703:135–140. doi: 10.1016/j.bbapap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [12].van der Vliet A, Eiserich JP, O'Neill CA, Halliwell B, Cross CE. Tyrosine modification by reactive nitrogen species: a closer look. Arch Biochem Biophys. 1995;319:341–349. doi: 10.1006/abbi.1995.1303. [DOI] [PubMed] [Google Scholar]
- [13].Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001;36:1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- [14].Robinson NE, Robinson AB. Deamidation of human proteins. Proc Natl Acad Sci U S A. 2001;98:12409–12413. doi: 10.1073/pnas.221463198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McCudden CR, Kraus VB. Biochemistry of amino acid racemization and clinical application to musculoskeletal disease. Clin Biochem. 2006;39:1112–1130. doi: 10.1016/j.clinbiochem.2006.07.009. [DOI] [PubMed] [Google Scholar]
- [16].Uversky VN, Eliezer D. Biophysics of Parkinson's disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci. 2009;10:483–499. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- [18].Ito Y, Kajkenova O, Feuers RJ, Udupa KB, Desai VG, Epstein J, Hart RW, Lipschitz DA. Impaired glutathione peroxidase activity accounts for the age-related accumulation of hydrogen peroxide in activated human neutrophils. J Gerontol A Biol Sci Med Sci. 1998;53:M169–M175. doi: 10.1093/gerona/53a.3.m169. [DOI] [PubMed] [Google Scholar]
- [19].Bancher C, Grundke-Iqbal I, Iqbal K, Fried VA, Smith HT, Wisniewski HM. Abnormal phosphorylation of tau precedes ubiquitination in neurofibrillary pathology of Alzheimer disease. Brain Res. 1991;539:11–18. doi: 10.1016/0006-8993(91)90681-k. [DOI] [PubMed] [Google Scholar]
- [20].Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Smith MA, Drew KL, Nunomura A, Takeda A, Hirai K, Zhu X, Atwood CS, Raina AK, Rottkamp CA, Sayre LM, Friedland RP, Perry G. Amyloid-beta, tau alterations and mitochondrial dysfunction in Alzheimer disease: the chickens or the eggs? Neurochem Int. 2002;40:527–531. doi: 10.1016/s0197-0186(01)00123-1. [DOI] [PubMed] [Google Scholar]
- [22].Williams A. Defining neurodegenerative diseases. BMJ. 2002;324:1465–1466. doi: 10.1136/bmj.324.7352.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- [25].Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- [26].Hayflick L. Biological aging is no longer an unsolved problem. Ann N Y Acad Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- [27].Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [28].Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- [29].Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- [31].Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van RH, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- [32].Wiesner RJ, Zsurka G, Kunz WS. Mitochondrial DNA damage and the aging process: facts and imaginations. Free Radic Res. 2006;40:1284–1294. doi: 10.1080/10715760600913168. [DOI] [PubMed] [Google Scholar]
- [33].Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580:2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- [34].Friguet B, Bulteau AL, Chondrogianni N, Conconi M, Petropoulos I. Protein degradation by the proteasome and its implications in aging. Ann N Y Acad Sci. 2000;908:143–154. doi: 10.1111/j.1749-6632.2000.tb06643.x. [DOI] [PubMed] [Google Scholar]
- [35].Schoneich C. Protein modification in aging: an update. Exp Gerontol. 2006;41:807–812. doi: 10.1016/j.exger.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [36].Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- [37].Farout L, Friguet B. Proteasome function in aging and oxidative stress: implications in protein maintenance failure. Antioxid Redox Signal. 2006;8:205–216. doi: 10.1089/ars.2006.8.205. [DOI] [PubMed] [Google Scholar]
- [38].Keller JN, Huang FF, Markesbery WR. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience. 2000;98:149–156. doi: 10.1016/s0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- [39].Petropoulos I, Conconi M, Wang X, Hoenel B, Bregegere F, Milner Y, Friguet B. Increase of oxidatively modified protein is associated with a decrease of proteasome activity and content in aging epidermal cells. J Gerontol A Biol Sci Med Sci. 2000;55:B220–B227. doi: 10.1093/gerona/55.5.b220. [DOI] [PubMed] [Google Scholar]
- [40].Grune T, Jung T, Merker K, Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and 'aggresomes' during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- [41].Sitte N, Merker K, Von ZT, Grune T, Davies KJ. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part I--effects of proliferative senescence. FASEB J. 2000;14:2495–2502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- [42].Sitte N, Merker K, Von ZT, Davies KJ, Grune T. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part II--aging of nondividing cells. FASEB J. 2000;14:2503–2510. doi: 10.1096/fj.00-0210com. [DOI] [PubMed] [Google Scholar]
- [43].Sitte N, Merker K, Von ZT, Grune T. Protein oxidation and degradation during proliferative senescence of human MRC-5 fibroblasts. Free Radic Biol Med. 2000;28:701–708. doi: 10.1016/s0891-5849(99)00279-8. [DOI] [PubMed] [Google Scholar]
- [44].Grune T, Merker K, Jung T, Sitte N, Davies KJ. Protein oxidation and degradation during postmitotic senescence. Free Radic Biol Med. 2005;39:1208–1215. doi: 10.1016/j.freeradbiomed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [45].Chondrogianni N, Stratford FL, Trougakos IP, Friguet B, Rivett AJ, Gonos ES. Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J Biol Chem. 2003;278:28026–28037. doi: 10.1074/jbc.M301048200. [DOI] [PubMed] [Google Scholar]
- [46].Picot CR, Perichon M, Cintrat JC, Friguet B, Petropoulos I. The peptide methionine sulfoxide reductases, MsrA and MsrB (hCBS-1), are downregulated during replicative senescence of human WI-38 fibroblasts. FEBS Lett. 2004;558:74–78. doi: 10.1016/S0014-5793(03)01530-8. [DOI] [PubMed] [Google Scholar]
- [47].Verbeke P, Clark BF, Rattan SI. Reduced levels of oxidized and glycoxidized proteins in human fibroblasts exposed to repeated mild heat shock during serial passaging in vitro. Free Radic Biol Med. 2001;31:1593–1602. doi: 10.1016/s0891-5849(01)00752-3. [DOI] [PubMed] [Google Scholar]
- [48].Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- [49].Uchida K. Lipofuscin-like fluorophores originated from malondialdehyde. Free Radic Res. 2006;40:1335–1338. doi: 10.1080/10715760600902302. [DOI] [PubMed] [Google Scholar]
- [50].Kurz T, Terman A, Brunk UT. Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch Biochem Biophys. 2007;462:220–230. doi: 10.1016/j.abb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [51].Terman A. Catabolic insufficiency and aging. Ann N Y Acad Sci. 2006;1067:27–36. doi: 10.1196/annals.1354.005. [DOI] [PubMed] [Google Scholar]
- [52].Krohne TU, Stratmann NK, Kopitz J, Holz FG. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res. 2010;90:465–471. doi: 10.1016/j.exer.2009.12.011. [DOI] [PubMed] [Google Scholar]
- [53].Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nakanishi H, Wu Z. Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res. 2009;201:1–7. doi: 10.1016/j.bbr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- [55].Tsay HJ, Wang P, Wang SL, Ku HH. Age-associated changes of superoxide dismutase and catalase activities in the rat brain. J Biomed Sci. 2000;7:466–474. doi: 10.1007/BF02253362. [DOI] [PubMed] [Google Scholar]
- [56].Siqueira IR, Fochesatto C, de AA, Santos M, Hagen M, Bello-Klein A, Netto CA. Total antioxidant capacity is impaired in different structures from aged rat brain. Int J Dev Neurosci. 2005;23:663–671. doi: 10.1016/j.ijdevneu.2005.03.001. [DOI] [PubMed] [Google Scholar]
- [57].Davies KJ, Delsignore ME, Lin SW. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987;262:9902–9907. [PubMed] [Google Scholar]
- [58].Cakatay U, Telci A, Kayali R, Tekeli F, Akcay T, Sivas A. Relation of oxidative protein damage and nitrotyrosine levels in the aging rat brain. Exp Gerontol. 2001;36:221–229. doi: 10.1016/s0531-5565(00)00197-2. [DOI] [PubMed] [Google Scholar]
- [59].Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A. 1994;91:5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Munch G, Thome J, Foley P, Schinzel R, Riederer P. Advanced glycation endproducts in ageing and Alzheimer's disease. Brain Res Brain Res Rev. 1997;23:134–143. doi: 10.1016/s0165-0173(96)00016-1. [DOI] [PubMed] [Google Scholar]
- [61].Munch G, Luth HJ, Wong A, Arendt T, Hirsch E, Ravid R, Riederer P. Crosslinking of alpha-synuclein by advanced glycation endproducts--an early pathophysiological step in Lewy body formation? J Chem Neuroanat. 2000;20:253–257. doi: 10.1016/s0891-0618(00)00096-x. [DOI] [PubMed] [Google Scholar]
- [62].Sasaki N, Fukatsu R, Tsuzuki K, Hayashi Y, Yoshida T, Fujii N, Koike T, Wakayama I, Yanagihara R, Garruto R, Amano N, Makita Z. Advanced glycation end products in Alzheimer's disease and other neurodegenerative diseases. Am J Pathol. 1998;153:1149–1155. doi: 10.1016/S0002-9440(10)65659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- [64].Browne SE, Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- [65].Nunomura A, Moreira PI, Lee HG, Zhu X, Castellani RJ, Smith MA, Perry G. Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS Neurol Disord Drug Targets. 2007;6:411–423. doi: 10.2174/187152707783399201. [DOI] [PubMed] [Google Scholar]
- [66].Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci U S A. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Beal MF. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992;31:119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- [68].Beal MF. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 2000;23:298–304. doi: 10.1016/s0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- [69].Jung T, Catalgol B, Grune T. The proteasomal system. Mol Aspects Med. 2009;30:191–296. doi: 10.1016/j.mam.2009.04.001. [DOI] [PubMed] [Google Scholar]
- [70].Ohshima H, Pignatelli B, Li CQ, Baflast S, Gilibert I, Boffetta P. Analysis of oxidized and nitrated proteins in plasma and tissues as biomarkers for exposure to reactive oxygen and nitrogen species. IARC Sci Publ. 2002;156:393–394. [PubMed] [Google Scholar]
- [71].Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- [72].Bose S, Mason GG, Rivett AJ. Phosphorylation of proteasomes in mammalian cells. Mol Biol Rep. 1999;26:11–14. doi: 10.1023/a:1006969517958. [DOI] [PubMed] [Google Scholar]
- [73].Ciechanover A, Schwartz AL. The ubiquitin-mediated proteolytic pathway: mechanisms of recognition of the proteolytic substrate and involvement in the degradation of native cellular proteins. FASEB J. 1994;8:182–191. doi: 10.1096/fasebj.8.2.8119489. [DOI] [PubMed] [Google Scholar]
- [74].Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J. 1998;335(Pt 3):637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Reinheckel T, Ullrich O, Sitte N, Grune T. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys. 2000;377:65–68. doi: 10.1006/abbi.2000.1717. [DOI] [PubMed] [Google Scholar]
- [76].Jariel-Encontre I, Bossis G, Piechaczyk M. Ubiquitin-independent degradation of proteins by the proteasome. Biochim Biophys Acta. 2008;1786:153–177. doi: 10.1016/j.bbcan.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [77].Medicherla B, Goldberg AL. Heat shock and oxygen radicals stimulate ubiquitin-dependent degradation mainly of newly synthesized proteins. J Cell Biol. 2008;182:663–673. doi: 10.1083/jcb.200803022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Orr HT. Neurodegenerative disease: neuron protection agency. Nature. 2004;431:747–748. doi: 10.1038/431747a. [DOI] [PubMed] [Google Scholar]
- [79].Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, Von ZT, Davies KJ. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14:1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- [80].Hohn A, Jung T, Grimm S, Grune T. Lipofuscin-bound iron is a major intracellular source of oxidants: role in senescent cells. Free Radic Biol Med. 2010;48:1100–1108. doi: 10.1016/j.freeradbiomed.2010.01.030. [DOI] [PubMed] [Google Scholar]
- [81].Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- [83].Bogdanov MB, Andreassen OA, Dedeoglu A, Ferrante RJ, Beal MF. Increased oxidative damage to DNA in a transgenic mouse model of Huntington's disease. J Neurochem. 2001;79:1246–1249. doi: 10.1046/j.1471-4159.2001.00689.x. [DOI] [PubMed] [Google Scholar]
- [84].Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- [85].Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Brown DR. Brain proteins that mind metals: a neurodegenerative perspective. Dalton Trans. 2009:4069–4076. doi: 10.1039/b822135a. [DOI] [PubMed] [Google Scholar]
- [87].Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer's disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- [88].Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- [89].Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- [90].Iqbal K, Liu F, Gong CX, Alonso AC, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118:53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Alonso AC, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004;279:34873–34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- [92].Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- [93].Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- [94].Baum L, Chen L, Ng HK, Pang CP. Apolipoprotein E isoforms in Alzheimer's disease pathology and etiology. Microsc Res Tech. 2000;50:278–281. doi: 10.1002/1097-0029(20000815)50:4<278::AID-JEMT5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- [95].Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- [96].Gotz J, Chen F, van DJ, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- [97].Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- [98].Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- [99].Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Piccini A, Russo C, Gliozzi A, Relini A, Vitali A, Borghi R, Giliberto L, Armirotti A, D'Arrigo C, Bachi A, Cattaneo A, Canale C, Torrassa S, Saido TC, Markesbery W, Gambetti P, Tabaton M. beta-amyloid is different in normal aging and in Alzheimer disease. J Biol Chem. 2005;280:34186–34192. doi: 10.1074/jbc.M501694200. [DOI] [PubMed] [Google Scholar]
- [101].Mandrekar-Colucci S, Landreth GE. Microglia and inflammation in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2010;9:156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- [103].Venisti Zarom L, Chen J, Regan RF. The oxidative neurotoxictiy of clioquinol. Neuropharmacology. 2005;49:687–694. doi: 10.1016/j.neuropharm.2005.04.023. [DOI] [PubMed] [Google Scholar]
- [104].Curtain CC, Ali F, Volitakis I, Cherny RA, Norton RS, Beyreuther K, Barrow CJ, Masters CL, Bush AI, Barnham KJ. Alzheimer's disease amyloid-beta binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J Biol Chem. 2001;276:20466–20473. doi: 10.1074/jbc.M100175200. [DOI] [PubMed] [Google Scholar]
- [105].Opazo C, Huang X, Cherny RA, Moir RD, Roher AE, White AR, Cappai R, Masters CL, Tanzi RE, Inestrosa NC, Bush AI. Metalloenzyme-like activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2) J Biol Chem. 2002;277:40302–40308. doi: 10.1074/jbc.M206428200. [DOI] [PubMed] [Google Scholar]
- [106].Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- [107].Sayre LM, Perry G, Harris PL, Liu Y, Schubert KA, Smith MA. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer's disease: a central role for bound transition metals. J Neurochem. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- [108].Yan SD, Stern DM. Mitochondrial dysfunction and Alzheimer's disease: role of amyloid-beta peptide alcohol dehydrogenase (ABAD) Int J Exp Pathol. 2005;86:161–171. doi: 10.1111/j.0959-9673.2005.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Szaingurten-Solodkin I, Hadad N, Levy R. Regulatory role of cytosolic phospholipase A2alpha in NADPH oxidase activity and in inducible nitric oxide synthase induction by aggregated Abeta1-42 in microglia. Glia. 2009;57:1727–1740. doi: 10.1002/glia.20886. [DOI] [PubMed] [Google Scholar]
- [110].Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- [113].Montine KS, Reich E, Neely MD, Sidell KR, Olson SJ, Markesbery WR, Montine TJ. Distribution of reducible 4-hydroxynonenal adduct immunoreactivity in Alzheimer disease is associated with APOE genotype. J Neuropathol Exp Neurol. 1998;57:415–425. doi: 10.1097/00005072-199805000-00005. [DOI] [PubMed] [Google Scholar]
- [114].Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer's disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- [115].Ahmed N, Ahmed U, Thornalley PJ, Hager K, Fleischer G, Munch G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer's disease and link to cognitive impairment. J Neurochem. 2005;92:255–263. doi: 10.1111/j.1471-4159.2004.02864.x. [DOI] [PubMed] [Google Scholar]
- [116].Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L. Oxidative modifications and aggregation of Cu,Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J Biol Chem. 2005;280:11648–11655. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- [117].Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- [118].Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- [119].Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mattson MP. Advances fuel Alzheimer's conundrum. Nat Genet. 1997;17:254–256. doi: 10.1038/ng1197-254. [DOI] [PubMed] [Google Scholar]
- [121].Mark RJ, Blanc EM, Mattson MP. Amyloid beta-peptide and oxidative cellular injury in Alzheimer's disease. Mol Neurobiol. 1996;12:211–224. doi: 10.1007/BF02755589. [DOI] [PubMed] [Google Scholar]
- [122].Rosenberg RN. The molecular and genetic basis of AD: the end of the beginning: the 2000 Wartenberg lecture. Neurology. 2000;54:2045–2054. doi: 10.1212/wnl.54.11.2045. [DOI] [PubMed] [Google Scholar]
- [123].Wong A, Luth HJ, uther-Conrad W, Dukic-Stefanovic S, Gasic-Milenkovic J, Arendt T, Munch G. Advanced glycation endproducts co-localize with inducible nitric oxide synthase in Alzheimer's disease. Brain Res. 2001;920:32–40. doi: 10.1016/s0006-8993(01)02872-4. [DOI] [PubMed] [Google Scholar]
- [124].Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Poppek D, Keck S, Ermak G, Jung T, Stolzing A, Ullrich O, Davies KJ, Grune T. Phosphorylation inhibits turnover of the tau protein by the proteasome: influence of RCAN1 and oxidative stress. Biochem J. 2006;400:511–520. doi: 10.1042/BJ20060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Keck S, Nitsch R, Grune T, Ullrich O. Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer's disease. J Neurochem. 2003;85:115–122. doi: 10.1046/j.1471-4159.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- [127].Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, Hisatomi T, Mochizuki M, Morita I. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest. 2005;115:2793–2800. doi: 10.1172/JCI24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Fratta P, Engel WK, McFerrin J, Davies KJ, Lin SW, Askanas V. Proteasome inhibition and aggresome formation in sporadic inclusion-body myositis and in amyloid-beta precursor protein-overexpressing cultured human muscle fibers. Am J Pathol. 2005;167:517–526. doi: 10.1016/s0002-9440(10)62994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tank SJ, Chima RS, Shah V, Malik S, Joshi S, Mazumdar RH. Renal amyloidosis following tuberculosis. Indian J Pediatr. 2000;67:679–681. doi: 10.1007/BF02762183. [DOI] [PubMed] [Google Scholar]
- [130].Cunnane G. Amyloid precursors and amyloidosis in inflammatory arthritis. Curr Opin Rheumatol. 2001;13:67–73. doi: 10.1097/00002281-200101000-00011. [DOI] [PubMed] [Google Scholar]
- [131].Rajkumar SV, Gertz MA, Kyle RA. Primary systemic amyloidosis with delayed progression to multiple myeloma. Cancer. 1998;82:1501–1505. [PubMed] [Google Scholar]
- [132].Thomas B, Beal MF. Parkinson's disease. Hum Mol Genet. 2007;16(Spec No. 2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- [133].Baloyannis SJ, Costa V, Baloyannis IS. Morphological alterations of the synapses in the locus coeruleus in Parkinson's disease. J Neurol Sci. 2006;248:35–41. doi: 10.1016/j.jns.2006.05.006. [DOI] [PubMed] [Google Scholar]
- [134].Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- [135].Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- [136].Dexter DT, Holley AE, Flitter WD, Slater TF, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord. 1994;9:92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- [137].Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- [138].Hanson JC, Lippa CF. Lewy body dementia. Int Rev Neurobiol. 2009;84:215–228. doi: 10.1016/S0074-7742(09)00411-5. [DOI] [PubMed] [Google Scholar]
- [139].Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson's disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology. 2007;27:494–506. doi: 10.1111/j.1440-1789.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- [140].Engelender S, Kaminsky Z, Guo X, Sharp AH, Amaravi RK, Kleiderlein JJ, Margolis RL, Troncoso JC, Lanahan AA, Worley PF, Dawson VL, Dawson TM, Ross CA. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet. 1999;22:110–114. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- [141].Wheeler TC, Chin LS, Li Y, Roudabush FL, Li L. Regulation of synaptophysin degradation by mammalian homologues of seven in absentia. J Biol Chem. 2002;277:10273–10282. doi: 10.1074/jbc.M107857200. [DOI] [PubMed] [Google Scholar]
- [142].Nussbaum RL, Ellis CE. Alzheimer's disease and Parkinson's disease. N Engl J Med. 2003;348:1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- [143].Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- [144].Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- [145].Waxman EA, Giasson BI. Molecular mechanisms of alpha-synuclein neurodegeneration. Biochim Biophys Acta. 2009;1792:616–624. doi: 10.1016/j.bbadis.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- [147].Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- [149].Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- [150].Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di IG, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- [151].Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez TE, del ST, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- [152].Kirik D, Annett LE, Burger C, Muzyczka N, Mandel RJ, Bjorklund A. Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson's disease. Proc Natl Acad Sci U S A. 2003;100:2884–2889. doi: 10.1073/pnas.0536383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157:401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- [155].Li W, Lee MK. Antiapoptotic property of human alpha-synuclein in neuronal cell lines is associated with the inhibition of caspase-3 but not caspase-9 activity. J Neurochem. 2005;93:1542–1550. doi: 10.1111/j.1471-4159.2005.03146.x. [DOI] [PubMed] [Google Scholar]
- [156].Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- [157].Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- [158].Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, Lee VM. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- [159].Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- [160].Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VM, Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- [161].Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- [162].Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- [163].Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J Biol Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- [164].Castegna A, Thongboonkerd V, Klein J, Lynn BC, Wang YL, Osaka H, Wada K, Butterfield DA. Proteomic analysis of brain proteins in the gracile axonal dystrophy (gad) mouse, a syndrome that emanates from dysfunctional ubiquitin carboxyl-terminal hydrolase L-1, reveals oxidation of key proteins. J Neurochem. 2004;88:1540–1546. doi: 10.1046/j.1471-4159.2003.02288.x. [DOI] [PubMed] [Google Scholar]
- [165].Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- [166].Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- [167].Valentine JS, Doucette PA, Zittin PS. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- [168].Crow JP, Sampson JB, Zhuang Y, Thompson JA, Beckman JS. Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J Neurochem. 1997;69:1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- [169].Subramaniam JR, Lyons WE, Liu J, Bartnikas TB, Rothstein J, Price DL, Cleveland DW, Gitlin JD, Wong PC. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat Neurosci. 2002;5:301–307. doi: 10.1038/nn823. [DOI] [PubMed] [Google Scholar]
- [170].Dupuis L, Gonzalez de Aguilar JL, Echaniz-Laguna A, Loeffler JP. Mitochondrial dysfunction in amyotrophic lateral sclerosis also affects skeletal muscle. Muscle Nerve. 2006;34:253–254. doi: 10.1002/mus.20566. [DOI] [PubMed] [Google Scholar]
- [171].Mitsumoto H, Santella RM, Liu X, Bogdanov M, Zipprich J, Wu HC, Mahata J, Kilty M, Bednarz K, Bell D, Gordon PH, Hornig M, Mehrazin M, Naini A, Flint BM, Factor-Litvak P. Oxidative stress biomarkers in sporadic ALS. Amyotroph Lateral Scler. 2008;9:177–183. doi: 10.1080/17482960801933942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Brennan WA, Jr., Bird ED, Aprille JR. Regional mitochondrial respiratory activity in Huntington's disease brain. J Neurochem. 1985;44:1948–1950. doi: 10.1111/j.1471-4159.1985.tb07192.x. [DOI] [PubMed] [Google Scholar]
- [173].Tabrizi SJ, Workman J, Hart PE, Mangiarini L, Mahal A, Bates G, Cooper JM, Schapira AH. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann Neurol. 2000;47:80–86. doi: 10.1002/1531-8249(200001)47:1<80::aid-ana13>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- [174].Ashley CT, Jr., Warren ST. Trinucleotide repeat expansion and human disease. Annu Rev Genet. 1995;29:703–728. doi: 10.1146/annurev.ge.29.120195.003415. [DOI] [PubMed] [Google Scholar]
- [175].Bates G. Huntingtin aggregation and toxicity in Huntington's disease. Lancet. 2003;361:1642–1644. doi: 10.1016/S0140-6736(03)13304-1. [DOI] [PubMed] [Google Scholar]
- [176].Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- [177].Perez-Severiano F, Rios C, Segovia J. Striatal oxidative damage parallels the expression of a neurological phenotype in mice transgenic for the mutation of Huntington's disease. Brain Res. 2000;862:234–237. doi: 10.1016/s0006-8993(00)02082-5. [DOI] [PubMed] [Google Scholar]
- [178].Rotig A, de LP, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet. 1997;17:215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- [179].Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, von BA, Schulz-Schaeffer W, Giese A, Westaway D, Kretzschmar H. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- [180].Singh A, Kong Q, Luo X, Petersen RB, Meyerson H, Singh N. Prion protein (PrP) knock-out mice show altered iron metabolism: a functional role for PrP in iron uptake and transport. PLoS One. 2009;4:e6115. doi: 10.1371/journal.pone.0006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Wong BS, Liu T, Li R, Pan T, Petersen RB, Smith MA, Gambetti P, Perry G, Manson JC, Brown DR, Sy MS. Increased levels of oxidative stress markers detected in the brains of mice devoid of prion protein. J Neurochem. 2001;76:565–572. doi: 10.1046/j.1471-4159.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- [182].Shacka JJ, Roth KA. Cathepsin deficiency as a model for neuronal ceroid lipofuscinoses. Am J Pathol. 2005;167:1473–1476. doi: 10.1016/S0002-9440(10)61233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].van Diggelen OP, Thobois S, Tilikete C, Zabot MT, Keulemans JL, van Bunderen PA, Taschner PE, Losekoot M, Voznyi YV. Adult neuronal ceroid lipofuscinosis with palmitoyl-protein thioesterase deficiency: first adult-onset patients of a childhood disease. Ann Neurol. 2001;50:269–272. doi: 10.1002/ana.1103. [DOI] [PubMed] [Google Scholar]
- [184].Wisniewski KE, Kida E, Walus M, Wujek P, Kaczmarski W, Golabek AA. Tripeptidyl-peptidase I in neuronal ceroid lipofuscinoses and other lysosomal storage disorders. Eur J Paediatr Neurol. 2001;5(Suppl A):73–79. doi: 10.1053/ejpn.2000.0439. [DOI] [PubMed] [Google Scholar]
- [185].Gurney ME, Cutting FB, Zhai P, Doble A, Taylor CP, Andrus PK, Hall ED. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol. 1996;39:147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- [186].Gotz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- [187].Beal MF, Matthews RT, Tieleman A, Shults CW. Coenzyme Q10 attenuates the 1-methyl-4-phenyl-1,2,3,tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res. 1998;783:109–114. doi: 10.1016/s0006-8993(97)01192-x. [DOI] [PubMed] [Google Scholar]
- [188].Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]