Abstract
The aim of this study was to determine if a mixed microbial community from the bovine rumen would respond to excess carbohydrate by accumulating reserve carbohydrate, energy spilling (dissipating excess ATP energy as heat), or both. Mixed microbes from the rumen were washed with N-free buffer and dosed with glucose. Total heat production was measured by calorimetry. Energy spilling was calculated as heat production not accounted by (i) endogenous metabolism (heat production before dosing glucose) and (ii) synthesis of reserve carbohydrate (heat from synthesis itself and reactions yielding ATP for it). For cells dosed with 5 mM glucose, synthesis of reserve carbohydrate and endogenous metabolism accounted for nearly all heat production (93.7%); no spilling was detected (P = 0.226). For cells dosed with 20 mM glucose, energy spilling was not detected immediately after dosing, but it became significant (P < 0.05) by approximately 30 min after dosing with glucose. Energy spilling accounted for as much as 38.7% of heat production in one incubation. Nearly all energy (97.9%) and carbon (99.9%) in glucose were recovered in reserve carbohydrate, fermentation acids, CO2, CH4, and heat. This full recovery indicates that products were measured completely and that spilling was not a methodological artifact. These results should aid future research aiming to mechanistically account for variation in energetic efficiency of mixed microbial communities.
INTRODUCTION
When ruminant livestock are fed grain, rumen microbes often encounter large excesses of carbohydrate (1). Pure cultures of rumen bacteria can respond to these carbohydrate excesses with different strategies (see Fig. S1 in the supplemental material). Some species use excess energy and carbohydrate to synthesize reserve carbohydrate and other reserve materials (1, 2). Other species spill energy, whereby they dissipate excess energy (ATP) through futile or substrate cycles (1, 3). Such cycles may involve flux of ions through the cell membrane (3) or simultaneous synthesis and degradation of glycogen (4, 5). Other responses include reducing ATP yield by releasing metabolic intermediates (overflow metabolites) and shifting to catabolic pathways that yield less ATP (1). Responses are similar for nonrumen microbes (2, 3, 6, 7).
Agriculturally, energy spilling may be detrimental because of its potential to reduce growth of rumen microbes—an important source of protein for ruminant livestock (8). Environmentally, energy spilling may be detrimental because its net products are heat and fermentation products, the latter of which may include the greenhouse gas methane (9). Reserve carbohydrate would be less detrimental because it may be mobilized later for growth (10) or pass from the rumen without being fermented (11), albeit ATP energy is spent on its synthesis and cannot be recovered. There thus exist agricultural and environmental incentives to study the magnitude of these responses in rumen microbes.
Although many studies have examined pure cultures of rumen microbes, few have examined mixed cultures to determine how they respond to excess carbohydrate. Indeed, responses such as energy spilling have seldom been investigated in any mixed community (6, 7), despite the suggestion that spilling evolved to confer competitive advantage in these communities (12). We know of 2 studies that investigated energy spilling in mixed communities. Chen et al. (13) induced spilling in activated sludge by adding an exogenous protonophore, but spilling was not demonstrated under conditions that are physiologically relevant to typical livestock production. Van Kessel and Russell (14) suggested that rumen bacteria spilled energy when grown under ammonia-N limitation, but they did not measure reserve carbohydrate. Some energy may have in fact been directed to reserve carbohydrate synthesis, not spilling—a possibility that the current study will investigate and account for.
Further, studies have generally examined only one response to excess carbohydrate at a time by, for example, examining species that spill energy but do not accumulate reserve carbohydrate (3). They have not examined if multiple responses can occur simultaneously among species in a mixed community.
The aim of this study was to determine how mixed rumen microbes would respond to excess carbohydrate (glucose). The hypothesis was that rumen microbes would direct small amounts of glucose to reserve carbohydrate but that larger amounts would be progressively directed to spilling. To test this hypothesis, the study deploys a new method to quantify energy directed toward reserve carbohydrate synthesis versus spilling. To our knowledge, this study is the first to examine responses to excess carbohydrate in a mixed community and the relative magnitudes of these responses.
MATERIALS AND METHODS
Preparation of mixed cultures and sampling.
Rumen fluid was collected from 1 of 4 cannulated Jersey cows fed a lactation diet (50% corn silage, 4.5% alfalfa hay, 21% corn wet milling product [Cargill Corn Milling, Dayton, OH], 9.05% ground corn, 4.64% soybean meal, 1.30% Amino Plus [Ag Processing Inc., Hiawatha, KS], 1.30% soy hulls, 0.38% fat, 2.01% vitamin and minerals) ad libitum in two equal meals. At 2.5 h after feeding, rumen contents were strained through 4 layers of cheesecloth. The strained fluid was diluted 1:1 with N-free buffer (Simplex type, pH 6.8 [3]) and added to a separatory funnel. All glassware was prewarmed to 39°C and pregassed with O2-free CO2. Plant particles, which rose to the top, were removed by aspiration after 45 min of incubation at 39°C. To prepare mixed rumen microbes, particle-free rumen fluid (40 ml) was centrifuged at 10,000 × g for 10 min (washing once with Simplex buffer) on a prewarmed rotor (JA-17 rotor, J2-21 centrifuge; Beckman, Brea, CA) under anaerobic conditions. Cells were anaerobically resuspended in Simplex buffer and transferred to a culture bottle, which was capped with a butyl rubber stopper and incubated at 39°C. Cells were dosed with glucose (20 mM final concentration). Cell pellets were harvested by centrifuging 1 ml culture (10,000 × g, 10 min, 4°C), washed once in 0.9% NaCl, and stored at −20°C. Pellets were harvested at intervals to give 3 points prior to dosing with glucose, at least 3 points during glucose excess, and at least 3 points after glucose was exhausted. Cell-free supernatant was prepared by combining supernatant from cell harvesting and washing.
Based on direct counts (15), recovery of prokaryotes during centrifugation was high (96.8% [standard error of the mean {SEM}], 6.0%, n = 8, total across 2 cows) and not different from 100% (P = 0.610). Direct counts (16) indicated that recovery of the protozoa was likewise high (98.0% [SEM, 4.4%], n = 8, total across 2 cows; P = 0.663). Unpaired t tests were used in this comparison and all subsequent ones. In additional preliminary experiments, protozoal counts were 3.92 × 105/ml cell suspension (SEM, 6.58 × 104/ml) or 1.05 × 108/g total microbial protein (SEM, 1.19 × 107/g) (n = 4, total across 3 cows). Composition of protozoa was 93.71% (SEM, 0.84%) genus Entodinium, 2.44% (SEM, 0.99%) genus Isotricha, 1.91% (SEM, 0.53%) genus Dasytricha, 1.07% (SEM, 0.75%) genus Epidinium, 0.74% (SEM, 0.22%) subfamily Diplodinae, and 0.13% (SEM, 0.13%) genus Ophryoscolex. Direct cell counts of prokaryotes were 4.26 × 109/ml cell suspension (SEM, 1.05 × 109/ml) or 1.12 × 1012/g total microbial protein (SEM, 1.75 × 1011/g) (n = 4, total across 3 cows). Protein was determined as described below.
Chemical analyses.
Pellets were analyzed for reserve carbohydrate using the anthrone method (17). Protein was determined using Pierce BCA protein assay kit (product 23227; Thermo Scientific, Rockford, IL) after hydrolyzing the pellet in NaOH (0.2 N final concentration, 100°C, 15 min). DNA was determined using the diphenylamine method, and RNA was analyzed using the orcinol method after extracting nucleotides with 0.5 N perchloric acid (20 min, 70°C) (17). For reserve carbohydrate and protein, pellets were first resuspended to 1 ml with distilled water and then immediately boiled (15 min) to inactive degradative enzymes. For DNA and RNA, pellets were resuspended directly in 0.5 N perchloric acid and then immediately extracted.
Cell-free supernatant was prepared for analysis of short-chain fatty acids by combining 0.85 ml with 0.15 ml 10 N H3PO4 and 0.1 ml of a 2-ethylbutyric acid (10 mM) internal standard. Short-chain fatty acids were analyzed by gas chromatography. The gas chromatograph was a Hewlett-Packard 5890 A with a 6-ft by 2-mm glass column packed with GP 15% SP-1220–1% H3PO4 stationary phase on a 100/120 Chromosorb W AW support. The carrier gas was N2 (20 ml/min). The temperature of the oven was initially 113°C and increased 2°C/min over 14 min, the temperature of the injector was 150°C, and the temperature of the detector was 180°C.
Separate aliquots of supernatant were analyzed for d-/l-lactic acid with a kit from R-Biopharm (Marshall, MI; product code 11112821035). Free glucose was analyzed with glucose oxidase-peroxidase (18). N-Ethylmaleimide was added to prevent interference with this method due to cysteine-HCl (19); 2 mol N-ethylmaleimide per mol cysteine-HCl was found to be adequate to prevent interference (data not shown).
Reaction properties.
Stoichiometry and thermodynamic properties of reactions were tabulated (Table 1) for further calculations. Thermodynamic symbols and units are summarized in Text S1 in the supplemental material. Reaction stoichiometry and change in ATP [ΔrATP(l); mol/mol] were from Russell (20). This stoichiometry, along with measured concentrations of reactants (glucose, reserve carbohydrate, fermentation acids), was used to infer concentrations of reactants not measured (CH4, CO2, H2O). The stoichiometric number [ν′(l); mol/mol] was also calculated for reaction stoichiometry. For ΔrATP(l) of glucose-utilizing reactions, it was assumed that glucose transport required no net ATP.
Table 1.
Properties of reactions relevant to glucose use in rumen microbesa
| Reaction | Abbreviation | Stoichiometryf | νi′b (mol/mol) | ΔrG′0 (kJ/mol) | ΔrH′0 (kJ/mol) | ΔrNH (mol/mol) | ΔrATP (mol/mol) |
|---|---|---|---|---|---|---|---|
| Fermentation | |||||||
| Glucose to acetate | GlcAc | Glucose + 2 H2O = 2 acetate + 4 H2(g) + 2 CO2(g) | 2 | −231.83 | 75.33 | −1.99 | 4 |
| Glucose to propionate | GlcPr | Glucose + 2 H2(g) = 2 propionate + 2 H2O | 2 | −367.14 | −333.66 | −1.99 | 4 |
| Glucose to butyratec | GlcBu | Glucose = butyrate + 2 H2(g) + 2 CO2(g) | 1 | −277.09 | −62.86 | −0.99 | 3 |
| Glucose to valerated | GlcVa | Glucose + H2(g) = valerate + CO2(g) | 1 | −342.56 | −264.31 | −0.99 | 2 |
| Glucose to lactate | GlcLa | Glucose = 2 lactate | 2 | −202.76 | −107.63 | −2.00 | 2 |
| CO2 to CH4 | CO2CH4 | CO2(g) + 4 H2(g) = CH4(g) + 2 H2O | 1 | −125.02 | −252.50 | −4.00 | 1 |
| Synthesis of reserve carbohydrate | GlcRc | Glucose + (reserve carbohydrate)n residues = (reserve carbohydrate)n + 1 residues + H2O | NAg | −231.83 | 75.33 | 0.00 | −2 |
| Dissociation of buffere | Buff | HBuff = Buff− | NA | NDh | 2.79 | −1.00 | 0 |
See the text for symbols and source of values. Conditions were as follows unless otherwise noted: T = 39°C, pH 6.8, I = 0.25 M.
Values of νi′ are for the major fermentation product (e.g., acetate in GlcAc reaction).
Reaction with isobutyrate was considered identical to that with GlcBu because properties for isobutyrate were not found.
Reaction with isovalerate was considered identical to that with GlcVa because properties for isovalerate were not found.
Measured under conditions described in the text.
g, gaseous; HBuff, protonated buffer; Buff−, deprotonated buffer.
NA, not applicable.
ND, not determined.
Thermodynamic properties for reactions included standard transformed enthalpy of reaction l [ΔrH′0(l); kJ/mol], standard transformed Gibbs energy of reaction l [ΔrG′0(l); kJ/mol], and change in binding of hydrogen atoms in biochemical reaction l [ΔrNH(l); mol/mol]. Properties were calculated at a temperature (T) of 39°C, pH 6.8 (that of Simplex buffer), and an ionic strength (I) of 0.25 M according to the Alberty method (21).
To calculate the properties of reactions, first the thermodynamic properties of chemical species at standard conditions (T = 25°C, pH = 0, and I = 0) were compiled from the literature (see Table S1 in the supplemental material). Second, the properties of species were adjusted to a T of 39°C, pH 6.8, and an I of 0.25 (see Table S2 in the supplemental material). Third, the properties of chemical reactants (made up of species) were calculated (see Table S3 in the supplemental material), yielding the standard transformed Gibbs energy of formation of reactant i (ΔfG′0i), standard transformed enthalpy of formation of a reactant i (ΔfH′0i), and number of hydrogen atoms in reactant i [N̄H(i)]. Finally, properties for reactions (Table 1) were calculated according to the following equations:
| (1) |
| (2) |
| (3) |
For chemical species, correction of properties to a T of 39°C assumed that heat capacity was constant over temperature. For reactants that were gases (CH4, CO2, H2), the gaseous state was assumed. For reserve carbohydrate, carbohydrate was assumed to be glycogen [glucan with (α1→4) and (α1→6) linkages] with a chain length of 15 residues (typical of rumen bacteria and protozoa [22–27]). The reaction with isobutyrate was considered identical to that with butyrate because properties for isobutyrate were not found; reactions with isovalerate and valerate were also considered identical because properties for isovalerate were not found.
Measurement of heat production.
Rate of heat production (dqt/Vdt, W/liter) was measured using isothermal microcalorimetry (μRC; Thermal Hazard Technology, Piscataway, NJ). This calorimeter measures heat production by power compensation. According to this principle (28), the measurement cell is heated or cooled to compensate for heat consumed or produced by the sample within. The power needed to maintain constant temperature within the cell is related to heat production by a calibration constant. A reference cell is present and corrects for thermal perturbations from the environment.
The cell suspension (1 ml) was added to a 2-ml autosampler vial with a rubber septum and placed in the measurement cell. Water (1 ml) was placed in the reference cell. A glass syringe (250 μl) was filled with 1 M glucose and placed in the syringe tower of the calorimeter. The calorimeter was set to 39.00°C. Heat production was recorded at intervals of 1 s.
The rate of heat production was corrected for a baseline obtained with water in the sample and reference cells before and after each experiment. Calibration was done using an internal electric heater. This calibration was verified by injecting 0.1 N HCl into 0.01 N NaOH (28), which, when corrected for the apparent heat of injecting 0.1 N HCl, produced 102.2% (SEM, 1.4%) of the heat expected (n = 6) (29).
The rate of heat production was integrated to give integrated heat production (kJ/liter) according to the equation qt/V = . Here and throughout, integration was done using the rectangle method with 1-s intervals. Integrated heat production was expressed as an enthalpy change in the calorimetric experiment (kJ/mol) by dividing by the change in glucose concentration according to the equation ΔrHt (cal) = (qt/V)/(dcglucose,t/dt)/103, where cglucose,t is the concentration of glucose (mol/liter) at time t. Here and throughout, differentiation was done using the finite difference method with 1-s intervals.
Heat is released when fermentation acids protonate buffer, and the quantities above require correction for this heat release. ΔrHt(cal) was corrected for this heat release with the equation ΔrHt = ΔrHt(cal) + ΔrNH ΔrH(Buff), where ΔrHt is the enthalpy of reaction at time t (kJ/mol) and ΔrH(Buff) is the enthalpy of dissociation of buffer (kJ/mol H+). ΔrH(Buff) (Table 1) was measured by injecting 0.01 N HCl into Simplex buffer at 39°C. ΔrNH was calculated from values in Table 1, weighting according to the relative rates of individual reactions. Subsequently, qt/V and dqt/Vdt were corrected for heat release from buffer (giving qt*/V and dqt*/Vdt) by back calculation.
Heat production and cellular functions.
Three functions were assumed to account for total integrated heat production (qt/V): (i) endogenous metabolism, (ii) synthesis of reserve carbohydrate, and (iii) energy spilling. Following an approach similar to that of Cook and Russell (30), we calculated the amount of total heat production accounted for by each of these 3 functions over the incubation.
For the first function, the rate of heat production from endogenous metabolism was assumed to be equal to the rate measured prior to dosing with glucose. This rate was assumed not to remain constant over the incubation, but rather to decline by 7.3%/h (as for control experiments where cells were never dosed with glucose; see Results). The rate of heat production was then integrated.
For the second function, the rate of heat production from synthesis of reserve carbohydrate was the absolute value of multiplying the rate of accumulation of reserve carbohydrate (mol glucose equivalents/s) and the molar heat of reserve carbohydrate synthesis (kJ/mol). Molar heat of reserve carbohydrate synthesis was calculated as
 |
where ci,t is the concentration of reactant i at time t (M), Glc is glucose, Ac is acetate, Pr is propionate, Bu is butyrate and isobutyrate, La is lactate, and Va is valerate and isovalerate. Abbreviations for reactions are provided in Table 1; for example, GlcAc refers to glucose fermentation to acetate. The rate of heat production was then integrated.
For the third function, heat production accounted for by energy spilling was calculated as total integrated heat production (qt/V) minus (i) integrated heat production accounted for by endogenous metabolism and (ii) integrated heat production accounted for by reserve carbohydrate synthesis.
Energy and carbon recovery.
Energy recovery and carbon recovery were calculated from concentrations of reactants (glucose, reserve carbohydrate, fermentation acids, CO2, CH4, H2O) and rate of heat production (see below). First, the amounts of energy and carbon in the culture were calculated according to the equations energy (kJ/liter) = and carbon (M) = ci,tN̄C(i), where N′ is the number of reactants and N̄C(i) is the number of carbon atoms in reactant i (see Table S3 in the supplemental material). Next, increases in energy and carbon after dosing glucose were calculated; energy and carbon after dosing were subtracted from the average of their respective values before dosing with glucose. The amount of energy in the glucose dose (kJ/liter) is given by cglucose,dosing · ΔfH′0glucose, and the amount of carbon in the glucose dose is given by cglucose,dosing · N̄C(glucose).
For recovery of 100%, the increase in energy and carbon after dosing with glucose equals the energy and carbon in dosed glucose. The actual increase (averaged over time) was compared to the expected increase for 100% recovery.
Sensitivity analysis.
Sensitivity analysis (31) was performed to test if energy spilling changed appreciably after changing the assumed parameter values used in its calculation. For any calculation for which some parameter values are assumed and subject to error, a sensitivity analysis investigates how much those errors impact the results of that calculation (31). In this study, assumed parameter values were those that concerned (i) endogenous metabolism as an estimate of maintenance functions, (ii) rate of decline in endogenous metabolism, (iii) ATP requirements for glucose transport, (iv) ATP yield of fermentation reactions, (v) identity of reserve carbohydrate (constituent monomers and chain length), (vi) physical state of gases for calculation of thermodynamic properties, and (vii) pH for calculation of thermodynamic properties. Assumed values were changed one at a time to alternate values, and spilling was recalculated. These alternate values and the reason for their choice are given in Table 2.
Table 2.
Amount of energy spilling following changes in assumed parameter values for spilling calculations
| Current assumption | Alternate assumption | Note (reference for alternate assumption) | Energy spilling (% of heat production) for dosage with: |
Differences | |
|---|---|---|---|---|---|
| 5 mM glucose | 20 mM glucose | ||||
| All | None (no changes) | 6.3 | 21.1 | 14.8 | |
| Maintenance functions estimated by endogenous metabolisma | Functions estimated by energy use of rumen bacteria extrapolated to growth rate = 0b | See text | 14.7 | 25.4 | 10.6 |
| Endogenous metabolism declines by 7.3%/h | Metabolism is constant | Hypothetical | 4.5 | 18.3 | 13.8 |
| 0 mol ATP/mol glucose transported | 0.33 mol ATP/molc | Cost of ion–driven transport (20) | 2.6 | 16.4 | 13.7 |
| 4 mol ATP/2 mol propionated | 2 mol ATP/2 mole | Yield of acrylate pathway (20) | 2.5 | 16.2 | 13.7 |
| 1 mol ATP/mol CH4f | 0.3 mol ATP/molg | Lowest yield in reference 48 | 3.5 | 17.9 | 14.4 |
| 1.5 mol ATP/molh | Highest yield in reference 48 | 8.0 | 23.1 | 15.1 | |
| Reserve polysaccharide has chain length of 15 residues and is polymer of glucosei | Polymer of fructosej | Hypothetical | 7.8 | 22.8 | 15.1 |
| Polymer of galactosek | Hypothetical | 9.0 | 24.3 | 15.3 | |
| Polymer of mannosel | Hypothetical | 8.0 | 23.1 | 15.1 | |
| 8 residuesm | Lowest measurement (26) | 6.1 | 20.8 | 14.7 | |
| 25 residuesn | Highest measurement (24) | 6.4 | 21.2 | 14.8 | |
| Thermodynamic properties of gas reactants assume reactants in gaseous stateo | Aqueous statep | Vaporization of aqueous state can be slow (49) | 2.4 | 16.3 | 13.9 |
| Thermodynamic properties of species assume pH 6.80q | pH 6.65r | Lowest pH measured in current study | 6.3 | 21.1 | 14.8 |
33.7 mW/g cellular protein (SEM = 2.7 mW/g; n = 8; see text).
28.0 mW/g cellular protein (see text) (adapted from reference 33).
ΔrATP for glucose-utilizing reactions decrease by 0.33; see Table 1 for current values and equation 4 for application.
ΔrATP(GlcPr) = 4; see Table 1 for current value and equation 4 for application.
ΔrATP(GlcPr) = 2; see equation 4 for application.
ΔrATP(CO2CH4) = 1; see Table 1 for current value and equation 4 for application.
ΔrATP(CO2CH4) = 0.3; see equation 4 for application.
ΔrATP(CO2CH4) = 1.5; see equation 4 for application.
ΔfH′0RC = −973.94; see Table S3 in the supplemental material for the current value and equation 1 for application.
ΔfH′0RC = −970.21; see equation 1 for application.
ΔfH′0RC = −967.01; see equation 1 for application.
ΔfH′0RC = −969.71; see equation 1 for application.
ΔfH′0RC = −974.47; see equation 1 for application.
ΔfH′0RC = −973.68; see equation 1 for application.
See current values in Table S3 in the supplemental material; see equations 1, 2, and 3 for application.
See alternate values in Table S3 in the supplemental material; see equations 1, 2, and 3 for application.
See the current values of properties in Table S2 in the supplemental material; see text for application.
For brevity, alternate values are not shown.
Value for 20 mM glucose − value for 5 mM glucose.
Statistics.
Data were analyzed using unpaired t tests. Local regression (LOCFIT package of R [32]) was used to fit time series data to smooth curves. The model chosen was a local quadratic with Gaussian kernel and nearest-neighbor bandwidths between 0.4 and 0.7, which reduced noise in the data without oversmoothing (e.g., truncating peaks). Original data are presented alongside the smooth curves in the figures, and smooth curves were used for calculations (e.g., for heat production accounted for by cellular functions and recoveries of energy and carbon). First-order rates of exponential decline were calculated for time series data as the linear decline after log transformation.
RESULTS
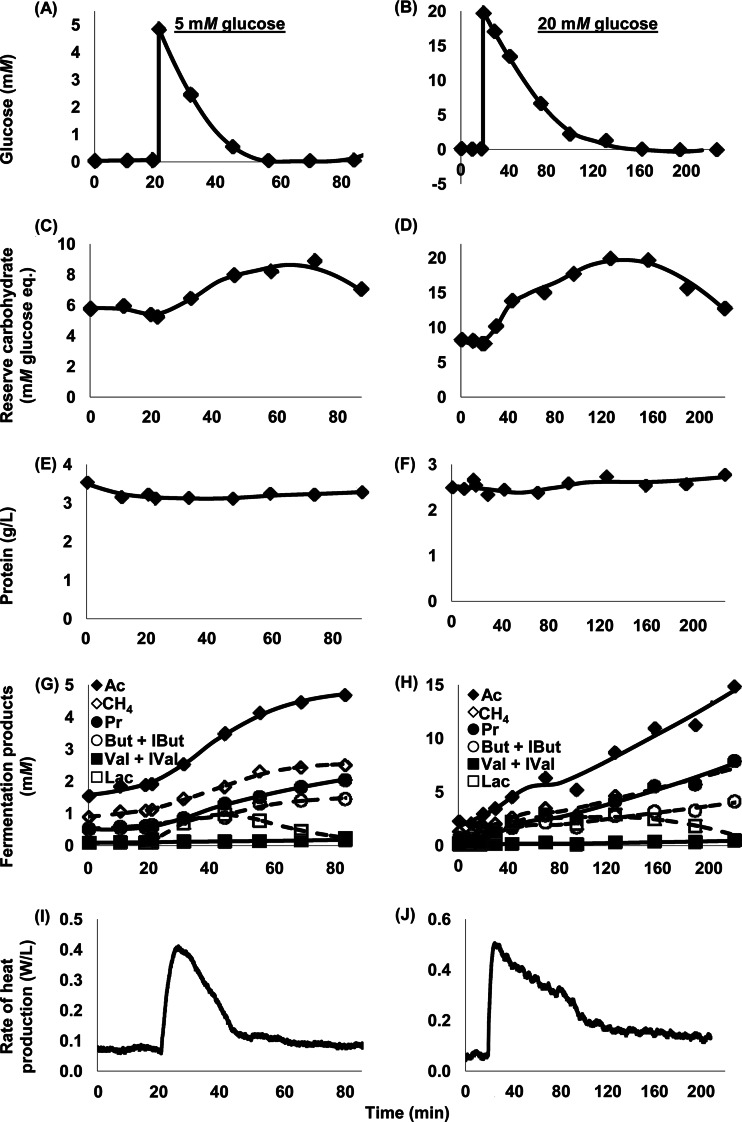
When rumen microbes were washed with N-free buffer and dosed with glucose, they responded immediately by consuming glucose, accumulating reserve carbohydrate, and producing heat (Fig. 1). From glucose dosing to glucose exhaustion, cells dosed with 20 mM glucose (final concentration) accumulated 356% more reserve carbohydrate than did cells dosed with 5 mM glucose (final concentration) (SEM = 65%; P = 0.002). In the same interval, cells dosed with 20 mM glucose produced 467% more heat than did cells dosed with 5 mM glucose (SEM = 69%; P < 0.001).
Fig 1.
Response of mixed rumen microbes to 5 mM (A, C, E, G, I) or 20 mM (B, D, F, H, J) glucose dosed at 20 min. Data are from cow 472 and represent 1 experiment per concentration of glucose; data for 3 other cows (representing 3 additional experiments per concentration of glucose) were similar (data not shown). (A, B) Glucose in media. (C, D) Reserve carbohydrate. (E, F) Cell protein. (G, H) Fermentation products, including acetate (Ac), methane (CH4), propionate (Pr), butyrate and isobutyrate (But + IBut), valerate and isovalerate (Val + IVal), and lactate (Lac). (I, J) Rate of heat production. Reserve carbohydrate was expressed in mM monomeric glucose equivalents to be in same units as glucose in media. Each data point represents one sample that was analyzed in triplicate. Heat production was measured at 1-s intervals as described in the text.
At the point of glucose exhaustion, reserve carbohydrate and rate of heat production began to decline (Fig. 1). For cells dosed with 5 mM glucose, the rate of heat production at glucose exhaustion was slightly higher than that prior to dosing with glucose (133% [SEM, 12%] of the predosing rate; P = 0.068; n = 4). It declined to predosing values relatively quickly (Fig. 1I). For cells dosed with 20 mM glucose, the rate of heat production at glucose exhaustion was much higher than that prior to dosing (202% [SEM, 20%]; P = 0.014; n = 4), and it persisted above predosing values long after glucose was exhausted (Fig. 1J). Reserve carbohydrate declined at an increasing rate after glucose was exhausted (Fig. 1C and D).
Over the duration of individual experiments, no large changes in protein were obvious (Fig. 1E and F). Across experiments (n = 8), an exponential decline was detected numerically (1.08%/h [SEM, 0.93%/h]; n = 8), but it was not significant (P = 0.283), and protein averaged 3.82 g/liter (SEM, 0.43 g/liter). No large changes in DNA or RNA were observed in a preliminary experiment (see Fig. S2 in the supplemental material), and these components were not measured subsequently.
Lactate initially accumulated (Fig. 1G and H), and concentrations peaked at 1.17 mM (SEM, 0.15 mM) and 3.99 mM (SEM, 0.67 mM) for cells dosed with 5 and 20 mM glucose (n = 4 for each), respectively. Lactate began to decline before glucose was exhausted (Fig. 1G and H), and concentrations by the end of incubation were low: 0.25 mM (SEM, 0.11 mM) and 0.74 mM (SEM, 0.32 mM) for cells dosed with 5 and 20 mM glucose (n = 4 for each), respectively. Initial pH (that of Simplex buffer) was 6.8, and final pH was never below 6.65.
For control incubations in which cells were starved for carbon (washed with N-free buffer and not dosed with glucose), heat production, reserve carbohydrate, and protein declined (Fig. 2). Across experiments (n = 4), the decline in reserve carbohydrate was rapid (−12.8%/h [SEM, 0.7%/h]) and significant (P < 0.001). The decline in heat production was slow (7.3%/h [SEM, 1.0%/h]; P = 0.006). The decline in protein was even slower (2.0%/h [SEM, 0.5%/h]; P = 0.031).
Fig 2.
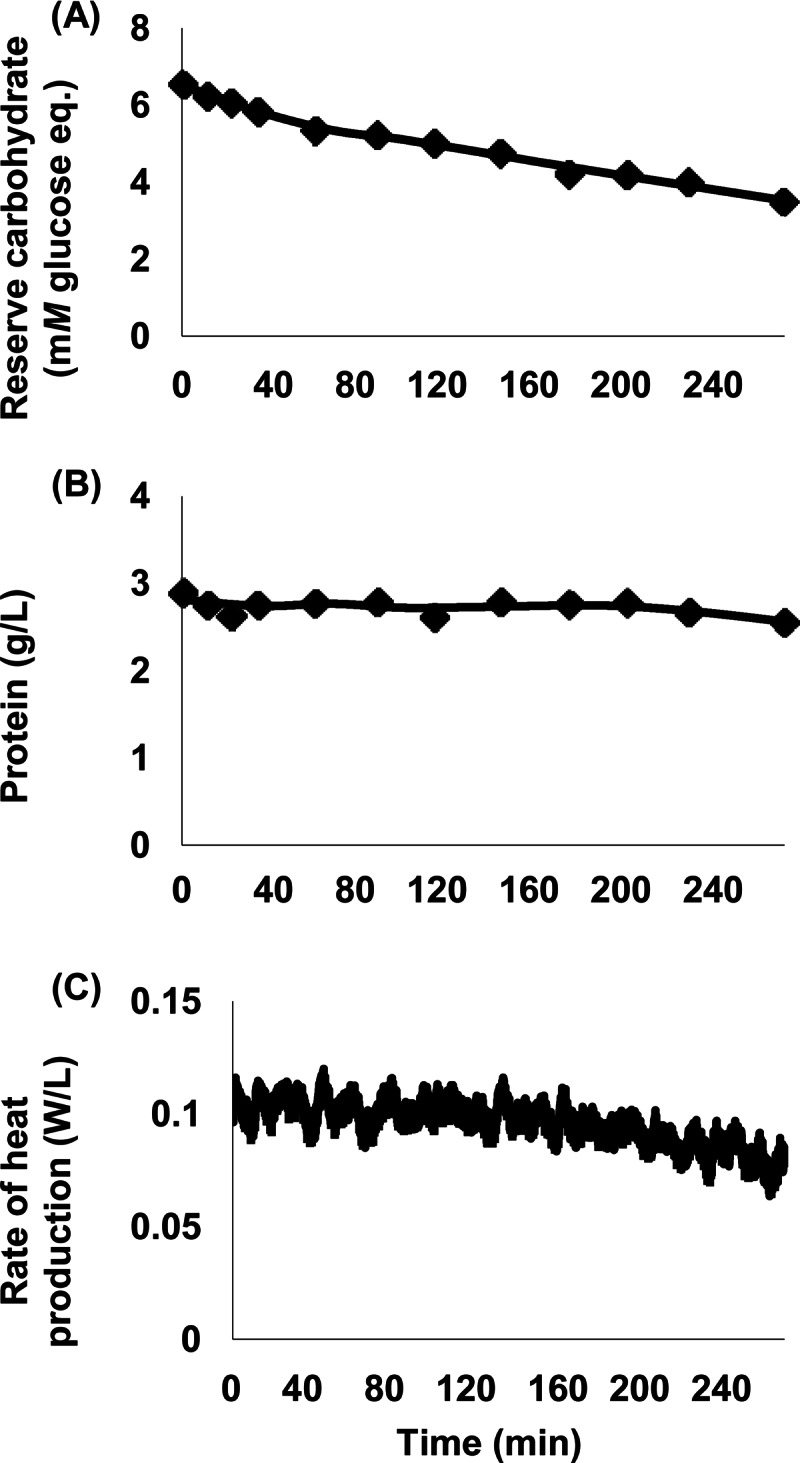
Response of mixed rumen microbes to starvation. (A) Reserve carbohydrate. (B) Cell protein. (C) Rate of heat production. Data are from cow 472 and represent one experiment; data for 3 other cows (representing 3 additional experiments) were similar (data not shown). Reserve carbohydrate was expressed in mM monomeric glucose equivalents. Each data point represents one sample that was analyzed in triplicate. Heat production was measured at 1-s intervals as described in the text.
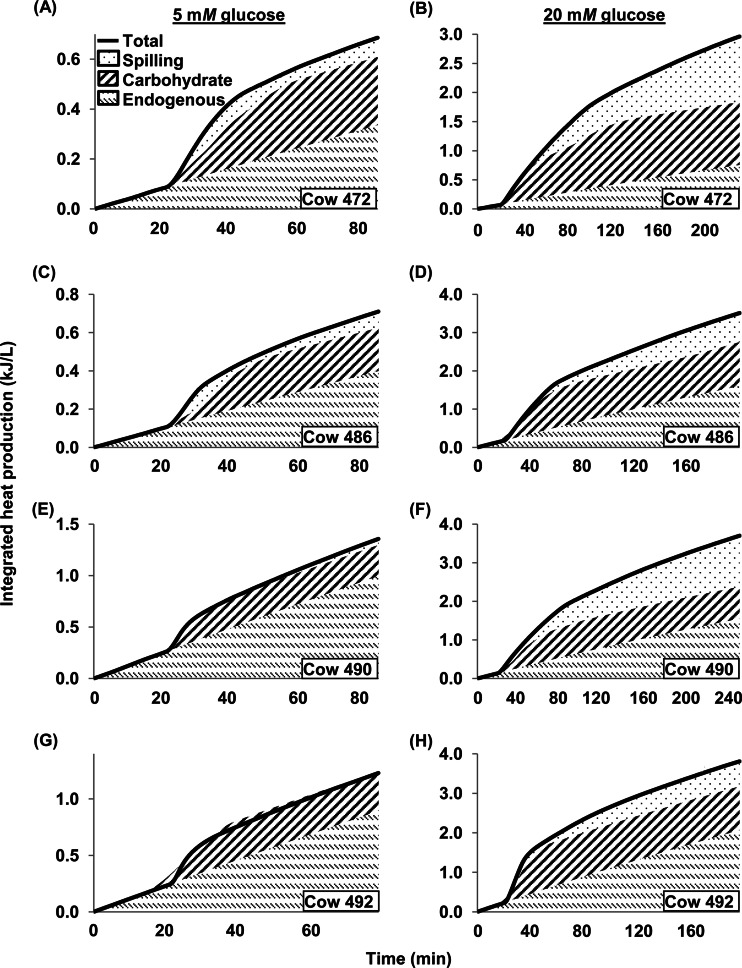
For the earlier experiments in which cells were dosed with glucose, we calculated how much heat production was accounted for by 3 cellular functions: endogenous metabolism, synthesis of reserve carbohydrate, and energy spilling (Fig. 2). Figure S3 in the supplemental material illustrates these calculations with example data. Heat production accounted for by endogenous metabolism was calculated (i) from the rate of heat production measured prior to dosing with glucose and (ii) assuming a decline of 7.3%/h (that measured above for cells not dosed with glucose) (cf. Fig. 2C). Heat production accounted for by synthesis of reserve carbohydrate was calculated by multiplying the rate of reserve carbohydrate accumulation by the molar heat of reserve carbohydrate synthesis. Heat production for energy spilling was that not accounted for by endogenous metabolism or reserve carbohydrate synthesis.
When cells were dosed with 5 mM glucose, endogenous metabolism and synthesis of reserve carbohydrate accounted for nearly all of the heat production (Fig. 3). Across experiments (n = 4), these 2 functions accounted for 93.7% (SEM, 4.1%) of the heat production when glucose was dosed. The remaining 6.3% of heat production, accounted for by energy spilling, differed from 0 numerically but not statistically (P = 0.226). When cells were dosed with a higher concentration of glucose (20 mM), endogenous metabolism and synthesis of reserve carbohydrate accounted completely for heat production, but only initially (Fig. 3). Across experiments (n = 4), energy spilling became detectable (P < 0.05) by 49 min from the start of the incubation and averaged 21.1% (SEM, 3.8%) after dosing with glucose. It eventually accounted for as much as 38.7% of heat production in one incubation (cow 472) (Fig. 3).
Fig 3.
Integrated heat production of mixed rumen microbes in response to 5 mM (A, C, E, G) or 20 mM (B, D, F, H) glucose. Shown are values of heat production accounted for by endogenous metabolism, synthesis of reserve carbohydrate, and energy spilling for cows 472, 486, 490, and 492. Each panel represents 1 experiment. Methods of calculation are described in the text and Fig. S3 in the supplemental material.
At glucose exhaustion, spilling was 4.0% (SEM, 3.0%) of total heat production for cells dosed with 5 mM glucose and did not differ (P = 0.271) from 0. It was 21.5% (SEM, 6.2%) for cells dosed with 20 mM glucose and differed (P = 0.0404) from 0. At the end of incubations (which were of variable length), spilling was 7.0% (SEM, 3.1%; P = 0.107) for cells dosed with 5 mM glucose and 28.6% (SEM, 5.3%; P = 0.0124) for cells dosed with 20 mM glucose.
Molar heat of reserve carbohydrate synthesis averaged −86.62 kJ/mol (SEM, 0.75 kJ/mol) across experiments (n = 8). Related quantities were −196.7 kJ/mol utilized hexose equivalents (SEM, 2.3 kJ/mol) (6 mol carbon in products) and 4.32 mol ATP/mol hexose equivalents (SEM, 0.07 mol ATP/mol hexose equivalents) (n = 8).
In a sensitivity analysis, assumed parameter values for calculating spilling were successively changed from their current values to alternate ones. After making this change, the calculated amount of spilling changed little (<5% for all but one case) (Table 2), even as the alternate values often represented the extreme range of possible values. A central aim of this study was comparing energy spilling for cells dosed with 5 versus 20 mM glucose. In the sensitivity analysis, the difference between 20 and 5 mM glucose ranged narrowly between 13.7 and 15.3% for all but one case (Table 2), indicating that the calculated amounts of spilling for 5 mM and 20 mM glucose were impacted similarly by changing parameter values. Thus, there is little opportunity for compounding errors from parameterization of equations used to calculate energy spilling.
Although energy spilling generally changed little in the sensitivity analysis, one exception occurred when changing the estimate of maintenance functions. When changing from the current to the alternate assumed parameter value, the calculated amount of spilling changed by >5% for cells dosed with 5 mM glucose (Table 2). Further, the difference between 5 and 20 mM glucose was only 10.6% (Table 2). The current parameter value was based on the assumption that maintenance functions were equal to endogenous metabolism. This value averaged 33.7 mW/g protein (SEM, 2.7 mW/g protein) in our study (average across 8 experiments in which glucose was dosed). The alternate parameter value was based on the assumption that maintenance functions were equal to energy use of rumen bacteria extrapolated to a growth rate of 0 (not measured in our study). The value of energy use of rumen bacteria extrapolated to a growth rate of 0 was set equal to 28.0 mW/g protein, which was calculated from Isaacson et al. (33) (2.6 × 10−4 mol glucose/cell dry matter/h reported by Isaacson et al. [33] × 196.7 kJ/mol hexose reported in this study × 1 g dry matter/0.507 g protein reported by Hackmann et al. [34] × 1 h/3,600 s × 106 mJ/kJ).
For experiments in which cells were dosed with glucose (n = 8), carbon recovery (99.9% [SEM, 5.0%]) and energy recovery (97.6% [SEM, 5.2%]) were not different from 100% (P = 0.658 and 0.985, respectively). Components included in the calculation of recovery included reserve carbohydrate, fermentation acids, CO2, CH4, and heat. DNA, RNA, and protein were excluded from the calculation because these components did not change over the duration of experiments where glucose was dosed (see above). Complete recovery confirmed that appropriate components were included and that these were measured completely.
DISCUSSION
When limited for N, some pure cultures of microbes respond to excess carbohydrate by accumulating reserve carbohydrate (35), whereas others spill energy (3). This study determined whether mixed cultures of rumen microbes would respond by accumulating reserve carbohydrate, spilling, or both. Ruminal protozoa rapidly convert consumed feed into reserve carbohydrate and can potentially profoundly influence growth efficiency measurements (36). Based on isotopic flux or precursor/product analyses, both the Gram-positive Clostridium cellulolyticum (37) and Gram-negative Fibrobacter succinogenes (5) changed catabolite usage and end product formation extensively when substrate availability or the source was modified. Even though the strain of C. cellulolyticum tested synthesizes little glycogen relative to F. succinogenes, both reports noted the important regulatory role of glycogen formation and cycling in those primary cellulolytic bacteria. The current approach is based on a previous study to improve the quantification of reserve carbohydrate to measure energy and carbon recovery (34); however, the current results extend conditions to providing excess glucose for a novel method to quantify energy spilling in a mixed community of rumen microbes.
In that previous study (34), mixed rumen microbes synthesized large amounts of reserve carbohydrate when washed in N-free buffer and given excess glucose. This reserve carbohydrate was identified as glycogen from thin-layer chromatography, enzymatic analysis, and iodine staining. Changes in reserve carbohydrate were quantitatively measured by the anthrone method, as suggested by carbon and energy recovery. That study did not quantify energy spilling; however, its verification of our assay's quantitative measurement of reserve carbohydrate supports the current study's accuracy of energy spilling calculation.
The current study determined the extent of energy spilling by measuring heat production by calorimetry. After first measuring heat production, we calculated the amount of total heat production accounted for by (i) synthesis of reserve carbohydrate and (ii) endogenous metabolism. Finally, unaccounted heat, if any, was attributed to energy spilling. Heat production accounted for by growth was ignored because cells were washed in N-free buffer and growth was absent (protein, DNA, and RNA did not change). Cook and Russell (30) used a similar conceptual approach to calculate how much total glucose consumption by Streptococcus bovis was accounted for by maintenance, growth, and spilling. These authors measured heat production also, but they expressed the final results in terms of glucose consumption using the constant conversion factor ΔrH0 of −88.2 kJ/mol glucose. S. bovis formed only 1 product (lactate) in that study. In our study, multiple fermentation products were formed; ΔrH′0 was therefore not constant, and results could not be expressed as directly in terms of glucose consumption.
Based our accounting of heat production, energy spilling did occur when the mixed-cell cultures were incubated under large excesses of glucose (20 mM). Spilling did not occur immediately after the cells were dosed with glucose. By approximately 30 min after dosing, however, a significant proportion of heat production could not be explained by synthesis of reserve carbohydrate and endogenous metabolism, indicating energy spilling. Some spilling occurred before the point of glucose exhaustion, and spilling continued after glucose was exhausted. Carbon and energy recoveries were complete, and energy spilling was therefore not an artifact of incompletely recovering reserve carbohydrate, an overflow metabolite, or other product. When cells were dosed with less glucose (5 mM), energy spilling was not significantly detected at any point in the incubations.
To calculate the magnitude of spilling, this study required a number of assumed parameter values (e.g., estimate of maintenance functions). How much are our calculations impacted by changes to these values? When these parameter values were systematically changed in a sensitivity analysis, the impact on our calculations was small (Table 2). The impacts on calculations were also similar for 5 and 20 mM glucose (Table 2). Note that changes investigated in this sensitivity analysis were often extreme, such as the assumption that glucose transport was 100% ion driven (instead of 0% as originally assumed). For rumen bacteria, only some transport is ion driven at low glucose concentrations (1 mM) and none is ion driven at high concentrations (5 mM) (38). Because changes in the simulation analysis were often extreme, ranges of values in Table 2 probably overestimate the errors in our calculations.
Although the sensitivity analysis generally supported our conclusion that our calculations were robust, spilling was impacted moderately when the estimate for maintenance functions was changed. Estimating energetic requirements for these functions (e.g., establishment of ion gradients or turnover of macromolecules [3, 6]) remains elusive (7, 39), and multiple approaches exist. Following the definition of Dawes (40), this study estimated these requirements from endogenous metabolism prior to dosing with glucose (during starvation and absence of growth). Following Pirt (41), others (see, e.g., reference 30) have estimated requirements by extrapolating energy use to a growth rate of 0 (during fed conditions and growth). This study's estimate was 20% larger than an earlier estimate for rumen bacteria where energy use was extrapolated to a growth rate of 0 (Table 2). Consequently, spilling increased moderately when this alternate estimate for rumen bacteria was used instead of our current estimate.
Some studies have suggested that spilling may occur or be induced in mixed communities. Van Kessel and Russell (14) reduced growth efficiency of mixed rumen microbes by replacing amino-N with ammonia-N, and they suggested that the reduction resulted from spilling. However, they did not account for energy used in synthesis of reserve carbohydrate and could have overestimated spilling. When adding the exogenous protonophore 3,3′,4′5-tetrachlorosalicylanilide (TCS), Chen et al. (13) reduced the growth efficiency of microbes in activated sludge. The rate of substrate consumption did not change, and they inferred that TCS addition induced energy spilling. However, spilling under more-physiological conditions (in the absence of the protonophore) was not demonstrated. Our study confirms that spilling can occur in mixed microbial communities from the rumen and under simple excess of carbohydrate.
Excesses of carbohydrate generated in this study should approach the upper bound of excesses encountered in the rumen. Whereas we dosed with 5 and 20 mM glucose, concentrations of glucose in the rumen rarely exceed 1 mM for animals fed high-forage diets or those adapted to grain (38). Still, concentrations of ca. 5 mM glucose can be reached after feeding unadapted animals large quantities of grain (42, 43). The highest concentration of glucose reported was 18 mM (after feeding glucose) (44). The highest concentration of soluble sugars was 69 mM glucose equivalents (after feeding beet pulp) (45). These concentrations pertain to the bulk fluid, and concentrations may be higher for microenvironments (e.g., around starch particles to which amylolytic bacteria attach) (38). In our study, carbohydrate excess was reinforced by removing N (cells were washed with N-free buffer). For animals fed grain, N in the rumen is present but low, creating carbohydrate excess (46). If N is chiefly in the form of ammonia, carbohydrate excess could be intensified (14) because rumen microbes grow far slower with ammonia-N than amino-N (14, 47). Energy spilling detected here probably falls in the upper ranges in the rumen encountered for high-sugar diets and microenvironments, especially when N is in the form of ammonia.
Energy spilling is not restricted to pure laboratory cultures; it can also occur in mixed ruminal communities that include bacteria, archaea, protozoa, and fungi. This finding supports the suggestion that spilling evolved to confer competitive advantage in environments, such as the rumen, that are periodically exposed to nutrient excesses (12). Rumen microbes respond to carbohydrate predominantly by synthesis of reserve carbohydrate, without spilling, under small excesses of carbohydrate. Future work will identify the microbial groups and biochemical mechanisms responsible for the spilling observed under large excesses. In particular, glycogen cycling (4, 5) should be a suspected mechanism because reserve carbohydrate was central in the response to excess carbohydrate.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jo Ann Van Kessel (USDA-ARS, Beltsville, MD) and Shujin Yang for reviewing the manuscript, Gönül Kaletunç for discussions on calorimetry, Daniel Bond (University of Minnesota) for discussions on energy spilling, Stephanie Metzger and Donna Wyatt for technical assistance with short-chain fatty acid analysis, Bethany Keyser and Katherine Backus for additional technical assistance, Reagen Bluel and the farm staff at Waterman Dairy Farm for animal care, and Normand St. Pierre for reviewing statistical methods. Unless otherwise noted, all persons acknowledged are from The Ohio State University.
Research was supported by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University. Manuscript number 08/13AS. Additional funds were from the OARDC Director's Associateship Program Award, University Distinguished Fellowship, OARDC Graduate Research Competition Competitive Grants Program 2010-114, and USDA Cooperative State Research, Education, and Extension Service USDA/NRICGP grant 2008-35206-18847.
Footnotes
Published ahead of print 12 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00482-13.
REFERENCES
- 1. Russell JB. 1998. Strategies that ruminal bacteria use to handle excess carbohydrate. J. Anim. Sci. 76:1955–1963 [DOI] [PubMed] [Google Scholar]
- 2. Preiss J, Romeo T. 1989. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv. Microb. Physiol. 30:183–238 [DOI] [PubMed] [Google Scholar]
- 3. Russell JB. 2007. The energy spilling reactions of bacteria and other organisms. J. Mol. Microbiol. Biotechnol. 13:1–11 [DOI] [PubMed] [Google Scholar]
- 4. Portais JC, Delort AM. 2002. Carbohydrate cycling in micro-organisms: what can 13C-NMR tell us? FEMS Microbiol. Rev. 26:375–402 [DOI] [PubMed] [Google Scholar]
- 5. Forano E, Delort AM, Matulova M. 2008. Carbohydrate metabolism in Fibrobacter succinogenes: what NMR tells us. Microb. Ecol. Health Dis. 20:94–102 [Google Scholar]
- 6. Russell JB, Cook GM. 1995. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol. Rev. 59:48–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tempest DW, Neijssel OM. 1984. The status of YATP and maintenance energy as biologically interpretable phenomena. Annu. Rev. Microbiol. 38:459–486 [DOI] [PubMed] [Google Scholar]
- 8. Clark JH, Klusmeyer TH, Cameron MR. 1992. Microbial protein synthesis and flows of nitrogen fractions to the duodenum of dairy cows. J. Dairy Sci. 75:2304–2323 [DOI] [PubMed] [Google Scholar]
- 9. Martin C, Morgavi DP, Doreau M. 2010. Methane mitigation in ruminants: from microbe to the farm scale. Animal 4:351–365 [DOI] [PubMed] [Google Scholar]
- 10. Wilkinson J. 1959. The problem of energy-storage compounds in bacteria. Exp. Cell Res. 7(Suppl):111–130 [Google Scholar]
- 11. McAllan AB, Smith RH. 1974. Carbohydrate metabolism in the ruminant. Bacterial carbohydrates formed in the rumen and their contribution to digesta entering the duodenum. Br. J. Nutr. 31:77–88 [DOI] [PubMed] [Google Scholar]
- 12. Tempest D. 1978. The biochemical significance of microbial growth yields: a reassessment. Trends Biochem. Sci. 3:180–184 [Google Scholar]
- 13. Chen GH, Mo HK, Saby S, Yip WK, Liu Y. 2000. Minimization of activated sludge production by chemically stimulated energy spilling. Water Sci. Technol. 42:189–200 [Google Scholar]
- 14. Van Kessel JS, Russell JB. 1996. The effect of amino nitrogen on the energetics of ruminal bacteria and its impact on energy spilling. J. Dairy Sci. 79:1237–1243 [DOI] [PubMed] [Google Scholar]
- 15. Holdeman L, Moore W. (ed). 1972. Anaerobe laboratory manual, 2nd ed Virginia Polytechnic Institute and State University, Blacksburg, VA [Google Scholar]
- 16. Dehority BA. 1993. Laboratory manual for classification and morphology of rumen ciliate protozoa. CRC Press, Boca Raton, FL [Google Scholar]
- 17. Herbert D, Phipps P, Strange R. 1971. Chemical analysis of microbial cells. Methods Microbiol. 5B:209–344 [Google Scholar]
- 18. Karkalas J. 1985. An improved enzymic method for the determination of native and modified starch. J. Sci. Food Agric. 36:1019–1027 [Google Scholar]
- 19. Haugaard N, Cutler J, Ruggieri MR. 1981. Use of N-ethylmaleimide to prevent interference by sulfhydryl reagents with the glucose oxidase assay for glucose. Anal. Biochem. 116:341–343 [DOI] [PubMed] [Google Scholar]
- 20. Russell JB. 2002. Rumen microbiology and its role in ruminant nutrition. James B. Russell, Ithaca, NY [Google Scholar]
- 21. Alberty RA. 2003. Thermodynamics of biochemical reactions. John Wiley & Sons, Hoboken, NJ [Google Scholar]
- 22. Forsyth G, Hirst E. 1953. Protozoal polysaccharides. Structure of the polysaccharide produced by the holotrich ciliates present in sheep's rumen. J. Chem. Soc. 2132–2135 [Google Scholar]
- 23. Eadie J, Manners D, Stark J. 1963. The molecular structure of a reserve polysaccharide from Entodinium caudatum. Biochem. J. 89:91 [Google Scholar]
- 24. Wakita M, Hoshino S. 1980. Physicochemical properties of a reserve polysaccharide from sheep rumen ciliates genus Entodinium. Comp. Biochem. Physiol. B 65:571–574 [Google Scholar]
- 25. Wallace RJ. 1980. Cytoplasmic reserve polysaccharide of Selenomonas ruminantium. Appl. Environ. Microbiol. 39:630–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lou J, Dawson KA, Strobel HJ. 1997. Glycogen formation by the ruminal bacterium Prevotella ruminicola. Appl. Environ. Microbiol. 63:1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown RG, Lindberg B, Cheng KJ. 1975. Characterization of a reserve glucan from Megasphaera elsdenii. Can. J. Microbiol. 21:1657–1659 [DOI] [PubMed] [Google Scholar]
- 28. Wadsö I, Goldberg RN. 2001. Standards in isothermal microcalorimetry. Pure Appl. Chem. 73:1625–1639 [Google Scholar]
- 29. Wagman DD, Evans WH, Parker VB, Schumm RH, Halow I, Bailey SM, Churney KL, Nuttall RL. 1982. The NBS tables of chemical thermodynamic properties. Selected values for inorganic and C1 and C2 organic substances in SI units. J. Phys Chem. Ref. Data 11(Suppl 2):1–392 [Google Scholar]
- 30. Cook GM, Russell JB. 1994. Energy-spilling reactions of Streptococcus bovis and resistance of its membrane to proton conductance. Appl. Environ. Microbiol. 60:1942–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pannell DJ. 1997. Sensitivity analysis of normative economic models: theoretical framework and practical strategies. Agric. Econ. 16:139–152 [Google Scholar]
- 32. Loader C. 1999. Local regression and likelihood. Springer-Verlag, New York, NY [Google Scholar]
- 33. Isaacson HR, Hinds FC, Bryant MP, Owens FN. 1975. Efficiency of energy utilization by mixed rumen bacteria in continuous culture. J. Dairy Sci. 58:1645–1659 [DOI] [PubMed] [Google Scholar]
- 34. Hackmann TJ, Keyser BL, Firkins JL. 2013. Evaluation of methods to detect changes in reserve carbohydrate for mixed rumen microbes. J. Microbiol. Methods 93:284–291 [DOI] [PubMed] [Google Scholar]
- 35. Preiss J. 1989. Chemistry and metabolism of intracellular reserves, p 189–258 In Poindexter JS, Leadbetter ER. (ed), Bacteria in nature, vol 3 Plenum Press, New York, NY [Google Scholar]
- 36. Hall MB. 2011. Isotrichid protozoa influence conversion of glucose to glycogen and other microbial products. J. Dairy Sci. 94:4589–4602 [DOI] [PubMed] [Google Scholar]
- 37. Desvaux M. 2006. Unravelling carbon metabolism in anaerobic cellulolytic bacteria. Biotechnol. Prog. 22:1229–1238 [DOI] [PubMed] [Google Scholar]
- 38. Kajikawa H, Amari M, Masaki S. 1997. Glucose transport by mixed ruminal bacteria from a cow. Appl. Environ. Microbiol. 63:1847–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Bodegom P. 2007. Microbial maintenance: a critical review on its quantification. Microb. Ecol. 53:513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dawes E. 1985. Starvation, survival and energy reserves, p 43–79 In Fletcher M, Floodgate G. (ed), Bacteria in their natural environments. Society for General Microbiology, New York, NY [Google Scholar]
- 41. Pirt SJ. 1965. The maintenance energy of bacteria in growing cultures. Proc. R. Soc. Lond. B Biol. Sci. 163:224–231 [DOI] [PubMed] [Google Scholar]
- 42. Mackie R, Gilchrist F, Robberts A, Hannah P, Schwartz H. 1978. Microbiological and chemical changes in the rumen during the stepwise adaptation of sheep to high concentrate diets. J. Agric. Sci. 90:241–254 [Google Scholar]
- 43. Ryan RK. 1964. Concentrations of glucose and low-molecular-weight acids in the rumen of sheep changed gradually from a hay to a hay-plus-grain diet. Am. J. Vet. Res. 25:653–659 [PubMed] [Google Scholar]
- 44. Piwonka EJ, Firkins JL, Hull BL. 1994. Digestion in the rumen and total tract of forage-based diets with starch or dextrose supplements fed to Holstein heifers. J. Dairy Sci. 77:1570–1579 [DOI] [PubMed] [Google Scholar]
- 45. Clapperton JL, Czerkawski JW. 1969. Methane production and soluble carbohydrates in the rumen of sheep in relation to the time of feeding and the effects of short-term intraruminal infusions of unsaturated fatty acids. Br. J. Nutr. 23:813–826 [DOI] [PubMed] [Google Scholar]
- 46. National Research Council 2000. Nutrient requirements of beef cattle, 7th ed National Academies Press, Washington, DC [Google Scholar]
- 47. Argyle JL, Baldwin RL. 1989. Effects of amino acids and peptides on rumen microbial growth yields. J. Dairy Sci. 72:2017–2027 [DOI] [PubMed] [Google Scholar]
- 48. Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. 2008. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6:579–591 [DOI] [PubMed] [Google Scholar]
- 49. Pauss A, Andre G, Perrier M, Guiot SR. 1990. Liquid-to-gas mass transfer in anaerobic processes: inevitable transfer limitations of methane and hydrogen in the biomethanation process. Appl. Environ. Microbiol. 56:1636–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.