Abstract
The 2009 pandemic H1N1 (pH1N1) influenza virus carried a swine-origin hemagglutinin (HA) that was closely related to the HAs of pre-1947 H1N1 viruses but highly divergent from the HAs of recently circulating H1N1 strains. Consequently, prior exposure to pH1N1-like viruses was mostly limited to individuals over the age of about 60 years. We related age and associated differences in immune history to the B cell response to an inactivated monovalent pH1N1 vaccine given intramuscularly to subjects in three age cohorts: 18 to 32 years, 60 to 69 years, and ≥70 years. The day 0 pH1N1-specific hemagglutination inhibition (HAI) and microneutralization (MN) titers were generally higher in the older cohorts, consistent with greater prevaccination exposure to pH1N1-like viruses. Most subjects in each cohort responded well to vaccination, with early formation of circulating virus-specific antibody (Ab)-secreting cells and ≥4-fold increases in HAI and MN titers. However, the response was strongest in the 18- to 32-year cohort. Circulating levels of HA stalk-reactive Abs were increased after vaccination, especially in the 18- to 32-year cohort, raising the possibility of elevated levels of cross-reactive neutralizing Abs. In the young cohort, an increase in MN activity against the seasonal influenza virus A/Brisbane/59/07 after vaccination was generally associated with an increase in the anti-Brisbane/59/07 HAI titer, suggesting an effect mediated primarily by HA head-reactive rather than stalk-reactive Abs. Our findings support recent proposals that immunization with a relatively novel HA favors the induction of Abs against conserved epitopes. They also emphasize the need to clarify how the level of circulating stalk-reactive Abs relates to resistance to influenza.
INTRODUCTION
A critical determinant of clinical protection against influenza is the presence of preexisting functional antibodies (Abs) specific for viral hemagglutinin (HA), as detected in the traditional hemagglutination inhibition (HAI) assay. This is emphasized by the influenza pandemic of 1968, when an HA shift to H3 in a circulating H2N2 strain was the only structural protein change required to generate the pandemic virus. In 2009, the initial cases of human infection with a novel H1N1 influenza A virus of swine origin were soon followed by the rapid global spread of the virus and the declaration of an influenza pandemic (1, 2). The 2009 pandemic H1N1 (pH1N1) virus carried a classical swine H1 HA that was highly divergent from the HAs of seasonal H1N1 influenza A viruses circulating at the time. As a result, the neutralizing Abs generated by infection or vaccination with the recent seasonal H1N1 viruses had little activity against the pH1N1 virus (3, 4).
The classical swine H1 HA (as carried by the 2009 pH1N1 virus) and that of the 1918 human pandemic virus are antigenically similar (5), likely reflecting the establishment of an avian-source H1N1 virus in human and swine populations in 1918 (6). Since then, H1N1 viruses have been maintained in North American swine with little change in the HA. The H1N1 viruses that commenced circulating in humans in 1918 were subjected to the selective pressures of host immunity, resulting in a progressive antigenic drift in the HA. However, significant antigenic relatedness with the H1 of the 1918 virus was probably maintained in circulating H1N1 viruses until at least 1947 (7). Consequently, a high proportion of individuals over the age of about 60 years carried Abs that neutralized the 2009 pH1N1 virus and were largely spared significant disease in the 2009 pandemic (8). Recipients of the A/New Jersey/76 (H1N1) swine influenza vaccine in 1976 also carried pH1N1-neutralizing Abs because of the close antigenic relatedness of the H1 HA molecules (3, 9).
Studies of responses to inactivated pH1N1 vaccines have demonstrated that a single unadjuvanted dose induces protective HAI Ab levels in the majority of adults of all ages (10–13). These include young adults without preexisting HAI activity against pH1N1, indicating that exposure to recent seasonal H1N1 viruses is sufficient to prime for a B cell response to the pH1N1 virus. A recent analysis identified qualitative differences between the responses of young and elderly adults to pH1N1 vaccination consistent with differences in the history of exposure to influenza virus antigens (13). In the elderly subjects, Abs induced against the HA globular head domain displayed a broader epitope repertoire and bound with higher affinity. This probably reflects the selection and activation of memory B cells (MBCs) present only in elderly adults and generated by past exposure to related HA molecules.
In recent studies, pH1N1 vaccination preferentially induced broadly cross-reactive Abs, including HA head-reactive Abs that neutralized a range of H1N1 strains and HA stalk-reactive Abs with neutralizing activity against viruses of different subtypes (14–17). This contrasted with the largely strain-specific Ab response to seasonal influenza vaccination (16, 18, 19). In models proposed to explain these observations (14–16), the Ab response to the seasonal vaccine largely reflects activation of abundant memory B cells that recognize immunodominant (and variable) epitopes in the HA head. The lower number of memory B cells specific for conserved epitopes in the HA head and stem are outcompeted when the antigen is limiting, and they do not contribute to the response. The activation of memory B cells that recognize conserved subdominant HA epitopes is likely following pH1N1 vaccination in individuals who had not been exposed previously to related viruses because the dominant HA epitopes in the pH1N1 virus are substantially novel and the memory B cells responsive to the epitopes are lacking. Responses to the conserved HA epitopes in the pH1N1 virus are thus likely to be pronounced in individuals born in the period after about 1947, when related viruses were not circulating in humans. Consistent with this, Miller and colleagues (17) analyzed sera from non-pH1N1-exposed individuals born after about 1947 and identified higher levels of HA stalk-reactive Abs in recipients of the 1976 swine influenza vaccine than in individuals who did not receive the vaccine.
The current study was undertaken to relate age and immune history to the B cell response to an inactivated pH1N1 vaccine. We demonstrate a strong pH1N1-specific response to vaccination in adult subjects of all ages, including a young adult cohort selected to minimize the likelihood of prior exposure to a pH1N1-like virus. Consistent with current models, the response in the young cohort in particular included the production of stalk-reactive Abs. However, increased neutralizing activity against the seasonal H1N1 virus in the young cohort after pH1N1 vaccination appeared to be mediated primarily by HA head-reactive rather than stalk-reactive Abs.
MATERIALS AND METHODS
Study design and participants.
Healthy adult men and nonpregnant women were enrolled in 3 age cohorts: 18 to 32 years (n = 20), 60 to 69 years (n = 20), and ≥70 years (n = 18). The upper age limit of 32 years for subjects in the youngest cohort ensured that they had not received the antigenically cross-reactive A/New Jersey/76 vaccine. Older subjects with a known history of immunization with the A/New Jersey/76 vaccine were excluded. Subjects with a history of laboratory-documented infection with pH1N1 virus or immunization with a pH1N1 vaccine were ineligible for the study. There had been pH1N1 activity in the local community prior to commencement of the study, and we cannot exclude the possibility that the pH1N1-specific Abs in some subjects might have been generated by subclinical infection. Most subjects in each cohort reported receiving the 2009-2010 seasonal trivalent influenza vaccine 2 to 4 months before administration of the pH1N1 vaccine (13 of 20, 16 of 20, and 17 of 18 subjects in the 18- to 32-year, 60- to 69-year, and ≥70-year cohorts, respectively). The study was conducted under a protocol approved by the University of Rochester Research Subjects Review Board. Informed written consent was obtained from each participant.
Subjects received a single intramuscular (i.m.) injection of inactivated influenza A/California/07/2009 (H1N1) monovalent subunit vaccine (Novartis). Each 0.5-ml dose contained 15 μg of HA antigen. Administration of the vaccine (study day 0) took place from January to March 2010. Blood was collected from each subject on days 0, 7 (range, 6 to 8), and 28. The day 0 and day 28 samples provided sera for analysis of circulating Abs. Nasal secretions were sampled on days 0 and 28 by spraying saline into a nostril and absorbing fluid by nasal wick for 5 min. B cells were enriched from the day 7 blood samples and analyzed by an enzyme-linked immunosorbent spot (ELISpot) assay and flow cytometry. Baseline values for the cellular analysis were generated from a selection of day 0 samples.
ELISpot assay for Ab-secreting cells.
B cells were negatively enriched from heparinized whole blood by treatment with a RosetteSep human blood enrichment cocktail (Stem Cell Technologies, Vancouver, Canada), followed by density gradient separation according to the manufacturer's instructions. Vaccine-specific IgG and IgA Ab-secreting cells (ASCs) were enumerated by an ELISpot assay as described previously (20). Briefly, Immobilon P membrane-based 96-well plates (Millipore, Billerica, MA) were coated overnight at 4°C with a 1:10 dilution of the inactivated vaccine in phosphate-buffered saline (PBS) (100 μl/well). PBS only was added to the negative-control wells. Plates were blocked with complete medium (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 100 units/ml penicillin G, and 100 μg/ml streptomycin) before use. Enriched B cells were resuspended in complete medium containing either alkaline phosphatase-conjugated goat anti-human IgG (H + L) (KPL, Gaithersburg, MD) at 0.2 μg/ml or alkaline phosphatase-conjugated goat anti-human IgA (KPL) at 0.2 μg/ml for the detection of IgG or IgA ASCs, respectively. Serial 2-fold dilutions of the cell suspensions were prepared in the coated/blocked plates, and the plates were incubated for at least 4 h at 37°C in 5% CO2. The plates were washed, and spots representing IgG or IgA ASCs were developed with a Vector Blue alkaline phosphatase substrate kit (Vector Laboratories, Burlingame, CA). Spots were counted using a CTL ImmunoSpot plate reader and counting software (Cellular Technology Limited, Cleveland, OH). Counts are expressed as a frequency of CD19+ B cells.
Flow cytometry.
Enriched B cells were stained with the following panel of directly conjugated reagents at previously determined optimal concentrations: anti-CD3 PE (UCHT-1), anti-CD19 fluorescein isothiocyanate (FITC) (HIB19), anti-CD20 V450 (L27), anti-CD27 allophycocyanin (APC)-H7 (M-T271), and anti-CD138 APC (MI15) (all from BD Biosciences, San Jose, CA) and anti-CD38 phycoerythrin (PE)-Cy7 (HIT2) (eBioscience, San Diego, CA). A LIVE/DEAD fixable violet staining kit (Invitrogen, Camarillo, CA) was used to discriminate dead cells. Data were acquired using an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Hemagglutination inhibition and microneutralization assays.
HAI and microneutralization (MN) assays were performed against the 2009 pH1N1 isolate A/California/04/09 and the seasonal H1N1 virus A/Brisbane/59/07 (Bris/07). Viruses were obtained from the Centers for Disease Control and Prevention (CDC) (Atlanta, GA) and were expanded in eggs prior to use. HAI titers were also measured against a reassortant H6N1 virus that expressed the HA of A/mallard/Sweden/81/02. A stock of the H6N1 reassortant virus was grown in eggs and inactivated prior to use. Anti-H6 mouse serum served as a positive control. Serum samples were pretreated by overnight incubation at 37°C with receptor-destroying enzyme (Denka Seiken, Tokyo, Japan), followed by heating at 56°C for 30 min.
The HAI assay was based on a standard procedure (20). Briefly, serial 2-fold dilutions of pretreated sera in 96-well V-bottom microtiter plates were mixed with 4 HA units of virus. After incubation at room temperature for 1 h, 50 μl of 0.75% turkey red blood cells was added to each well and mixed gently. Plates were then held at 4°C for a minimum of 45 min before the HAI titers were read. The HAI titer was defined as the reciprocal of the highest serum dilution with no HA activity. For each sample, the baseline HAI activity was determined by incubating only serum and red blood cells. These titers ranged from 4 to 8 in all cohorts and were used for calculating the fold increase in the HAI titer.
The MN assay was performed according to a protocol provided by the CDC. Briefly, 100 50% tissue culture infective doses (TCID50s) of virus (50 μl/well) was added to serial 2-fold dilutions of pretreated sera (10 replicate wells/dilution; 50 μl/well) in 96-well flat-bottom tissue culture plates. Dilutions of virus and sera were prepared in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% bovine serum albumin (BSA), antibiotics, and 20 mM HEPES (designated virus diluent). After gentle mixing, the plates were incubated at 37°C for 1 h. MDCK cells (1.5 × 105 cells/ml) in virus diluent were then added (100 μl/well), and the plates were incubated at 37°C for 18 to 20 h. After incubation, the wells were washed with PBS and fixed with 80% acetone in PBS. An enzyme-linked immunosorbent assay (ELISA) to detect viral nucleoprotein (NP) expression in MDCK cells was used to identify viral replication. Briefly, the plates were washed after fixing, and an anti-NP monoclonal antibody (MAb) (Millipore) was added to the wells. Bound Ab was detected by the addition of horseradish peroxidase-conjugated goat anti-mouse IgG (KPL), followed by o-phenylenediamine dihydrochloride substrate tablets in citrate buffer (Sigma-Aldrich, St. Louis, MO). The reaction was stopped with 0.5 M sulfuric acid, and the absorbance was read at 490 nm. Wells were scored as positive for neutralizing activity if the absorbance was less than the sum of the median absorbance of the virus control wells (virus plus MDCK cells only) and the median absorbance of cell control wells (MDCK cells only). The 50% neutralizing Ab titer (MN titer) was determined from the proportions of positive and negative wells. An MN titer of 5 was assigned to the samples in which the lowest dilution in the assay (1/10) was negative.
Kinetic ELISA for nasal Abs.
HA-specific IgA in nasal secretions collected by the nasal wick procedure was measured by a kinetic ELISA (21). Briefly, 96-well ELISA plates were coated with purified 2009 pH1N1 HA (Novartis, Liverpool, United Kingdom) and incubated at 4°C for 18 h. The plates were washed and blocked, and then the samples extracted from the nasal wicks were added to duplicate wells. The samples added to uncoated wells were used to determine the assay background. After incubation at 4°C for 18 h, the assay was completed by adding, in sequence, biotinylated goat anti-human IgA (Invitrogen), streptavidin-conjugated horseradish peroxidase (Sigma-Aldrich), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) substrate (Sigma-Aldrich). The rate of color development (milliunits of optical density [mOD]/min) was determined by readings at 414 nm taken every 9 s for 5 min using a SpectraMax Plus microplate reader (Molecular Devices, Sunnyvale, CA) and analyzed using SoftMax for Windows software (Molecular Devices). A similar kinetic ELISA using plates coated with anti-human IgA (SouthernBiotech, Birmingham, AL) was used to measure the total IgA levels in the nasal samples. The concentration of total IgA was calculated from a curve constructed with an IgA standard from human colostrum (Sigma-Aldrich). HA-specific IgA titers were adjusted for the levels of total IgA and are expressed as mOD/min/μg total IgA.
ELISA for serum Abs.
Abs reactive with the HA stalk or the intact HA of pH1N1 were detected by an ELISA. A chimeric HA consisting of the head domain of an H6 HA and the stalk domain of PR8 (H1) virus (cH6/1) was generated as described previously (22). The head domain of the chimeric HA was derived from the H6 HA of A/mallard/Sweden/81/02. Purified full-length recombinant HA from influenza A/California/04/09 (H1N1) (NR-15258) was obtained from NIH/NIAID Biodefense and Emerging Infections (BEI) Resources. Immulon 4HBX 96-well plates were coated with recombinant HA (0.05 μg/well) or with BSA as a negative control. Briefly, ELISAs were completed by the addition of serial 3-fold dilutions of the test sample and then horseradish peroxidase-conjugated goat anti-human IgG (SouthernBiotech), followed by 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB) substrate (BioLegend, San Diego, CA). The reaction was stopped with 1 M H2SO4, and the well absorbance was read at 450 nm. Titers are expressed as the reciprocal of the highest dilution giving an absorbance of greater than twice the value for a similar titration in the negative-control wells.
Statistics.
For statistical analyses, data were transformed to stabilize variances and approximate normal distributions. Log transformation was used for IgG and IgA ASC frequencies, HAI titers, and MN titers; inverse hyperbolic transformation was used for the percentages of CD38high CD27high and CD38high CD138+ cell subsets. Analysis of variance (ANOVA) was used for multiple between-cohort comparisons. Repeated ANOVA was used for paired comparisons of titers collected over time. Correlations within the cohorts were evaluated using the Spearman correlation test. Linear regression modeling with model selection based on the likelihood ratio test was used to assess (i) the relationship between prevaccination pH1N1-specific Ab titers and the fold change in titer and (ii) the relationship between early cellular measurements and the fold change in the HAI titer. P values of <0.05 were considered statistically significant.
RESULTS
Antigen-specific B cell response to pH1N1 vaccination.
The antigen-specific B cell response to vaccination was evaluated in subjects in three age cohorts: 18 to 32 years, 60 to 69 years, and ≥70 years. The pre- and postvaccination levels of pH1N1-specific Abs in the circulation and at the nasal mucosa were determined. In addition, the early appearance of circulating ASCs specific for vaccine components was monitored, since a transient wave of these cells peaking on approximately day 7 is a feature of the response to i.m. influenza vaccination in adults (23).
Circulating and nasal wash Ab levels.
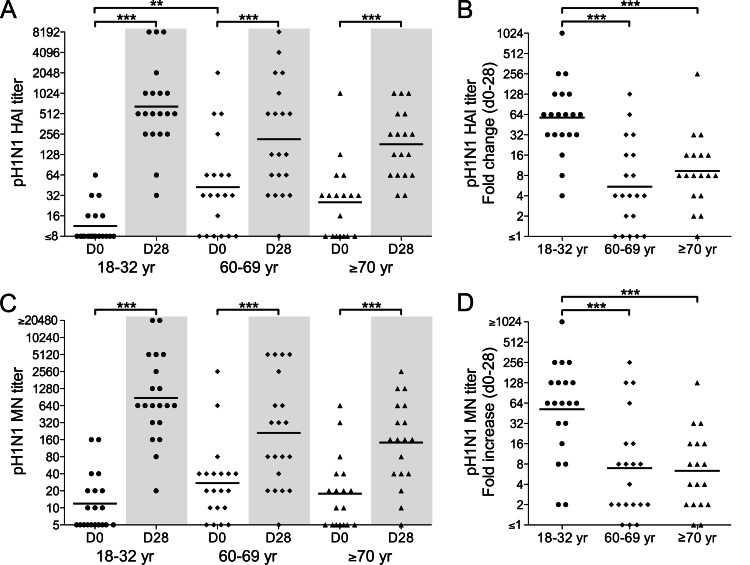
Sera collected on study days 0 and 28 were analyzed for pH1N1-specific Abs by HAI and MN assays (Fig. 1). On day 0, the trend was for HAI titers that were higher in the older cohorts than in the 18- to 32-year cohort, but the difference was only significant for the 60- to 69-year cohort. This pattern was less marked for the MN titers on day 0. Overall, the day 0 titers were consistent with greater prevaccination exposure to pH1N1-like viruses in the older cohorts.
Fig 1.
Ab responses to an inactivated pH1N1 vaccine in the 18- to 32-year (circles), 60- to 69-year (diamonds), and ≥70-year (triangles) age cohorts. The titers of pH1N1-specific Abs in serum on days 0 and 28 after vaccination were measured by HAI (A and B) and MN (C and D) assays. The titers on days 0 and 28 (A and C) and fold changes in titers from days 0 to 28 (B and D) are shown for individual subjects. Bars identify geometric mean titers (GMTs) (A and C) or geometric means of fold increases (B and D). Within-cohort statistical analyses represent paired comparisons of day 0 and day 28 titers. Only statistically significant differences are indicated: **, P < 0.01, and ***, P < 0.001.
Vaccination resulted in a highly significant increase in the HAI and MN titers in all cohorts (P < 0.0001 for paired comparisons of day 0 and day 28 titers). HAI titers increased ≥4-fold in the majority of subjects in each cohort, indicating an effective seroresponse (Fig. 1B and D). This included 14 of the 15 subjects in the older cohorts with HAI titers of ≤16 on day 0. Notably, the fold increase in HAI and MN titers in the 18- to 32-year cohort was significantly greater than those in the other cohorts.
We used linear regression modeling to evaluate whether the pH1N1-specific Ab response to vaccination related to the prevaccination titers. For each cohort, there was a negative correlation between the HAI titer on day 0 and the fold increase in the HAI titer after vaccination (P = 0.009), suggesting a dampening of the response by preexisting Abs. A similar trend was evident for MN titers, but the correlations were not significant (P = 0.1697).
The nasal secretions sampled on days 0 and 28 were tested by ELISAs for IgA Abs specific for the 2009 pH1N1 HA. Day 0 titers were similar in all cohorts and were not significantly increased on day 28 (Fig. 2).
Fig 2.
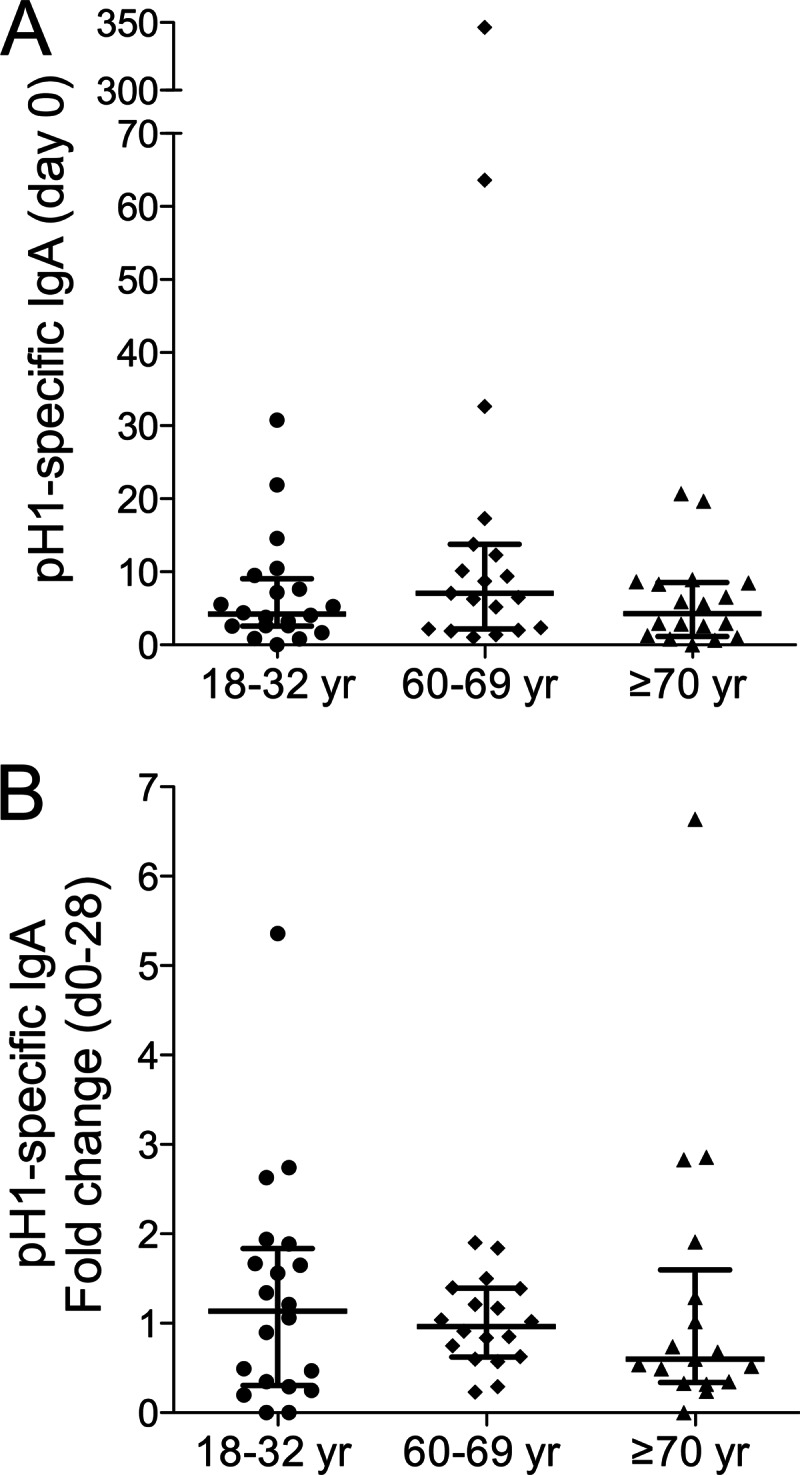
Virus-specific IgA in nasal secretions after pH1N1 vaccination. Nasal secretions sampled on days 0 and 28 after vaccination of the indicated age cohorts (18 to 32 years, 60 to 69 years, and ≥70 years) were tested by an ELISA for pH1-specific IgA. Titers of pH1-specific IgA (mOD/min/μg total IgA) on day 0 (A) and fold changes in the titers from days 0 to 28 (B) are shown for individual subjects. The median values (bar) and interquartile ranges are identified.
Cellular responses in peripheral blood.
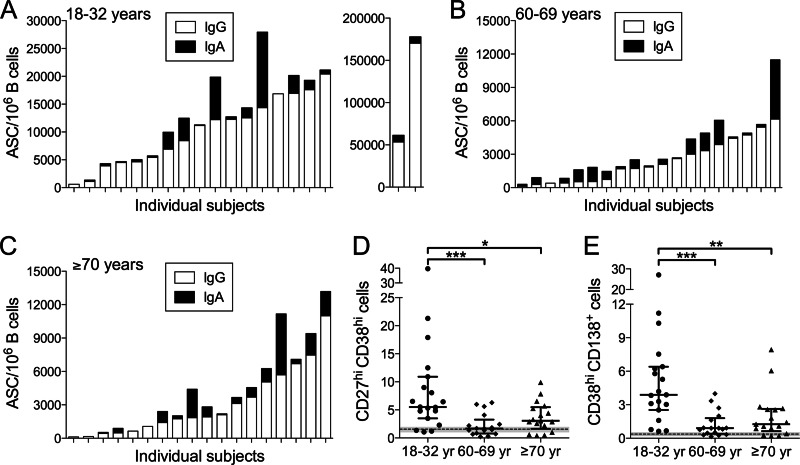
To evaluate early ASC formation, B cells in blood collected on day 7 were enriched to >90% purity and analyzed by an ELISpot assay and flow cytometry. The majority of subjects in all cohorts responded to vaccination with vaccine-specific IgG and IgA ASC formation (Fig. 3A to C). However, the IgG ASC frequency (per 106 B cells) of 9,640 (geometric mean) (95% confidence interval, 5,299 to 17,537) in the 18- to 32-year cohort was significantly higher than the frequencies in the 60- to 69-year cohort (1,440 [829 to 2,501], P < 0.0001) and the ≥70-year cohort (1,649 [861 to 3,160], P < 0.0001). The IgA ASC frequencies in the 18- to 32-year (591 [162 to 2,151]), 60- to 69-year (362 [150 to 871]), and ≥70-year (168 [38 to 749]) cohorts were not significantly different.
Fig 3.
Circulating ASCs induced by pH1N1 vaccination. B cells enriched from blood were analyzed by an ELISpot assay for vaccine-specific ASCs and by flow cytometry for ASC phenotypes. (A to C) Vaccine-specific ASCs. IgG and IgA ASC frequencies on days 6 to 8 after vaccination are shown for individual subjects in the 18- to 32-year (A), 60- to 69-year (B), and ≥70-year (C) age cohorts. Vaccine-specific ASCs were not detected in the day 0 samples. (D and E) Flow cytometric identification of ASCs. Frequencies of CD38high CD27high cells (plasmablasts) (D) and CD38high CD138+ cells (plasma cells) (E) represent the percentages of CD3− CD19+ cells. Frequencies on days 6 to 8 after vaccination are shown for individual subjects in the indicated age cohorts (18 to 32 years, 60 to 69 years, and ≥70 years). The median values (bars) and interquartile ranges are identified. Day 0 values did not differ between cohorts and are shown as the means (dashed lines) and 95% confidence intervals (CIs) (shaded zones). Only statistically significant differences in the ASC frequencies are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Flow cytometric analysis of enriched CD19+ B cells from the blood focused on the plasmablast population (CD19+ CD38high CD27high) and a subset of plasmablasts (designated plasma cells) that expressed the differentiation marker CD138 (CD19+ CD38high CD138+) (18, 24). These cell populations were uniformly CD20low/−. The day 0 plasmablast and plasma cell frequencies were similar between age cohorts and consistent with the very low numbers of these cells present under steady-state conditions in the peripheral blood of healthy adults (24). On day 7 after vaccination, the frequencies of circulating plasmablasts and plasma cells were significantly higher in the 18- to 32-year cohort than in the 60- to 69-year and the ≥70-year cohorts (Fig. 3D and E), consistent with the pattern for vaccine-specific IgG ASCs.
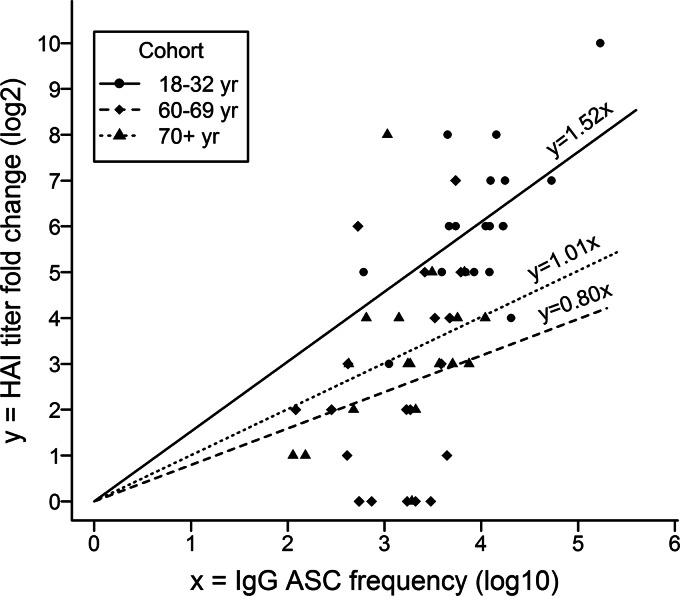
We applied linear modeling to evaluate whether the day 7 analysis of circulating cells by an ELISpot assay and flow cytometry was predictive of the HAI Ab response to vaccination. In each cohort, there was a strong positive correlation between the virus-specific IgG ASC frequency and the fold increase in the HAI titer (Fig. 4). The intercepts for all regression lines were zero, but the slope for the 18- to 32-year cohort was significantly greater than those for the 60- to 69-year cohort (P < 0.0001) and the ≥70-year cohort (P = 0.0018), indicating a greater fold increase in the HAI titer for a given IgG ASC frequency in the 18- to 32-year cohort than in the older cohorts. Plasmablast and plasma cell frequencies were positively correlated with the fold increase in the HAI titer in the 18- to 32-year cohort (P = 0.0351 and 0.0218 for plasmablasts and plasma cells, respectively) but not in the older cohorts.
Fig 4.
Relationship between vaccine-specific IgG ASCs and the HAI response. A linear regression model assuming different coefficients for different age cohorts was fitted to evaluate the relationship between the frequency (log 10) of circulating vaccine-specific IgG ASCs (per 106 B cells) on days 6 to 8 after pH1N1 vaccination and the fold change (log 2) in the pH1N1-specific HAI titer from days 0 to 28. The likelihood ratio test indicated zero intercepts for all regression lines (P = 0.88). The fitted model explained 87% of the variability of the data (R2 ≈ 0.87). For each cohort, a strong positive correlation existed between the virus-specific IgG ASC frequency and the fold change in the HAI titer (P < 0.0001). The slope was significantly greater for the 18- to 32-year cohort than for the 60- to 69-year cohort (P < 0.0001) and the ≥70-year cohort (P = 0.0018); the slopes for the 60- to 69-year and ≥70-year cohorts were not significantly different.
pH1N1 vaccination induces HA stalk-reactive Abs in young adults.
We used an ELISA strategy to investigate the production of stalk-binding Abs in the three age cohorts after pH1N1 vaccination. A chimeric HA molecule (cH6/1) with an exotic head domain that was novel to most of the human population was used as a coating reagent so that bound Abs were most likely directed against the stalk region. Consistent with this reasoning, all samples collected on day 28 after vaccination were HAI negative (titers < 4) against an H6N1 virus that carried the same H6 head domain as cH6/1. Pica and colleagues (22) found little reactivity with cH6/1 in sera from non-pH1N1-infected adults. However, we found a greater range of cH6/1-binding activity, including low activity, in a preliminary analysis of sera from adults in a healthy-donor program (data not shown).
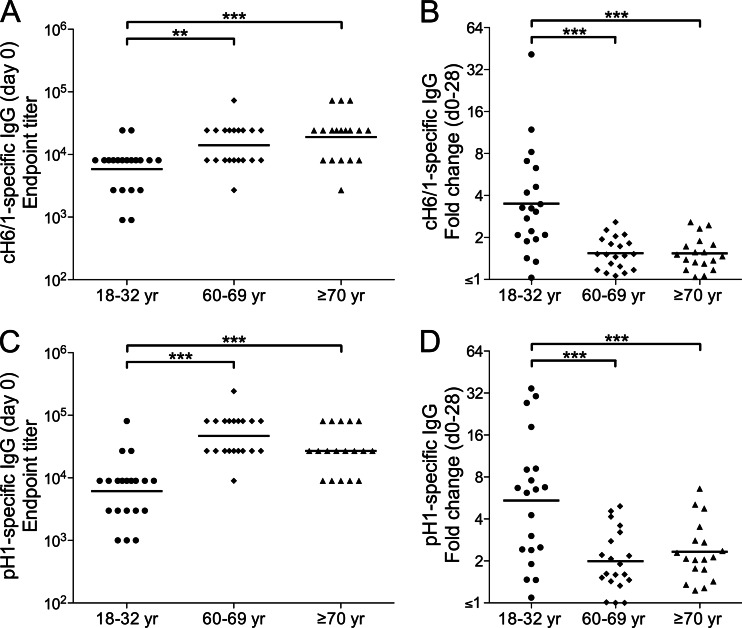
IgG Abs that bound to cH6/1 were present in all subjects on day 0, with significantly higher titers in the older cohorts (Fig. 5A). The increase in anti-cH6/1 IgG titers on day 28 was highly significant in the 18- to 32-year cohort (P < 0.0001 for a paired comparison of day 0 and day 28 titers), indicating the induction of Abs reactive with the HA stalk. In addition, the fold increase in anti-cH6/1 IgG titers in the 18- to 32-year cohort was significantly greater than those in the other cohorts (Fig. 5B). There was a strong positive correlation between the fold increase in anti-cH6/1 IgG titers and the fold increase in pH1N1-specific HAI titers in the 18- to 32-year cohort (r = 0.724, P = 0.0003), indicating parallel development of responses against HA head and stalk regions. Although cH6/1-specific IgG titers were also significantly increased on day 28 in the older cohorts (P = 0.0009 and 0.0490 for the 60- to 69-year and ≥70-year cohorts, respectively, in paired comparisons of day 0 and day 28 titers), the fold increases were relatively small.
Fig 5.
Induction of HA stalk-reactive Abs by pH1N1 vaccination. Serum titers of IgG reactive with the chimeric HA molecule cH6/1 (A and B) or with the intact HA (pH1) of the vaccine virus (C and D) were measured by ELISAs on days 0 and 28 after vaccination of the indicated age cohorts (18 to 32 years [circles], 60 to 69 years [diamonds], and ≥70 years [triangles]). The titers were determined by endpoint titration and are expressed as the reciprocal of the serum dilution. Fold changes in the titer were calculated from OD ratios for dilutions in the linear regions of serum titration curves. The titers on day 0 (A and C) and the fold changes in titer from days 0 to 28 (B and D) are shown for individual subjects. Bars identify geometric mean titers (GMTs) (A and C) or geometric means of the fold increase (B and D). Only statistically significant differences are indicated: **, P < 0.01, and ***, P < 0.001.
We also performed ELISAs using plates coated with pH1, the entire HA molecule of the pH1N1 virus. In all cohorts, the levels of anti-pH1 IgG before and after vaccination reflected the situation for pH1N1-specific HAI and MN titers. On day 0, pH1-specific IgG titers were significantly higher in the older cohorts than in the 18- to 32-year cohort (Fig. 5C). After vaccination, there was a clear increase in pH1-specific IgG in all cohorts (P < 0.0001, P = 0.0029, and P < 0.0001 for the 18- to 32-year, 60- to 69-year, and ≥70-year cohorts, respectively, in paired comparisons of day 0 and day 28 titers), but this increase was most marked in the young cohort (Fig. 5D). Notably, pH1-specific IgG was readily measurable by ELISAs in all subjects in the 18- to 32-year cohort on day 0, even though pH1N1-specific HAI titers in the majority of these subjects were below the limit of detection.
pH1N1 vaccination enhances serum Ab activity against a seasonal H1N1 influenza virus in young adults.
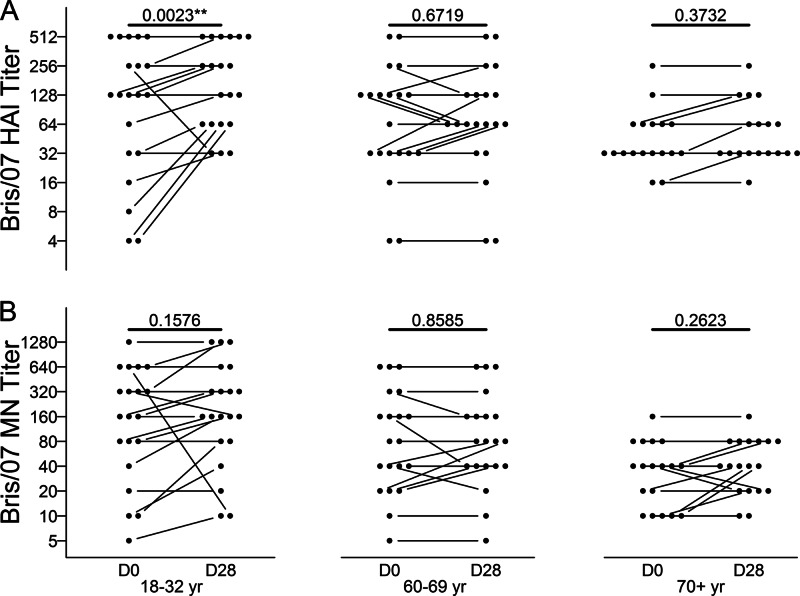
To investigate the induction of broad anti-influenza activity by pH1N1 vaccination, we assessed the effect of vaccination on HAI and MN titers against the heterovariant H1N1 influenza virus Bris/07, a recent circulating seasonal strain. A more pronounced effect of vaccination on MN than on HAI activity would be consistent with the activity of stalk-reactive Abs, since these Abs are not detected in the HAI assay. In all cohorts on day 0, the mean HAI and MN titers against Bris/07 were relatively high and likely reflected recent infection or vaccination with the homologous virus (Fig. 6). Overall, the effect of pH1N1 vaccination on anti-Bris/07 HAI or MN titers was small. However, the increase in anti-Bris/07 HAI titers after vaccination was statistically significant in the 18- to 32-year cohort. Although this was not also the case for anti-Bris/07 MN titers in the 18- to 32-year cohort, there was a positive correlation between the fold increases in anti-Bris/07 HAI and MN titers (r = 0.641, P = 0.0023). Taken together, our results point to a major contribution of HA head-reactive, rather than stalk-reactive, Abs to any increase in anti-Bris/07 MN titers in the young cohort. Notably perhaps, the anti-Bris/07 HAI and MN titers on day 0 in the 18- to 32-year cohort were inversely correlated with the fold increases in these titers after pH1N1 vaccination (r = −0.747 and P = 0.0002 for HAI titers; r = −0.565 and P = 0.0094 for MN titers), raising the possibility that high preexisting levels of anti-Bris/07 Abs masked small increases in the titers of these Abs.
Fig 6.
HAI and MN titers against the seasonal H1N1 virus Bris/07 after pH1N1 vaccination. Bris/07-specific HAI (A) and MN (B) titers in serum were determined on days 0 and 28 after vaccination of the indicated age cohorts (18 to 32 years, 60 to 69 years, and ≥70 years). Titers are shown for individual subjects; paired titers are connected. The P values for paired comparisons of day 0 and day 28 titers are shown.
DISCUSSION
The goal of the current study was to relate age (and associated differences in immune history) to the B cell response to a single dose of inactivated 2009 pH1N1 vaccine. The subjects were analyzed in three age cohorts representing young adults through the elderly. The cohort of young adults (aged 18 to 32 years) had little likelihood of prior exposure to pH1N1-like viruses, which had not circulated in human populations from approximately 1950 until 2009. An upper age limit of 32 years for this cohort ensured that the subjects had not received the pH1N1-like swine flu vaccine in 1976. Subjects with laboratory-documented pH1N1 infection or a recent influenza-like illness were ineligible for the study, but there had been pH1N1 activity at the study site, and we cannot exclude the possibility that some subjects experienced subclinical infection. However, the baseline levels of pH1N1-specific HAI and MN Abs were very low in the young subjects in the current study, consistent with previous studies of sera collected from young adults prior to the emergence of the pH1N1 virus (3, 4). Thus, the expectation was that the young cohort would have little B cell memory for the HA of the pH1N1 virus, especially for determinants in the head region of the molecule (25). Nevertheless, a single dose of pH1N1 vaccine elicited an effective seroresponse (a ≥4-fold increase in the pH1N1-specific HAI or MN titers) in the majority of subjects in each of the age cohorts in the current study. Indeed, the response was significantly greater in the young cohort than in the older cohorts. Similar results were reported elsewhere (10–13).
A vigorous Ab response to influenza vaccination results from the activation of preexisting memory B cells (MBCs) (15, 23). It follows that the strong increase in the pH1N1-specific HAI titers in the 18- to 32-year cohort after pH1N1 vaccination reflected the activation of MBCs reactive with the HA head region. Such cells are clearly present in these subjects (15, 26), even though preexisting pH1N1-specific HAI Abs might not be detectable. Notably, Li and colleagues (15) demonstrated that many IgG-secreting plasmablasts generated early in the response to pH1N1 vaccination produced Abs that bound to the HA of the H1N1 strain Bris/07, indicating activation of cross-reactive MBCs generated by seasonal influenza viruses. Our demonstration of circulating IgG reactive with the pH1N1 HA (pH1) in all subjects in the 18- to 32-year cohort prior to pH1N1 vaccination is consistent with the presence of cross-reactive MBCs capable of responding to pH1, although we did not measure the prevaccination levels of IgG reactive only with the head of pH1. There is evidence that MBCs have broader reactivity than long-lived ASCs generated in the response to the same antigen (27). Thus, the characteristics of circulating Abs prior to vaccination might not necessarily reflect the potential for an MBC response. In addition, activated MBCs might undergo additional rounds of somatic hypermutation and selection to increase affinity for target epitopes, further distinguishing Abs accumulating in response to vaccination from those generated previously (23, 28). Because of past exposures to pH1N1-like viruses, the older subjects theoretically carried an even greater number of MBCs reactive with the pH1N1 HA head than did the young subjects. It is unclear to what extent the response to vaccination in the older cohorts was reduced by age-related factors (29) or higher preexisting pH1N1-specific Ab levels (30).
An early indicator of the MBC response to i.m. influenza vaccination in humans is the transient wave of circulating ASCs that peaks after approximately 7 days (23). We evaluated this early cellular response with the goal of relating it to the standard measurement of vaccine effectiveness, namely, the fold increase in the HAI titer. A strong positive correlation was identified between the peak frequency of circulating virus-specific IgG ASCs after vaccination and the magnitude of the HAI response (15). Using linear modeling, we found that this correlation was equally strong in the three age cohorts in our analysis. However, a given IgG ASC frequency predicted a greater fold increase in the HAI titer in the young cohort than in the older cohorts. This relationship reflects the response magnitude and the Ab affinity. The amount of Ab secreted by an individual ASC is approximately the same in young and elderly subjects (31). There is evidence that ASCs induced by pH1N1 vaccination produce higher-affinity HA head-reactive Abs in older subjects (13, 16), but this difference probably diminishes with affinity maturation of the response in the younger subjects. Overall, it is likely that the difference between age cohorts in the relationship between the ASC frequency and HAI fold increase primarily reflects a numerically greater ASC response to vaccination in the young cohort. It has been proposed that the ability of the bone marrow to support plasma cell survival decreases with age (32), but any impact of this on the vaccine-induced circulating Ab levels in our analysis is unclear. Non-antigen-specific measurements of circulating plasmablast and plasma cell frequencies by flow cytometry were much less reliable predictors of an effective HAI response to vaccination.
The circulating virus-specific ASCs on day 7 after pH1N1 vaccination frequently included a substantial number of IgA producers in all cohorts. However, there was no associated increase in IgA specific for the viral HA in the nasal secretions sampled on day 28. This is consistent with evidence that virus-specific ASC numbers in the nasal mucosa do not increase in response to i.m. influenza vaccination (33). Mucosal homing molecules might not be expressed by IgA ASCs generated in association with the predominantly IgG response to i.m. vaccination (34). Interestingly, day 0 levels of pH1-specific IgA in nasal secretions were similar in all cohorts, in marked contrast to the significantly higher levels of circulating pH1-specific IgG in the older cohorts than in the young cohort. In part, this might reflect a difference in the duration of systemic IgG and mucosal IgA production after influenza infection or prior vaccination (35).
Recent studies demonstrated that infection or vaccination with the 2009 pH1N1 virus favors induction of broadly cross-reactive HA-specific Abs, including Abs reactive with the HA stalk (14–18). Importantly, our analysis adds to the limited available information on circulating levels of stalk-reactive Abs before and after pH1N1 exposure. We measured the stalk-reactive Abs by ELISAs using the chimeric cH6/1 HA (consisting of the H6 head domain and the H1 stalk domain) as a coating reagent. Other studies using this strategy found little serum reactivity to cH6/1 in young adults without exposure to pH1N1 or an antigenically related virus (17, 22), whereas our preliminary experiments demonstrated a range of cH6/1 binding levels in sera from healthy donors. On day 0 in the current analysis, stalk-reactive IgG was clearly present in all subjects in the 18- to 32-year cohort, and the titers were significantly higher in the older cohorts. Consistent with this, Khurana and colleagues (13) identified Abs reactive with the HA stalk region in subjects prior to pH1N1 vaccination, including subjects seronegative for the virus. Sui and colleagues (36) showed that sera from non-H5-exposed subjects contained H5-reactive IgG as well as Abs that bound to group 1 HA stalks. Notably, the titers of H5-reactive IgG, which might largely reflect binding to the HA stalk (37), were comparable to the anti-cH6/1 titers in our analysis. H5-reactive and group 1 HA stalk-reactive Abs have also been demonstrated in intravenous immunoglobulin (IVIG), consisting of pooled IgG from thousands of (most likely) H5-naive individuals (36, 38). The presence of H5-binding Abs in pooled sera from adults but not from children suggests progressive acquisition with increasing age and influenza exposure (38). There is evidence that seasonal influenza viruses induce at least some stalk-reactive Ab production (16, 39, 40). Occasionally, a more marked increase in titers might occur, depending on the form of exposure and degree of HA novelty. This process is likely to contribute to the day 0 levels of stalk-reactive Abs measured in our analysis and also to account for the higher titers in older cohorts. It might be relevant that many study subjects received the seasonal influenza vaccine 2 to 5 months prior to the pH1N1 vaccine. Notably perhaps, in the 18- to 32-year cohort, there was a modest correlation between day 0 HAI titers against Bris/07 (a component of the seasonal vaccine) and day 0 titers of anti-stalk Abs (r = 0.458, P = 0.042), consistent with some anti-stalk Ab induction by the seasonal vaccine.
The pH1N1 vaccine elicited a highly significant increase in anti-stalk Ab levels in the 18- to 32-year cohort, but this effect was much less pronounced in the older cohorts. These findings are consistent with current models relating the strength of the responses to conserved epitopes, such as stalk determinants, with the novelty of the HA (14–16). It is suggested that an individual exposed to a succession of related HAs develops a growing population of MBCs reactive with immunodominant (and variable) head epitopes. As these cells become abundant, they outcompete less frequent MBCs specific for conserved epitopes and come to dominate the Ab responses to related HAs, accounting for the largely strain-specific responses to seasonal influenza viruses. The situation is altered when an individual is exposed to an HA with head epitopes that are substantially different from those experienced previously; MBCs specific for conserved epitopes are then freed from competitive restraints and contribute to the HA-specific Ab response. Our results for the young cohort after pH1N1 vaccination fit with the latter scenario, whereas the HA head-focused responses in the older cohorts are consistent with activation of predominantly head-reactive MBCs generated by past exposures to pH1N1-like viruses. It is noteworthy that the 18- to 32-year cohort not only produced stalk-reactive Abs but also generated a vigorous response to the head of the pH1N1 HA (as measured by HAI titers). Thus, stalk-reactive Ab production is not completely inhibited by the presence of an apparently substantial MBC population reactive to HA head determinants.
The vigorous response to pH1N1 vaccination with the production of anti-stalk Abs in the 18- to 32-year cohort suggested that broad anti-influenza activity might also be increased. We assessed this by evaluating changes in the HAI and MN activity against the seasonal H1N1 strain A/Bris/07. The sensitivity of this analysis was probably limited in subjects with high preexisting HAI and MN titers against Bris/07. However, in the subjects with low preexisting anti-Bris/07 HAI and MN titers, the trend was for an increase in anti-Bris/07 activity after pH1N1 vaccination, consistent with a broadening of protection. Notably, an increase in the anti-Bris/07 MN titer was generally associated with an increase in the anti-Bris/07 HAI titer. This suggests an effect due predominantly to cross-reactive Abs against the HA head determinants, since stalk-reactive Abs would be detected only by the MN assay, whereas the head-reactive Abs that mediate HAI are also likely to be neutralizing. Our observations fit with recent studies demonstrating that the HA-specific Abs generated by pH1N1 vaccination include not only stalk-reactive Abs but also head-reactive Abs with activity against a range of H1N1 variants (14, 15).
In summary, our analysis provides a comprehensive picture of the B cell response to a pH1N1 vaccine in age cohorts differing in their history of exposure to pH1N1-like viruses. One goal was to establish associations between the serological, cellular, and mucosal aspects of the response. Importantly, we analyzed the pre- and postvaccination levels of Abs reactive with the HA stalk and asked whether changes in these levels related to a broadening of anti-influenza activity. It has been proposed that the induction of cross-reactive anti-stalk Abs by pH1N1 during the 2009 pandemic boosted immunity to seasonal H1N1 viruses and contributed to their disappearance (41). In our analysis, stalk-reactive Ab levels in the young cohort were significantly increased after pH1N1 vaccination, but this was not associated with increased neutralizing activity against a seasonal influenza virus. Perhaps the increase in anti-stalk Ab levels is greater following pH1N1 infection than following vaccination. Another possibility is that the MN assay used in our study was less sensitive for detecting influenza neutralization by stalk-reactive than by head-reactive Abs (39). In addition, the MN assay strategy allowed for only a single cycle of viral replication and thus was limited to the detection of Abs that block cell infection. This approach would not have detected anti-stalk Abs that act at later stages in the production of infectious viruses (42). Functional analyses of anti-stalk Abs will be facilitated by the recent development of recombinant viruses expressing chimeric HA molecules with exotic head domains (22). Nevertheless, our analysis emphasizes the need to clearly establish the relationship between circulating stalk-reactive Ab levels and resistance to influenza. This is particularly important in light of the recent intense interest in the development of broadly protective influenza vaccines based on the induction of stalk-reactive Abs (43).
ACKNOWLEDGMENTS
We thank the staff of the University of Rochester Vaccine Research Unit for subject enrollment, vaccine administration, and sample collection, Lu Wang (University of Rochester) for assistance with statistical analyses and figure preparation, and Kristin Scheible (University of Rochester) for reviewing the manuscript.
This research was supported by the Intramural Research Program of the NIH (NIAID), the Centers of Excellence for Influenza Research and Surveillance (CEIRS) at the University of Rochester (grant HHSN266200700008C) and the Mount Sinai School of Medicine (grant HHSN266200700010C), and the Center for Biodefense Immune Modeling at the University of Rochester (grant HHSN27221000055C).
Footnotes
Published ahead of print 10 April 2013
REFERENCES
- 1. Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neumann G, Noda T, Kawaoka Y. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz JM. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361:1945–1952 [DOI] [PubMed] [Google Scholar]
- 4. Skowronski DM, Hottes TS, McElhaney JE, Janjua NZ, Sabaiduc S, Chan T, Gentleman B, Purych D, Gardy J, Patrick DM, Brunham RC, De Serres G, Petric M. 2011. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. J. Infect. Dis. 203:158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE, Jr, Wilson IA. 2010. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 328:357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmer SM, Burke DS. 2009. Historical perspective—emergence of influenza A (H1N1) viruses. N. Engl. J. Med. 361:279–285 [DOI] [PubMed] [Google Scholar]
- 7. O'Donnell CD, Wright A, Vogel LN, Wei CJ, Nabel GJ, Subbarao K. 2012. Effect of priming with H1N1 influenza viruses of variable antigenic distances on challenge with 2009 pandemic H1N1 virus. J. Virol. 86:8625–8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Girard MP, Tam JS, Assossou OM, Kieny MP. 2010. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine 28:4895–4902 [DOI] [PubMed] [Google Scholar]
- 9. McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM. 2010. Recipients of vaccine against the 1976 “swine flu” have enhanced neutralization responses to the 2009 novel H1N1 influenza virus. Clin. Infect. Dis. 50:1487–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, Zhang XF, Pan HX, Meng FY, Hu YM, Liu WD, Li CG, Li W, Zhang X, Hu JM, Peng WB, Yang BP, Xi P, Wang HQ, Zheng JS. 2009. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 361:2414–2423 [DOI] [PubMed] [Google Scholar]
- 11. Talaat KR, Greenberg ME, Lai MH, Hartel GF, Wichems CH, Rockman S, Jeanfreau RJ, Ghosh MR, Kabongo ML, Gittleson C, Karron RA. 2010. A single dose of unadjuvanted novel 2009 H1N1 vaccine is immunogenic and well tolerated in young and elderly adults. J. Infect. Dis. 202:1327–1337 [DOI] [PubMed] [Google Scholar]
- 12. Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, Basser RL. 2009. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 361:2405–2413 [DOI] [PubMed] [Google Scholar]
- 13. Khurana S, Verma N, Talaat KR, Karron RA, Golding H. 2012. Immune response following H1N1pdm09 vaccination: differences in antibody repertoire and avidity in young adults and elderly populations stratified by age and gender. J. Infect. Dis. 205:610–620 [DOI] [PubMed] [Google Scholar]
- 14. Thomson CA, Wang Y, Jackson LM, Olson M, Wang W, Liavonchanka A, Keleta L, Silva V, Diederich S, Jones RB, Gubbay J, Pasick J, Petric M, Jean F, Allen VG, Brown EG, Rini JM, Schrader JW. 2012. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front. Immunol. 3:87 doi: 10.3389/fimmu.2012.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, Mulligan M, Das SR, Yewdell JW, Mehta AK, Wilson PC, Ahmed R. 2012. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc. Natl. Acad. Sci. U. S. A. 109:9047–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He XS, Sasaki S, Baer J, Khurana S, Golding H, Treanor JJ, Topham DJ, Sangster MY, Jin H, Dekker CL, Subbarao K, Greenberg HB. 2013. Heterovariant cross-reactive B-cell responses induced by the 2009 pandemic influenza A/H1N1 vaccine. J. Infect. Dis. 207:288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, Palese P. 2013. 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J. Infect. Dis. 207:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856 [DOI] [PubMed] [Google Scholar]
- 20. He XS, Sasaki S, Narvaez CF, Zhang C, Liu H, Woo JC, Kemble GW, Dekker CL, Davis MM, Greenberg HB. 2011. Plasmablast-derived polyclonal antibody response after influenza vaccination. J. Immunol. Methods 365:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boyce TG, Gruber WC, Coleman-Dockery SD, Sannella EC, Reed GW, Wolff M, Wright PF. 1999. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine 18:82–88 [DOI] [PubMed] [Google Scholar]
- 22. Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, Garcia-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl. Acad. Sci. U. S. A. 109:2573–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caraux A, Klein B, Paiva B, Bret C, Schmitz A, Fuhler GM, Bos NA, Johnsen HE, Orfao A, Perez-Andres M. 2010. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138− and CD138+ plasma cells. Haematologica 95:1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, Salimi N, Vita R, Ponomarenko J, Scheuermann RH, Sette A, Peters B. 2009. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. U. S. A. 106:20365–20370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faenzi E, Zedda L, Bardelli M, Spensieri F, Borgogni E, Volpini G, Buricchi F, Pasini FL, Capecchi PL, Montanaro F, Belli R, Lattanzi M, Piccirella S, Montomoli E, Ahmed SS, Rappuoli R, Del Giudice G, Finco O, Castellino F, Galli G. 2012. One dose of an MF59-adjuvanted pandemic A/H1N1 vaccine recruits pre-existing immune memory and induces the rapid rise of neutralizing antibodies. Vaccine 30:4086–4094 [DOI] [PubMed] [Google Scholar]
- 27. Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. 2011. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J. Exp. Med. 208:2599–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC. 2009. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 10:1292–1299 [DOI] [PubMed] [Google Scholar]
- 29. Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, Sambhara S. 2012. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging Dis. 3:68–90 [PMC free article] [PubMed] [Google Scholar]
- 30. Nimmerjahn F, Ravetch JV. 2010. Antibody-mediated modulation of immune responses. Immunol. Rev. 236:265–275 [DOI] [PubMed] [Google Scholar]
- 31. Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, Nishtala M, Wrammert J, Smith K, James JA, Dekker CL, Davis MM, Wilson PC, Greenberg HB, He XS. 2011. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J. Clin. Invest. 121:3109–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siegrist CA, Aspinall R. 2009. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9:185–194 [DOI] [PubMed] [Google Scholar]
- 33. Brokstad KA, Eriksson JC, Cox RJ, Tynning T, Olofsson J, Jonsson R, Davidsson A. 2002. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J. Infect. Dis. 185:878–884 [DOI] [PubMed] [Google Scholar]
- 34. Dullaers M, Li D, Xue Y, Ni L, Gayet I, Morita R, Ueno H, Palucka KA, Banchereau J, Oh S. 2009. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity 30:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clements ML, Murphy BR. 1986. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J. Clin. Microbiol. 23:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sui J, Sheehan J, Hwang WC, Bankston LA, Burchett SK, Huang CY, Liddington RC, Beigel JH, Marasco WA. 2011. Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies. Clin. Infect. Dis. 52:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, Golding H. 2010. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2:15ra15 doi: 10.1126/scitranslmed.3000624 [DOI] [PubMed] [Google Scholar]
- 38. Lynch GW, Selleck P, Sullivan JS. 2009. Acquired heterosubtypic antibodies in human immunity for avian H5N1 influenza. J. Mol. Genet. Med. 3:205–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corti D, Suguitan AL, Jr, Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, Weiss RA, Sallusto F, Subbarao K, Lanzavecchia A. 2010. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 120:1663–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Staneková Z, Mucha V, Sladkova T, Blaskovicova H, Kostolansky F, Vareckova E. 2012. Epitope specificity of anti-HA2 antibodies induced in humans during influenza infection. Influenza Other Respi. Viruses 6:389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palese P, Wang TT. 2011. Why do influenza virus subtypes die out? A hypothesis. mBio 2(5):e00150-11 doi: 10.1128/mBio.00150-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ekiert DC, Wilson IA. 2012. Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr. Opin. Virol. 2:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nabel GJ, Fauci AS. 2010. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat. Med. 16:1389–1391 [DOI] [PubMed] [Google Scholar]