Summary
Tendons and ligaments are similar structures in terms of their composition, organisation and mechanical properties. The distinction between them stems from their anatomical location; tendons form a link between muscle and bone while ligaments link bones to bones. A range of overlapping functions can be assigned to tendon and ligaments and each structure has specific mechanical properties which appear to be suited for particular in vivo function. The extracellular matrix in tendon and ligament varies in accordance with function, providing appropriate mechanical properties. The most useful framework in which to consider extracellular matrix differences therefore is that of function rather than anatomical location. In this review we discuss what is known about the relationship between functional requirements, structural properties from molecular to gross level, cellular gene expression and matrix turnover. The relevance of this information is considered by reviewing clinical aspects of tendon and ligament repair and reconstructive procedures.
Keywords: musculoskeletal, tendon, ligament, biomechanics, extracellular matrix, collagen
Introduction
Tendons and ligaments are essential components of the skeletal system and their precise mechanical properties are required for efficient locomotion. Both structures are examples of dense regular connective tissues and have relatively similar structural and mechanical properties; the distinction between tendons and ligaments is based on anatomical location. Tendons lie between a muscle belly and the target bone for muscle-generated force, thereby providing a mechanical connection between muscle and bone. Ligaments are situated between two bones and serve to connect different parts of the bony skeleton. Rather than focusing on anatomical differences, a more useful framework to consider the precise composition and organisation of matrix components in tendons and ligaments is to consider their functional requirements. In this way it can be seen that there is a continuum of mechanical properties, rather than tendons and ligaments forming two discrete groups.
The demand to understand the relationship between in vivo function, mechanical properties and the structural composition and organisation to achieve specific mechanical properties in tendon and ligament has never been greater. Epidemiological studies show that the incidence of tendon and ligament injuries is increasing1, 2 and these frequently require surgical intervention. Surgery can involve a direct repair of the damaged tendon or ligament, where the torn ends of the damaged structure are sutured or fixed together as closely as possible to allow them to heal. Alternatively, surgery can involve a reconstruction, where healthy tendon or ligament tissue from another site or donor is harvested and transplanted to replace the damaged structures3. Synthetic materials have also been used, with mixed results, whilst newer approaches using tissue engineering attempt to recreate tendon or ligament-like tissue outside the body4 to replace the damaged natural structures there by avoiding donor site morbidity. The expectation that full functional recovery is achieved is increasing, especially as people are encouraged to maintain health by participating in sporting activities into middle and old age. Understanding the significance of specific tendon or ligament properties is essential to meet the expectations of restoring former function, rather than the less ambitious goal of a return to a sedentary lifestyle.
Function of tendons and ligaments
The principal function of tendons is to transmit the force generated by skeletal muscle contraction to the bony skeleton, which usually results in movement of a joint. Ligaments work in synergy with tendons by limiting joint movement. Both tendons and ligaments however, have additional more specialised functions.
Some tendons have a spring-like function and contribute towards reducing the energetic cost of high speed locomotion5. These energy-storing tendons are stretched under load and then recoil, returning a large proportion of the stored elastic energy and thereby reducing the muscular effort needed to return the limb to the starting position. The human Achilles tendon is a good example of a tendon adapted for a spring-like function. An in vivo study has shown that during one-legged hopping the elastic recoil of the Achilles tendon contributes 16% of the total average mechanical work of the hop6. Calculation of total energy saving by the Achilles tendon and plantar ligaments of the foot in running humans has been calculated at 50% per step7. Another tendon showing a high degree of adaptation for an energy storing role is the superficial digital flexor tendon (SDFT) in the horse. Other tendons show what might be considered to be a more specialised form of energy storage which can be used to amplify muscle power. This can be seen in a jumping frog when elastic energy stored in tendon is rapidly released producing a power that exceeds the initial muscle power output8. A similar function has been described for the elastic components (tendinous tissue) of the biceps muscle in the equine forelimb9. Under different conditions, which result in delayed recoil of the tendon, power attenuation can result and the tendon therefore has a braking effect8. In addition tendons, as viscoelastic materials, absorb kinetic energy and act as a force dampener; a property which may protect other skeletal tissues from potentially damaging high peak forces.
While the restriction of joint movement is considered to be the main role of ligament, their true role is in guiding joint movement; allowing motion in specific planes while restricting movement in others. Ligaments also have additional functions similar to those of tendon in energy storage and as mechanical dampeners. A further important aspect of ligament function is in proprioception; providing feedback regarding joint position in space and contributing to coordinated movement and joint stability.
Gross structural properties of tendons and ligaments
Mechanical properties
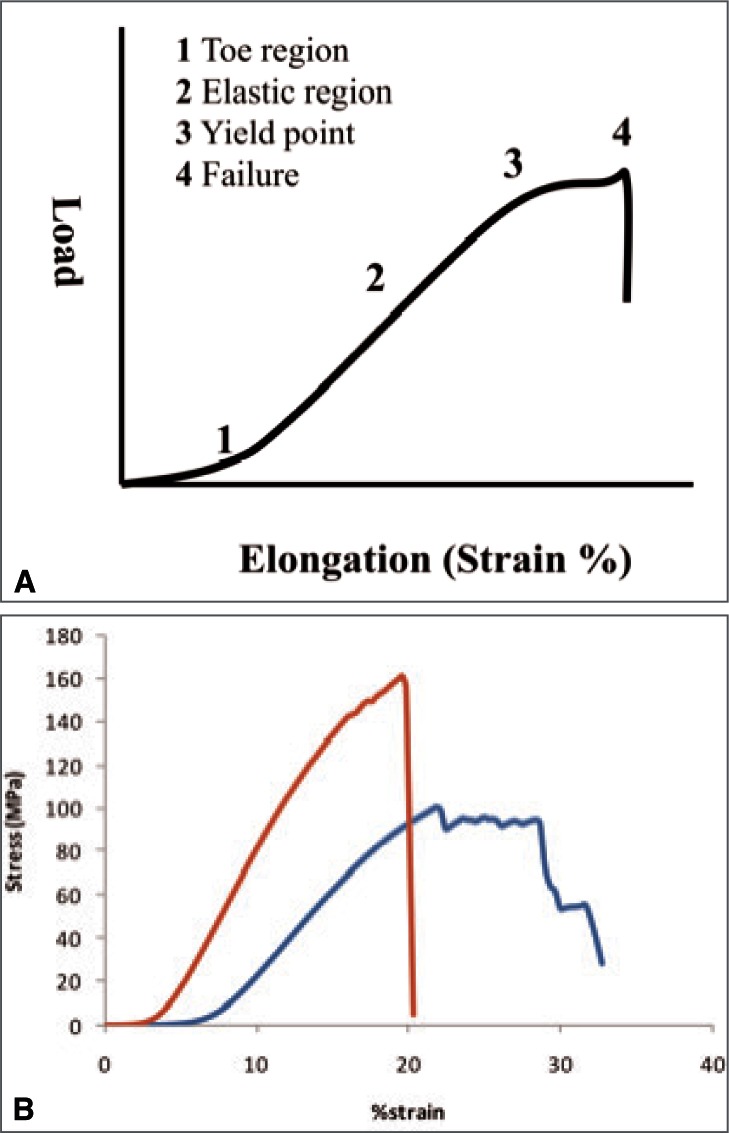
Tendons and ligaments are viscoelastic materials, evolved to resist predominantly tensile forces. As they are primarily mechanical instruments, it is not surprising that the mechanical properties of these structures have been the subject of many studies and the characteristic force elongation relationship was described many years ago10. Initial application of load results in a relatively large elongation of the tissue (Fig. 1A) followed by a gradual transition into a linear response to increases in load. As further load is applied the structure begins to yield giving rise to a sigmoidal shaped curve, before the structure finally fails completely. There is a number of properties that can be deduced from the force elongation curve and used to describe the particular structure being studied.
Figure 1.
Graph showing characteristic relationship between applied force and elongation of tendon and ligaments (A) and an example of data collected for the equine SDFT and SL (B).
The force at which the tendon or ligament fails (ultimate load) is obviously an important property, although this is largely related to the size of the structure. Large tendons and ligaments such as the equine deep digital flexor tendon (DDFT) and suspensory ligament (SL) are capable of supporting a mass of up to 2 tonnes before failure. The human Achilles tendon (AT) has an ultimate tensile strength of approximately 4 kN while the anterior tibialis tendon (ATT) fails at about 1.5 kN11. A more interesting comparison is that of material strength (ultimate stress) and studies suggest that this is generally lower for ligaments than for tendons (SL − 91 ± 19 MPa; SDFT − 131 ± 27 MPa) and lower in tendons designed to store and release elastic energy (SDFT) than in positional tendons such as the equine common digital extensor tendon (CDET − 189 ± 37 MPa)12. This may be as a consequence of other adaptations which provide more physiologically important specialisation of mechanical properties.
The differences that may be considered to be of more physiological relevance with regard to normal activity relate to stiffness, ultimate strain, fatigue properties and hysteresis. Positional tendons such as the human ATT and the CDET in the horse are required to be relatively inextensible when subjected to physiological loads. Excessive stretching in these positional tendons would decrease efficiency as movement of the joint would be reduced and furthermore control of precise movements would be problematic. Concurrent with this, in vivo measurements of strain in the human ATT have recorded maximum values of only 3.1%13; likewise the equine CDET experiences maximum strains in vivo of approximately 3%, based on muscle architectural measurements11. Energy storing tendons, such as the AT and equine SDFT are required to undergo large strains in response to physiological loads to allow storage and return of energy at an appropriate usable rate. In vivo measurements in the human AT have recorded strains of up to 10.3% during one-legged hopping6 and strains of up to 16.6% have been recorded for the equine SDFT in vivo at gallop14. Ligaments tend to be more similar to energy storing tendons in this respect, as stretching is required to allow joint movement to take place. These requirements are demonstrated by significant differences in material stiffness (Fig. 1B) with the SDFT, CDET and SL having elastic moduli of 1165 ± 178 MPa, 1510 ± 291 MPa and 643 ± 130 MPa respectively12.
Energy storing tendons also require fatigue resistance as they may be subjected to a large number of loading and unloading cycles at relatively high strains during locomotion. Not all ligaments that are adapted for lower stiffness and high strains (like energy storing tendons) however require high fatigue resistance as they are not necessarily subjected to repetitive loading and unloading cycles if not associated with running type activities (for example human spinal ligaments). While fatigue resistance at low strains is important for positional tendons involved in locomotion, a more challenging problem is to provide fatigue resistance at the high strains experienced by energy storing tendons. Our recent work suggests that recovery of structure after application and removal of high strain is greater in the SDFT than the CDET indicating that the energy storing tendon may indeed be more fatigue resistant15. A low level of hysteresis is another property which is intuitively more important for energy storing tendons, both in terms of efficiency by reducing the energetic cost of locomotion, and to reduce heat generation due to energy loss. Again, a low level of hysteresis is not necessarily a requirement for ligaments with high extensibility. Our recent work has demonstrated that bundles of fibres from the SDFT have significantly lower hysteresis at 4 and 8% strain than bundles of fibres from the CDET when tested in vitro15.
As illustrated above, to allow for specific functions in tendons and ligaments precise mechanical properties are required. To understand how these differences are achieved at whole structure level it is necessary to have an understanding of the sub-structure of tendon and ligaments.
Architectural properties
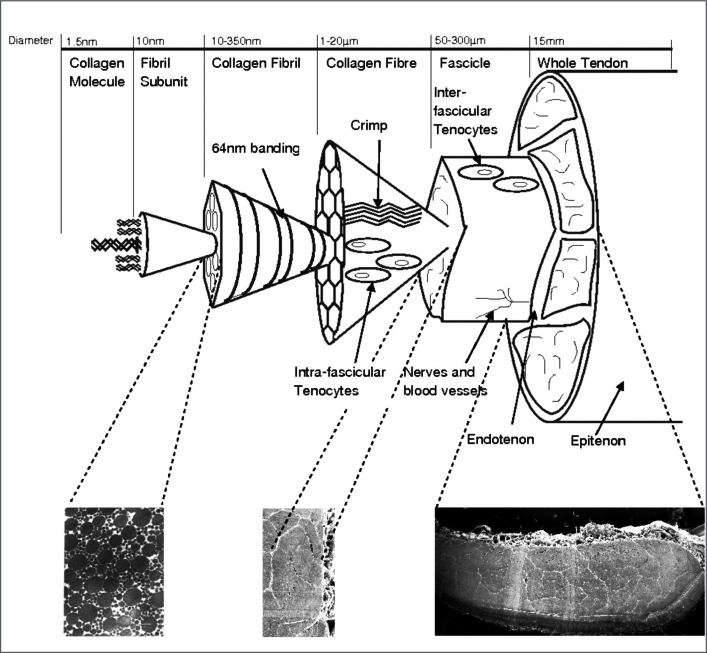
Tendons and ligaments are composite materials composed of collagen and non-collagenous proteins. The collagenous component of tendon and ligaments has a complex hierarchical arrangement which follows the same basic pattern in both structures. This has been described and depicted diagrammatically by a number of authors16–18 to illustrate the arrangement of the collagen component (Fig. 2). The predominant collagen type is the fibril-forming type I collagen. Collagen molecules align in a highly ordered parallel arrangement with each molecule staggered relative the neighbouring molecule giving rise to overlap and gap zones to form pentamer microfibrils with a characteristic banded pattern repeating every 67 nm. Microfibrils group together to form collagen fibrils with diameters of 40–300 nm, which can be viewed under an electron microscope in transverse and longitudinal sections of tissue. Many studies have described the populations of collagen fibrils in different tendons and ligaments from various species. The collagen fibrils then group together to form fibres which are visible under light microscopy, fibres form sub-fascicles and finally fascicles group together to form the whole structure. A thin connective tissue membrane known as the epitenon surrounds the whole tendon and extends into the tendon separating fascicles where it is known as the endotenon or interfascicular matrix. It is the combination of mechanical properties at each of these substructural levels that result in the overall mechanical behaviour of the tendon or ligament.
Figure 2.
Hierarchical structure of tendon and ligament (reproduced from Thorpe et al., 201049).
Tendon and ligament sub-structure
Collagen molecules
Collagen is the major protein in both tendons and ligaments, accounting for approximately 75% of the dry weight. The remainder of the weight is composed of other non-collagenous matrix proteoglycans and glycoproteins, the fibrous protein elastin and cellular proteins. Evidence suggests that the ratio of collagen to non-collagenous components differs between different structures. Our work19 has shown that the collagen content of the equine SDFT and DDFT is 75.8% ± 1.5 and 76.9% ± 2.1 respectively and is significantly higher than that of the suspensory ligament (65.1% ± 1.6). The positional CDET has significantly higher collagen content (80.4% ± 1.3) than the energy storing SDFT. Previous work on tendon and ligament tissue from the sheep knee joint demonstrated a similar pattern, with the cruciate ligaments showing a lower collagen content (57%) than the tendons studied (lateral digital extensor tendon (73.2%); SDFT (78.8%); patellar tendon (73.7%). Interestingly the collateral ligaments had a higher collagen content (70%) than the cruciate ligaments, demonstrating inter-ligament variation20. Although type I collagen is the predominant collagen in both tendon and ligament small amounts of other collagen types are present, the second most abundant collagen type is type III collagen; another fibril-forming collagen. Structures requiring greater compliance, such as skin and blood vessels have a higher type III collagen content21. In accordance, we have found that type III collagen levels are higher in the energy storing SDFT and SL in the horse than in the DDFT and CDET (unpublished data). Higher levels of type III collagen have also been observed in cruciate and collateral ligament from the rabbit knee joint compared to the patellar and Achilles tendon22.
The mechanical properties of single collagen molecules have been calculated using atomistic modelling giving values of 11.2 GPa for break stress, which occurs at 50% strain, and an elastic modulus of 7 GPa23. These computed predictions compare well with experimental results obtained using X-ray diffraction24 and Brillouin light scattering25 to measure stretching of tropocollagen molecules.
Type I collagen molecules are formed of three polypeptide chains (α chains) wrapped around each other to form a right-handed super-helix. Each polypeptide chain has a primary sequence of repeating triplets of amino acids formed of glycine and two other amino acids, most commonly proline followed by hydroxyproline, giving rise to the helical domain of the molecule which is flanked at the N- and C-termini by non-helical domains known as telopeptides. The hydroxylation of proline residues is a post-translational modification; the initial gene product undergoes extensive post-translational modification prior to formation of the triple helix. As a result, differences may exist in type I collagen molecules in different tissues despite an identical gene product, due to differences in the extent and nature of post-translational modifications.
The extent of proline hydroxylation determines the stability of the collagen molecule. An increase in hydroxylation increases stability, as demonstrated by an increase in the thermal denaturation temperature26. Differences in proline hydroxylation levels have been observed in disease conditions where there is a delay in triple helix formation resulting in over-modification27. There is no evidence however to suggest that hydroxylation levels differ between tendons and ligaments. In addition to hydroxylation of proline residues, lysine residues are also hydroxylated by a family of lysyl hydroxylase enzymes. Lysine and hydroxylysine residues take part in crosslink formation between collagen molecules and therefore have an important influence on mechanical properties of the collagen fibril.
The type of crosslink formed depends on the degree of lysine hydroxylation and whether this occurs in the helical part of the molecule or the non-helical telopeptide region (for a review of collagen cross-linking see Bailey et al. 199828). Lysine and hydroxylysine residues are converted into aldehydes by the extra-cellular enzyme lysyl oxidase; if hydroxylation is extensive in the telopeptide region more hydroxylysine aldehyde will be formed. A spontaneous reaction then takes place between the lysine or hydroxylysine aldehydes and a lysine or hydroxylysine residue in the helical region of neighbouring molecules to form the immature bivalent cross-links. Immature cross-links undergo further spontaneous reactions to form mature trivalent pyridinoline and pyrrole cross-links. Extensive hydroxylation of lysine residues throughout the collagen molecule results in the formation of hydroxylysyl-pyridinoline (HL-Pyr). In tissues where less hydroxylation of the helical or telopeptide lysine residues occurs, the mature cross-links lysyl-pyridinoline (L-Pyr) and pyrrole form respectively. A further mature crosslink, histidino-hydroxy lysinonorleucine (HHL), found predominantly in skin but also present in some tendons, can form from the interaction between an immature cross-link and histidine residue. Little is known about variation in post-translational lysine hydroxylation between tendon and ligaments or tendons with different functions however the collagen crosslink pattern is quite different (see below) suggesting that these must be considerable.
Collagen fibrils
Collagen fibrils form by a highly ordered aggregation of collagen molecules into microfibrils and then fibrils. The modelling work of Buehler (2006) shows a ‘softening’ of mechanical properties from collagen molecule to fibril. These studies suggest an elastic fibril strength of 0.3 GPa with failure at 0.5 GPa and a Young’s modulus of 5 GPa. Experimental work using a microelectromechanical systems device29 or atomic force microscopy30 for measuring mechanical properties of collagen fibrils directly suggests somewhat lower values for Young’s modulus of around 0.2 – 0.8 GPa. The presence of crosslinks between collagen molecules increases the range of elastic fibril deformation with much higher yield stresses occurring with high crosslink densities31. Although all mature tendons and ligaments appear to have high crosslink densities, our work has shown that the crosslink profile differs significantly between different tendons and ligaments. The predominant mature crosslink detected in the SDFT, DDFT and SL was HL-Pyr, with much lower levels in the CDET; the main crosslink found here being histidinohydroxymesodesmosine (HHMD). The SDFT and SL had similar levels of HL-Pyr (SDFT – 0.47 ± 0.06 moles/mole collagen; SL − 0.46 ± 0.05 moles/mole collagen) which were significantly higher than those found in the DDFT (0.35 ± 0.05 moles/mole collagen) and the CDET (0.04 ± 0.01 moles/mole collagen). HHMD was detected in high amounts in the CDET samples (1.24 ± 0.15 moles/mole collagen) and at trace levels in the DDFT but not in the SDFT or SL. HHL was detected in all the CDET samples (0.069 ± 0.005 moles/mole collagen) and some of the DDFT samples at trace levels but not in the SDFT or SL. The divalent immature aldimine crosslink dehydro-hydroxy lysinonorleucine (dehydro-HLNL) was detected in the CDET (0.04 ± 0.01 moles/mole collagen) but none of the other structures11. In the rabbit the cruciate and collateral ligaments from the knee joint differ from the patellar and Achilles tendon in terms of reducible crosslink levels suggesting levels of mature crosslinks are also different22. The consequence of these differences in the crosslink profiles between tendon and ligaments is not known at present but is likely to impact on the mechanical properties of the matrix tissue.
Collagen fibrils diameters have been measured in several studies and these show considerable morphological variation between tendon and ligament types. In mature tendon and ligament a bimodal distribution of collagen fibril diameters is seen. Ligaments tend to have a greater proportion of smaller diameter fibrils than tendons. The mass average diameter, a measure that takes into account the fact that large fibrils occupy more area than the same number of smaller fibrils, was shown to be higher in the sheep SDFT (240 ± 6 nm) than the lateral collateral ligament (126 ± 17 nm), medial collateral ligament (157 ± 22 nm) and anterior cruciate ligament (181 ± 14 nm)20. In our studies on equine tissue we have also shown differences in the fibril diameter distribution between functionally distinct tendons and between tendons and ligaments. The SDFT (169 nm ± 19) had a significantly lower mass average fibril diameter than the CDET (229 nm ± 36) suggesting that larger fibril diameters might be responsible for a stiffer matrix in the CDET12. In keeping with this, the suspensory ligament; an energy storing structure with a significantly lower elastic modulus than both the SDFT and CDET had the lowest mass average fibril diameter (122 nm ± 14). Smaller diameter fibrils in tendons and ligaments subjected to high strains support the idea suggested by Parry et al.32 that this may be an adaptation to withstand creep as a greater number of smaller fibrils increases the potential for inter-fibrillar crosslinks. A further feature that becomes evident at the fibril level is a sudden change in direction along the length of the fibril. Scanning electron microscopy studies have shown that collagen fibrils in tendon show ‘knots’ or fibrillar crimps. These ‘knots’ are formed as the fibrils first twist leftwards, changing their plane of running, and then bend sharply also changing the direction of coursing33. The fibrillar crimp functions as a biological hinge, opening when tensional load is applied and recoiling when load is removed33. It is not clear why crimps form or what controls the angle and frequency of such crimps however it is clear that differences exist between different tissues.
Collagen fibres
Collagen fibres in tendon and ligament have a diameter of approximately 10 μm. The crimps originating at the fibril level align, resulting in the waveform seen in longitudinal histologic sections of tendon and ligament at the fibre level. Measurements of crimp morphology suggest that structures with an energy storing function are more crimped, having large crimp angles and a small crimp base length. For example, the work of Franchi et al. on rat tissues34 found that the vastus intermedius tendon, which plays a role during quadriceps extension and requires greater elastic recoil, has larger crimp angles than the positional rectus femoris and patellar tendon. Our own data support this finding as the SL and SDFT have greater crimp angles than the DDFT and CDET and shorter crimp lengths than the CDET (unpublished data).
Collagen fibre mechanical properties have been investigated in a number of studies using confocal microscopy. The cells, which lie between collagen fibres in tendon, have been stained using fluorescent dyes and used as markers to track movement between collagen fibres35. Using this technique, it has been found that fibre extension is less than the applied strain indicating that fibre sliding is the main mechanism for tendon fascicle extension in rat tail tendon and bovine extensor tendon35, 36. More recently an alternative technique has been used to study fibre response to load. This technique involves staining the collagen and then photo bleaching a grid into the dye. The deformation of the grid can then be quantified, allowing direct assessment of the matrix response to applied strain37. Using this technique it has been shown that fibre extension is considerably less than applied strain and does not differ significantly between the high strain equine SDFT and low strain CDET tendon fascicles15.
Collagen fascicles
Collagen fibres group together to form fascicles; these fascicles are surrounded by inter-fascicular matrix and are visible to the naked eye. Fascicles can be dissected free from tendons and studied as independent units. Recent work has shown that fascicles dissected from the equine SDFT are significantly smaller in cross sectional area (0.12 ± 0.006 mm2) than fascicles obtained from the CDET (0.16 ± 0.09 mm2)38. In both tendon types the fascicles have an irregular shape; fascicles in the CDET appear to be more tightly packed than those in the SDFT38. Micromechanical testing of these isolated fascicles gives surprising results; despite the lower ultimate stress and elastic modulus of the whole SDFT, fascicles from this tendon did not differ significantly in these properties compared to fascicles from the CDET. Furthermore, despite the SDFT withstanding higher strains before failure, fascicles from this tendon failed at a lower strain than those from the CDET38. Characterisation of the interfascicular matrix explains this apparent contradiction; at physiological loads the interfascicular matrix is much more easily deformed in the SDFT than the CDET and thus the greater extension in the SDFT appears to stem from sliding between fascicles38, a mechanism which may protect high strain tendons and ligaments from damage.
Fascicle extension mechanisms have been investigated in functionally distinct tendons using the technique mentioned above, staining the collagen fibres with a fluorescent dye and photo bleaching a grid into the stain to allow quantification of fibre movement. Recent work has shown that the amount of fibre stretch for SDFT and CDET fascicles was low compared to the applied strain but did not differ; the mechanism to achieve the overall fascicle extension was however different between tendon types. In the CDET fibre sliding was the dominant mechanism while in the SDFT rotation of the grid suggests an unwinding of a helical substructure15. These experiments also demonstrated that the SDFT fascicles have a greater ability to recover following applied strain and experience significantly lower levels of energy loss (hysteresis) following loading and unloading15; both properties which are desirable for energy storing structures.
Non-collagenous components
Water constitutes the main component of the wet weight of tendon and ligament tissue and has a significant relationship with tendon tissue stiffness. Tendons with a lower elastic modulus have higher water contents as illustrated by a comparison of equine tendons. The energy storing SDFT and SL had the highest water contents (SDFT – 64.2% ± 0.8; SL – 67.5% ± 1.2) followed by the DDFT (61.6% ± 1.2), with the lowest water content in the positional CDET (57.1% ± 0.9) (11). A similar pattern is seen in the sheep where the cruciate and collateral ligament of the knee joint has significantly higher water contents than the tendons studied20.
Although collagen is the major constituent of tendon and ligament and provides the essential tensile properties, other non-collagenous proteins play an important role in organising the collagen component and providing adhesion between the collagen subunits. The small leucine-rich proteoglycans such as decorin, fibromodulin and biglycan are the most abundant in tendon and ligament. Proteoglycans are composed of a protein core and glycosaminoglycan (GAG) sidechain and therefore a measure of the GAG content gives an indication of the levels of proteoglycan present. Studies in the rabbit22 have shown that ligaments (collateral and cruciate) have higher levels of GAG than tendons (patellar and Achilles) in agreement with the finding for ligaments and tendons around the sheep knee joint20. In our studies we found that the levels of sulphated GAG were significantly lower in the CDET (2.5 μg/mg dry weight tissue ± 0.4) than the SDFT (9.1 μg/mg ± 1.5), DDFT (10.6 μg/mg ± 3.0) and SL (13.2 μg/mg ± 1.6) while the levels of sulphated GAG in the SDFT were significantly lower than those in the SL but not significantly different to the DDFT (11). These differences in sulphated GAG levels indicate variations in the type and amount of proteoglycan in the tendon matrix in functionally distinct tendons. As proteoglycans play a role in regulating collagen fibril diameters and interactions between collagen subunits throughout the tendon hierarchy, differences are important contributors to mechanical specialisation.
Another matrix molecule which has received considerable attention in tendon and ligament studies is collagen oligomeric matrix protein (COMP). COMP binds to fibrillar collagens; each COMP molecule can bind to five collagen molecules, possibly for assembly into a collagen fibril. Recent studies have shown that COMP can accelerate collagen fibrillogenesis in vitro and it has been suggested that COMP has an organizational rather than in a structural role39. The levels of COMP appear to be much higher in structures subjected to high strains. In our work on equine tissues, levels of COMP were much higher in the SDFT (22.1 ± 6.1 μg/mg) than the CDET (1.9 ± 0.6 μg/mg) with highest levels present in the SL (32.1 ± 6.0 μg/mg) (unpublished data).
Cellular specialization
Cell density
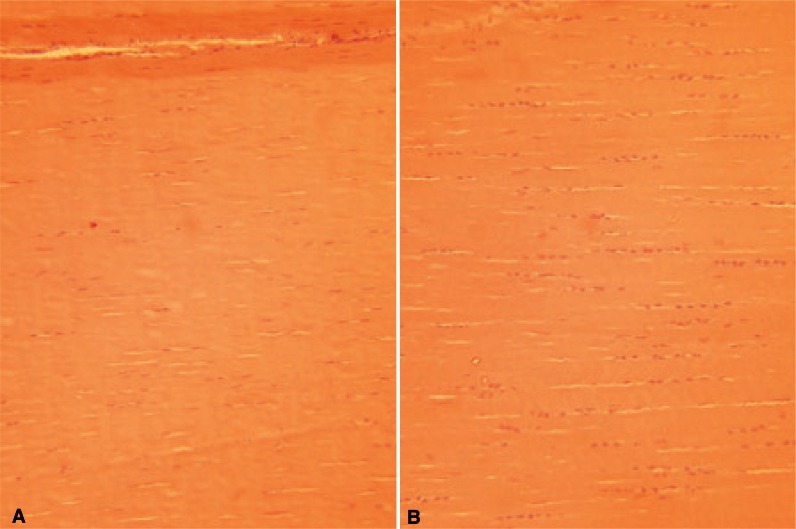
Given the marked variation in matrix composition, it would appear intuitive that gene expression differs between functionally distinct tendons and ligaments. Our work is indeed consistent with this, and in fact, even the density of the cells within the matrix shows a consistent variation. Those structures which experience high strains in vivo appear to be most cellular as demonstrated by higher DNA contents. In our analysis of equine tissue the SL had the highest levels of DNA (2.27 ± 0.29 μg DNA/mg dry weight tissue) followed by the SDFT (1.51 ± 0.16 μg/mg) then the DDFT (0.80 ± 0.15 μg/mg) while the CDET (0.47 ± 0.18 μg/mg) had the lowest levels11. These differences are also apparent on histology sections of tendons and ligaments (Fig. 3 A, B).
Figure 3.
H&E histologic longitudinal sections from the equine SDFT (A) and SL (B) showing the higher cellularity in the SL and different morphologies of tenocyte nuclei.
Expression of matrix proteins
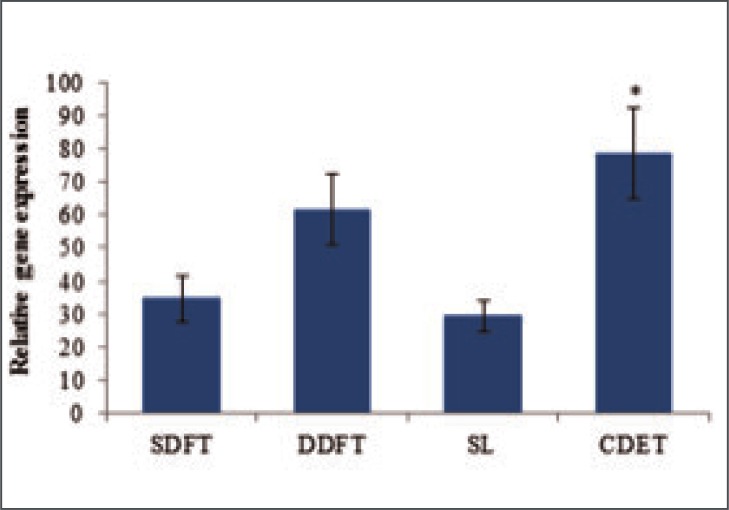
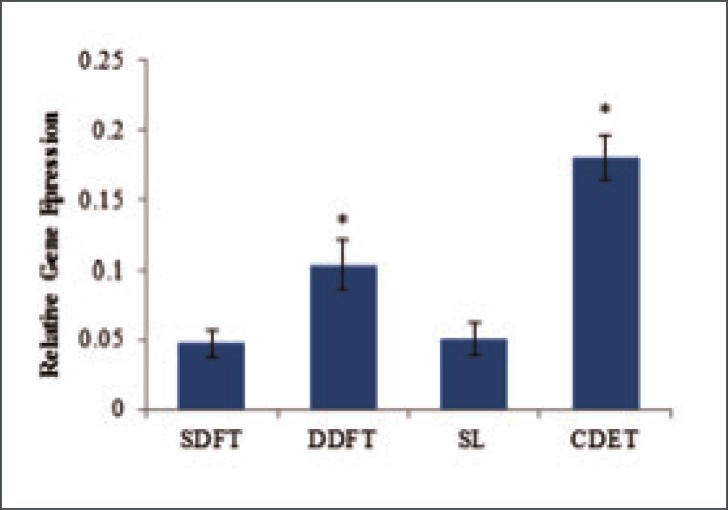
Studies of the expression of collagen genes in equine SDFT, DDFT, CDET and SL showed that type I (Col 1 A2), type III (Col 3 A1), type V (Col 5 A1) and type X11 (Col 12 A1) collagens are all synthesised by cells from these structures19. As expected, the type I collagen gene was most highly expressed in all tendons with levels approximately two fold greater than those for the type III collagen gene and 4 fold greater than the type XII gene expression levels. Type V collagen gene expression levels were the lowest with levels approximately 20 fold less than those for expression of the type I collagen gene. The expression of type I collagen gene did not differ between tendon types when considered relative to tissue weight however, due to a lower cellularity in the CDET, expression was significantly higher per cell (Fig. 4). Furthermore, using Western blot analysis, our studies found that the levels of type I pro-collagen that could be extracted from the tendon tissue were significantly higher in the CDET (4.6 times) than the SDFT providing further evidence for higher levels of collagen synthesis in the positional CDET19.
Figure 4.
Relative expression of COL1A2 per cell in equine forelimb tendons and ligament. Data are shown as mean ± SEM, n = 32. *Indicates significant difference relative to the SDFT.
In our studies, genes for aggrecan, biglycan, decorin, fibromodulin and lumican proteoglycans were expressed in all the equine tendons and ligament studied19. The expression of decorin was greater than expression of other proteoglycans in all tendons in keeping with the levels of proteoglycan present in the tissue. The large aggregating proteoglycan aggrecan was expressed at the lowest level in all tendons compared to the other proteoglycans studied. Comparison of the different structures showed that the positional CDET had significantly lower levels of expression than the SDFT for all of the measured proteoglycans. Levels of decorin expression were also lower in the DDFT than the SDFT but showed no difference between the SDFT and SL. Perhaps more interesting is consideration of the ratio of the predominant collagen type (type I) to the predominant proteoglycan (decorin) for each structure. This ratio for the SDFT is significantly lower than that for the CDET and DDFT but not significantly different to the SL (Fig. 5), reflecting the higher proteoglycan content in high strain structures.
Figure 5.
Ratio of Col1A2 to decorin expression in equine forelimb tendons and ligament. Data are shown as mean ± SEM, n = 32. *Indicates significant difference relative to the SDFT.
Other non-collagenous components play an important role in the matrix of tendon and ligaments. One such component is COMP which represents the most abundant glycoprotein in tendon and has a structure specific distribution in equine tendon and ligament tissue (see above). In our gene expression studies in equine tissue, we found that the levels of COMP messenger RNA were significantly higher in the high-strain SDFT and SL than the DDFT and CDET19. Scleraxis is a transcription factor and consider to be a marker for a tendon and ligament phenotype. The equine SDFT, DDFT, CDET and SL all expressed the scleraxis gene and levels of expression were significantly higher in the SDFT and SL19 suggesting that scleraxis may be a marker of uniaxial strain.
Expression of matrix degrading enzymes and their inhibitors
We have studied the expression of matrix metalloproteinases 1, 3, 9, 10, 13 and 23 at the mRNA level and found expression of all enzymes in the SDFT, DDFT, CDET and SL19. The collagenases (MMP 1 and MMP 13) and the gelatinase (MMP 9) were expressed at higher levels in the CDET than in the SDFT. Higher expression of MMP13 was confirmed by a fluorogenic substrate assay which showed more enzyme activity in the CDET than the SDFT. The higher expression level for MMP 9 in the CDET was confirmed at the protein level using gelatin zymography. Levels of collagenase and gelatinase expression did not differ significantly between the SDFT and SL. Expression levels for the stromolysins (MMP 3 and MMP10) were significantly lower in the CDET than the SDFT perhaps reflecting the lower levels of substrate present in this tendon type although stromolysin levels of expression were also significantly lower in the SL than the SDFT even though proteoglycan levels are high in this ligament. TIMP-3, a naturally occurring inhibitor of the MMPs, showed a lower level of expression in the DDFT and CDET compared to the SDFT but no difference between the SDFT and SL was observed.
Matrix turnover rates
Despite a higher cellularity, recent work40, 41 suggests that the SDFT has a lower collagen turnover rate than the CDET. Aspartic acid racemisation was used to calculate protein half life in tissue from the functionally distinct tendon types. A collagen half-life of 200 years for the SDFT was significantly higher than that for the CDET (34 years). However the half-life of non-collagenous protein was 2 years in the SDFT and was significantly lower than the value of 3.5 years measured for the CDET, in agreement with a more influential role of the non-collagenous interfascicular matrix in the energy storing tendon. A higher rate of collagen turnover in the CDET was supported by the finding of higher levels of collagenase generated neoepitope and cross-linked C-terminal telopeptide of type I collagen (ICTP) in the CDET tissue compared to the SDFT41. The finding that a tendon subjected to high stresses and strains has a lower rate of collagen turnover than a low stressed tendon is unexpected, however, it may be that structures that operate with low safety margins are protected from remodelling which may weaken the structure.
Clinical aspects
The incidence of tendon and ligament disease and injury is extremely large and represents a major health-care burden. For example, rotator cuff tendon tears of the shoulder are present in approximately a third of people over 60 years of age and the incidence of anterior cruciate and medial collateral ligament injury is estimated at 2 per 1000 people per year. In addition, Achilles tendon problems account for 10% of all running injuries, and tendinopathy is implicated in half of all work-related maladies. Tendinopathy is a term used to describe painful chronic tendon injury that is often work or activity related. Although the exact mechanism is still somewhat unclear, one hypothesis is that repetitive microtrauma causes rupture of a small number of collagen fibrils, thus increasing the load on the remainder. This increased load may result in production of matrix metalloproteinases, which degrade collagen and cause degenerative changes42. The uncertainty over the hierarchical level at which over load damage occurs illustrates the need to understand tendon and ligament ultrastructure and response to load at all levels of the hierarchy.
Tendons and ligaments are mechano-responsive, meaning that they alter their composition and mechanical properties in response to the loads to which they are subjected. A better understanding of the relationship between composition and mechanical properties may allow this response to be used to advantage. Cells sense changes in their mechanical environment by a process known as mechano-transduction and respond by modulation of the biochemical mediators they produce. This can lead to a catabolic, homeostatic or anabolic state, in which extracellular matrix production and the material and structural properties can decrease, be maintained or increased43. Understanding these responses is crucial in developing appropriate injury prevention and treatment strategies. For example, immobilization of the knee after medial collateral ligament injury has been shown to lead to decreased mechanical properties of the ligament substance and a greater percentage of disorganized collagen fibrils. This knowledge has led to a shift in clinical management from surgical repair and immobilization to functional rehabilitation with early knee motion42.
The healing process of an acute tendon or ligament injury can be divided into three phases; an initial inflammatory phase, with haematoma formation immediately after injury, leading to the second, reparative phase of fibroblastic proliferation and production of a proteoglycan and collagen matrix that bridges the site of injury. This process leads to the formation of scar tissue or disorganized matrix of collagen fibres at the repair site resulting in decreased mechanical properties compared to the intact structure and so there is a risk of re-rupture. The final phase is that of remodelling with alignment of collagen fibers and this collagen maturation can last for several years. Another problem caused by the healing process is the formation of adhesions and scarring around the tendon sheath. This is especially the case in injured digital flexor tendons in the hand, where such adhesions can stop the smooth gliding of the tendon and cause significantly reduced finger function. Early mobilization, rather than immobilization, has been shown to be beneficial for healing flexor tendons by increasing their tensile strength while also reducing the formation of adhesions. It is therefore important that when a surgical repair of flexor tendons is performed, the repair method and materials used are strong enough to allow such mobilization without the repair itself failing or coming apart44.
In the shoulder, however, rotator cuff injuries usually involve injury to the tendon at its site of insertion to the bone, rather than its mid-substance, and so in contrast to flexor tendon repair, surgical rotator cuff repair involves reattachment of the torn end of the tendon back onto the bone. Studies have suggested that immobilization actually increases tendon-to-bone healing, with increased expression of aggrecan and collagen types II and XII, decreased collagen type III associated with scar formation, and improved collagen organization at the repair site44.
Anterior cruciate ligament (ACL) reconstruction has become a common procedure for injuries of the ACL that result in instability of the knee. The injured ACL is replaced by a graft that is fixed into tunnels in the distal femur and tibia. Appropriate selection of graft tissue relies, in part, on knowledge of the mechanical properties required; patellar, hamstring and quadriceps tendon autografts (i.e. harvested from the same patient), together with Achilles tendon allograft (i.e. harvested from a donor) have all been used successfully45. A hamstring graft needs to be composed of three or four strands to achieve the necessary strength. The structure of the native ACL and transplanted graft tissue differs significantly. The tendon grafts therefore undergo a gradual biological process of “ligamentization”, whereby they gradually lose tendon-specific features and start exhibiting more ligamentous-type histologic features46. During the first two weeks after implantation there is evidence for central necrosis and hypocellularity of the graft, followed by a phase of vascularization and repopulation. The biomechanical strength of the graft is significantly poorer during this phase and gradually recovers over four to six months as the graft continues to remodel. A “mature” graft is one in which no further changes are observed, and some studies report no differences on light microscopy between native ACLs and mature grafts. However, on an ultrastructural level, every graft specimen showed depletion of large diameter collagen fibrils and a unimodal collagen fibril distribution, in contrast to the bimodal distribution found in native ACLs46, 47.
The outcome of ACL reconstruction therefore depends on two biological events – secure fixation and integration of the graft into the osseous tunnels, and maturation of the graft, therefore the rehabilitation after surgery needs to be tailored accordingly. Immobilization has been shown to be beneficial in promoting integration of the tendon graft into the bone tunnels of the tibia and femur but has a deleterious effect, resulting in increased knee joint stiffness48. Modern methods of graft fixation have developed such as interference screws or cortical buttons, and are strong enough to hold the graft in place whilst allowing controlled joint motion, while return to full sporting activities is not allowed for several months until the graft recovers its strength.
Advances in our understanding of the relationship between function and structure in tendon and ligaments, and translation of knowledge into clinical applications will enable more sophisticated procedures to be developed to improve functional outcome following injury.
References
- 1.Kammerlander C, Braito M, Kates S, Jeske C, Roth T, Blauth M, et al. The Epidemiology of Sports-Related Injuries in Older Adults: A Central European Epidemiologic Study. Aging clinical and experimental research. 2012 doi: 10.3275/8273. [DOI] [PubMed] [Google Scholar]
- 2.Nyyssonen T, Luthje P, Kroger H. The increasing incidence and difference in sex distribution of Achilles tendon rupture in Finland in 1987–1999. Scandinavian journal of surgery: SJS: official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2008;97(3):272–275. doi: 10.1177/145749690809700312. [DOI] [PubMed] [Google Scholar]
- 3.Woo SL, Debski RE, Zeminski J, Abramowitch SD, Saw SS, Fenwick JA. Injury and repair of ligaments and tendons. Annual review of biomedical engineering. 2000;2:83–118. doi: 10.1146/annurev.bioeng.2.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Rodeo SA, Delos D, Weber A, Ju X, Cunningham ME, Fortier L, et al. What’s new in orthopaedic research. The Journal of bone and joint surgery American volume. 2010;92(14):2491–2501. doi: 10.2106/JBJS.J.01174. [DOI] [PubMed] [Google Scholar]
- 5.Alexander RM. Tendon elasticity and muscle function. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2002;133(4):1001–1011. doi: 10.1016/s1095-6433(02)00143-5. [DOI] [PubMed] [Google Scholar]
- 6.Lichtwark GA, Wilson AM. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. The Journal of experimental biology. 2005;208(Pt 24):4715–4725. doi: 10.1242/jeb.01950. [DOI] [PubMed] [Google Scholar]
- 7.Ker RF, Bennett MB, Bibby SR, Kester RC, Alexander RM. The spring in the arch of the human foot. Nature. 1987;325(7000):147–149. doi: 10.1038/325147a0. [DOI] [PubMed] [Google Scholar]
- 8.Richards CT, Sawicki GS. Elastic recoil can either amplify or attenuate muscle-tendon power, depending on inertial vs. fluid dynamic loading. Journal of theoretical biology. 2012;313:68–78. doi: 10.1016/j.jtbi.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Wilson AM, Watson JC, Lichtwark GA. Biomechanics: A catapult action for rapid limb protraction. Nature. 2003;421(6918):35–36. doi: 10.1038/421035a. [DOI] [PubMed] [Google Scholar]
- 10.Gratz CMB. S.N. Engineering methods in medical research. Mech Engng. 1935;57:217–220. [Google Scholar]
- 11.Birch HL. Tendon matrix composition and turnover in relation to functional requirements. International journal of experimental pathology. 2007;88(4):241–248. doi: 10.1111/j.1365-2613.2007.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith T. The relationship between tendon morphology and function. University College London; 2006. [Google Scholar]
- 13.Maganaris CN, Paul JP. In vivo human tendinous tissue stretch upon maximum muscle force generation. Journal of biomechanics. 2000;33(11):1453–1459. doi: 10.1016/s0021-9290(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 14.Stephens PR, Nunamaker DM, Butterweck DM. Application of a Hall-effect transducer for measurement of tendon strains in horses. American journal of veterinary research. 1989;50(7):1089–1095. [PubMed] [Google Scholar]
- 15.Thorpe CT, Klemt C, Birch HL, Clegg PD, Screen HRC. Helical sub-structure in energy storage tendons: a mechanism for efficient energy storage and return? Acta Biomaterialia. 2013 doi: 10.1016/j.actbio.2013.05.004. (in press) [DOI] [PubMed] [Google Scholar]
- 16.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connective tissue research. 1978;6(1):11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 17.Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. Journal of biomechanics. 2003;36(10):1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 18.Fratzl PW. R. Nature’s hierarchical materials. Progress in Materials Science. 2007;52(8):1263–1334. [Google Scholar]
- 19.Thorpe C. Extracellular matrix synthesis and degradation in functionally distinct tendons. University college London; 2010. [Google Scholar]
- 20.Rumian AP, Wallace AL, Birch HL. Tendons and ligaments are anatomically distinct but overlap in molecular and morphological features-a comparative study in an ovine model. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2007;25(4):458–464. doi: 10.1002/jor.20218. [DOI] [PubMed] [Google Scholar]
- 21.Silver FH, Horvath I, Foran DJ. Viscoelasticity of the vessel wall: the role of collagen and elastic fibers. Critical reviews in biomedical engineering. 2001;29(3):279–301. doi: 10.1615/critrevbiomedeng.v29.i3.10. [DOI] [PubMed] [Google Scholar]
- 22.Amiel D, Frank C, Harwood F, Fronek J, Akeson W. Tendons and ligaments: a morphological and biochemical comparison. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1984;1(3):257–265. doi: 10.1002/jor.1100010305. [DOI] [PubMed] [Google Scholar]
- 23.Buehler MJ. Atomistic and continuum modeling of mechanical properties of collagen: Elasticity, fracture, and self-assembly. Journal of Materials Research. 2006;21(8):1947–1961. [Google Scholar]
- 24.Sasaki N, Odajima S. Elongation mechanism of collagen fibrils and force-strain relations of tendon at each level of structural hierarchy. Journal of biomechanics. 1996;29(9):1131–1136. doi: 10.1016/0021-9290(96)00024-3. [DOI] [PubMed] [Google Scholar]
- 25.Harley R, James D, Miller A, White JW. Phonons and the elastic moduli of collagen and muscle. Nature. 1977;267(5608):285–287. doi: 10.1038/267285a0. [DOI] [PubMed] [Google Scholar]
- 26.Miles CA, Sims TJ, Camacho NP, Bailey AJ. The role of the alpha2 chain in the stabilization of the collagen type I heterotrimer: a study of the type I homotrimer in oim mouse tissues. Journal of molecular biology. 2002;321(5):797–805. doi: 10.1016/s0022-2836(02)00703-9. [DOI] [PubMed] [Google Scholar]
- 27.Pyott SM, Schwarze U, Christiansen HE, Pepin MG, Leistritz DF, Dineen R, et al. Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and result in perinatal lethal to moderate osteogenesis imperfecta phenotypes. Human molecular genetics. 2011;20(8):1595–1609. doi: 10.1093/hmg/ddr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mechanisms of ageing and development. 1998;106(1–2):1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 29.Eppell SJ, Smith BN, Kahn H, Ballarini R. Nano measurements with micro-devices: mechanical properties of hydrated collagen fibrils. Journal of the Royal Society, Interface/the Royal Society. 2006;3(6):117–121. doi: 10.1098/rsif.2005.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Rijt JA, van der Werf KO, Bennink ML, Dijkstra PJ, Feijen J. Micromechanical testing of individual collagen fibrils. Macromolecular bioscience. 2006;6(9):697–702. doi: 10.1002/mabi.200600063. [DOI] [PubMed] [Google Scholar]
- 31.Buehler MJ. Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. Journal of the mechanical behavior of biomedical materials. 2008;1(1):59–67. doi: 10.1016/j.jmbbm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978;203(1152):305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- 33.Franchi M, Ottani V, Stagni R, Ruggeri A. Tendon and ligament fibrillar crimps give rise to left-handed helices of collagen fibrils in both planar and helical crimps. Journal of anatomy. 2010;216(3):301–309. doi: 10.1111/j.1469-7580.2009.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franchi M, Quaranta M, Macciocca M, De Pasquale V, Ottani V, Ruggeri A. Structure relates to elastic recoil and functional role in quadriceps tendon and patellar ligament. Micron. 2009;40(3):370–377. doi: 10.1016/j.micron.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Screen HR, Lee DA, Bader DL, Shelton JC. Development of a technique to determine strains in tendons using the cell nuclei. Biorheology. 2003;40(1–3):361–368. [PubMed] [Google Scholar]
- 36.Screen HR, Lee DA, Bader DL, Shelton JC. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proceedings of the Institution of Mechanical Engineers Part H, Journal of engineering in medicine. 2004;218(2):109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- 37.Cheng VWTS. H.R.C. The micro-structural strain response of tendon. Journal of Material Science. 2007;42:8957–8965. [Google Scholar]
- 38.Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HR. Specialization of tendon mechanical properties results from interfascicular differences. Journal of the Royal Society, Interface/the Royal Society. 2012;9(76):3108–3117. doi: 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halasz K, Kassner A, Morgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. The Journal of biological chemistry. 2007;282(43):31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 40.Birch HL, Worboys S, Eissa S, Jackson B, Strassburg S, Clegg PD. Matrix metabolism rate differs in functionally distinct tendons. Matrix biology: journal of the International Society for Matrix Biology. 2008;27(3):182–189. doi: 10.1016/j.matbio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD, Birch HL. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. The Journal of biological chemistry. 2010;285(21):15674–15681. doi: 10.1074/jbc.M109.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung HJ, Fisher MB, Woo SL. Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports medicine, arthroscopy, rehabilitation, therapy & technology: SMARTT. 2009;1(1):9. doi: 10.1186/1758-2555-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JH, Guo Q, Li B. Tendon biomechanics and mechanobiology-a minireview of basic concepts and recent advancements. Journal of hand therapy: official journal of the American Society of Hand Therapists. 2012;25(2):133–140. doi: 10.1016/j.jht.2011.07.004. quiz 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons et al. 2012;21(2):228–237. doi: 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dheerendra SK, Khan WS, Singhal R, Shivarathre DG, Pydisetty R, Johnstone D. Anterior cruciate ligament graft choices: a review of current concepts. The open orthopaedics journal. 2012;6:281–286. doi: 10.2174/1874325001206010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claes S, Verdonk P, Forsyth R, Bellemans J. The “ligamentization” process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. The American journal of sports medicine. 2011;39(11):2476–2483. doi: 10.1177/0363546511402662. [DOI] [PubMed] [Google Scholar]
- 47.Vavken P, Sadoghi P, Murray MM. The effect of platelet concentrates on graft maturation and graft-bone interface healing in anterior cruciate ligament reconstruction in human patients: a systematic review of controlled trials. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2011;27(11):1573–1583. doi: 10.1016/j.arthro.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dagher E, Hays PL, Kawamura S, Godin J, Deng XH, Rodeo SA. Immobilization modulates macrophage accumulation in tendon-bone healing. Clinical orthopaedics and related research. 2009;467(1):281–287. doi: 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thorpe CT, Clegg PD, Birch HL. A review of tendon injury: why is the equine superficial digital flexor tendon most at risk? Equine veterinary journal. 2010;42(2):174–180. doi: 10.2746/042516409X480395. [DOI] [PubMed] [Google Scholar]