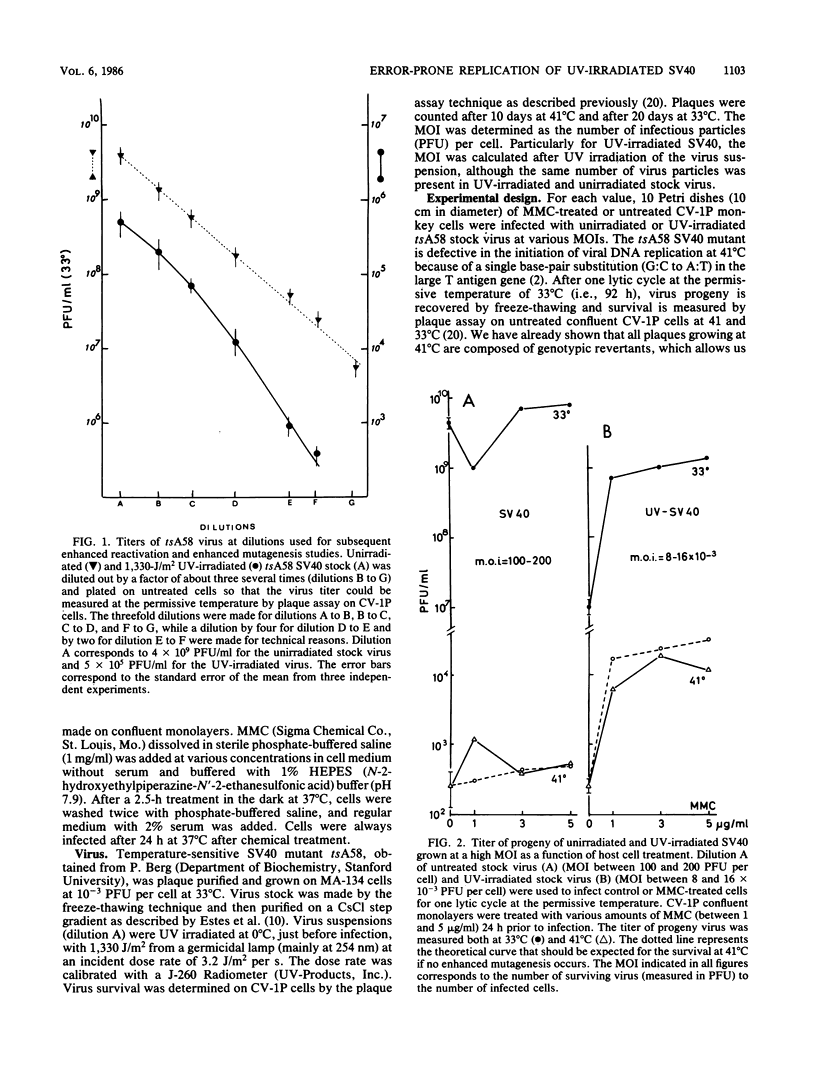
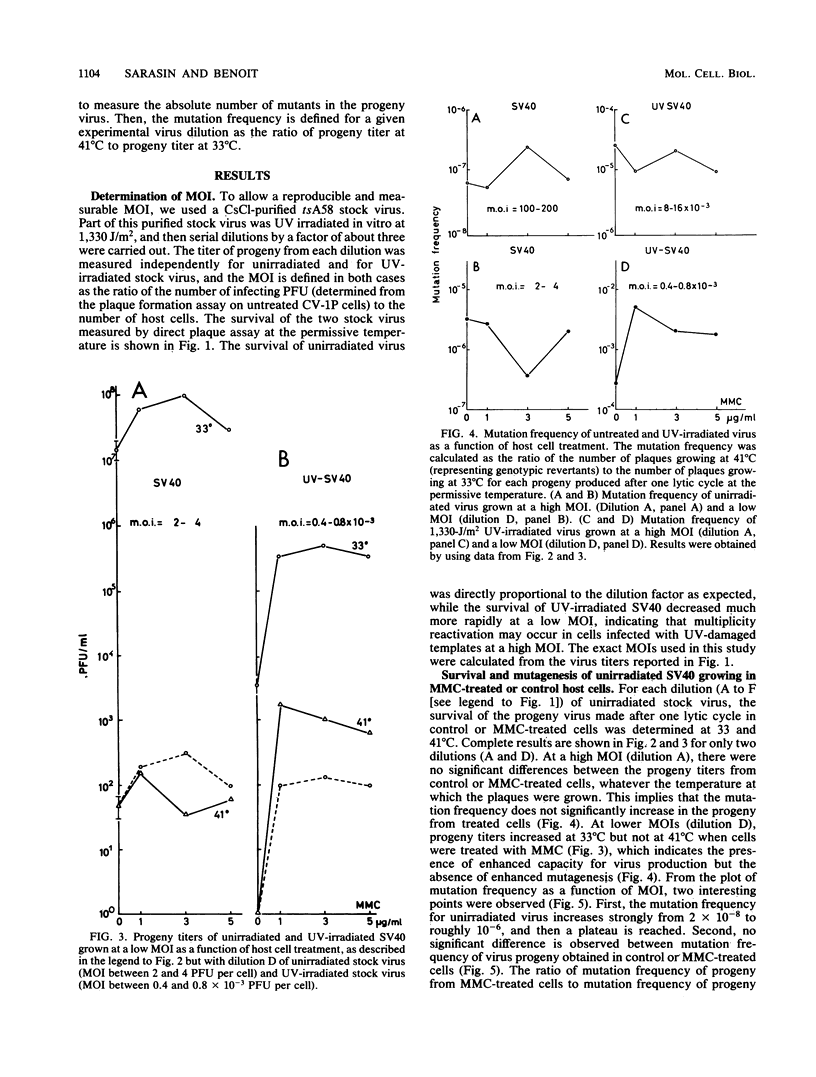
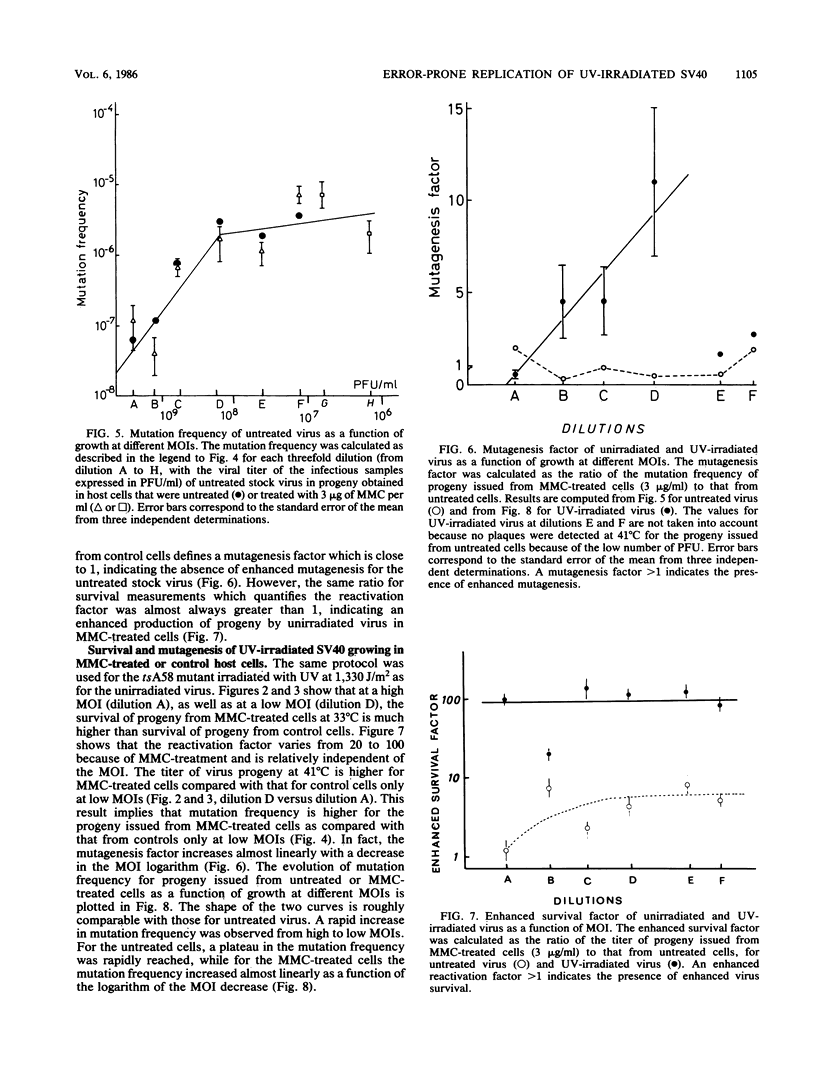
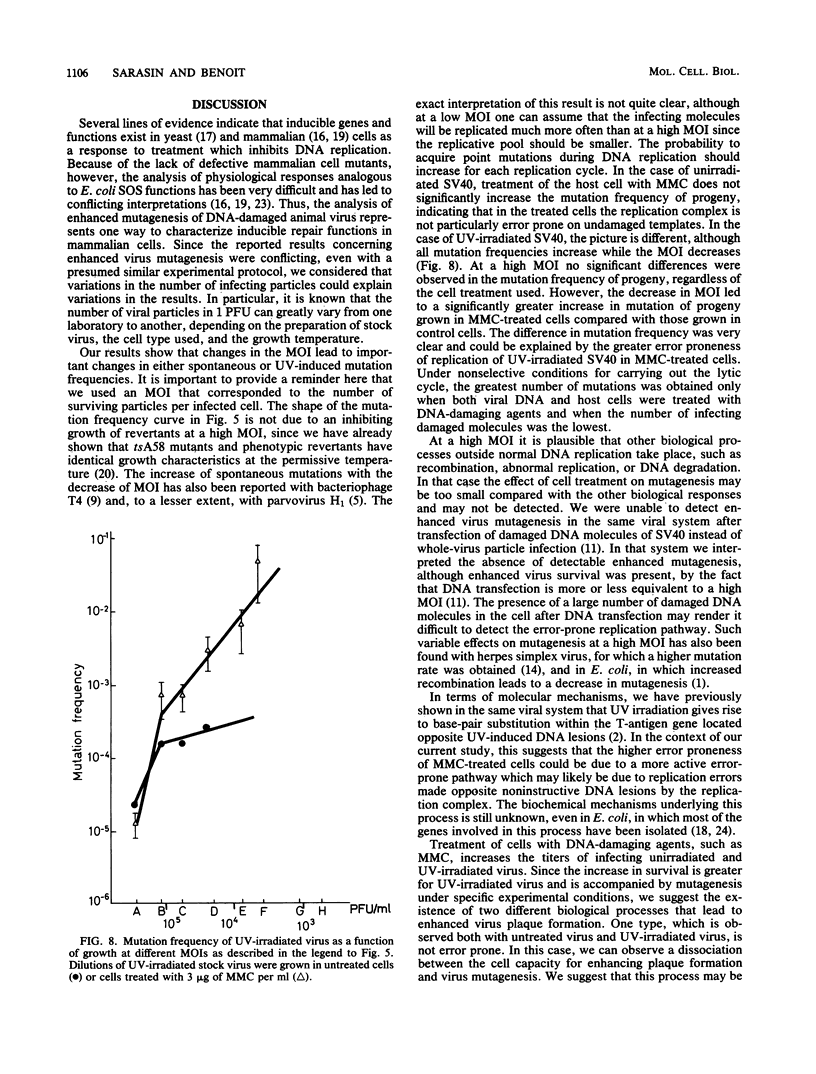
Abstract
Treatment of monkey kidney cells with mitomycin C (MMC) 24 h prior to infection with UV-irradiated simian virus 40 (SV40) enhanced both virus survival and virus mutagenesis. The use of SV40 as a biological probe has been taken as an easy method to analyse SOS response of mammalian cells to the stress caused by DNA damage or inhibition of DNA replication. The mutation assay we used was based on the reversion from a temperature-sensitive phenotype (tsA58 mutant) to a wild-type phenotype. The optimal conditions for producing enhanced survival and mutagenesis in the virus progeny were determined with regard to the multiplicity of infection (MOI). Results showed that the level of enhanced mutagenesis observed for UV-irradiated virus grown in MMC-treated cells was an inverse function of the MOI, while enhanced survival was observed at nearly the same level regardless of the MOI. For the unirradiated virus, almost no increase in the mutation of virus progeny issued from MMC-treated cells was observed, while a small amount of enhanced virus survival was obtained. These results show that enhanced virus mutagenesis and enhanced virus survival can be dissociated under some experimental conditions. Enhanced virus mutagenesis, analogous to the error-prone replication of phages in SOS-induced bacteria, was observed, at least for SV40, only when DNA of both virus and host cells was damaged and when infection occurred with a small number of viral particles. We therefore hypothesize that an error-prone replication mode of UV-damaged templates is observed in induced monkey kidney cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanco M., Devoret R. Repair mechanisms involved in prophage reactivation and UV reactivation of UV-irradiated phage lambda. Mutat Res. 1973 Mar;17(3):293–305. doi: 10.1016/0027-5107(73)90001-8. [DOI] [PubMed] [Google Scholar]
- Bourre F., Sarasin A. Targeted mutagenesis of SV40 DNA induced by UV light. Nature. 1983 Sep 1;305(5929):68–70. doi: 10.1038/305068a0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Decline in mutation frequency in temperature-sensitive SV40 viruses before viral DNA synthesis. Mutat Res. 1977 Sep;44(3):291–298. doi: 10.1016/0027-5107(77)90088-4. [DOI] [PubMed] [Google Scholar]
- Cornelis J. J., Lupker J. H., van der Eb A. J. UV-reactivation, virus production and mutagenesis of SV40 in VU-irradiated monkey kidney cells. Mutat Res. 1980 Jun;71(1):139–146. doi: 10.1016/0027-5107(80)90014-7. [DOI] [PubMed] [Google Scholar]
- Cornelis J. J., Su Z. Z., Rommelaere J. Direct and indirect effects of ultraviolet light on the mutagenesis of parvovirus H-1 in human cells. EMBO J. 1982;1(6):693–699. doi: 10.1002/j.1460-2075.1982.tb01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta U. B., Summers W. C. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammlian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2378–2381. doi: 10.1073/pnas.75.5.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H. UV-induced reversion of adenovirus 5ts2 infecting human cells. Photochem Photobiol. 1981 Sep;34(3):403–406. doi: 10.1111/j.1751-1097.1981.tb09015.x. [DOI] [PubMed] [Google Scholar]
- Drake J. W. Ultraviolet mutagenesis in bacteriophage T-4. I. Irradiation of extracellular phage particles. J Bacteriol. 1966 May;91(5):1775–1780. doi: 10.1128/jb.91.5.1775-1780.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentil A., Margot A., Sarasin A. Enhanced reactivation and mutagenesis after transfection of carcinogen-treated monkey kidney cells with UV-irradiated simian virus 40 (SV40) DNA. Biochimie. 1982 Aug-Sep;64(8-9):693–696. doi: 10.1016/s0300-9084(82)80112-0. [DOI] [PubMed] [Google Scholar]
- Lavi S. Carcinogen-mediated amplification of viral DNA sequences in simian virus 40-transformed Chinese hamster embryo cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6144–6148. doi: 10.1073/pnas.78.10.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle C. D., Goddard J. G., Lin C. H. Repair and mutagenesis of herpes simplex virus in UV-irradiated monkey cells. Mutat Res. 1980 Apr;70(2):139–149. doi: 10.1016/0027-5107(80)90153-0. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Knott D. C. Enhanced mutagenesis parallels enhanced reactivation of herpes virus in a human cell line. EMBO J. 1982;1(6):701–703. doi: 10.1002/j.1460-2075.1982.tb01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. Is there SOS induction in mammalian cells? Photochem Photobiol. 1980 Dec;32(6):823–830. doi: 10.1111/j.1751-1097.1980.tb04062.x. [DOI] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Ruby S. W., Szostak J. W. Specific Saccharomyces cerevisiae genes are expressed in response to DNA-damaging agents. Mol Cell Biol. 1985 Jan;5(1):75–84. doi: 10.1128/mcb.5.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Carcinogens enhance survival of UV-irradiated simian virus 40 in treated monkey kidney cells: induction of a recovery pathway? Proc Natl Acad Sci U S A. 1978 Jan;75(1):346–350. doi: 10.1073/pnas.75.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A., Benoit A. Induction of an error-prone mode of DNA repair in UV-irradiated monkey kidney cells. Mutat Res. 1980 Mar;70(1):71–81. doi: 10.1016/0027-5107(80)90059-7. [DOI] [PubMed] [Google Scholar]
- Sarasin A., Bourre F., Benoit A. Error-prone replication of ultraviolet-irradiated simian virus 40 in carcinogen-treated monkey kidney cells. Biochimie. 1982 Aug-Sep;64(8-9):815–821. doi: 10.1016/s0300-9084(82)80135-1. [DOI] [PubMed] [Google Scholar]
- Sarasin A. SOS response in mammalian cells. Cancer Invest. 1985;3(2):163–174. doi: 10.3109/07357908509017499. [DOI] [PubMed] [Google Scholar]
- Taylor W. D., Bockstahler L. E., Montes J., Babich M. A., Lytle C. D. Further evidence that ultraviolet radiation-enhanced reactivation of simian virus 40 in monkey kidney cells is not accompanied by mutagenesis. Mutat Res. 1982 Nov;105(5):291–298. doi: 10.1016/0165-7992(82)90095-1. [DOI] [PubMed] [Google Scholar]
- Walker G. C., Dobson P. P. Mutagenesis and repair deficiencies of Escherichia coli umuC mutants are suppressed by the plasmid pKM101. Mol Gen Genet. 1979 Apr 17;172(1):17–24. doi: 10.1007/BF00276210. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


