Abstract
Contemporary claims that mitotically active female germ line or oogonial stem cells (OSCs) exist and support oogenesis during postnatal life in mammals have been debated in the field of reproductive biology since March 2004, when a mouse study posed the first serious challenge to the dogma of a fixed pool of oocytes being endowed at birth in more than 50 years. Other studies have since been put forth that further question the validity of this dogma, including the isolation of OSCs from neonatal and adult mouse ovaries by 4 independent groups using multiple strategies. Two of these groups also reported that isolated mouse OSCs, once transplanted back into ovaries of adult female mice, differentiate into fully functional eggs that ovulate, fertilize, and produce healthy embryos and offspring. Arguably, one of the most significant advances in this emerging field was provided by a new research study published this year, which reported the successful isolation and functional characterization of OSCs from ovaries of reproductive age women. Two commentaries on this latest work, one cautiously supportive and one highly skeptical, were published soon afterward. This article evaluates the current literature regarding postnatal oogenesis in mammals and discusses important next steps for future work on OSC biology and function.
Keywords: oogenesis, oocyte, ovary, germ cell, stem cell, human
Introduction
A recent study by White et al1 published in Nature Medicine describes the successful isolation and characterization of oogonial stem cells (OSCs; also referred to as female germ line stem cells, female GSCs or fGSCs) from ovarian cortical tissue of women in their 20s and 30s. In addition to validating a sensitive fluorescence-activated cell sorting (FACS)-based method for purifying OSCs from adult ovary tissue of mice and women, this study provides evidence from, among other experiments, in vivo xenografting approaches showing that human OSCs introduced into adult human ovarian tissue rapidly undergo differentiation into oocytes that appear to arrest at the diplotene stage of meiosis I and orchestrate folliculogenesis.1 These observations essentially mirror the outcomes reported from fate mapping experiments following intraovarian transplantation of mouse OSCs into adult female recipients.1,2 From a much broader perspective, the identification of OSCs in ovaries of women not only extends a growing body of work supporting the existence of OSCs in mice1–8 but also raises the possibility that germ cells are subject to active renewal during reproductive life in women.9 Such a concept is already universally accepted for adult females of less evolved species, such as flies and teleost fish,10–13 and for adult males of essentially all animal species.14,15
Perhaps not surprisingly given that the existence of OSCs in mammals has been debated for years,16,17 two commentaries with distinctly opposite tones and viewpoints on this new study were published within 2 weeks of its online release.18,19 The first of these, which appeared in Nature Medicine along with the study by White et al,1 concluded that while questions remain regarding the role of OSCs in female reproductive function under normal physiological conditions, “this study [White et al] will change the tone of future discourse on the subject [of OSCs and postnatal oogenesis in mammals] toward measured enthusiasm . . .”18 The second commentary was expedited for online publication in Biology of Reproduction within only 10 days after online release of the White et al1 study. This commentary not only contrasted sharply with the Nature Medicine opinion piece in both tone and overall message, but it also raised what the authors perceived as significant flaws in the White et al1 study, which precluded any firm conclusions to be drawn.19 Further underscoring the divided nature of the field, 2 more publications have since followed this work, one offering additional evidence for,8 and another disputing the existence of,20 mitotically active germ cells in mammals during postnatal life. In the sections to follow, we evaluate the current body of literature on mammalian OSCs and offer suggestions for how best to more clearly define the properties and function of these rare cells that several laboratories have now independently isolated by different strategies.
A Method to Purify OSCs
The study of mammalian OSCs depends on validation of a reliable strategy for the purification of these cells from postnatal ovary tissue. A key step toward this objective was published in 2009, in a groundbreaking study that used an immunomagnetic bead–based assay (also referred to as magnetic-assisted cell sorting or MACS) to isolate fractions of cells from dispersed ovaries of neonatal and young adult mice based on cell surface expression of the germ line–specific marker Ddx4 (DEAD box polypeptide 4; also commonly referred to as mouse vasa homolog or Mvh).2 The cells obtained exhibited a genetic signature consistent with primitive germ cells and were successfully established as stable cultures for long-term propagation. Their functional identity as oocyte-producing progenitor germ cells was confirmed through intragonadal transplantation assays, essentially identical to those used for almost 20 years to test the functionality of male germ cell preparations containing spermatogonial stem cells (SSCs).21,22 These experiments demonstrated that transplanted OSCs, carrying a virally introduced green fluorescent protein (GFP) reporter gene for cell fate tracking, differentiate into oocytes which give rise to eggs that fertilize and produce viable offspring.2 This work was followed 2 years later by another article from this same group using OSCs carrying randomly integrated transgenes.6
While exciting, the cell isolation approach reported by Zou et al2 was questioned by some,23 since the protein used to retrieve viable OSCs from dispersed mouse ovaries was historically viewed as being cytoplasmic protein in germ cells.24,25 To evaluate this possibility in more detail, we immediately attempted to repeat the OSC isolation protocol described by Zou et al2 but were unsuccessful, primarily because of an absence of clear methodological details in their article. After months of troubleshooting various steps, our attempts at immunomagnetic bead–based sorting of OSCs from mouse ovaries consistently yielded a final cell fraction containing debris and dead cells along with contaminating oocytes.1 We therefore abandoned MACS and switched to assessment of cell-surface expression of Ddx4 protein in mouse OSCs by FACS, since this method permits one to exclude dead or damaged cells and to collect a homogenous population of cells of a specific size. The latter ability to perform cell size-selection ensures that small oocytes are excluded from the final cell fraction. Through extensive validation experiments using antibodies against both ends of the Ddx4 protein, exposure of the C-terminus of Ddx4 on the cell surface of mouse OSCs but not on the cell surface of oocytes, which possess only cytoplasmic Ddx4, was confirmed. The resultant Ddx4-positive viable cell fraction—defined by the presence of immunologically detectable Ddx4 protein on the extracellular surface of the plasma membrane of a population of cells between 5 and 8 μm in diameter—was shown to be free of contaminating immature oocytes, which are not only significantly larger in size but also lack externalized Ddx4 protein.1 This same strategy was then successfully used to purify OSCs from dispersed ovarian cortical tissue obtained with written informed consent from reproductive-age women.1
Even with independent confirmation of cell-surface expression of the C-terminus of Ddx4 in OSCs,1,2 some scientists continue to question these results and, in turn, the usefulness of Ddx4 antibody-based methods for the purification of OSCs,20 simply based on traditional beliefs that Ddx4 is a cytoplasmic protein in germ cells. Aside from the fact that rigorous experimental evidence has been published proving this incorrect,1 it bears mention that a second germ cell lineage marker termed Ifitm3 (interferon-induced transmembrane protein 3; also commonly referred to as Fragilis) can also be used to isolate OSCs from postnatal mouse ovaries, and the cells obtained are indistinguishable from those isolated in parallel using antibodies against the C-terminus of Ddx4.7 Unlike Ddx4, which had no prior precedent for extracellular expression in germ cells until the publication from Zou et al,2 Ifitm3 is a documented transmembrane protein involved in early germ cell specification.26 In fact, antibodies against the extracellular domain of Ifitm3 work extremely well for identification of premeiotic germ cells and their purification from mixed cell preparations using immunological sorting methods.27,28 Accordingly, while those skeptical of the existence of OSCs continue to dismiss reports of the purification of these rare cells because the isolation strategies used are discordant with traditional beliefs that Ddx4 is a cytoplasmic protein in all germ cells, it is unclear how these concerns can be reconciled with observations that antibodies against a different germ cell-specific protein, with confirmed extracellular expression and utility for premeiotic germ cell sorting,27,28 efficiently purify the same cells from postnatal mouse ovaries as those isolated by Ddx4 antibody-based protocols.7
What Cells Are Actually Isolated From Ovaries by Ddx4-based FACS?
Following FACS-based purification, viable Ddx4-positive cells from adult ovary tissue were subjected to gene expression profiling analysis before culture. Freshly isolated mouse and human OSCs express high levels of Prdm1 (PR domain containing 1 with ZNF domain; also commonly referred to as Blimp1), Dppa3 (developmental pluripotency-associated 3; also commonly referred to as Stella), and Ifitm3,1 which definitively identify primitive germ cells in mammals.26–30 In addition, mouse and human OSCs express high levels of the catalytic subunit of telomerase (Tert),1 which is a hallmark feature of pluripotent stem cells and germ cells.31 Importantly, Prdm1, Dppa3, Ifitm3, and Tert remain highly expressed in cultured OSCs, even after months of propagation.1 Such findings are important to consider when evaluating a concern raised by others that the ability of OSCs isolated by White et al1 to establish actively dividing germ cell colonies in culture “smacks of in vitro transformation.”19 Aside from the unnecessary negativity of the tone of this statement, these authors do not go on to provide any details regarding what specific cell type they feel is undergoing this claimed transformation event, what the claimed transformation event is, and what specific cell type they feel is produced by the claimed transformation event. Irrespective, the fact that White et al1 showed OSCs express the same definitive markers of primitive germ cells both before and after culture argues against this. And while OSCs placed in culture at very low initial seeding densities in the absence of mouse embryonic fibroblast (MEF) feeder cells can take several weeks (human) or more (mouse) to establish visibly dividing colonies of germ cells,1 growth of mouse and human OSCs established on MEFs from the start is, not surprisingly, much more vigorous, and these cells can form actively dividing germ cell colonies in less than 2 weeks.
This comment is actually reminiscent of a prior criticism voiced by some over the Zou et al2 study published in 2009. It was argued that since the mouse OSCs used for functionality testing through intragonadal transplantation assays were expanded ex vivo before being returned to the ovaries, the ability of these cells to generate oocytes that mature, fertilize, and produce viable offspring was due to some undefined culture-induced change/changes in the initially purified cells prior to transplantation. This seems ill conceived for several reasons. First and foremost, such a criticism ignores the fact that fully functional eggs were obtained from the transplanted cells, cultured or otherwise. This has not been achieved with any other cell tested to date, including embryonic stem cells or induced pluripotent stem cells, and it has been independently confirmed.1 Second, many of those who perform functional testing of SSCs rely on transplantation of germ cells that have been obtained from testes and cultured for weeks to months before reintroduction of the expanded cells into the testes of recipient male mice. If those critical of the existence and functional capacity of mammalian OSCs believe that some in vitro transformation event conveys germ line competency to Ddx4-postive cells purified from adult ovaries, it is unclear why such a caveat is not similarly applied to a multitude of published studies using heterogeneous germ cell preparations prepared from testes and cultured in vitro before in vivo testing of spermatogenic potential.
One possible reason for this may be that comparable outcomes have been observed using freshly isolated versus ex vivo expanded germ cells in SSC transplantation experiments in mice.32,33 Studies such as these indicate that in vitro culture of male germ cell preparations enriched for SSCs does not in general affect their spermatogenic potential once injected back into the testis; in turn, it is reasonable to assume at this stage that OSCs possess the ability to differentiate into fully functional eggs, irrespective of whether freshly isolated or cultured cells are used for intraovarian transplantation experiments. It is noteworthy that only 0.04% to 1.26% of presumptive male GSCs maintained in long-term cultures are, however, capable of reconstituting spermatogenesis following intratesticular transplantation into infertile male mice.34 These findings indicate there is significant cellular heterogeneity in SSC cultures that are used for in-depth characterization of these cells, and very few of the cells present in these cultures actually represent bona fide male GSCs as defined by spermatogenic colony formation following transplantation. Although recent analyses of long-term cultures of mouse and human OSCs indicate that expression of Prdm1, Dppa3, and Ifitm3, at the protein level, remain uniformly detectable in essentially every cell,1 it will still be important in the future to comparatively map the genetic and epigenetic profiles of freshly isolated versus cultured OSCs.
In addition, scientists who study mammalian OSCs will need to assess whether the freshly isolated cells, like their ex vivo expanded counterparts, can generate functional oocytes once transplanted back into adult ovary tissue immediately after purification. Our laboratory has already started to pursue such work, and while the mating trials have just been initiated, OSCs freshly isolated from the ovaries of adult TgOG2 transgenic female mice, which express GFP under control of a germ line–specific Pou5f1 gene promoter, generate GFP-expressing oocytes enclosed within follicles following injection into adult wild type mouse ovaries (D.C. Woods, Y.A.R. White and J. L. Tilly, unpublished data; Figure 1). These observations, which are fully consistent with published outcomes observed from 2 different laboratories using ex vivo expanded OSCs,1,2,6 further discount the validity of the statement from Oatley and Hunt that the White et al1 study “smacks of in vitro transformation.”19
Figure 1.
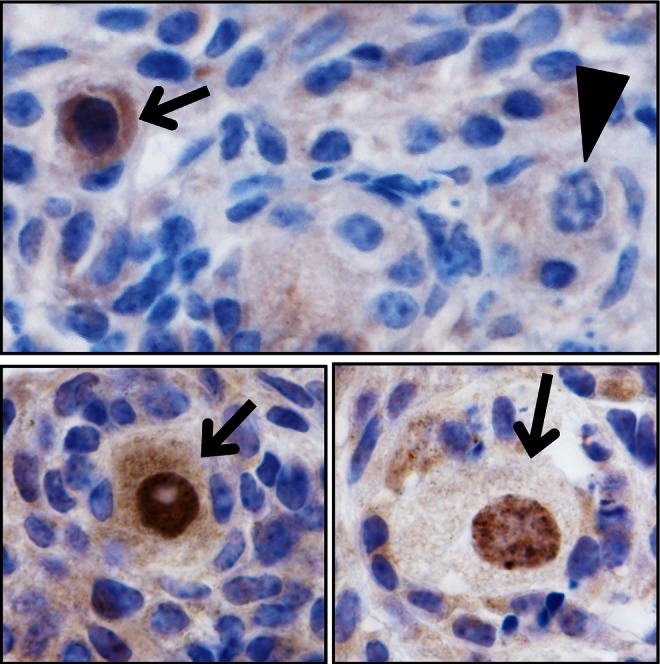
Freshly isolated OSCs generate oocytes after immediate reintroduction into adult ovaries. Following FACS-based purification of OSCs from ovaries of young adult female TgOG2 transgenic mice, the isolated cells were injected into ovaries of young adult wild-type female recipients (2-5 × 103 cells injected per ovary). Five days later, immunohistochemical assessment of GFP expression was used to identify GFP-positive oocytes originating from the injected OSCs (arrows), contained in follicles, adjacent to host oocyte-containing follicles (GFP negative; arrowhead). See the article by White et al.1 for details.
In Vitro Oogenesis Studies: Are Haploid Cells a Problem?
A defining feature of OSCs isolated by various laboratories using different techniques is the ability of these cells to stably proliferate in vitro for months.1,2,5,7,8 This property not only draws yet another parallel to an approach used routinely in the study of SSCs33,34 but also confirms that these germ cells, present in ovaries of adult mice and women, are mitotically active and thus premeiotic. One of the more intriguing observations made by both Pacchiarotti et al5 and White et al1 during their in vitro characterization studies relates to the spontaneous formation of what appear to be oocytes from cultured OSCs. Interestingly, if oocytes formed by mouse OSCs in vitro are aggregated with granulosa cells from neonatal mouse ovaries, structures closely resembling immature follicles rapidly form in culture.5 These findings indicate that OSC-derived oocytes naturally recruit, or are a natural target of, endogenous granulosa cells. The ensuing process of in vitro folliculogenesis aligns with the observations that OSCs returned to adult ovarian tissue in an in vivo setting rapidly generate oocytes that become surrounded by granulosa cells to form follicles.1,2
In pure cultures of mouse and human OSCs (viz lacking somatic cells of any kind, including MEFs), White et al1 also showed the oocytes that form from these cells in vitro express all of the appropriate markers of endogenous oocytes, including Kit, the meiotic diplotene stage–specific marker Ybx2 (also commonly referred to as Msy2), Nobox (newborn ovary homeobox), Lhx8 (LIM homeobox protein 8), Gdf9 (growth differentiation factor 9), and all 3 zona pellucida glycoprotein genes (Zp1–Zp3). Moreover, FACS-based analysis of cellular chromosomal content in cultures of both mouse and human OSCs revealed the presence of a 1n (haploid) cell peak that was not detected in dividing somatic cells analyzed as a negative control in parallel. However, Oatley and Hunt have subsequently questioned this latter observation in stating “the reported increase in apparent haploid cells [in cultures of mouse and human OSCs] indicates not only meiotic entry, but also completion of both meiotic divisions. This is surprising since oocytes never reach a haploid state in vivo. Thus, a population of haploid oocytes presents a problem.”19 Is the apparent formation of haploid cells in these cultures of premeiotic female germ cells really a problem? In evaluating literature not discussed by these authors, spontaneous oocyte activation does in fact occur in vivo, and meiotic activation and progression occurs apace, often quite normally.35,36 Thus, published data clearly show that oocytes, in the absence of sperm penetration, can spontaneously exit meiotic arrest and complete both meiotic divisions in vivo, at least under conditions in which regulatory input from surrounding somatic granulosa cells is physically disrupted by ovulation.
The bigger problem here is that parallels are mistakenly being drawn by these authors between in vitro outcomes reported by White et al1 using pure germ cell cultures with meiotic events that normally occur as oocytes mature inside follicles in vivo and following their ovulatory release. In other words, the apparent formation of haploid cells in cultures of OSCs lacking normal regulatory input from granulosa cells, the latter of which have been known for decades to actively repress meiotic resumption in oocytes,37 is not really surprising or a problem if one does not fall into the trap of trying to compare apples (in vitro germ cell behavior without somatic cell interaction) to oranges (in vivo germ cell behavior with normal somatic cell interaction). This is not meant to infer that the oocytes generated by pure cultures of OSCs in vitro are normal or developmentally competent by any means; rather, the true value of these findings is rooted in the utility of cultured OSCs as a novel screening platform for identification of hormones, agents, and signaling pathways capable of increasing or decreasing the basal rate of oocyte formation. Such information may lead not just to a more detailed understanding of the process of human oogenesis but also to new potential therapeutic approaches for management of the ovarian reserve based on targeting OSCs or their intraovarian niches in vivo.9
In Vivo Studies: The Litmus Test of Germ Line Stem Cell Identity
For nearly two decades, studies of mammalian SSCs have utilized intratesticular transplantation as the gold standard method to unequivocally establish the identity of these cells.21,22 Many reports have used testicular germ cell preparations that were cultured ex vivo prior to functionality testing, partly due to the fact that the germ cells retrieved for study are a mix of true SSCs, which are extremely rare, along with more differentiated spermatogonial cell subtypes. In fact, no method has been validated to date that permits the purification of only bona fide male GSCs.38 Even with these caveats, SSCs are defined primarily by the ability of small numbers of cells in transplanted male germ cell preparations to engraft, institute spermatogenic colonies in the seminiferous tubules, and generate functional sperm as assessed by fertility testing. To facilitate this, the fate of transplanted germ cells is tracked by monitoring expression of a foreign reporter gene product, such as LacZ or GFP, or a randomly integrated transgene, introduced into the cells to be studied prior to the transplantation procedure.
In a recent opinion-based critique of the White et al1 article, Oatley and Hunt emphasize that the “litmus test of the putative SSC cells obtained—their capacity to regenerate continual sperm production following transplantation into a recipient testis—has been firmly established.”19 These authors went on to state, “for OSCs, doubt will persist until clear evidence is provided that they give rise to genetically normal, developmentally competent eggs.” If this is truly the litmus test for mammalian GSC identity, then OSCs have already passed this requirement in published studies from 2 different laboratories, at least as defined by the ability of OSCs to differentiate into fully mature eggs that fertilize to yield embryos and normal viable offspring.1,2,6 While more studies are of course needed to rigorously evaluate the genetic normalcy of oocytes produced by transplanted OSCs, there should at minimum no longer be a persistence of doubt over the existence of oocyte-producing progenitor germ cells in adult mammalian ovaries. Aside from the fact that 4 different groups have now reported OSCs can be isolated and stably propagated long term,1,2,5,8 2 have independently shown that OSCs retain their primary functional directive in vivo following intraovarian transplantation—the long-term formation of oocytes that mature and fertilize to produce viable embryos and offspring.1,2,6
Insights From Genetically Recombined Germ Cell “Reporter” Mice: What Have We Actually Learned?
Recently, a study by Zhang et al20 from the laboratory of Kui Liu reported observations from an assessment of Ddx4-Cre transgenic mice crossed with Rosa26rbw/+ mice, which triggers a genetic recombination event in any cell that has at some point during development activated the Ddx4 gene promoter. The germ cells in resultant offspring of this cross switch on expression of a specific fluorescent marker gene as a consequence of the Ddx4 promoter-driven recombination event, which is not detected in cells lacking Ddx4 gene activation. Zhang et al20 state, as their rationale for using this approach to test for the existence of mitotically active germ cells in ovaries of postnatal female mice, that “it was of great importance to validate the conclusions drawn by Zou et al [ref 2] and White et al [ref 1]” regarding the isolation of OSCs from postnatal mouse ovaries “with an experimental approach other than an antibody-based cell purification.” At the outset, 2 major points need to be considered when evaluating the data presented in this new study, and more importantly the validity of the principal conclusion drawn by these authors that “no mitotically active Ddx4-expressing female germ line progenitors exist in postnatal mouse ovaries.”20
The first of these is that the genetic approach employed by Zhang et al20 results in a permanent genetic alteration in any cell that at any point has activated the Ddx4 gene promoter region used to drive Cre recombinase expression in this transgenic mouse line. As a result, such cells will maintain expression of the recombined (activated) fluorescent reporter gene, even if the endogenous Ddx4 gene is no longer active. Under no circumstances does this approach take into account whether the recombined cells still possess endogenous Ddx4 protein or, if they do, where within the recombined cells the Ddx4 protein is localized. This is not a small matter since both oocytes and OSCs express the Ddx4 gene. Thus, both types of female germ cells will be genetically recombined in a permanent manner and, accordingly, will show identical patterns of expression of the same fluorescent reporter gene. If careful efforts are not made to identify exactly what type of female germ cell is being assessed, or to separate one type of female germ cell from another prior to assessment, there is no way of knowing whether the outcomes obtained reflect analysis of OSCs or oocytes. In this regard, it is noteworthy that Zhang et al20 did not separate different types of recombined germ cells obtained from ovaries of their mice, nor did they offer any clues as to the identity of the “Ddx4-expressing” cells shown in figure 2B of their article through even simple polymerase chain reaction (PCR)-based analysis of gene expression patterns specific to OSCs versus oocytes. The second point of note is that these authors made no attempt to isolate OSCs from the ovaries of their genetically recombined mice using methods reported by others but instead simply dismissed antibody-based sorting approaches as being “troublesome” since “Ddx4 is known to be an intracellular cytoplasmic protein that is not expressed on the cell surface.” Interestingly, neither of the 2 reference citations offered by Zhang et al to support this statement actually shows that Ddx4 protein is always found inside germ cells (see A Method to Purify OSCs above for more discussion on this topic as well as on non-Ddx4 antibody-based methods for isolating OSCs).
In looking at this article more closely, 3 general sets of experiments were conducted by Zhang et al.20 The first of these was based on transplantation of dissociated fetal ovarian cells, collected at 12.5 days postcoitum, from Rosa26rbw/+ mice into ovaries of young adult wild-type female mice without or with prior chemotherapy conditioning. Under both conditions, follicles containing oocytes and granulosa cells derived from the transplanted fetal ovarian Rosa26rbw/+ cells were detected, but none of the follicles shown in the article were chimeric (viz derived from a mix of transplanted and host cells). Based on this, Zhang et al20 conclude that adult mouse ovaries can support de novo folliculogenesis, but only if both germ line progenitors (embryonic primordial germ cells or PGCs) and follicular somatic cell progenitors (embryonic pregranulosa cells) are provided together. In other words, they claim that the adult ovary host environment does not contain nor provide either of these cell types. Unfortunately, several key controls are missing here, which preclude accurate interpretation of the data shown. First, it is unclear why nonrecombined reporter mice (Rosa26rbw/+) were used as a source of fetal ovarian cells for transplantation in this experiment, since both cell types in the absence of Ddx4-Cre-mediated excision express enhanced GFP (EGFP). Germ cell–specific detection, which results from Ddx4-Cre-driven recombination at the Rosa26rbw/+ locus, leads to a permanent switch from EGFP to red, yellow, or cyan fluorescent protein expression only in germ cells. This would have allowed assessment of the posttransplantation fate of each cell type independently. An even more basic question concerns the rationale for transplanting the entire cell fraction prepared from fetal ovaries, rather than separate fractions of purified PGCs and fetal somatic cells evaluated independently. If the goal here was to truly assess whether both cell lineages must be transplanted together for de novo folliculogenesis to be observed in adult mouse ovaries, then each cell fraction must be transplanted separately to “force” the adult ovary to potentially utilize its own progenitor somatic (fetal PGC transplants) or germ (fetal somatic cell transplants) cells to support new follicle formation. Indeed, in mouse and human OSC fate mapping studies, pure preparations of these premeiotic germ cells are sufficient to drive de novo folliculogenesis in recipient ovarian tissue, the latter of which supplies the needed pregranulosa cells.1,2
The second experimental design is the only one that actually deals with an assessment of postnatal ovary-derived germ cells from Ddx4-Cre/Rosarbw/+ female mice. To obtain “Ddx4-expressing” cells for analysis, ovaries of genetically recombined female mice at postnatal day 8 were collected for mechanical and trypsin-based enzymatic dispersion. The cell suspension was then filtered through a 40-μm pore cell strainer to “remove large oocytes and cell aggregates,” and the entire resultant fraction of cells less than 40-μm in diameter was placed in culture to monitor cells with red fluorescent protein (RFP) expression indicative of Ddx4-Cre-mediated recombination at the Rosarbw/+ locus (viz germ cells). The outcomes of these experiments, which are shown in figure 2B of the article from Zhang et al,20 indicate that RFP-positive (Ddx4-expressing) cells present in the dispersed postnatal ovaries examined do not proliferate after 72 hours of culture but instead degenerate. Based solely on these findings and no other supporting data, these authors conclude that “Ddx4-expressing cells from postnatal mouse ovaries were mitotically inactive.” Given that this statement essentially refutes the findings of 4 other laboratories who have successfully isolated and studied mouse OSCs,1,2,5,8 a careful examination of the data shown to support such a bold statement is warranted. Unfortunately, this reveals several major problems.
The most concerning is that all of the RFP-positive postnatal ovarian cells shown by Zhang et al20 are significantly larger in diameter than OSCs, the latter of which range in size from 5 to 6 μm, and no larger than 8 μm, in diameter.1 In fact, based on comparison to the 20-μm scale bars shown in figure 2B of this new study, and the fact that the size cutoff of the cells placed in culture was 40 μm—which would, as Liu and colleagues state, remove “large oocytes” but certainly would not filter out the thousands of smaller immature oocytes that represent the bulk majority of oocytes present in postnatal day 8 mouse ovaries—the “Ddx4-expressing” cells evaluated in this study were in all likelihood small oocytes and not OSCs. This easily explains why the RFP-positive cells of postnatal mouse ovaries cultured by Zhang et al20 would, as surmised by John Eppig who edited the article for the journal in which it appeared, “just sit there and go tum-tiddly-tum.”39 In other words, it is entirely expected that immature oocytes, which would without question be RFP positive in these genetically recombined mice and thus identified as “Ddx4-expressing cells,” would fail to proliferate in culture. There is no other possible outcome. This caveat then leads to another major problem with the interpretation of this critical experiment: why did Liu and colleagues not perform, and why were these authors not asked to perform, any analysis of the RFP-expressing cells shown in figure 2B of their article to determine the identity of these cells as anything but oocytes? A simple PCR-based screen of germ line versus immature oocyte markers, as has been required for published studies of the OSCs that Liu and colleagues question,1,2,5 would have addressed this clearly; and yet these data, which are crucial to any interpretation of the outcomes of this experiment, are missing. Based simply on the large size of the RFP-positive cells shown in figure 2B of this article (all >10 μm, most 15 μm or more, in diameter), there is little doubt that the Ddx4-expressing cells in postnatal mouse ovaries examined would be positive for a number of oocyte-specific genes, such as Nobox, Gdf9, and Zp3, that are absent in freshly isolated OSCs.1 This being the case, the only data set presented by Zhang et al20 that actually pertains to “Ddx4-expressing cells” in postnatal mouse ovaries is reflective of how immature oocytes behave in culture, not OSCs, the existence of which this new study claims to refute based on ambiguous and experimentally unsupported observations.
The third and final experimental design, the outcomes of which represent the majority of data presented by Zhang et al,20 is perhaps the most perplexing since its stated objective is to establish that “Ddx4-negative ovarian clonal cells in culture are not germ line progenitors.” While these authors conducted several different experiments to rigorously show that Ddx4-negative clonal cells present in the ovaries of their recombined mice are not germ cells, it is unclear why Liu and colleagues expected otherwise since any nonrecombined cells in their Ddx4-Cre/Rosarbw/+ mice do not express the Ddx4 gene and are thus inherently not germ lineage cells to begin with. Even so, these authors attempt to draw a link between these non–germ line cells to OSCs reported by others in stating that colonies formed by their Ddx4-negative ovarian clonal cells “had a morphology similar to those reported for OSC colonies.”20 Why this was done is unclear; however, if the inference is that other groups have mistakenly identified these Ddx4-negative ovarian clonal cells as OSCs, this is not the case. Aside from the fact that multiple groups have independently shown freshly isolated and cultured OSCs express Ddx4 at both the mRNA and protein levels,1,2,5 which these Ddx4-negative ovarian clonal cells do not, there are many other properties not shared by the 2 cell types. Finally, Zhang et al20 also reported that their Ddx4-negative ovarian clonal cells generate “oocyte-like” cells in vitro. This conclusion was deduced, however, only by morphological appearance of the aforementioned cells. Unlike oocytes formed by cultured mouse and human OSCs, which retain expression of Ddx4,1 the “oocyte-like” cells reported by Liu and colleagues are Ddx4 negative.20 This fundamental difference aside, these authors once again did not attempt, and were not asked before acceptance of this work for publication, to more clearly characterize their claimed “oocyte-like” cells by simple PCR-based gene expression analysis. This basic expectation would have allowed one to determine whether the purported “oocyte-like” cells described by these authors are positive for a spectrum of oocyte-specific markers shown to be expressed by mouse and human OSC-derived oocytes in vitro, including the Kit, Ybx2, Nobox, Lhx8, Gdf9, Zp1, Zp2, and Zp3 genes.1 Based on the Ddx4-negative genetic character of the starting pool of cells used by Zhang et al20 to give rise to these “oocyte-like” cells in vitro, the answer would most likely be no.
With so many questions surrounding this new study, and the likelihood that no one else in the field will attempt to address them, valuable time and resources are now being redirected to pursuit of unplanned experiments in our laboratory using Ddx4-Cre mice crossed with Rosa26 fluorescent gene reporter mice. Our objective is not to repeat the work of Zhang et al20 but to clarify what was reported from the Liu laboratory by performing 2 critical experiments missing from their study. The first of these is straightforward gene expression analysis to actually determine the identity of the postnatal ovary-derived Ddx4-expressing cells studied by these authors, as shown in figure 2B of their article.20 The second, which is equally important, is to purify OSCs from the ovaries of genetically recombined adult female mice, using both Ddx4 antibody–and Ifitm3 antibody–based sorting protocols validated by us and others.1,2,7 This will provide an opportunity to directly test the mitotic potential of postnatal ovary-derived germ cells (Ddx4 positive, based on both Ddx4-Cre-mediated genetic recombination and expression of the endogenous Ddx4 gene) when immature oocytes are not used as starting material for the cultures. As for the remainder, there is really no need to document that Ddx4-negative cells in the ovaries of these genetically recombined mice are not germ line cells of any variety since that is already an inherent and indisputable feature of this genetic mouse model.
Conclusions and Future Considerations for Moving Forward
The field of mammalian OSC biology has arguably been in existence for less than a decade, compared with the SSC field that dates back to at least the late 1960s into the 1970s.40,41 Shortly following the initial article in 2004, first claiming the existence of female GSCs in mice,3 those skeptical of the potential for active follicular renewal in postnatal mouse ovaries quickly demanded purification of the cells and proof of their ability to generate functional oocytes before a challenge to the dogma of a fixed pool of oocytes being endowed at birth could even be considered.16,17 Those demands were subsequently met, and independently confirmed, over the past 8 years using protocols that mirror those universally accepted for determination of SSC identity.1,2,5–8 However, this rapid progress is still viewed as insufficient by some scientists, which is reflected by recent statements in opinion-based pieces that, even with the discovery of human OSCs by White et al,1 “skeptics are plagued by several nagging questions: What do these cells do in the ovary? Where do they come from?”19
Such statements fail to take into account that those who study mammalian OSCs have not had the luxury of four decades of research time, like those who study SSCs, much less four decades of time without the often rampant negativity that has embroiled the study of OSCs since 2004.16,17 If that was the case, the “nagging questions” recently voiced by Oatley and Hunt in their assessment of the White et al1 study would have been addressed long ago. We and others are evaluating the in vivo significance of OSCs to adult ovarian function and are exploring their origins. While time will be needed to complete these types of experiments, the current absence of such information in published form should not be construed as a source of skepticism over OSCs or something that our fellow scientists are “plagued” by. As has been the case since 2004, those skeptical of the existence of these cells and postnatal oogenesis in mammals are often quick to demand answers to what they perceive are “nagging questions” before being satisfied; however, these same individuals are apparently reluctant to devote their own time, money, and resources toward completion of the experiments they feel are necessary to address these questions. Devising experiments for others to do is quick and easy; performing such experiments to completion is an entirely different thing, and progress from those who choose to do this should not be subject to outright criticism by those who choose not to.
Putting all of this aside, there is consensus on both sides of this debate that the physiological role/roles of OSCs in adult mammalian ovaries and the regulatory networks that control OSC self-renewal capacity versus differentiation into oocytes, remain to be elucidated. In addition, considerable effort must be made to understand the relevance of these newly discovered cells to the process of ovarian aging. In fact, this latter area of work is a topic that some scientists have posed as one of the most significant hurdles in accepting that OSCs exist and support postnatal oogenesis in mammals. For example, Oatley and Hunt recently asked, “if they [OSCs] can and do give rise to viable eggs in the adult ovary, why is female reproduction of such limited duration?”19 While seemingly a fair question, the reproductive period in women normally spans almost 30 years, which is shorter than that of men but still far from limited in duration. At a more basic level, these types of criticisms fail to take into account published observations from others. For example, in Drosophila, OSC function is widely accepted as a fundamental aspect of both daily egg formation in adult ovaries and female reproductive capacity.10 However, oogenesis in Drosophila peaks early in reproductive life and then declines to the point of failure before death of the female fly.11 If age-related oogenic failure in this species is not put forth as evidence of an absence of OSCs and de novo oogenesis in flies during peak reproductive life, why then is a comparable situation in mammals, viz age-related depletion of the ovarian reserve, portrayed as evidence arguing against OSC-supported oocyte renewal earlier in life? Similarly, in male mice SSCs persist in testes that have undergone atrophy with age and exhibit spermatogenic failure.42,43 Yet, these observations do not raise questions about the existence of SSCs or their physiological importance to male gonadal function earlier in adult life. In fact, just like presumably quiescent SSCs harvested from aged atrophic mouse testes can resume sperm production after transplantation into young adult testes,42 dormant premeiotic germ cells in aged atrophic mouse ovaries can form new oocytes that become enclosed in follicles if these cells are exposed to a young adult ovarian environment.4 Why, then, should mammalian females be automatically held to a different standard, especially when not a single piece of evidence exists proving that adult mammalian ovaries do not possess OSCs or are incapable of de novo oocyte formation? In fact, the preponderance of experimental evidence discussed above, emanating from independent laboratories around the world, actually argues otherwise.
In closing, the controversy over the existence of OSCs in mammals continues, despite substantial progress made over just the past few years in purifying these cells from both mouse and human ovaries for characterization and functionality testing. Unfortunately, those skeptical of these cells continue to propound very negative views,19 which only serves to make it more difficult for scientists who study OSCs to obtain the funding needed to address the many questions about these cells that remain. And while similar critical commentaries, also rooted in negative tones, speculation, and omission of key published articles,16,17 have been commonplace in the literature since the 2004 publication concluding that OSCs exist and support oocyte renewal in adult mouse ovaries,3 there is one key difference now. More than 8 years have since passed, offering ample time for anyone interested in these cells or the concept of postnatal oogenesis, whether they believe in the existence of OSCs or not, to design and conduct their own experiments. Yet, highly critical and often outright negative perspectives are still published in scientific journals from individuals who have not performed a single experiment pertaining to these cells in their own laboratories to substantiate their claims.19 While these scientists may be viewed as experts in reproductive or stem cell biology, it is unclear how people who have never worked with these cells can justifiably question the findings of others, who have devoted tremendous energy and resources to study these cells on a daily basis, as being invalid or incorrect. Granted there is much more work to be done, but there is now simply too much information on OSCs from multiple laboratories to so easily ignore or dismiss.
Additionally, it is crucial that any new studies that make bold claims regarding a lack of evidence for the existence of mitotically active progenitor germ cells in postnatal mammalian ovaries, such as the recent article by Zhang et al,20 be carefully reviewed, dissected, and held to the same rigors of scientific proof as those applied to studies of OSCs concluding otherwise. Based on the discussions above (see Insights From Genetically Recombined Germ Cell “Reporter” Mice: What Have We Actually Learned?) and comments on the Zhang et al20 study from some members of the scientific community who have historically been skeptical of OSCs and postnatal oogenesis in mammals,39,44 this does not seem to be the case. Nevertheless, it is encouraging to hear that other scientists do feel “measured enthusiasm” for this work is now, on the heels of the White et al study,1 spreading deeper into the scientific community.18 This will hopefully lead to a much more positive and constructive collaborative spirit that supports and promotes, rather than criticizes, questions, and impedes, continued progress in this new and fundamentally important area of mammalian reproductive biology. Another advantage of keeping an open mind is that reassessment of prior experimental observations, such as those recently obtained from cell lineage analysis of oocytes in female mice with age,45 may lead to quite different and more logical interpretations of outcomes that simultaneously help explain historical studies and add to the growing body of evidence supporting the existence of active oocyte renewal in adult mammalian ovaries.46
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. D.C.W. declares interest as a scientific consultant for OvaScience, Inc. (Cambridge, MA); J.L.T. declares interest in intellectual property described in U.S. Patent 7,955,846 and is a co-founder of OvaScience, Inc.; Y.A.R.W. has no interests to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Work conducted by the authors discussed herein was supported by a Method to Extend Research in Time (MERIT) Award from the National Institute on Aging (NIH R37-AG012279 to J.L.T.), a Ruth L. Kirschstein National Research Service Award (NIH F32-AG034809 to D.C.W.), a Glenn Foundation Award for Research in the Biological Mechanisms of Aging, the Henry and Vivian Rosenberg Philanthropic Fund, the Sea Breeze Foundation, and Vincent Memorial Hospital Research Funds.
References
- 1. White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–636 [DOI] [PubMed] [Google Scholar]
- 3. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150 [DOI] [PubMed] [Google Scholar]
- 4. Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany NY). 2009;1(12):971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pacchiarotti J, Maki C, Ramos T, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79(3):159–170 [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Yang Z, Yang Y, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3(2):132–141 [DOI] [PubMed] [Google Scholar]
- 7. Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20(12):2197–2204 [DOI] [PubMed] [Google Scholar]
- 8. Hu Y, Bai Y, Chu Z, et al. GSK3 inhibitor-BIO regulates proliferation of female germline stem cells from the postnatal mouse ovary. Cell Prolif. 2012;45(4):287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woods DC, Tilly JL. The next (re)generation of human ovarian biology and female fertility: is current science tomorrow’s practice? Fertil Steril. 2012;98(1):3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17(1):15–25 [DOI] [PubMed] [Google Scholar]
- 11. Zhao R, Xuan Y, Li X, Xi R. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell. 2008;7(3):344–354 [DOI] [PubMed] [Google Scholar]
- 12. Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328(5985):1561–1563 [DOI] [PubMed] [Google Scholar]
- 13. White YAR, Woods DC, Woods AW. A transgenic zebrafish model of targeted oocyte ablation and de novo oogenesis. Dev Dyn. 2011;240(8):1929–1937 [DOI] [PubMed] [Google Scholar]
- 14. Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Cuevas M, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development. 2011;138(14):2861–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Powell K. Going against the grain. PloS Biol. 2007;5(12):e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80(1):2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Telfer EE, Albertini DF. The quest for human ovarian stem cells. Nat Med. 2012;18(3):353–354 [DOI] [PubMed] [Google Scholar]
- 19. Oatley JM, Hunt PA. Of mice and (wo)men: purified oogonial stem cells from mouse and human ovaries. Biol Reprod. 2012;86(6):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci U S A. 2012;109(31):12580–12585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–11307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Normile D. Reproductive biology. Study suggests a renewable source of eggs and stirs more controversy. Science. 2009;324(5925):320. [DOI] [PubMed] [Google Scholar]
- 24. Fujiwara Y, Komiya T, Kawabata H, et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci U S A. 1994;91(25):12258–12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev. 2000;93(1-2):1390–149 [DOI] [PubMed] [Google Scholar]
- 26. Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418(6895):293–300 [DOI] [PubMed] [Google Scholar]
- 27. Saitou M, Surani A. Cell surface expressed marker of pluripotency. United States Patent 7,226,994 (issued 5 June 2007)
- 28. Saitou M, Surani A. Antibodies for identification of murine Fragilis extracellular domain and methods for identifying pluripotent cells. United States Patent 7,884,193 (issued 8 February 2011)
- 29. Ohinata Y, Payer B, O'Carroll D, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436(7048):207–213 [DOI] [PubMed] [Google Scholar]
- 30. Saitou M, Yamaji M. Germ cell specification in mice: signaling, transcription regulation, and epigenetic consequences. Reproduction. 2010;139(6):931–942 [DOI] [PubMed] [Google Scholar]
- 31. Flores I, Benetti R, Blasco MA. Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol. 2006;18(3):254–260 [DOI] [PubMed] [Google Scholar]
- 32. Ogawa T, Aréchaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminferous tubules. Int J Dev Biol. 1997;41:111–122 [PubMed] [Google Scholar]
- 33. Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69(2):612–616 [DOI] [PubMed] [Google Scholar]
- 34. Kanatsu-Shinohara M, Miki H, Inoue K, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–991 [DOI] [PubMed] [Google Scholar]
- 35. Longo FJ. An ultrastructural analysis of spontaneous activation of hamster eggs aged in vivo. Anat Rec. 1974;179(1):27–55 [DOI] [PubMed] [Google Scholar]
- 36. Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol Reprod. 1997;57(4):743–750 [DOI] [PubMed] [Google Scholar]
- 37. Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro. I. The activation of ovarian eggs. J Exp Med. 1935;62(5):665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hermann BP, Phillips BT, Orwig KE. The elusive spermatogonial stem cell marker? Biol Reprod. 2011;85:221–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Underwood E. Stem cell study scrambles egg debate, again. http://news.sciencemag.org/sciencenow/2012/07/stem-cell-study-scrambles-egg-de.html
- 40. Clermont Y, Bustos-Obregon E. Re-examination of spermatogonial renewal in the rat by means of seminiferous tubules mounted ‘‘in toto.’’ Am J Anat. 1968;122:237–247 [DOI] [PubMed] [Google Scholar]
- 41. Schulze C. Morphological characteristics of the spermatogonial stem cells in man. Cell Tissue Res. 1979;198:191–199 [DOI] [PubMed] [Google Scholar]
- 42. Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X, Ebata KT, Robaire B, Nagano MC. Aging of male germ line stem cells in mice. Biol Reprod. 2006;74(1):119–124 [DOI] [PubMed] [Google Scholar]
- 44. Yong E. Ovarian stem cell debate. http://the-scientist.com/2012/07/09/ovarian-stem-cell-debate/
- 45. Reizel Y, Itzkovitz S, Adar R, et al. Cell lineage analysis of the mammalian female germline. PLoS Genet. 2012;8(2):e1002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woods DC, Telfer EE, Tilly JL. Oocyte family trees: old branches or new stems? PLoS Genet. 2012;8(7):e1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]


