Abstract
Experimental research over the past decade has supported the critical role of astrocytes activated by different types of injury and the pathophysiological processes that underlie the development of epilepsy. In both experimental and human epileptic tissues astrocytes undergo complex changes in their physiological properties, which can alter glio-neuronal communication, contributing to seizure precipitation and recurrence. In this context, understanding which of the molecular mechanisms are crucially involved in the regulation of glio-neuronal interactions under pathological conditions associated with seizure development is important to get more insight into the role of astrocytes in epilepsy.
This article reviews current knowledge regarding the role of glial adenosine kinase as a neuropathological marker of the epileptic brain. Both experimental findings in clinically relevant models, as well as observations in drug-resistant human epilepsies will be discussed, highlighting the link between astrogliosis, dysfunction of adenosine homeostasis and seizure generation and therefore suggesting new strategies for targeting astrocyte-mediated epileptogenesis.
Keywords: ADK, astrocytes, rodents, human, epilepsy
Adenosine kinase, the key enzyme in adenosine metabolism
Astrocytes serve as a key regulator of adenosine tone in the brain through adenosine (ADK) mediated metabolic clearance (Boison, 2008; Boison et al., 2010). As a consequence, an increase in astrogliosis, as observed across multiple disease processes including epilepsy (Sofroniew and Vinters, 2010) and Alzheimer’s disease (Cagnin et al., 2001; Nagele et al., 2004), has profound effects on extracellular adenosine levels and adenosine mediated signalling (Boison, 2008). The significance of pathological changes in adenosine tone become readily apparent in light of adenosine’s potent anticonvulsant (Dragunow, 1986) and neuroprotective (Dragunow and Faull, 1988) actions that are mediated by the G protein-coupled adenosine A1 receptor (A1R) (Fredholm et al., 2005b; Fredholm et al., 2005a; Fedele et al., 2006; Boison et al., 2010).
Independent of the adenosine tone, excessive network activity causes an adenosine surge that induces a state of synaptic depression and inhibits excitatory neurons (Dunwiddie, 1980; Mitchell et al., 1993; Manzoni et al., 1994), which in the context of seizures or epilepsy is anticonvulsant (Dragunow, 1991; Gouder et al., 2003). Therefore, adenosine augmentation strategies, in particular those restricted to a hyperexcitable brain area, hold promise as a rational approach for epilepsy therapy (Boison, 2009). Both astrocytes and neurons have been established as a source for extracellular adenosine (Figure 1). In regards to astrocytes, high frequency stimulation of the CA1 pyramidal neurons induces a Ca2+ mediated release of ATP from astrocytes (Cotrina et al., 1998; Zhang et al., 2003; Pascual et al., 2005) through either vesicular transport (Pascual et al., 2005) or hemichannels (Kang et al., 2008). Once in the synaptic cleft ATP is rapidly converted to adenosine by a series of ectonucleotidases (Zimmermann, 2000). Recently, neurons have also been implicated as a source for synaptic adenosine, whereby stimulation of postsynaptic CA1 neurons evokes a release of adenosine that suppresses excitatory transmission (Lovatt et al., 2012). Adenosine is removed from the extracellular space by equilibrative (Baldwin et al., 2004) and concentrative (Gray et al., 2004) nucleoside transporters, which are expressed in both astrocytes and neurons (Guillen-Gomez et al., 2004; Peng et al., 2005; Alanko et al., 2006). Importantly, expression of ADK in astrocytes (Studer et al., 2006) allows the influx and metabolic clearance of adenosine (Boison et al., 2010), whereas the lack of ADK in neurons permits those cells to release adenosine directly (Lovatt et al., 2012). Within the astrocyte cytoplasm adenosine is rapidly phosphorylated by ADK, which converts the ribonucleoside into 5′-adenosine monophosphate (AMP) (Mimouni et al., 1994). As a consequence, neuropathological changes that cause astrogliosis and an associated increase in ADK, as observed in epilepsy, can reduce synaptic adenosine levels thereby increasing network excitability and the propensity for ictogenesis (Fedele et al., 2005; Etherington et al., 2009).
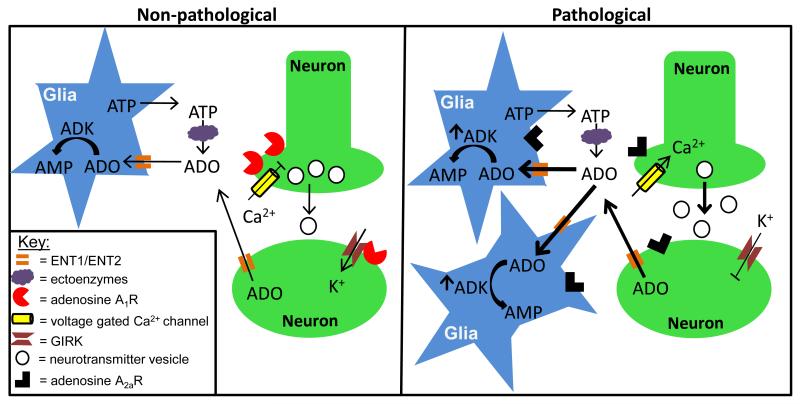
Figure 1. Astrocyte based ADK modulates network excitation by controlling the extracellular adenosine tone.
Non-pathological state (left panel): Extracellular adenosine (ADO) arises from two sources including: (i) Ca2+ mediated release of ATP from astrocytes followed by catabolism to ADO through a series of ectoenzymes that include nucleoside triphosphate diphosphohydrolases, ectonucleotide pyrophosphatase/phosphodiesterases and ecto-5′-nucleotidases; and (ii) Direct ADO release into the extracellular space upon postsynaptic stimulation of neurons. Once in the extracellular space ADO inhibits neuron excitation through activation of A1Rs localized to the pre- and postsynaptic neuron membrane. On the presynaptic neuron, A1R activation inhibits Ca2+ dependent vesicular release of excitatory neurotransmitters by inhibiting P/Q- and N-type voltage gated Ca2+ channels; while on the postsynaptic neuron A1R hyperpolarizes the cell by activating G-protein coupled inwardly rectifying K+ channels (GIRKS). ADO is cleared from the extracellular space by passive propagation through concentrative and equilibrative transporters (ENT1/ENT2) on the astrocyte membrane. Once in the astrocyte cytoplasm ADO is metabolized into AMP by ADK, which sets the ambient ADO tone. Pathological state (right panel): Sustained neuronal excitation, as observed in epilepsy, induces a shift in adenosine receptor expression levels with A1R being superseded by A2AR. As a consequence, there is a loss of A1R activity, which translates to increased network excitability due to increased Ca2+ dependent vesicular release of excitatory neurotransmitters and attenuated GIRK mediated hyperpolarization. Furthermore, A2AR activation causes an increase in astrogliosis that is accompanied by increased ADK expression and activity. Pathological levels of ADK will drive ADO influx and metabolism; thereby, decreasing the extracellular ADO tone.
Expression of ADK in the normal brain
In adult brain, ADK is primarily expressed in astrocytes. Immunocytochemical analysis of adult rat and mouse brain revealed predominant astrocytic expression throughout the hippocampus and cortex. Two isoforms of ADK have been identified in mammals, a long nuclear isoform and a short cytoplasmic isoform (Cui et al., 2009). Nuclear ADK immunoreactive material (IR) was observed in a subpopulation of resting astroglial cells, whereas cytoplasmic expression was weak or below detectable levels (Fedele et al., 2005; Li et al., 2008b; Aronica et al., 2011). A similar cellular expression pattern was detected in the temporal cortex of rats with predominantly nuclear ADK in resting glial cells (Aronica et al., 2011). Although the specific role of these isoforms in brain has still to be established, the nuclear ADK is likely involved in epigenetic mechanisms as regulator of methyltransferase reactions, whereas the cytoplasmic isoform is thought to regulate the extracellular levels of adenosine [(discussed above; for review see (Boison, 2008)]. Accordingly, reduced adenosine tone has been detected in mice constitutively overexpressing a transgene for the cytoplasmic isoform of ADK (Fedele et al., 2005; Pignataro et al., 2007; Li et al., 2008a). Moreover a recent study indicates a role for the cytoplasmic isoform in sleep regulation (Palchykova et al., 2010).
The function of ADK isoforms in developing human brain is still unclear. Developmental studies performed in mouse brain indicate a switch from neuronal expression during the perinatal period to a near exclusive astrocytic expression in adult brain (Studer et al., 2006). These observations point to a dual functionality of this enzyme and suggest a key role for ADK in the brain that may affect important cellular functions of neural progenitor cells, such as proliferation, survival and neural plasticity. Interestingly, strong expression of ADK has been detected in human fetal brain (gestational week, GW 13; temporal cortex); with these high levels observed by Western blot analysis in total cortical homogenates being a possible reflection of the enzyme’s expression in the deep compartments of the cortical wall (VZ/SVZ; ventricular/subventricular zone) at early stages of corticogenesis (unpublished observations). Whether a dysregulation of ADK expression/function early during development could contribute to cognitive dysfunction in children with epilepsy deserves further investigation.
Expression of ADK in the epileptic brain
Astrogliosis is a pathological hallmark of various types of medically refractory focal epilepsy, including epilepsy that develops following traumatic, ischemic or infectious brain injury (Sofroniew and Vinters, 2010). Astrogliosis is also the prominent morphological feature of hippocampal sclerosis (HS), which represents the most common neuropathological finding in adult patients undergoing surgery for intractable temporal lobe epilepsy (TLE) (Thom, 2009). Activation of astrocytes is also observed in focal malformations of cortical development (such as focal cortical dysplasia [FCD] and cortical tubers in tuberous sclerosis complex [TSC]), which are recognized causes of chronic medically intractable epilepsy in children and young adults [for review see (Aronica et al., 2012a)]. In addition, astroglial tumors (particularly slow-growing, low-grade tumors) represents a common cause of epilepsy in both adults and pediatric patients (van Breemen et al., 2007).
Over the past decade, an increasing number of observations have shown the existence of rapid regulatory cross-talks between neurons and glia during synaptic transmission, suggesting an astrocytic basis for epilepsy (Seifert and Steinhauser, 2011). Astrocytes can influence network excitability in epilepsy through different mechanisms, including a dysfunctional adenosine homeostasis, which may result from changes in ADK expression levels (Boison, 2008).
Experimental studies: animal models of epilepsy
The ADK hypothesis of epileptogenesis (Boison, 2008) is based on the observation that ADK is upregulated in reactive astrocytes in experimental models of TLE. Upregulation of ADK in astrocytes has been reported in the mouse hippocampus at 1 and 4 weeks after kainic acid (KA)-induced status epilepticus [SE; (Gouder et al., 2004); Table I]. At these time points a cytoplasmic expression of ADK in reactive astrocytes was also evident. In line with the immunocytochemical data, ADK activity was also found to be significantly increased 8 weeks after KA (Gouder et al., 2004). In contrast, a rapid, but transient, reduction of ADK was detected during the initial KA-induced SE (Gouder et al., 2004).
Table I. Adenosine kinase changes in the epileptic brain.
| ADK | References | |
|---|---|---|
| Animal models | ||
| Kindling model/rat | * | (Li et al., 2007b) |
| Post-SE model: Chemical/KA/mouse a |
+/astrocytes | (Gouder et al., 2004) |
| Post-SE model: Electrical/hippocampus/rat b |
+/astrocytes | (Aronica et al., 2011) |
| Model of focal epileptogenesis/mouse c |
+/astrocytes | (Li et al., 2008b) (Li et al., 2012) |
| Patient tissue | ||
| Temporal lobe epilepsy | +/astrocytes | (Aronica et al., 2011) (Masino et al., 2011) |
| Astrocytomas | +/tumor astrocytes | (de Groot et al., 2012) |
| Gangliogliomas | +/tumor astrocytes | (de Groot et al., 2012) |
| Focal cortical dysplasia | - | - |
+: increased immunoreactivity (IR) expression compared to controls.
therapeutic implants of adenosine kinase deficient stem cells
Post-SE: status epilepticus
unilateral injection of kainate (KA) into the dorsal hippocampus of adult mice: increased IR 1 and 4 weeks after KA injection; increased adenosine kinase activity 8 weeks after KA.
Electrical stimulation of the hippocampus: increased IR 1 week and 3-4 months after SE.
unilateral intraamygdaloid injections of KA: increased IR 3 weeks after KA injection (Li et al., 2008b); three weeks or two months after KA (Li et al., 2012).
In a subsequent study, an intra-amygdaloid injection of KA in mice was used to model focal epileptogenesis [(Li et al., 2008b); table I]. In this model, which is characterized by highly localized astrogliosis in the CA3 region, both focal seizures and upregulation of astroglial ADK was restricted to the injured region (3 weeks after KA injection), suggesting that a focal upregulation of ADK may be sufficient to induce highly confined hyperexcitability (Li et al., 2008b). In a more recent study, this mouse model of focal epileptogenesis was used to evaluate the contribution of a local disruption of glial adenosine homeostasis to ictogenesis in mice (Li et al., 2012). The authors identified 2 independent foci (amygdala and CA3 region; 3 weeks or 2 months after KA), characterized by astrogliosis and upregulation of ADK and corresponding to the focal origin of subclinical seizure activity [(Li et al., 2012); see also below discussion on functional consequences of ADK regulation on neuronal excitability].
In order to understand the dynamics of ADK expression during development and progression of epilepsy, expression and cellular distribution of ADK was also studied in a rat model of TLE (post-SE model induced by electrical stimulation of the hippocampus) [(Aronica et al., 2011); Table I]. In this model (both in the hippocampus and temporal cortex) there was an upregulation of ADK protein expression during the latent phase (1 week post SE), which preceded the development of spontaneous electrographic seizures and was characterized by prominent astrogliosis. Similar to the post-SE mouse model, immunocytochemical analysis showed ADK expression in reactive astrocytes with prominent cytoplasmic labeling. The upregulation of ADK in activated astrocytes persisted in both the hippocampus and temporal cortex well into the chronic epileptic phase (3-4 months post-SE) in rats with a progressive form of epilepsy. However, ADK gene expression has been shown to be down-regulated 24 hrs after induction of SE (Gorter et al., 2006). This transient down-regulation (observed also in the post-SE mouse model (Gouder et al., 2004), may likely represent an attempt to increase the protective levels of adenosine (Pignataro et al., 2008). In addition, a down-regulation of ADK levels post-injury could also contribute, together with the regulation of glial adenosine receptor (AR) expression, to the development of astrogliosis (Hask et al., 2005; Boison, 2010).
Data generated in rodent models of epilepsy infer that astrogliosis and the associated increase in ADK expression contribute to epileptogenesis. However, epilepsy is a complex disorder whereby multiple factors (i.e. granule cell dispersion, neuronal cell loss, mossy fiber sprouting) contribute to the disease process. Unfortunately, these factors confound interpreting the definitive role of astrocyte based ADK in epilepsy. Through a series of mechanistic studies using genetic approaches, pathological levels of ADK have been identified as sufficient for epileptogenesis. First, transgenic mice (Adk-tg) were engineered to overexpress the cytoplasmic ADK-S isoform under control of the human ubitquitin promoter on an Adk null background that does not express either the endogenous ADK-S or ADK-L isoform. These mice have a 141% overexpression of ADK-S throughout the brain and corresponding 2.2 fold increase in ADK activity compared to wild-type controls. Importantly, the Adk-tg mice develop spontaneous cortical and hippocampal electrographic seizure-like activity by 8-9 weeks of age (Fedele et al., 2005; Li et al., 2008b). Because the human ubiquitin promoter drives expression in both neurons and astrocytes conclusions pertaining to cell specificity are limited. To expand on these findings, targeted gene therapy using adeno-associated viral vectors designed to selectively overexpress (AAV8-pGFA-Adk-sense) or knockdown (AAV8-pGFA-Adk-antisense) the ADK-S isoform within astrocytes have been employed. A single intrahippocampal injection of AAV8-pGFA-Adk-sense into the CA3 subregion of wild-type mice yielded a 144% overexpression of ADK-S isoform within astrocytes, which was sufficient to induce spontaneous electrographic seizures. Finally, targeted downregulation (AAV8-pGFA-Adk-antisense virus) of the ADK-S isoform in the astrocytes of Adk-tg mice yielded a complete (100%) suppression of focal seizure activity (Theofilas et al., 2011). Together these findings indicate that astrocyte based ADK overexpression is sufficient to induce seizures and highlight ADK and more generally adenosine augmentation as a tangible therapeutic target (discussed below) in epilepsy.
Human studies: common causes of refractory epilepsy
As discussed above, astrogliosis represents a major pathological feature of HS (Blümcke et al., 2009; Thom, 2009) and recent data suggest a role for astroglial cells in seizure development and progression (Binder and Steinhauser, 2006; Wetherington et al., 2008; Seifert et al., 2010). Overexpression of ADK has recently been reported in specimens of patients undergoing surgery for pharmacologically refractory TLE [(Aronica et al., 2011); Table I]. In both HS and temporal cortex of TLE patients, ADK IR was detected in reactive astrocytes with a characteristic cytoplasmic localization (Aronica et al., 2011). This increase in astroglial ADK levels observed in human specimens could explain the relatively low adenosine baseline levels detected in microdialysis samples of epileptic patients, compared to control human hippocampus (During and Spencer, 1992).
The cellular distribution and expression of ADK has also been investigated in astroglial brain tumors [(de Groot et al., 2012); Table I]. ADK IR is detected in the tumor astrocytes with a predominant cytoplasmic localization as well as peritumoral tissue containing infiltrating tumor cells. Interestingly, a significantly higher expression of ADK is detected in the peritumoral infiltrated tissue of patients with epilepsy compared to patients without epilepsy (de Groot et al., 2012). The peritumoral region has been suggested to play a critical role in the generation and propagation of seizure activity (van Breemen et al., 2007; de Groot et al., 2012). Thus, the observed over-expression of ADK may potentially contribute to the epileptogenicity of this region, suggesting a surgical approach that should aim to maximize simultaneous resection of both the tumor and (if possible) the peritumoral epileptic focus (de Groot et al., 2012). Additional studies of a series of tumors, including a large cohort of long-term epilepsy associated tumors [LEAT; (Thom et al., 2012)], which could be stratified on the basis of the duration and/or severity of epilepsy, are essential to further assess the value of ADK expression/activity as a biomarker of epileptogenicity.
Evaluation of ADK expression in other pathologies associated with chronic intractable epilepsy and characterized by astrogliosis, deserves further investigation. Interestingly, in focal cortical dysplasia (FCD), we observed strong ADK expression in reactive astrocytes within the dysplastic cortex (unpublished observations). In addition, it could be interesting to take into consideration the potential role of focal deregulation of ADK expression/function in reactive astrocytes within the context of epilepsy associated with Alzheimer disease (AD) (Palop and Mucke, 2009; Roberson, 2011).
Functional consequences of ADK regulation on astrocyte function
As outlined above (see introduction), ADK critically regulates the extracellular adenosine levels in brain (Boison, 2006; Etherington et al., 2009). Changes in the levels of adenosine, as a result of the regulation of ADK expression/function in epileptic tissue, may influence astroglial function through activation of different ARs, which have been detected in astrocytes [for reviews see (Boison et al., 2010; Aronica et al., 2012b)].
Activation of A1R on astrocytes has been shown to reduce their proliferation in vitro (Rathbone et al., 1991; Ciccarelli et al., 1994), as well as to mediate cytoprotective effects (Ciccarelli et al., 2007; D’Alimonte et al., 2007; Bjorklund et al., 2008). In addition, astrocyte function and proliferation may secondarily influence the activation of A1Rs expressed on microglial cells, which has been shown to attenuate neuroinflammation (Tsutsui et al., 2004; Synowitz et al., 2006).
Adenosine mediates its actions on astrocytes also through the A2ARs, which are also expressed in microglia and induced following different types of brain injury or inflammation (Cunha, 2005; Rebola et al., 2011). Activation of glial A2ARs has been suggested to control neuroinflammation (Nishizaki et al., 2002; Rebola et al., 2011). Acting via the astroglial A2ARs, adenosine may also regulate the extracellular concentration of glutamate (Li et al., 2001; Nishizaki et al., 2002) and inhibit the production of nitric oxide [NO;(Brodie et al., 1998)]. In contrast to the A1Rs, activation of A2ARs increases astrocyte proliferation (Hindley et al., 1994; Brambilla et al., 2003). Thus, activation of A2ARs may play a critical role in promoting astrogliosis in epileptic brain, particularly when occurring in concert with changes in A1Rs expression (Figure 1). Interestingly, a downregulation of A1Rs (which negatively modulates astrocyte proliferation) has been observed during epileptogenesis (Ekonomou et al., 2000; Rebola et al., 2003). As discussed above, induction of astrogliosis is asso ciated with an upregulation of ADK (Figure 2), leading to a reduction in extracellular levels of adenosine [(Boison, 2006); Figure 1]. Inflammatory molecules, such as IL-1β, up-regulated in epileptogenic tissue from TLE patients [for review see (Aronica and Crino, 2011; Vezzani et al., 2011)] may also play a role in the regulation of adenosine cycle in astrocytes. Accordingly, IL-1β has been shown to increase the expression of ADK in human astrocytes in culture, suggesting a potential modulatory crosstalk between the astrocyte-based adenosine cycle and inflammation (Aronica et al., 2011).
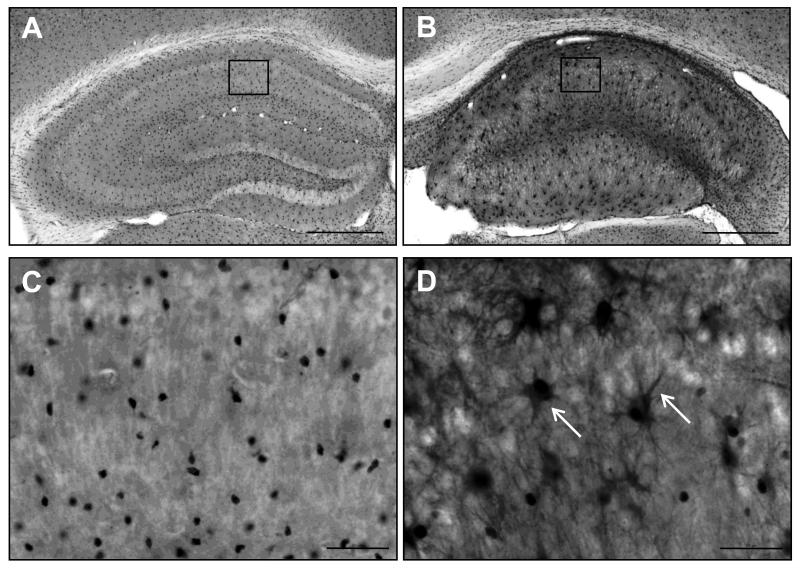
Figure 2. Increased ADK expression in a rodent model for mesial temporal lobe epilepsy.
A,B ADK immunohistochemistry images from the contra- and ipsilateral hemisphere of a C57BL/6 mouse 10 weeks following intrahippocampal KA injection. A single unilateral injection of KA (400 ng/100 nL) into the CA1 subregion (coordinates relative to Bregma AP: −2.18; ML: −1.8, DV: −1.7) induces focal astrogliosis and associated ADK upregulation within the ipsilateral hippocampus (panel B), compared to the non-injured contralateral hemisphere (panel A). C,D High magnification images of the regions demarcated by boxes in panels A and B. Note that in the non-injured hemisphere (panel C) the nuclear isoform of ADK is predominantly expressed, while in the injured hemisphere there is a robust increase in cytoplasmic ADK expression within astrocytes (arrows, panel D).
Increasing evidence points towards a critical role of ARs in neuron-glia communication and neuroinflammation (Boison, 2010; Gomes et al., 2011). Activation of A2BRs has been shown to induce the release of IL-6 from astrocytes and the activation of the A3Rs to promote the synthesis of the chemokine MCP-1 [monocyte chemotactic protein-1; for reviews see (Hasko et al., 2005; Abbracchio and Ceruti, 2007; Gomes et al., 2011)]. Thus, it is tempting to hypothesize that a dysfunction in adenosine’s homeostasis may critically influence balance of pro- and anti-inflammatory processes, leading to uncontrolled inflammation that may contribute to the development of the epileptic process and/or cognitive dysfunction [for reviews see (Vezzani et al., 2011; Boison et al., 2012)].
ADK expression levels may also play a role in the regulation of tumor growth and apoptotic cell death in astrocytomas (Abbracchio et al., 1997; Synowitz et al., 2006; Dehnhardt et al., 2007; Gessi et al., 2010; Gessi et al., 2011). Accordingly, increased ADK mRNA expression has been detected in human cancer samples outside the brain, such as in colorectal cancer (Giglioni et al., 2008). Moreover, it has been demonstrated that extracellular adenosine reduced the viability of cultured astrocytoma cells (Sai et al., 2006), suggesting that induction of ADK might represent a strategy to improve survival of tumor cells.
Functional consequences of ADK regulation on neuronal excitability
Adenosine modulates neuronal excitability via activation of the high affinity A1 or A2A, low-affinity A2B, or low abundance A3 adenosine receptors that feed into a multitude of different neuronal and astrocytic pathways (Blum et al., 2003; Sebastiao and Ribeiro, 2009b, a; Boison et al., 2010). In the context of epilepsy, the predominant research focus has been on adenosine signalling via inhibitory A1 and facilitory A2A receptors (Figure 1). In comparison to the A2AR where expression is primarily localized to the striatum (Rosin et al., 1998; Ferre et al., 2007), the A1R is widespread throughout the limbic system with greatest expression levels in the hippocampus and cortex [(Reppert et al., 1991); Allen Brain Atlas, www.brain-map.org]. However, seizure activity has been shown to alter the expression levels of both the A1R and A2AR. Rats that were either stimulated by intraamygdalar kindling or received an intraperitoneal injection of KA had a robust increase in cortical A2A expression and activity, while the A1R was downregulated (Rebola et al., 2005). Likewise in the hippocampus there is a decrease in A1R following chemical (Cremer et al., 2009) or electrical kindling (Aden et al., 2004).
Depending on the subcellular localization, the A1R has the capabilities to modulate both the pre- and postsynaptic activity of neurons (Figure 1). Numerous lines of research have established that adenosine activation of the A1R inhibits excitatory post-synaptic potentials [for review see (Dunwiddie and Masino, 2001)]. More specifically, in the mossy fiber synapse, A1R activation inhibits P/Q- and N-type voltage gated Ca2+ channels that subsequently attenuate synaptic transmission by reducing the release probability of excitatory neurotransmitters (Gundlfinger et al., 2007). On the post-synaptic membrane of excitatory neurons, A1R modulates the activity of inwardly rectifying K+ channels, which can directly stabilize the membrane potential or hyperpolarize the cell (Luscher et al., 1997; Takigawa and Alzheimer, 2002). Recently, the A1R has also been found to reduce GABAA-receptor dependent depolarizations that occur during a seizure (Ilie et al., 2012). Thus, A1R modulation of network excitability has the capability to exert a profound anticonvulsant effect.
The pathological state of network hyperexcitability leading to seizures in epilepsy is in part due to an ADK-mediated reduction in adenosine tone leading to diminished A1R activity (Figure 1). Injection of KA in either the hippocampus (Gouder et al., 2004) or amygdala (Li et al., 2007a; Li et al., 2008b; Li et al., 2012) causes a focal injury that is confined to the ipsilateral hemisphere and is characterized by overt astrogliosis and increased ADK expression (Figure 2). These epileptic hallmarks are accompanied by increased network excitability and electrographic seizures (Gouder et al., 2004; Li et al., 2007a; Li et al., 2008b; Li et al., 2012). Importantly, seizure activity in KA injected mice is attenuated by either 5-iodotubercidin, an ADK inhibitor (Gouder et al., 2004) or adenosine that is focally delivered by transplanted ADK-deficient embryonic stem cells (Li et al., 2008b). Further evidence that dysregulation of the adenosine system aggravates an epileptic phenotype comes from a series of studies that employ A1R-knockout mice or antagonists. First, independent of a focal injury, A1R knockout mice have spontaneous electrographic seizures that occur in the CA3 subregion of the hippocampus despite normal wild type levels of ADK expression (Li et al., 2007a). Second, using a low dose of KA (1 nmol) injected into the hippocampus of A1R knockout mice, seizure severity is escalated from non-convulsive (observed in wild type mice injected with the same dose) to convulsive during SE. Moreover, the A1R knockout mice die within 5 days of KA injection and there is extensive neuronal cell death within both the ipsi- and contralateral hippocampus. Pathology in the wild type mice is confined to the ipsilateral injection site (Fedele et al., 2006). However, administration of a non-convulsive dose of an A1R antagonist to intraamygdaloid injected KA mice causes a synchronization of epileptic foci and subsequent generalization of seizures to the cortex (Li et al., 2012). To distinguish between ADK and A1R dependent effects on neuronal excitability in temporal lobe epilepsy, Li et al compared the seizure phenotype of epileptic wild type mice 4 weeks after the intraamygdaloid KA injection, and the spontaneous seizures in the Adk-tg mice that overexpress ADK in brain and have normal A1R expression and in the A1R knockout mice which have normal ADK expression. Remarkably, all three models displayed a similar seizure phenotype indicating that either overexpression of ADK alone or loss of the A1R alone is sufficient to trigger spontaneous electrographic seizures (Li et al., 2007a; Li et al., 2008a). Together, these data implicate that disruption of adenosine signaling can affect neuronal excitability at different levels. However, considering that ADK acts upstream of the A1R; overexpression of ADK is expected to exert dominant effects over A1R expression changes.
Conclusions and clinical relevance
Astrogliosis and an increase in ADK is a pathologcial hallmark of epilepsy. The consequence of increased ADK in the epileptic brain is a decrease in the ambient adenosine tone and A1R activity. As a result, systemic administration of A1R agonists or ADK inhibitors successfully attenuates seizure activity (Fredholm, 2003; Jacobson and Gao, 2006; Boison, 2011). However, the use of systemic A1R agonists, ADK inhibitors or adenosine as a therapeutic strategy for epilepsy treatment is limited due to negative side effects including bradycardia, vasoconstriction in the kidney and sedation (Albrecht-Kupper et al., 2012). Thus, it is imperative that novel therapeutic approaches, which focally restore normal adenosine levels, are developed for epilepsy treatment. The prospects for these new therapies are likely to be successful based on the efficacy of adenosine augmentation in rodents. Multiple studies have established that focal delivery of adenosine to the brain, via either transplantation of ADK deficient embryonic stem cells (Li et al., 2007b; Ren et al., 2007; Li et al., 2009; Ren and Boison, 2010) or biodegradable adenosine loaded silk based polymers (Wilz et al., 2008; Szybala et al., 2009), prevents seizures and epileptogenesis. Additionally, the ketogenic diet, which is effective in treating refractory epileptic patients, has been shown to mediate anticonvulsant actions through the A1R (Masino et al., 2011). By further developing adenosine augmentation therapies for clinical use it may be possible to revolutionize the neurocentric approach to treating epilepsy; thereby, finding a cure.
Highlights.
Adenosine is neuroprotective and the brain’s endogenous anticonvulsant.
ADK in astrocytes regulates brain adenosine tone by phosphorylating adenosine to AMP.
Astrogliosis and increased ADK are hallmarks of mesial Temporal Lobe Epilepsy.
The A1 receptor facilitates neuroprotection and inhibits excitatory neurotransmission.
Focal adenosine augmentation therapies prevent seizures and epileptogenesis.
Acknowledgements
EA is supported by National Epilepsy Funds (NEF 09-05) and EU FP7 project NeuroGlia (Grant Agreement N° 202167). DB is supported by grants from the National Institutes of Health (NS065957, NS061844, MH083973) and the U.S. Department of the Army (W81XWH-12-1-0283)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbracchio MP, Ceruti S. P1 receptors and cytokine secretion. Purinergic Signal. 2007;3:13–25. doi: 10.1007/s11302-006-9033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Rainaldi G, Giammarioli AM, Ceruti S, Brambilla R, Cattabeni F, Barbieri D, Franceschi C, Jacobson KA, Malorni W. The A3 adenosine receptor mediates cell spreading, reorganization of actin cytoskeleton, and distribution of Bcl-XL: studies in human astroglioma cells. Biochem Biophys Res Commun. 1997;241:297–304. doi: 10.1006/bbrc.1997.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aden U, O’Connor WT, Berman RF. Changes in purine levels and adenosine receptors in kindled seizures in the rat. Neuroreport. 2004;15:1585–1589. doi: 10.1097/01.wnr.0000133227.94662.c9. [DOI] [PubMed] [Google Scholar]
- Alanko L, Porkka-Heiskanen T, Soinila S. Localization of equilibrative nucleoside transporters in the rat brain. J Chem Neuroanat. 2006;31:162–268. doi: 10.1016/j.jchemneu.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Albrecht-Kupper BE, Leineweber K, Nell PG. Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic Signal. 2012;8:91–99. doi: 10.1007/s11302-011-9274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl 3):26–32. doi: 10.1111/j.1528-1167.2011.03033.x. [DOI] [PubMed] [Google Scholar]
- Aronica E, Becker AJ, Spreafico R. Malformations of cortical development. Brain pathology. 2012a;22:380–401. doi: 10.1111/j.1750-3639.2012.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Ravizza T, Zurolo E, Vezzani A. Astrocyte immune responses in epilepsy. GLIA. 2012b doi: 10.1002/glia.22312. [DOI] [PubMed] [Google Scholar]
- Aronica E, Zurolo E, Iyer A, de Groot M, Anink J, Carbonell C, van Vliet EA, Baayen JC, Boison D, Gorter JA. Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia. 2011;52:1645–1655. doi: 10.1111/j.1528-1167.2011.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. GLIA. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- Bjorklund O, Shang M, Tonazzini I, Dare E, Fredholm BB. Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. European journal of pharmacology. 2008;596:6–13. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Blum D, Hourez R, Galas MC, Popoli P, Schiffmann SN. Adenosine receptors and Huntington’s disease: implications for pathogenesis and therapeutics. Lancet Neurol. 2003;2:366–374. doi: 10.1016/s1474-4422(03)00411-3. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Kistner I, Clusmann H, Schramm J, Becker AJ, Elger CE, Bien CG, Merschhemke M, Meencke HJ, Lehman T, Buchfelder M, Weigel, Buslei R, Stefan H, Pauli E, Hildebrandt M. Towards a clinico-pathological classification of granule cell dispersion in human mesial temporal lobe epilepsies. Acta Neuropathologica. 2009;117:535–544. doi: 10.1007/s00401-009-0512-5. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends in Pharmacological Sciences. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Progress in Neurobiology. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine augmentation therapies (AATs) for epilepsy: prospect of cell and gene therapies. Epilepsy Res. 2009;85:131–141. doi: 10.1016/j.eplepsyres.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine dysfunction and adenosine kinase in epileptogenesis. Open Neurosci J. 2010;4:93–101. doi: 10.2174/1874082001004020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Modulators of nucleoside metabolism in the therapy of brain diseases. Curr Top Med Chem. 2011;11:1068–1086. doi: 10.2174/156802611795347609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 2010;17:1071–1082. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Singer P, Shen HY, Feldon J, Yee BK. Adenosine hypothesis of schizophrenia--opportunities for pharmacotherapy. Neuropharmacology. 2012;62:1527–1543. doi: 10.1016/j.neuropharm.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. GLIA. 2003;43:190–194. doi: 10.1002/glia.10243. [DOI] [PubMed] [Google Scholar]
- Brodie C, Blumberg PM, Jacobson KA. Activation of the A2A adenosine receptor inhibits nitric oxide production in glial cells. FEBS letters. 1998;429:139–142. doi: 10.1016/s0014-5793(98)00556-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, Ballerini P, Ambrosini G, Giuliani P, Tiboni GM, Caciagli F. Effects of exogenous ATP and related analogues on the proliferation rate of dissociated primary cultures of rat astrocytes. Journal of neuroscience research. 1994;39:556–566. doi: 10.1002/jnr.490390507. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R, D’Alimonte I, Ballerini P, D’Auro M, Nargi E, Buccella S, Di Iorio P, Bruno V, Nicoletti F, Caciagli F. Molecular signalling mediating the protective effect of A1 adenosine and mGlu3 metabotropic glutamate receptor activation against apoptosis by oxygen/glucose deprivation in cultured astrocytes. Molecular pharmacology. 2007;71:1369–1380. doi: 10.1124/mol.106.031617. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer CM, Palomero-Gallagher N, Bidmon HJ, Schleicher A, Speckmann EJ, Zilles K. Pentylenetetrazole-induced seizures affect binding site densities for GABA, glutamate and adenosine receptors in the rat brain. Neuroscience. 2009;163:490–499. doi: 10.1016/j.neuroscience.2009.03.068. [DOI] [PubMed] [Google Scholar]
- Cui XA, Singh B, Park J, Gupta RS. Subcellular localization of adenosine kinase in mammalian cells: The long isoform of AdK is localized in the nucleus. Biochemical & Biophysical Research Communications. 2009;388:46–50. doi: 10.1016/j.bbrc.2009.07.106. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic signalling. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alimonte I, Ballerini P, Nargi E, Buccella S, Giuliani P, Di Iorio P, Caciagli F, Ciccarelli R. Staurosporine-induced apoptosis in astrocytes is prevented by A1 adenosine receptor activation. Neuroscience letters. 2007;418:66–71. doi: 10.1016/j.neulet.2007.02.061. [DOI] [PubMed] [Google Scholar]
- de Groot M, Iyer A, Zurolo E, Anink J, Heimans JJ, Boison D, Reijneveld JC, Aronica E. Overexpression of ADK in human astrocytic tumors and peritumoral tissue is related to tumor-associated epilepsy. Epilepsia. 2012;53:58–66. doi: 10.1111/j.1528-1167.2011.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehnhardt M, Palm C, Vieten A, Bauer A, Pietrzyk U. Quantifying the A1AR distribution in peritumoural zones around experimental F98 and C6 rat brain tumours. J Neurooncol. 2007;85:49–63. doi: 10.1007/s11060-007-9391-6. [DOI] [PubMed] [Google Scholar]
- Dragunow M. Adenosine: the brain’s natural anticonvulsant? Trends Pharmacol Sci. 1986;7:128. [Google Scholar]
- Dragunow M. Adenosine and seizure termination. Ann Neurol. 1991;29:575. doi: 10.1002/ana.410290524. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull RLM. Neuroprotective effects of adenosine. Trends Pharmacol Sci. 1988;9:193–194. doi: 10.1016/0165-6147(88)90079-x. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV. Endogenously released adenosine regulates excitability in the in vitro hippocampus. Epilepsia. 1980;21:541–548. doi: 10.1111/j.1528-1157.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Annals of Neurology. 1992;32:618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- Ekonomou A, Sperk G, Kostopoulos G, Angelatou F. Reduction of A1 adenosine receptors in rat hippocampus after kainic acid-induced limbic seizures. Neuroscience letters. 2000;284:49–52. doi: 10.1016/s0304-3940(00)00954-x. [DOI] [PubMed] [Google Scholar]
- Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli B. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele DE, Li T, Lan JQ, Fredholm BB, Boison D. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol. 2006;200:184–190. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Ferre S, Diamond I, Goldberg SR, Yao L, Hourani SM, Huang ZL, Urade Y, Kitchen I. Adenosine A(2A) receptors in ventral striatum, hypothalamus and nociceptive circuitry Implications for drug addiction, sleep and pain. Prog Neurobiol. 2007 doi: 10.1016/j.pneurobio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine receptors as ta rgets for drug development. Drug News Perspect. 2003;16:283–289. doi: 10.1358/dnp.2003.16.5.829316. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: Insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005a;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005b;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808:1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Gessi S, Sacchetto V, Fogli E, Merighi S, Varani K, Baraldi PG, Tabrizi MA, Leung E, Maclennan S, Borea PA. Modulation of metalloproteinase-9 in U87MG glioblastoma cells by A3 adenosine receptors. Biochem Pharmacol. 2010;79:1483–1495. doi: 10.1016/j.bcp.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Giglioni S, Leoncini R, Aceto E, Chessa A, Civitelli S, Bernini A, Tanzini G, Carraro F, Pucci A, Vannoni D. Adenosine kinase gene expression in human colorectal cancer. Nucleosides Nucleotides Nucleic Acids. 2008;27:750–754. doi: 10.1080/15257770802145629. [DOI] [PubMed] [Google Scholar]
- Gomes CV, Kaster MP, Tome AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta. 2011;1808:1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Gorter JA, Van Vliet E, Aronica E, Rauwerda H, Breit T, Lopes da Silva FH, Wadman WJ. Potential New Antiepileptogenic Targets Indicated by Microarray Analysis in a Rat Model for Temporal Lobe Epilepsy. Journal of Neuroscience. 2006;26:11083–11110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouder N, Fritschy JM, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia. 2003;44:877–885. doi: 10.1046/j.1528-1157.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- Gouder N, Scheurer L, Fritschy J-M, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JH, Owen RP, Giacomini KM. The concentrative nucleoside transporter family, SLC28. Pflugers Arch. 2004;447:728–734. doi: 10.1007/s00424-003-1107-y. [DOI] [PubMed] [Google Scholar]
- Guillen-Gomez E, Calbet M, Casado J, de Lecea L, Soriano E, Pastor-Anglada M, Burgaya F. Distribution of CNT2 and ENT1 transcripts in rat brain: selective decrease of CNT2 mRNA in the cerebral cortex of sleep-deprived rats. J Neurochem. 2004;90:883–893. doi: 10.1111/j.1471-4159.2004.02545.x. [DOI] [PubMed] [Google Scholar]
- Gundlfinger A, Bischofberger J, Johenning FW, Torvinen M, Schmitz D, Breustedt J. Adenosine modulates transmission at the hippocampal mossy fibre synapse via direct inhibition of presynaptic calcium channels. Journal of Physiology-London. 2007;582:263–277. doi: 10.1113/jphysiol.2007.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hask x00F, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends in Pharmacological Sciences. 2005;26:511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005;26:511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley S, Herman MA, Rathbone MP. Stimulation of reactive astrogliosis in vivo by extracellular adenosine diphosphate or an adenosine A2 receptor agonist. Journal of neuroscience research. 1994;38:399–406. doi: 10.1002/jnr.490380405. [DOI] [PubMed] [Google Scholar]
- Ilie A, Raimondo JV, Akerman CJ. Adenosine release during seizures attenuates GABAA receptor-mediated depolarization. J Neurosci. 2012;32:5321–5332. doi: 10.1523/JNEUROSCI.5412-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lan JQ, Boison D. Uncoupling of astrogliosis from epileptogenesis in adenosine kinase (ADK) transgenic mice. Neuron Glia Biol. 2008a;4:91–99. doi: 10.1017/S1740925X09990135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ren G, Kaplan DL, Boison D. Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3-selective epileptogenesis. Epilepsy Res. 2009;84:238–241. doi: 10.1016/j.eplepsyres.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lan JQ, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology. 2007a;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lytle N, Lan JQ, Sandau US, Boison D. Local disruption of glial adenosine homeostasis in mice associates with focal electrographic seizures: a first step in epileptogenesis? Glia. 2012;60:83–95. doi: 10.1002/glia.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008b;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A, Segschneider M, Simon RP, Brustle O, Boison D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007b;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- Li XX, Nomura T, Aihara H, Nishizaki T. Adenosine enhances glial glutamate efflux via A2a adenosine receptors. Life sciences. 2001;68:1343–1350. doi: 10.1016/s0024-3205(00)01036-5. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci U S A. 2012;109:6265–6270. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D. A ketogenic diet suppresses seizures in mice through adenosine A(1) receptors. J Clin Invest. 2011;121:2679–2683. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimouni M, Bontemps F, Van den Berghe G. Kinetic studies of rat liver adenosine kinase. J Biol Chem. 1994;269:17820–17825. [PubMed] [Google Scholar]
- Mitchell JB, Lupica CR, Dunwiddie TV. Activity-dependent release of endogenous adenosine modulates synaptic responses in the rat hippocampus. J Neurosci. 1993;13:3439–3447. doi: 10.1523/JNEUROSCI.13-08-03439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Nishizaki T, Nagai K, Nomura T, Tada H, Kanno T, Tozaki H, Li XX, Kondoh T, Kodama N, Takahashi E, Sakai N, Tanaka K, Saito N. A new neuromodulatory pathway with a glial contribution mediated via A(2a) adenosine receptors. GLIA. 2002;39:133–147. doi: 10.1002/glia.10100. [DOI] [PubMed] [Google Scholar]
- Palchykova S, Winsky-Sommerer R, Shen HY, Boison D, Gerling A, Tobler I. Manipulation of adenosine kinase affects sleep regulation in mice. Journal of Neuroscience. 2010;30:13157–13165. doi: 10.1523/JNEUROSCI.1359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Peng L, Huang R, Yu AC, Fung KY, Rathbone MP, Hertz L. Nucleoside transporter expression and function in cultured mouse astrocytes. Glia. 2005;52:25–35. doi: 10.1002/glia.20216. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. Journal of Cerebral Blood Flow & Metabolism. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. Journal of Cerebral Blood Flow & Metabolism. 2008;28:17–23. doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, DeLuca B, Jovetich M. Extracellular guanosine increases astrocyte cAMP: inhibition by adenosine A2 antagonists. Neuroreport. 1991;2:661–664. doi: 10.1097/00001756-199111000-00007. [DOI] [PubMed] [Google Scholar]
- Rebola N, Porciuncula LO, Lopes LV, Oliveira CR, Soares-da-Silva P, Cunha RA. Long-term effect of convulsive behavior on the density of adenosine A1 and A 2A receptors in the rat cerebral cortex. Epilepsia. 2005;46(Suppl 5):159–165. doi: 10.1111/j.1528-1167.2005.01026.x. [DOI] [PubMed] [Google Scholar]
- Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonca A, Cunha RA. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. The European journal of neuroscience. 2003;18:820–828. doi: 10.1046/j.1460-9568.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- Rebola N, Simoes AP, Canas PM, Tome AR, Andrade GM, Barry CE, Agostinho PM, Lynch MA, Cunha RA. Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. Journal of neurochemistry. 2011;117:100–111. doi: 10.1111/j.1471-4159.2011.07178.x. [DOI] [PubMed] [Google Scholar]
- Ren G, Boison D. Engineering human mesenchymal stem cells to release adenosine using miRNA technology. Methods Mol Biol. 2010;650:225–240. doi: 10.1007/978-1-60761-769-3_17. [DOI] [PubMed] [Google Scholar]
- Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp Neurol. 2007;208:26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Stehle JH, Rivkees SA. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- Roberson ED. Contemporary approaches to Alzheimer’s disease and frontotemporal dementia. Methods Mol Biol. 2011;670:1–9. doi: 10.1007/978-1-60761-744-0_1. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- Sai K, Yang D, Yamamoto H, Fujikawa H, Yamamoto S, Nagata T, Saito M, Yamamura T, Nishizaki T. A(1) adenosine receptor signal and AMPK involving caspase-9/-3 activation are responsible for adenosine-induced RCR-1 astrocytoma cell death. Neurotoxicology. 2006;27:458–467. doi: 10.1016/j.neuro.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handb Exp Pharmacol. 2009a;471:534. doi: 10.1007/978-3-540-89615-9_16. [DOI] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. Tuning and fine-tuning of synapses with adenosine. Curr Neuropharmacol. 2009b;7:180–194. doi: 10.2174/157015909789152128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Steinhauser C. Neuron-astrocyte signaling and epilepsy. Experimental neurology. 2011 doi: 10.1016/j.expneurol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Seifert G, Carmignoto G, Steinhäuser C. Astrocyte dysfunction in epilepsy. Brain Res Rev. 2010;63:212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathologica. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy J-M, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Synowitz M, Glass R, Farber K, Markovic D, Kronenberg G, Herrmann K, Schnermann J, Nolte C, van Rooijen N, Kiwit J, Kettenmann H. A1 adenosine receptors in microglia control glioblastoma-host interaction. Cancer Res. 2006;66:8550–8557. doi: 10.1158/0008-5472.CAN-06-0365. [DOI] [PubMed] [Google Scholar]
- Szybala C, Pritchard EM, Lusardi TA, Li T, Wilz A, Kaplan DL, Boison D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp Neurol. 2009;219:126–135. doi: 10.1016/j.expneurol.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells. J Physiol. 2002;539:67–75. doi: 10.1113/jphysiol.2001.012883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilas P, Brar S, Stewart KA, Shen HY, Sandau US, Poulsen D, Boison D. Adenosine kinase as a target for therapeutic antisense strategies in epilepsy. Epilepsia. 2011;52:589–601. doi: 10.1111/j.1528-1167.2010.02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M. Hippocampal sclerosis: progress since Sommer. Brain Pathol. 2009;19:565–572. doi: 10.1111/j.1750-3639.2008.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Blumcke I, Aronica E. Long-term epilepsy-associated tumors. Brain pathology. 2012;22:350–379. doi: 10.1111/j.1750-3639.2012.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, Warren K, Power C. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurology. 2007;6:421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Experimental neurology. 2011 doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials. 2008;29:3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
Web Reference
- Allen Brain Atlas mouse A1R expression profile. http://mouse.brainmap.org/search/show?page_num=0&page_size=20&no_paging=false&exact_match=false&search_term=adenosine%20A1R&search_type=gene.