Acute lymphoblastic leukemia (ALL) has an age-adjusted incidence rate of 1.7 per 100 000 men and women per year with 40% of total cases occurring in adults.1 ALL is risk-stratified based upon genetic features and lineage (B cell versus T cell). It is traditionally treated based upon lineage (B versus T) and also in part based upon the cytogenetic or molecular features of the disease. For virtually all types of ALL this treatment includes intensive chemotherapy, sometimes accompanied by specific tyrosine kinase inhibitors (BCR-ABL+ ALL) and/or allogeneic stem cell transplant for ALL patients at highest risk of relapsing. Although these intensive treatment approaches have greatly improved outcome for children with ALL, adults with this diagnosis have a higher frequency of toxicity with therapy and significantly lower frequency of cure. ALL patients who relapse following receipt of intensive chemotherapy and/or allogeneic transplant have an extremely poor prognosis with survival measured in terms of months. Despite these grim statistics few new therapies have come forward that have greatly influenced outcome in this disease. Identifying such therapies represents a high priority.
One form of therapy that has greatly influenced outcome in other forms of B-cell malignancy has been CD20 therapeutic antibodies. Rituximab, a chimeric anti-C20 antibody, has been shown to prolong survival in virtually all more mature B-cell malignancies in which it has been tested. Several recent small studies with CD52 (alemtuzumab) or CD20 (rituximab) -directed therapy have been incorporated as single agents in relapsed patients or into traditional therapy for ALL, and have demonstrated some evidence of efficacy prompting several ongoing larger studies.2–5 Both CD52 and CD20 are variably expressed on ALL cell blasts at modest copy number making these less ideal for tumor-directed antibody therapy.6–8 Contrasting with this, CD19 is expressed at a very early pro-B phase of B-cell development and is expressed uniformly on virtually all B-ALL cases. Recently, several CD19 antibodies that are engineered either by directed mutagenesis of the Fc-binding domain (XmAb-5574)9,10 or by afucosylation,11,12 thereby enhancing their FcγRIIIa-binding affinity and antibody-dependent cytotoxicity, have been reported. We previously have shown that XmAb-5574 mediates superior antibody-dependent cellular cytotoxicity (ADCC) in vitro against primary CLL cells.10 Given the uniform expression of CD19 on B-ALL cells, we sought to compare this antibody with other CD20 and CD52 antibodies currently under study in this disease. Our data demonstrate that XmAb-5574 mediates robust ADCC and modest direct killing with a cross-linking antibody against primary ALL cells, as compared with all the CD20- and CD52-directed antibodies, making it an ideal candidate for future clinical trials in this disease.
Patient sample processing and cell culture
Blood was obtained from patients with informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the Institutional Review Board of The Ohio State University (OSU). All patients examined in this series had immunophenotypically-defined B-ALL. ALL cells were isolated from freshly donated bone marrow with Ficoll density-gradient centrifugation (Ficoll-Plaque Plus, Amersham Biosciences, Piscataway, NJ, USA). Isolated cells were incubated in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO, USA), 2 mM L-glutamine (Invitrogen, Carlsbad, CA, USA), and 56 μ/ml penicillin/56 μg/ml streptomycin (Invitrogen) at 37 °C in an atmosphere of 5% CO2. Normal cells were obtained from Red Cross partial leukocyte preparations, and natural killer (NK) cells were negatively selected with Rosette-Sep (StemCell, Vancouver, BC, Canada) kits according to the manufacturer’s instructions. The purity of enriched populations of normal cells was routinely checked with the use of CD19 and CD56-PE staining by flow cytometry.
Antigen Quantification by flow cytometry
Quantitative analysis of CD19, CD20 and CD52 surface density was done using the Quantum Simply Cellular kit (Bangs Laboratories, Fishers, IN, USA), according to the manufacturer’s instructions. XmAb-5574, ofatumumab, and alemtuzumab were directly labeled with Alexa Fluor 488 4-SDP Ester (Invitrogen) according to manufacturer’s instructions. FACS analysis was performed using a Beckman Coulter FC500 cytometer. Ten-thousand events were collected for each sample. Data were acquired in list mode and analyzed using CXP Analysis Software (Beckman Coulter, Indianapolis, IN, USA).
Assessment of apoptosis by flow cytometry
The apoptosis of cells was measured using annexin V-FITC/PI staining followed by FACS analysis according to the manufacturer’s protocol (BD Biosciences). Results are presented as percentage cytotoxicity, which is defined as (% annexinV± and/or PI± cells of treatment group)/(% annexinV± and/or PI± cells of media control) ×100. FACS analysis was performed using a Beckman Coulter FC500 cytometer (Beckman Coulter). Ten-thousand events were collected for each sample and data were acquired in list mode.
ADCC assay
ADCC activity was determined by standard 4-hour 51Cr-release assay. Briefly, 51Cr-labeled target cells (5 × 103 cells/well of ALL cells) were incubated for 30 min with 10 μg/ml of individual antibodies. Unbound antibodies were washed off and cells were placed in to 96-well plates. Effector cells (NK cells from healthy donors) were then added to the plates at the indicated effector-to-target (E:T) ratios. After 4-hour incubation, supernatants were removed and counted on a Perkin Elmer Wizard gamma counter. The percentage of specific cell lysis was determined by: % lysis =100 × (ER−SR)/(MR−SR), where ER, SR and MR represent experimental, spontaneous and maximum release, respectively.
Statistics
Data were analyzed by mixed-effect models, accounting for observational dependencies among various treatments. Holm’s method was used to adjust multiplicity for primary end points.
We first sought to determine the expression of target antigens CD19, CD52 and CD20 on primary adult ALL blast cells. As shown in Figure 1a, ALL patients express similar proportion of CD20 (80 000 average molecules/cell, ranging from 30 to 130 000) and CD19 (100 000 average molecules/cell, ranging from 60 to 150 000) on the cell surface with moderate variability among patients. In contrast, CD52 expression across all of the ALL cells was more varied, with an average of 400 000 molecules per cell, with a range of 40–500 000. However, the difference in surface antigen levels between CD19 and CD20 or CD52 is not statistically significant.
Figure 1.
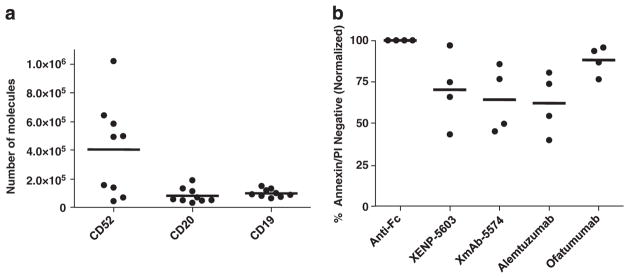
Surface antigen density on ALL cells and direct cytotoxicity. (a) Alexa-488-labeled alemtuzumab, ofatumumab or XmAb-5574 used to quantify the surface levels of CD52, CD20 and CD19, respectively, on primary ALL patient samples by flow cytometry (n = 9). (b) ALL cells treated with 10 μg/ml of each antibody with 50 μg/ml of goat anti-human anti-Fc cross-linking antibody for 24 h and viability assessed by flow cytometry (n = 4).
We next sought to determine the relative efficacy of different antibodies targeting CD20, CD19 and CD52 on CDC and direct killing against primary ALL cells. No CDC was observed with any of the antibodies tested (data not shown). In a similar manner, CD20-targeted antibodies did not mediate cell death against ALL cells with (Figure 1b) or without (data not shown) an anti-Fc cross-linking antibody. This contrasts with alemtuzumab, the non-engineered XENP-5603, and engineered XmAb-5574, which promote modest direct killing in the presence of an anti-Fc cross-linking antibody. These data suggest that CD19 and CD52-directed antibodies mediate direct cytotoxicity against primary ALL cells, whereas CD20-directed antibodies do not.
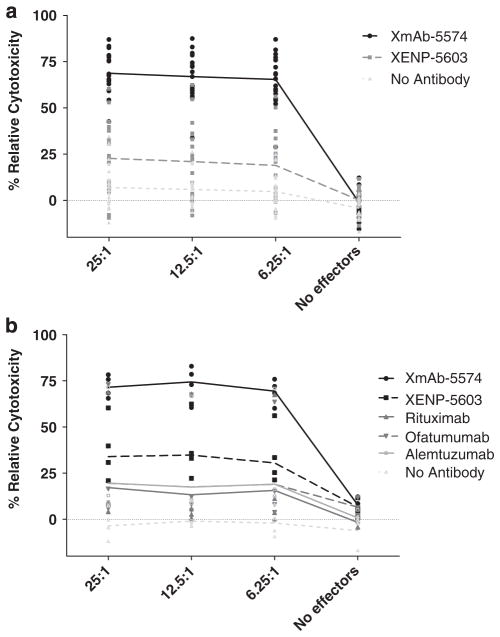
Given the importance of NK cell-mediated ADCC in other systems, we studied the ADCC potential of the anti-CD19 antibodies. XENP-5603 shows ADCC activity against ALL target cells but the engineered XmAb-5574 shows significantly enhanced activity at all E:T ratios tested (P = <0.0001) (Figure 2a). Finally, we wanted to expand on the data in Figure 2a and determine whether CD52, C20 and CD19 antibodies have differences in ADCC mediated toward ALL blast cells. Figure 2b demonstrates that the non-engineered XENP-5603 antibody directed at CD19 mediates greater ADCC as compared with rituximab, ofatumumab and alemtuzumab. Furthermore, we again validated that ADCC was dramatically increased toward ALL blast cells with the Fc-engineered antibody XmAb-5574. XmAb-5574 mediates significantly superior ADCC against ALL blast cells than alemtuzumab, rituximab and ofatumumab at all E:T ratios tested (P = <0.0001 for all). These studies suggest that XmAb-5574 mediates significant and superior ADCC against ALL cells as compared with other CD20 and CD52 antibodies currently under clinical investigation for this disease.
Figure 2.
XmAb-5574 mediates superior ADCC. (a) XmAb-5574 mediates enhanced ADCC compared with XENP-5603 against ALL patient cell targets with normal NK cell effectors (n = 7 ALL patient cell targets with three NK cell effectors each) with 10 μg/ml of antibody. The x axis shows effector:target ratios. (b) XENP-5603 mediates superior ADCC compared with anti-CD20 and anti-CD52 therapeutic antibodies against ALL patient cell targets with normal NK cell effectors (n = 2 ALL patient cell targets with three NK cell effectors each) with 10 μg/ml of each antibody.
Herein, we have demonstrated that XmAb-5574, an Fc region-engineered antibody, mediates potent ADCC toward human ALL cells at low effector-to-target cell numbers and modest direct killing of these cells with a cross-linking antibody. This effect is dramatic as compared with different CD20 antibodies that are FDA approved. Additionally, superiority is demonstrated for ADCC and direct killing when XmAb-5574 is compared with alemtuzumab, an antibody targeting CD52. Notably, rituximab and also alemtuzumab have demonstrated some evidence of clinical activity in ALL. The finding that XmAb-5574 mediates significantly better effector cell-mediated killing than these antibodies raises promise that this new antibody will have clinical activity in the disease.
Previous attempts to target CD19 with therapeutic antibodies or immune toxins have been unsuccessful. However, recent reports have suggested that a bispecific single-chain antibody Blinatumomab has impressive activity in several different CD19-positive malignancies by redirecting T cells (via CD3) to CD19-bearing targets. In particular, blinatumomab was shown to be effective in eliminating minimal residual disease from 16 of 21 patients with CD19-positive ALL.13 Other studies have shown blinatumomab has significant activity in different types of CD19+ B-cell malignancies including ALL.14,15 Although XmAb-5574 is predicted to efficiently recruit NK cells, monocytes, and macrophages to the ALL tumor cells, one might hypothesize that similar benefit observed with blinatumomab may be seen in the settings of both MRD and also active ALL. These data support phase I/II studies with XmAb-5574 in adult ALL.
Acknowledgments
We thank the patients for providing research samples used in this study and members of the CLL Experimental Therapeutics laboratory for critical comments. We are grateful for research support from The Leukemia and Lymphoma Society, P50-CA140158, PO1-CA95426, The Harry Mangurian Foundation, and The D Warren Brown Foundation. XENP-5603 and XmAb-5574 were provided by Xencor, Inc.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
SR designed and performed experiments, wrote the first draft of the manuscript, contributed to revisions of the paper, and approved the final submitted version. CC provided input into experimental design, performed experiments, and reviewed and approved the final version of the manuscript. DJ and XM assisted in design of experiments, performed the statistical analysis reported, reviewed and approved the final version of the manuscript. NM and JCB obtained funding to perform the research, designed the experiments, participated in the analysis of the data, reviewed multiple drafts of the manuscript and approved the final version for submission.
Contributor Information
N. Muthusamy, Email: raj.muthusamy@osumc.edu.
JC. Byrd, Email: john.byrd@osumc.edu.
References
- 1.Xie Y, Davies SM, Xiang Y, Robison LL, Ross JA. Trends in leukemia incidence and survival in the United States (1973–1998) Cancer. 2003;97:2229– 2235. doi: 10.1002/cncr.11316. [DOI] [PubMed] [Google Scholar]
- 2.Claviez A, Eckert C, Seeger K, Schrauder A, Schrappe M, Henze G, et al. Rituximab plus chemotherapy in children with relapsed or refractory CD20-positive B-cell precursor acute lymphoblastic leukemia. Haematologica. 2006;91:272– 273. [PubMed] [Google Scholar]
- 3.Thomas DA, O’Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28:3880– 3889. doi: 10.1200/JCO.2009.26.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccaluga PP, Martinelli G, Malagola M, Rondoni M, Bonifazi F, Bandini G, et al. Alemtuzumab in the treatment of relapsed acute lymphoid leukaemia. Leukemia. 2005;19:135. doi: 10.1038/sj.leu.2403578. author reply 136. [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo AL, Yu AL, Reaman G, Ingle AM, Secola R, Adamson PC. A phase II study of Campath-1H in children with relapsed or refractory acute lymphoblastic leukemia: a Children’s Oncology Group report. Pediatr Blood Cancer. 2009;53:978– 983. doi: 10.1002/pbc.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevallier P, Robillard N, Houille G, Ayari S, Guillaume T, Delaunay J, et al. Simultaneous study of five candidate target antigens (CD20, CD22, CD33, CD52, HER2) for antibody-based immunotherapy in B-ALL: a monocentric study of 44 cases. Leukemia. 2009;23:806– 807. doi: 10.1038/leu.2008.303. [DOI] [PubMed] [Google Scholar]
- 7.Nijmeijer BA, van Schie ML, Halkes CJ, Griffioen M, Willemze R, Falkenburg JH. A mechanistic rationale for combining alemtuzumab and rituximab in the treatment of ALL. Blood. 2010;116:5930– 5940. doi: 10.1182/blood-2010-01-262006. [DOI] [PubMed] [Google Scholar]
- 8.Piccaluga PP, Arpinati M, Candoni A, Laterza C, Paolini S, Gazzola A, et al. Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk Lymphoma. 2011;52:325– 327. doi: 10.3109/10428194.2010.529206. [DOI] [PubMed] [Google Scholar]
- 9.Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68:8049– 8057. doi: 10.1158/0008-5472.CAN-08-2268. [DOI] [PubMed] [Google Scholar]
- 10.Awan FT, Lapalombella R, Trotta R, Butchar JP, Yu B, Benson DM, Jr, et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood. 2010;115:1204– 1213. doi: 10.1182/blood-2009-06-229039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst R, Wang Y, Gallagher S, Mittereder N, Kuta E, Damschroder M, et al. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J Pharmacol Exp Ther. 2010;335:213– 222. doi: 10.1124/jpet.110.168062. [DOI] [PubMed] [Google Scholar]
- 12.Ward E, Mittereder N, Kuta E, Sims GP, Bowen MA, Dall’acqua W, et al. A glycoengineered anti-CD19 antibody with potent antibody-dependent cellular cytotoxicity activity in vitro and lymphoma growth inhibition in vivo. Br J Haematol. 2011;155:426– 437. doi: 10.1111/j.1365-2141.2011.08857.x. [DOI] [PubMed] [Google Scholar]
- 13.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493– 2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 14.Handgretinger R, Zugmaier G, Henze G, Kreyenberg H, Lang P, von Stackelberg A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011;25:181– 184. doi: 10.1038/leu.2010.239. [DOI] [PubMed] [Google Scholar]
- 15.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974– 977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]