Abstract
Brain inflammation in early life may enhance adult susceptibility to develop neurodegenerative disorders triggered by environmental toxins. Our previous studies show that perinatal lipopolysaccharide (LPS) exposure enhances adult susceptibility to rotenone-induced injury to the dopaminergic system in the substantia nigra (SN) of the adult rat brain. To further investigate the enhanced adult susceptibility by neonatal LPS exposure to rotenone neurotoxicity, we used our neonatal rat model of LPS exposure (1 mg/kg, intracerebral injection in postnatal day 5, P5, neonatal rats) to examine the protein levels of α-synuclein and dopamine transporters (DAT) in the adult rat. By P70, rats from the saline- or LPS-exposed group were challenged with rotenone, a commonly used pesticide, through subcutaneous mini-pump infusion at a dose of 1.25 mg/kg per day for 14 days. The accumulation of α-synuclein aggregation and increment of DAT protein content were found in the SN of LPS-exposed rats. Neonatal LPS exposure enhanced rotenone-stimulated accumulation of α-synuclein aggregation and increment in DAT protein expression in the cytoplasmic compartment of the SN, and in the synaptosomal compartment of the striatum of adult rats. Rotenone treatment also resulted in reduction of [3H]dopamine uptake and mitochondrial complex I activity in the striatum of rats with neonatal LPS exposure, but not in those without this exposure. The current study suggests possible roles of α-synuclein aggregate and DAT distribution in the cytoplasm and synaptosome triggered by environmental toxins in later life in the development of neurodegenerative disorders. Our model may be useful in studying mechanisms involved in the pathogenesis of nonfamilial Parkinson's disease and for developing potential therapeutic treatments for this disease.
Keywords: lipopolysaccharide, α-synuclein, dopamine transporter, neurodegenerative disorder, substantia nigra, rotenone
1. Introduction
Brain inflammation early in life has been reported to have long-term consequences and could speculatively modify the risk of a variety of neurological disorders in children and adults (Burd et al., 2012). Perinatal or early life exposure to an endotoxin, lipopolysaccharide (LPS), has been shown to increase the risk of dopaminergic disorders in animal models of Parkinson's disease (PD) (Fan et al., 2011a; Feleder et al., 2010; Ling et al., 2002, 2004, 2006). PD is a neurodegenerative disease and the pathological hallmarks of PD are a loss of dopamineproducing neurons in the substantia nigra (SN), causing dopamine depletion in the striatum, and the presence of neuronal cytoplasmic inclusions known as Lewy bodies (a major component: α-synuclein) (Sidhu et al., 2004a, 2004b). We have recently reported that exposure to LPS (postnatal day 5, P5, 1 mg/kg, intracerebral injection) during early development produces lesions in the nigrostriatal dopaminergic system. It also causes a functional disability in juvenile rats, suggesting possible involvement of dopaminergic impairment in this neonatal rat model of the central inflammation-induced neurological dysfunction (Fan et al., 2005b, 2008a, 2008c). Using this neonatal rat model, neonatal LPS exposure caused a chronic central inflammation and long-lasting injury to the dopaminergic system persistent into adult life (P70) (Fan et al., 2011b).
Our previous studies also show that perinatal LPS exposure enhances adult susceptibility to rotenone, a pesticide and a specific inhibitor of the mitochondria complex I, -induced injury to the dopaminergic system in the SN of the adult rat brain (Fan et al., 2011a). However, the mechanisms involved in the perinatal brain inflammation-enhanced adult susceptibility to the development of neurodegenerative disorders triggered by environmental toxins at an ordinarily non-toxic or sub-toxic dose are still unclear. Our preliminary study shows that neonatal LPS exposure increases α-synuclein protein and dopamine transporter (DAT) expression in the rat brain 24 hours after LPS injection. It has been proposed that neuroinflammation and α-synuclein dysfunction potentiate each other, and that may drive chronic progression of neurodegeneration (Gao et al., 2011). α-Synuclein is a neuronal cytosolic protein expressed primarily at presynaptic terminals in the central nervous system, and α-synuclein has been shown to associate, as a peripheral membrane protein, with synaptic vesicles, axonal transport vesicles, lipid droplets, and yeast vesicles at the outer membrane leaflet of the plasma membrane (Pacheco et al., 2012). A primary function of α-synuclein in dopaminergic neurons may be the modulation of DAT function to regulate the dopamine content and synaptic tone at the synapse, as the soluble α-synuclein decreasing the amount of DAT at the plasma member and the α-synuclein aggregation triggering DAT recruitment to the plasma membrane (Sidhu et al., 2004a, 2004b). Disruption of this modulatory process permits increased re-uptake of dopamine by DAT, causing high levels of intracellular dopamine with ensuing profound neurotoxicity (Sidhu et al., 2004a, 2004b). Moreover, α-synuclein can associate with the inner mitochondrial membrane and mitochondrial α-synuclein accumulation results in complex I impairment in dopaminergic neurons and increased free radical production (Chinta et al., 2010).
We are speculating a role of α-synuclein aggregation and DAT expression and their distribution in the perinatal brain inflammation-enhanced adult susceptibility to the development of neurodegenerative disorders triggered by environmental toxins. Therefore, the objective of this study was to investigate whether neonatal LPS exposure causes long-lasting changes in the α-synuclein aggregation and DAT expression and distribution as well as neurobehavioral deficits following the exposure to environmental toxins at an ordinarily non-toxic or sub-toxic dose (rotenone, 1.25 mg/kg per day, 14 days) in later life. In order to investigate the mechanism involved in the perinatal brain inflammation-enhanced adult susceptibility, new sets of animals were performed in the present study. Similar to the results reported in our previous study (Fan et al., 2011b), LPS model consistently demonstrated the neurobehavioral deficits in the present study.
2. Materials and Methods
2.1. Chemicals
Unless otherwise stated, all chemicals used in this study were purchased from Sigma (St. Louis, MO., USA). Monoclonal mouse antibodies against α-synuclein were purchased from BD Biosciences (San Jose, CA, USA). Polyclonal rat antibody against dopamine transporter (DAT) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). [3H]Dopamine ([3H]DA, specific activity 34.6 Ci/mmol) was purchased from PerkinElmer (Boston, MA, USA).
2.2. Animals
Timed pregnant Sprague-Dawley rats arrived in the laboratory on day 19 of gestation. Animals were maintained in a room with a 12-h light/dark cycle and at constant temperature (22 ± 2°C). The day of birth was defined as postnatal day 0 (P0). After birth, the litter size was adjusted to twelve pups per litter to minimize the effect of litter size on body weight and brain size. All procedures for animal care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center or Fu Jen Catholic University. Every effort was made to minimize the number of animals used and their suffering.
2.3. Surgery procedures and drug treatment
In order to eliminate a possible gender difference, only male rats were used in the present study. Intracerebral injection of LPS to 5-day old Sprague-Dawley male rat pups was performed as previously described (Cai et al., 2003; Fan et al., 2011a, 2011b; Pang et al., 2003). Under light anesthesia with isoflurane (1.5%), LPS (1 mg/kg body weight, from Escherichia coli, serotype 055: B5) in sterile saline (total volume of 2 µl) was administered to the rat brain at the location of 1.0 mm posterior to the bregma, 1.0 mm left to the sagittal suture and 2.0 mm deep to the scalp at the left hemisphere in a stereotaxic apparatus with a neonatal rat adapter. The injection site was aimed at the area just above the left cingulum. The injection was completed in 5 min and the needle was kept in this position for an additional 2 min and then retrieved slowly out of the brain. The wound was sutured and the pups were placed on a thermal blanket (34–35°C) for recovery before being returned to their dams. The dose of LPS was chosen based on the previously reported results that it produces reproducible brain injury (Cai et al., 2003; Fan et al., 2011a; Pang et al., 2003). The control rats were injected with the same volume of sterile saline. All animals survived the intracerebral injection. Each dam had the same litter size (12 pups) and equal numbers of LPS-treated and saline-treated rat pups were included in a litter. The pups were weaned at P21 and four rats (2 LPS-treated and 2 saline-treated) per cage were housed thereafter.
2.4. Minipump preparation and implantation
Rotenone was administered through a subcutaneous minipump infusion. The osmotic minipump preparation and subcutaneous implantation of minipumps were performed as described byFan et al. (2011a),Fleming et al. (2004) and Sherer et al. (2003) with modifications. On P70, rats pre-treated with LPS or saline (Sal) on P5 were randomly further divided into two groups: one group received rotenone (Rot) infusion and the other group received vehicle (Veh) infusion. Therefore, four experimental groups (24 male rats for each group) were included in the present study: Sal/Veh, Sal/Rot, LPS/Veh and LPS/Rot. Alzet osmotic minipumps (2ML4, Durect Corp., Cupertino, CA, USA) were aseptically filled one day before implantation with rotenone in equal amounts of dimethylsulfoxide (DMSO) and polyethylene glycol (PEG-300) [DMSO/PEG-300 (1:1)] or with DMSO/PEG-300 vehicle alone. The filled minipumps were placed into sterile 0.9% saline at 37°C overnight until implantation. Under anesthesia with 2% isoflurane in oxygen, minipumps were placed into subcutaneous burrows on the dorsal surface for infusion. The pumps were designed to deliver 1.25 mg rotenone/kg per day or vehicle for 14 days (from P70 to P84). At the end of 14 days (P84), the pumps were removed and the skin was closed with wound clips. Animals were allowed to recover for an additional 14 days (from P85 to P98) to provide sufficient time for the development of a stable lesion.
A total of seventy-two rats from six litters were used in the present study. One pup from each litter was assigned to each group, and then the n number was equal to six for each group. To verified the consistency of the LPS model as compared with previous studies (Fan et al., 2011a, 2011b), behavioral tests (the exposure rearing, vibrissa-elicited forelimb-placing test, and pole test) sensitive to varying degrees of dopamine loss in the striatum and substantia nigra were used in this study to assess motor function (Fleming et al., 2004; Schallert and Woodlee, 2005; Tillerson et al., 2002; Woodlee et al., 2005). Rats were sacrificed by decapitation to collect fresh brain tissue on P98. Brain tissues collected from the substantia nigra (SN) and striatum were used for determination of western blot analysis (6 rats for each group), and [3H]DA (dopamine) uptake study (6 rats for each group). Brain tissues collected from the substantia nigra (SN), dorsal midbrain, striatum, and cortex were used for determination of the mitochondrial complex I activity (6 rats for each group).
2.5. Immunoblotting analysis
Protein expression of α-synuclein and DAT was determined in the P98 rat brain by western blotting according to the methods of Fan et al. (2011a, 2011b) and Hadlock et al. (2009) with modifications. P98 rats were sacrificed by decapitation and tissues from different brain regions were isolated, frozen in liquid nitrogen, and stored at −80°C. Brain tissues were homogenized in ice-cold 0.32 M sucrose (50 mM Tris buffer, pH7.4) and centrifuged (800 × g for 12 min, 4°C). The supernatant (S1) was then centrifuged (20,000 × g for 15 min, 4°C), and the resulting supernatant (S2) (cytoplasmic compartment including endosomes) and pellets (P2) (synaptosomes) were used for immunoblotting analysis. Protein levels of homogenates were determined by the Bradford method. The homogenates were diluted with 1:2 (v/v) nonreducing loading buffer (Laemmli sample buffer, Bio-Rad Laboratories, Hercules, CA, USA) and subjected to Western blot analysis. Equal quantities of protein (10 µg/10 µl) were loaded into each well of a 4 to 20% SDS-polyacrylamide gradient gel (MINI-PROTEAN TGX, 4 to 20%, Bio-Rad Laboratories, Hercules, CA, USA). The separated proteins were transferred electrophoretically to PVDF membranes (Bio-Rad Laboratories, Hercules, CA, USA) at 100V for one hour. The blots were incubated with a blocking solution containing 5% non-fat milk and 0.1% Tween-20 in Tris-buffered saline (TBS) for one hour before incubation with the primary antibody (1:1000) in the blocking solution overnight at 4°C. The blots were then incubated with peroxidase-conjugated antibodies in the blocking solution (1:4000) for one hour at room temperature. The immunoreactivity was detected by the Enhanced Chemiluminescence Plus or advanced ECL system (GE Healthcare, Piscataway, NJ, USA) and then determined with the Chemidoc MP Imaging System followed by quantification using Image Lab software (both from Bio-Rad Laboratories, Hercules, CA, USA). To ensure that equal amounts of protein were applied to the immunoblot, the membranes were stripped with a stripping buffer (Thermo Scientific, Rockford, IL, USA) and re-probed for β-actin (1:4000, Sigma, St. Louis, MO., USA) to normalize the results.
2.6. Synaptosomal [3H]DA (dopamine) uptake
Uptake of [3H]DA was determined according to the method of Hadlock et al. (2009) and Nickell et al. (2010) with modifications. P98 rats were sacrificed by decapitation and tissues from different brain regions were isolated, frozen in liquid nitrogen, and stored at −80°C. Brain tissues were homogenized in ice-cold 0.32 M sucrose (50 mM Tris buffer, pH7.4) and centrifuged (800 × g for 12 min; 4°C). The supernatant (S1) was then centrifuged (20,000 × g for 15 min; 4°C), and the resulting pellets (P2) (synaptosomes) were resuspended in ice-cold water at concentrations of 2 mg/ml to lyse the synaptosomal membranes. Synaptosomal fractions were chilled at 4°C until DA uptake experiments commenced. Assays were performed in duplicate with a final volume of 250 µl. Aliquots of 25 µl synaptosomal fractions (50 µg of P2 protein) were added to tubes containing assay buffer (126 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 16 mM NaH2PO4, 1.4 mM MgSO4, 11 mM glucose, and 1 mM ascorbic acid, pH 7.4) and 1 µM pargyline, and then incubated at 37°C for 5 min. Nonspecific uptake was determined in the presence of 10 µM nomifensine. Samples were placed on ice, and 25 µl of 1 µM [3H]DA (100 nM final concentration) (specific activity 34.6 Ci/mmol; PerkinElmer, Boston, MA) was added to each tube, after which accumulation was permitted to proceed for 5 min at 37°C. DA concentration and time of uptake were chosen based on the reports by Hadlock et al. (2009) and Nickell et al. (2010). The reaction was terminated by the addition of 250 µl of ice-cold assay buffer and subsequent filtration, followed immediately by washing 2 times with ice-cold assay buffer. Radioactivity retained by the filters was counted using a liquid scintillation counter (PerkinElmer, Boston, MA, USA). Non-specific uptake, defined as the DA uptake in the presence of 10 µM nomifensine, was subtracted from total uptake to define DAT-mediated specific uptake.
2.7. Determination of mitochondrial complex I activity
Complex I activity was determined by a spectrophotometric assay based on the quantification of the rate of oxidation of the complex I substrate NADH to ubiquinone as described by Champy et al. (2004) and Hoglinger et al. (2005) with minor modifications. P98 rats were sacrificed by decapitation, and bilateral regions of the substantia nigra, dorsal midbrain, striatum, and cortex were isolated, frozen in liquid nitrogen, and stored at −80°C. The frozen brain tissue was homogenized mechanically, sonicated on ice in 10 mM Tris-HCl buffer (pH 7.2) containing 225 mM mannitol, 75 mM saccharose and 0.1 mM EDTA, and then centrifuged (600 × g) for 20 min at 4°C to obtain post-nuclear supernatants. The optical density of the supernatants (40 µg sample protein) in 1 ml of an assay mixture was spectrophotometrically recorded at a wavelength of 340 nm for 200 seconds at 37°C. The assay mixture was a potassium phosphate buffer (25 mM, pH 7.5) containing 2 mM potassium cyanide, 5 mM magnesium chloride, 2.5 mg/ml bovine serum albumin, 2 µM antimycin A, 100 µM decylubiquinone and 300 µM NADH. The proportion of NADH oxidation sensitive to an excess of rotenone (10 µM) was attributed to the complex I. This procedure minimizes the dissociation of rotenone from the complex I because of the use of small buffer volumes, maintenance at low temperatures and rapid analysis. The specific activity (nmol NADH oxidation/min/mg protein) of Complex I (NADH-ubiquinone oxidoreductase) was calculated using a molar extinction coefficient ε340nm = 6.22 mM−1cm−1 (Chen et al., 2005). Enzyme activities were expressed as nmol/min/mg of brain tissue. {Complex I activity = [Rate (min−1) / ε340nm(6.22 mM−1cm−1)] / 0.040 mg}
2.8. Quantification of data and statistics
Data from immunoblotting analysis, [3H]DA uptake, and mitochondrial complex I activity were presented as the mean ± SEM and analyzed by one-way ANOVA followed by the Student-Newman-Keuls test. Results with a p < 0.05 were considered statistically significant.
3. Results
Similar to the results reported in our previous study (Fan et al., 2011b), LPS model consistently demonstrated the neurobehavioral deficits in the present study including an increase in exposure rearing responses, reduction in success rate in the vibrissa-elicited forelimb-placing test, and elongation of mean latency times in the pole test in rats. The motor behavioral deficits in rats following LPS exposure were spontaneously reversible; by P70 all of the tested behaviors in the LPS-injected group reached the level of the control group. But, consistent with our previous finding (Fan et al., 2011a), the rotenone exposure on P70 enhanced neurobehavioral impairments in the neonatal LPS pre-exposure group, in the form of an increase in muscle tone or magnitude of stretch reflexes (determined by exposure rearing activity), prolongation of the reaction time (determined by vibrissa-elicited forelimb-placing test), and prolongation of the movement time (determined by pole test).
3.1 Neonatal LPS exposure affected the accumulation of α-synuclein aggregation following rotenone neurotoxicity in later life
α-Synuclein is a neuronal cytosolic protein expressed primarily at presynaptic terminals in the central nervous system, and α-synuclein has been shown to associate, as a peripheral membrane protein, with synaptic vesicles, axonal transport vesicles, lipid droplets, and yeast vesicles at the outer membrane leaflet of the plasma membrane (Pacheco et al., 2012). Our preliminary study showed that neonatal LPS exposure increased the α-synuclein expression in the rat brain 24 hours after LPS injection. In the current study, we determined α-synuclein expression in rat brain samples in the cytoplasmic (S2) and synaptosomal (P2) compartments of SN and striatum using immunoblotting analysis. The relative abundance of α-synuclein between striatum (10 µg total protein) and SN (10 µg total protein) in S1 (S2+P2) S2 and P2 fraction were around 180%, 200% and 145%, respectively.
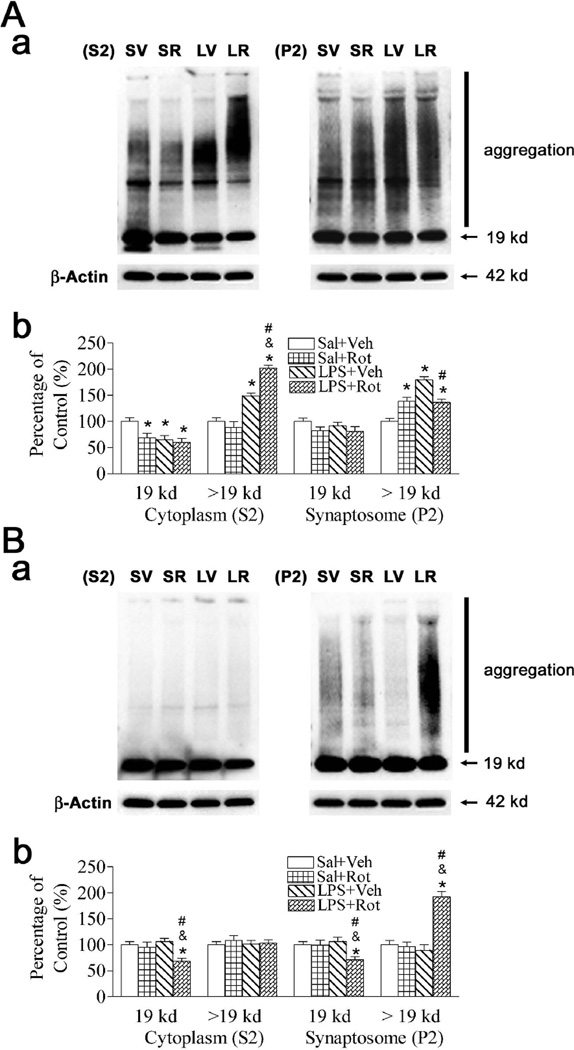
Neonatal LPS exposure caused an increase in the accumulation of α-synuclein aggregations (> 19 kd) (p < 0.05) and reduction of α-synuclein monomer (19 kd) in the cytoplasmic compartment (S2) of SN (p < 0.05) (Fig. 1A), but not in the striatum (Fig. 1B). Rotenone treatment in adult life did not affect the accumulation of α-synuclein aggregations in the cytoplasmic compartment (S2) in either the SN or the striatum of the Saline + rotenone treated animals, but resulted in an increased accumulation of α-synuclein aggregations in the synaptosomal compartment (P2) in the SN of LPS + rotenone treated animals (p < 0.05) (right panel of Fig. 1A). Rotenone treatment in adult life following neonatal LPS exposure (LPS + rotenone group) caused accumulation of α-synuclein aggregation in the cytoplasmic compartment (S2) of the SN (p < 0.05) (left panel of Fig. 1A), and the synaptosomal compartment (P2) of the striatum (p < 0.05) (right panel of Fig. 1B) of P98 rats, but not in the rats without neonatal LPS exposure (Saline + rotenone group). The increase in the accumulation of α-synuclein aggregations (> 19 kd) and reduction of α-synuclein monomer (19 kd) may reduce many molecular interactions, synaptic vesicle formation, axonal transport, and dopamine synthesis and metabolism (Sidhu et al., 2004a, 2004b).
Fig. 1.
Neonatal LPS exposure enhanced rotenone effects on the accumulation of α-synuclein aggregations in the SN (A) and striatum (B) of the P98 rat brain. The brain samples including cytoplasmic (S2) and synaptosomal (P2) compartments were used for immunoblotting analysis as described in methods. Aa and Ba, Western blotting of protein expression of α-synuclein in SN and striatum of P98 rats, respectively. Ab and Bb, Expression of α-synuclein is presented as the percentage of expression in the control group (Sal + Veh, SV) in SN and striatum of P98 rats, respectively. Neonatal LPS exposure (LPS + Veh, LV) caused an increase in the accumulation of α-synuclein aggregations (> 19 kd) and reduction of α-synuclein monomer (19 kd) in the cytoplasmic compartment (S2) of SN (A), but not in the striatum (B). Rotenone treatment in adult life did not affect the accumulation of α-synuclein aggregations in the cytoplasmic compartment (S2) in either the SN or the striatum of the Sal + Rot (SR) treated animals, but resulted in an increased accumulation of α-synuclein aggregations in the synaptosomal compartment (P2) in the SN of LPS + Rot (LR) treated animals. Neonatal LPS exposure enhanced rotenone-stimulated accumulation of α-synuclein aggregation (LR) in the cytoplasmic compartment (S2) of the SN (left panel of Aa and Ab), and in the synaptosomal compartment (P2) of the striatum (right panel of Ba and Bb) of P98 rats, but not in the rats without neonatal LPS exposure (SR). The results are expressed as the mean ± SEM of six animals in each group, and analyzed by one-way ANOVA. *P < 0.05 represents a significant difference for the Sal + Rot group, LPS + Veh group or LPS + Rot group compared with the Sal + Veh group. &P < 0.05 represents a significant difference for the LPS + Rot group as compared with the Sal + Rot group. #P < 0.05 represents a significant difference for the LPS + Rot group as compared with the LPS + Veh group.
3.2. Neonatal LPS exposure enhanced dopamine transporter protein expression following rotenone neurotoxicity in later life
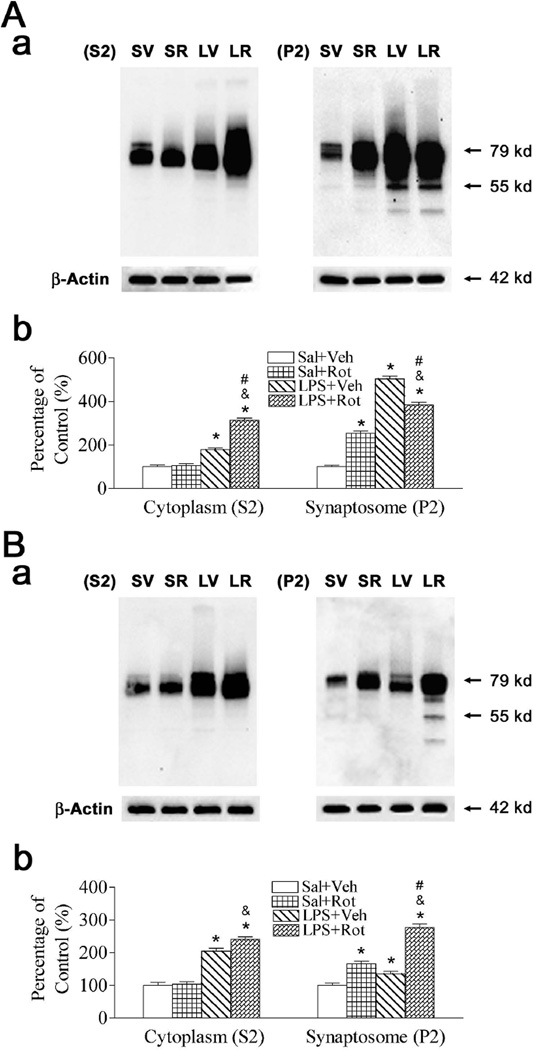
A primary function of α-synuclein in dopaminergic neurons may be the modulation of DAT function to regulate the dopamine content and synaptic tone at the synapse, as the soluble α-synuclein decreasing the amount of DAT at the plasma member and the α-synuclein aggregation triggering DAT recruitment to the plasma membrane (Sidhu et al., 2004a, 2004b). In the current study, the distribution of DAT in the cytoplasmic compartment (S2) and the synaptosomal compartment (P2), which may affect DA re-uptake in the DA neurons, was measured by immunoblotting analysis. Neonatal LPS exposure increased the expression of DAT in both the cytoplasmic compartment (S2) and the synaptosomal compartment (P2) of the SN (p < 0.05) (Fig. 2A) and striatum (p < 0.05) (Fig. 2B). Rotenone treatment in adult life did not affect DAT expression in cytoplasmic compartment (S2) in either the SN or the striatum of the saline + rotenone treated animals, but resulted in an increased DAT expression in synaptosomal compartment (P2) in both the SN (p < 0.05) and the striatum (p < 0.05) of saline + rotenone treated animals. Neonatal LPS exposure enhanced rotenone-stimulated induction of DAT expression in the cytoplasmic compartment (S2) of the SN (left panel of Fig. 2A), and in the synaptosomal compartment (P2) of the striatum (right panel of Fig. 2B) of P98 rats. LPS + rotenone group increased DAT expression in both the cytoplasmic compartment (S2) and synaptosomal compartment (P2) of the SN and striatum as compared with that of the saline + rotenone group (p < 0.05) (Figs. 2A and 2B). The increased DAT expression in synaptosomal compartment may increase the DA re-uptake to increase free cytoplasmic dopamine (Sidhu et al., 2004a, 2004b).
Fig. 2.
Neonatal LPS exposure enhanced rotenone effects on expression of DAT in the SN (A) and striatum (B) of the P98 rat brain. The brain samples including cytoplasmic compartment (S2) and synaptosomal compartment (P2) were used for immunoblotting analysis as described in methods. Aa and Ba, Western blotting of protein expression of DAT in SN and striatum of P98 rats, respectively. Ab and Bb, Expression of DAT is presented as the percentage of expression in the control group (Sal + Veh, SV) in SN and striatum of P98 rats, respectively. Neonatal LPS exposure (LPS + Veh, LV) increased the expression of DAT in the SN (A) and striatum (B). Rotenone treatment in adult life did not affect DAT expression in the cytoplasmic compartment (S2) in either the SN or the striatum of the Sal + Rot (SR) treated animals, but resulted in an increased DAT expression in the synaptosomal compartment (P2) in both the SN and the striatum of LPS + Rot (LR) treated animals. Neonatal LPS exposure enhanced rotenone-stimulated induction of DAT expression (LR) in cytoplasmic compartment (S2) of the SN (left panel of Aa and Ab), and in the synaptosomal compartment (P2) of the striatum (right panel of Ba and Bb) of P98 rats. The results are expressed as the mean ± SEM of six animals in each group, and analyzed by one-way ANOVA. *P < 0.05 represents a significant difference for the Sal + Rot group, LPS + Veh group or LPS + Rot group compared with the Sal + Veh group. &P < 0.05 represents a significant difference for the LPS + Rot group as compared with the Sal + Rot group. #P < 0.05 represents a significant difference for the LPS + Rot group as compared with the LPS + Veh group.
3.3. Rotenone treatment following neonatal LPS exposure reduced [3H]DA uptake in SN and striatum
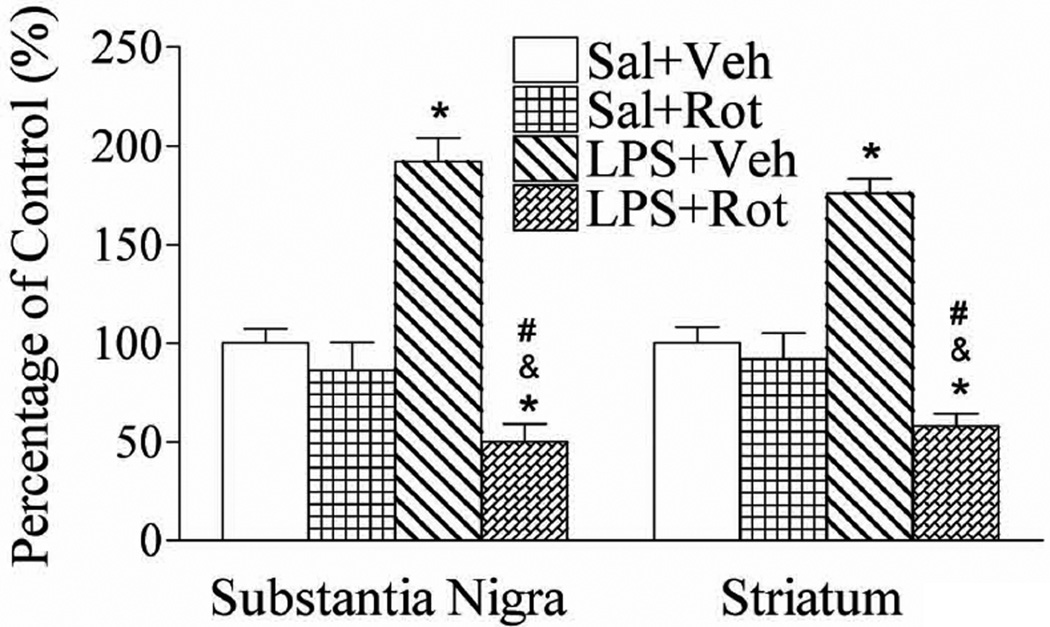
To determine the possible effect of neonatal LPS exposure on DAT function, dopamine uptake was measured by total [3H]DA uptake into brain synaptosomes as described in the method. Neonatal LPS exposure increased the [3H]DA uptake in the SN and striatum of adult rats (p < 0.05) (Fig. 3). These results revealed that LPS exposure may have a possible effect on DAT function. Rotenone treatment with a relatively low dose (1.25 mg/kg per day for 14 days) did not affect [3H]DA uptake in the SN and striatum of the saline + rotenone group (Fig. 3), but significantly reduced [3H]DA uptake into the SN and striatal synaptosomes of the LPS + rotenone group, as compared to that of the LPS + vehicle group (p < 0.05) and the saline + rotenone group (p < 0.05) (Fig. 3). The increase in DA re-uptake may overload extracellular dopamine into the neuronal cytoplasm and its possible transformation into a reactive and highly toxic molecule (Sidhu et al., 2004a, 2004b). However, the reduced DA uptake indicates the dopaminergic neuron dysfunction.
Fig. 3.
Neonatal LPS exposure enhanced rotenone-induced effects on [3H]DA uptake in the SN and striatum of the P98 rat brain. Dopamine uptake was measured by total [3H]DA uptake into brain synaptosomes and [3H]DA uptake is presented as the percentage of data in the control group (Sal + Veh). Neonatal LPS exposure increased [3H]DA uptake in the SN (left panel) and striatum (right panel) of P98 rat brains. Rotenone treatment in adult life did not affect [3H]DA uptake in either the SN or the striatum of the Sal + Rot treated animals, but resulted in a decreased [3H]DA uptake in both the SN and the striatum of LPS + Rot treated animals. The results are expressed as the mean ± SEM of six animals in each group, and analyzed by one-way ANOVA. *P < 0.05 represents a significant difference for the LPS + Veh group or LPS + Rot group compared with the Sal + Veh group. &P < 0.05 represents a significant difference for the LPS + Rot group as compared with the Sal + Rot group. #P < 0.05 represents a significant difference for the LPS + Rot group as compared with the LPS + Veh group.
3.4. Neonatal LPS exposure enhanced vulnerability of mitochondrial complex I activity to rotenone neurotoxicity in later life
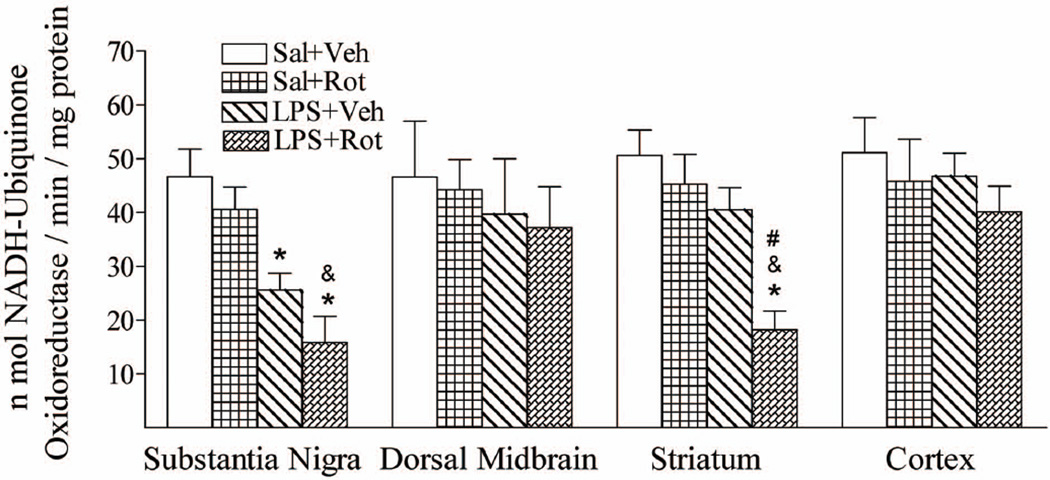
α-Synuclein can associate with the inner mitochondrial membrane and mitochondrial α-synuclein accumulation results in complex I impairment in dopaminergic neurons and increased free radical production (Chinta et al., 2010). Our previous studies have found that neonatal LPS exposure resulted in the decreased mitochondrial complex I activity in the SN and enhanced the vulnerability of mitochondrial complex I activity in the striatum in responses to rotenone challenge in later life (Fan et al., 2011a). However, whether this enhancement is regional specific in the rat brain still needs to be clarified. Brain tissues collected from the SN, dorsal midbrain, striatum, and cortex were used for determination of the mitochondrial complex I activity, which was determined as the amount of NADH oxidized per minute per milligram of protein in homogenates of P98 rat brain tissues. Neonatal LPS exposure decreased mitochondrial complex I activity in the SN (p < 0.05), but not in the dorsal midbrain, striatum, and cortex of P98 rat brain (Fig. 4). Treatment with a relatively low dose of rotenone (1.25 mg/kg per day for 14 days) at the adult stage did not significantly decrease the mitochondrial complex I activity in the SN, dorsal midbrain, striatum, and cortex of P98 rats without the neonatal LPS exposure (Fig. 4). However, rotenone treatment resulted in a significantly decreased mitochondrial complex I activity in the striatum area of the P98 rats with the neonatal LPS exposure (p < 0.05) (Fig. 4). Rotenone treatment at this dosage also further reduced mitochondrial complex I activity in the SN of the LPS + rotenone group as compared to that in the LPS + vehicle group, although the difference did not reach statistical significance. LPS + rotenone group reduced mitochondrial complex I activity in both the SN and striatum as compared with that of the saline + rotenone group (p < 0.05) (Fig. 4).
Fig. 4.
Neonatal LPS exposure enhanced rotenone-induced effects on reduction in the enzymatic activity of mitochondrial complex I regional specific in SN and striatum of the P98 rat brain. Neonatal LPS exposure reduced enzymatic activity of mitochondrial complex I in the SN, but not in the striatum, dorsal midbrain or cortex. Rotenone treatment in adult life did not affect mitochondrial complex I activity in either the SN or dorsal midbrain, striatum or cortex of the Sal + Rot treated animals, but resulted in a decreased mitochondrial complex I activity in both the SN and the striatum of LPS + Rot treated animals. LPS-induced and LPS-enhanced vulnerability of mitochondrial complex I activity is regional specific in SN and striatum, respectively, but not in dorsal midbrain or cortex. The similar data of SN and striatum in responses to rotenone challenge in later life have also been reported in our previous study (Fan et al., 2011a). The results are expressed as the mean ± SEM of six animals in each group, and analyzed by one-way ANOVA. *P < 0.05 represents a significant difference for the LPS + Veh group or LPS + Rot group compared with the Sal + Veh group. &P < 0.05 represents a significant difference for the LPS + Rot group as compared with the Sal + Rot group. #P < 0.05 represents a significant difference for the LPS + Rot group as compared with the LPS + Veh group.
As shown in Fig. 4, LPS-induced and LPS-enhanced vulnerability of mitochondrial complex I activity is regional specific in SN and striatum, respectively, but not in dorsal midbrain or cortex. The similar data of SN and striatum in responses to rotenone challenge in later life have also been reported in our previous study (Fan et al., 2011a). Decreased mitochondrial complex I activity reduces cellular ATP levels and may lead to reduced ATP-dependent axonal transport (Hoglinger et al., 2005).
4. Discussion
Nonfamilial PD is associated with advanced age, but it is still unclear whether dopaminergic neuronal death results from events initiated during development, adulthood, or represents a cumulative effect across the life span (Landrigan et al., 2005; Miller and O’Callaghan, 2008). Perinatal or early life exposure to LPS has been shown to increase the risk of dopaminergic disorders in animal models of PD (Feleder et al., 2010; Ling et al., 2002, 2004, 2006). Our previous finding also shows that perinatal brain inflammation may enhance adult susceptibility to the development of neurodegenerative disorders triggered later on by environmental toxins at an ordinarily non-toxic or sub-toxic dose (Fan et al., 2011a). Neonatal LPS exposure enhances vulnerability of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life, as indicated by motor neurobehavioral impairments, losses of tyrosine hydroxylase (TH) immunoreactive neurons in the substantia nigra, decreased mitochondrial complex I activity, and decreased retrogradely labeled nigrostriatal dopaminergic projecting neurons in adult rats (Fan et al., 2011a). Consistent with our previous study (Fan et al., 2011a), the present results indicated that neonatal LPS exposure enhances rotenone neurotoxicity in the neurobehavioral impairments including an increase in muscle tone or magnitude of stretch reflexes (determined by exposure rearing activity), prolongation of the reaction time (determined by vibrissa-elicited forelimb-placing test), and prolongation of the movement time (determined by pole test). In the current study, we also found that neonatal LPS exposure enhanced rotenonestimulated accumulation of α-synuclein aggregation and increment in DAT protein expression in the cytoplasmic compartment of the SN, and in the synaptosomal compartment of the striatum of adult rats (Figs. 1 and 2). Rotenone treatment also resulted in reduction of [3H]DA uptake (Fig. 3) and mitochondrial complex I activity (Fig. 4) in the striatum of rats with neonatal LPS exposure, but not in those without this exposure. The present results suggest the possible mechanisms involved in the perinatal brain inflammation augmented adult susceptibility to the development of neurodegenerative disorders triggered by environmental toxins.
Neonatal LPS exposure produced not only acute inflammatory response, but also chronic inflammation in the adult rat brain, as evidenced by an increase in microglia number, elevated IL-1β concentration, and cyclooxygenase-2 (COX-2) expression (Cai et al., 2012; Fan et al., 2005a, 2008b, 2008c, 2011b). The increased COX-2 in dopaminergic cells under stressful conditions can facilitate dopamine oxidation to quinone species, triggering oxidative stress (Bartels and Leenders, 2010). Additionally, COX-2 overexpression in dopaminergic cells may also play a role in α-synuclein accumulation (Chae et al., 2008). Our preliminary study shows that neonatal LPS exposure resulted in increases in protein levels of α-synuclein and dopamine transporter, and an increase in [3H]DA uptake in the rat brain 24 hours after LPS injection. The present results also indicated that neonatal LPS exposure resulted in the accumulation of α-synuclein aggregation (> 19 kd) and reduction of α-synuclein monomer (19 kd) in the cytoplasmic compartment (S2) of SN (Fig. 1A), an increment of DAT protein content, and increased [3H]DA uptake in the SN of adult rats. The α-synuclein is a cytosolic protein; however, the α-synuclein secretion may occur under physiological conditions but more prominently under pathological conditions such as those associated with mitochondrial and proteosomal dysfunction (Lee et al., 2008; Pacheco et al., 2012). The elevated oligomeric α-synuclein has been found in human cerebrospinal fluid and blood plasma of PD patients (El-Agnal et al., 2003; 2006; Pacheco et al., 2012; Sierks et al., 2011). The accumulation of α-synuclein oligomers in the extracellular space can activate microglia and alter the plasma membrane through the formation of pore/perforations with resultant increase in the levels of intracellular Ca2+ (Danzer et al., 2007; Pacheco et al., 2012). Extracellular α-synuclein aggregates can be removed by proteolytic degradation or endocytocis to favor the formation of Lewy body-like inclusions in neighboring cells (Lee et al., 2008; Desplats et al., 2009; Pacheco et al., 2012). It has also been suggested that microglia may be the major scavenger cells for extracellular α-synuclein aggregates in brain parenchyma (Lee et al., 2008; Pacheco et al., 2012). The microglia response is modulated by α-synuclein expression in SN (Sanchez-Guajardo et al., 2010) and extracellular aggregated α-synuclein activates microglia also (Zhang et al, 2005). Furthermore, microglial activation enhances dopaminergic neurodegeneration induced by aggregated alpha-synuclein (Zhang et al, 2005). Therefore, it has been proposed that neuroinflammation and α-synuclein dysfunction instigates a self-propelling progression and thus drives chronic advancement of neurodegeneration (Gao et al., 2011; Pacheco et al., 2012).
Cytoplasmic soluble α-synuclein (monomer) decreases TH activity, regulates the clearance of cytoplasmic dopamine through the vesicular monoamine transporter 2 (VMAT2) and holds the DAT into a cytoplasmic compartment, thus preventing overload of extracellular dopamine into the neuronal cytoplasm (Sidhu et al., 2004a, 2004b). It has been reported that reduction in the amount of soluble α-synuclein may increase free cytoplasmic dopamine and thus reactive oxygen species (ROS) formation. Formation of α-synuclein aggregates results in the increase in TH and DAT activities as well as reduction of vesicles formation and neuronal plasticity (Sidhu et al., 2004a, 2004b). Therefore, in the current study the neonatal LPS exposure-induced increases in DAT amount in the synaptosomal compartment (P2) (Fig. 2) and [3H]DA uptake (Fig. 3) may have resulted from the decreased cytoplasmic (S2) α-synuclein monomer and increased accumulation of α-synuclein aggregation. Neonatal LPS exposureinduced α-synuclein dysfunction in the brain doparminergic system may permit increased reuptake of dopamine by DAT, producing higher levels of intracellular dopamine with resultant neurotoxicity and thus chronic progression of neurodegeneration. The current results indicated that rotenone treatment in adult life did not affect DAT expression in the cytoplasmic compartment in either the SN or the striatum of the saline + rotenone treated animals, but resulted in an increased DAT expression in the synaptosomal compartment in both the SN and the striatum of saline + rotenone treated animals (Fig. 2). Neonatal LPS exposure enhanced rotenone-stimulated induction of DAT expression in the cytoplasmic compartment (S2) of the SN, and in the synaptosomal compartment (P2) of the striatum of P98 rats (Fig. 2). Moreover, the perinatal LPS exposure enhanced the adult susceptibility to rotenone-induced injury to the dopaminergic system in the SN of the adult rat brain, as evidenced by the dopaminergic neuronal loss, impairment of dendritic arborization and synaptic connectivity and eventually PD-like neurological deficits (Fan et al., 2011a).Ahmadi et al. (2008) also reported that DA neurons became selectively sensitized to rotenone when intracellular DA concentration was elevated. The rotenone-induced up-regulation of DAT was found in PC12 cells (Sai et al., 2008); however, the down-regulated DAT protein expression was found in the rotenone-treated rat (Lin et al., 2008). The change in the density of DAT has not been clarified in the different stage of PD. However, it has been found that the density of DA neuron related components, such as D2 receptors, increases in early untreated PD, but there is an accelerated rate of D2 loss as the disease advances (Cumming and Borghammer, 2012).
As we reported previously (Fan et al., 2011a, 2011b), mitochondrial complex I activity in the SN of P98 rat brain with neonatal LPS exposure was significantly reduced as compared to that of the control group (Fig. 4). The neonatal LPS exposure-induced impairment of mitochondrial complex I activity is regional specific in the rat brain and was not observed in the dorsal midbrain, striatum and cortex of P98 rats (Fig. 4). Mitochondrial dysfunction has been identified in PD, Huntington’s disease, Friedreich’s ataxia and hereditary spastic paraplegia, Alzheimer’s disease, and amyotrophic lateral sclerosis (Schapira 2011). The α-synuclein can associate with the inner mitochondrial membrane, inhibiting mitochondria fusion and leading to mitochondria fragmentation while mitochondrial α-synuclein accumulation results in complex I impairment in dopaminergic neurons and increased free radical production (Chinta et al., 2010; Schapira 2011). Therefore, the neonatal LPS exposure-induced lasting mitochondria impairment in SN may be caused by the chronic accumulation of α-synuclein in the rat brain. Treatment with relative high dose of rotenone (2.5 mg/kg/day, 28 days) reduced around 20% to 30% of mitochondrial complex I activity in the brain as compared with that in the control rats (Hoglinger et al., 2005). The present study also demonstrated that the small dose of rotenone (1.25 mg/kg/day, 14 days) did not significantly affect mitochondrial complex I activity in the SN and striatum without the neonatal LPS exposure, while reduction in mitochondrial complex I activity was observed in both the SN and striatum in the LPS + rotenone group (Fig. 4). Rotenone, a pesticide and a specific inhibitor of the mitochondria complex I, can inhibit energy-producing mitochondrial enzymes and lead to the diminished production of ATP, consequently inhibiting [3H]DA uptake into the rat striatal synaptosomes by noncompetitive inhibition of the DAT (Maragos et al., 2002). In the present study, neonatal LPS exposure enhanced rotenone-induced decreases in the [3H]DA uptake in both striatum and SN (Fig. 3), although the DAT expression was increased in both striatum and SN as compared with P98 control rats (Fig. 2). As we mentioned before, treatment with rotenone caused an increased DAT expression in the synaptosomal compartment (P2) in both the SN and the striatum of saline + rotenone treated animals (Fig. 2). These increased DAT expression following rotenone treatment may possible result from the adaption effect of the noncompetitive inhibition of the DAT or the α-synuclein aggregation triggering DAT recruitment to the plasma membrane (Sidhu et al., 2004a, 2004b). Chronic inhibition of the mitochondrial complex I even if distributed uniformly throughout the brain has been shown to damage dopaminergic neurons selectively (Uversky, 2004). Therefore, the persistently compromised mitochondrial function in the SN following neonatal LPS exposure could contribute to the enhanced vulnerability of the dopaminergic systems in this animal model.
Our present and previous studies in a rat model have shown that central inflammation in the neonatal period, an event commonly and frequently occurring in human infants, enhances susceptibility of the dopaminergic system to an ordinarily non- or sub-toxic dose of environmental toxins with subsequent development of PD-like neurological deficits in later life. Brain inflammation early in life has been reported to have long-term consequences and could speculatively modify the risk of a variety of neurological disorders in children and adults (Burd et al., 2012). Such early-life inflammation also has been hypothesized to be involved in the induction of neurodegenerative disorders, schizophrenia, anxiety/depression, cognitive dysfunction, and alterations of the normal aging process (Bilbo et al., 2006; Bilbo and Schwarz, 2009). The current study showed that the neonatal LPS exposure and subsequent environmental toxin exposure in a non-toxic or sub-toxic dose can produce long-lasting increases in the accumulation of α-synuclein aggregates and DAT expression in later life. Our findings suggest a role of α-synuclein aggregate and DAT distribution in the cytoplasm and synaptosome in the development of environmental toxin induced neurodegenerative disorders in later life following early brain inflammation in rats. In addition, our model is useful in studying mechanisms involved in the pathogenesis of nonfamilial PD and development of potential therapeutic treatments for this disease.
Acknowledgements
This work was supported by a grant NSC 99-2320-B-030-003-MY3 from National Science Council of Taiwan, a grant NS 54278 from NIH, and Newborn Medicine Funds from the Department of Pediatrics, University of Mississippi Medical Center, Jackson, MS, USA.
Abbreviations
- COX-2
cyclooxygenase-2
- DA
dopamine
- DAT
dopamine transporter
- LPS
lipopolysaccharide
- P5
postnatal day 5
- PD
Parkinson's disease
- SN
substantia nigra
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadi FA, Grammatopoulos TN, Poczobutt AM, Jones SM, Snell LD, Das M, Zawada WM. Dopamine selectively sensitizes dopaminergic neurons to rotenoneinduced apoptosis. Neurochem Res. 2008;33:886–901. doi: 10.1007/s11064-007-9532-5. [DOI] [PubMed] [Google Scholar]
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Animal Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav. Brain. Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav. Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels AL, Leenders KL. Cyclooxygenase and neuroinflammation in Parkinson's disease neurodegeneration. Curr. Neuropharmacol. 2010;8:62–68. doi: 10.2174/157015910790909485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am. J. Reprod. Immunol. 2012;67:287–294. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- Cai Z, Fan LW, Kaizaki A, Tien LT, Ma T, Pang Y, Lin S, Lin RCS, Simpson KL. Neonatal systemic exposure to lipopolysaccharide enhances vulnerability of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Dev. Neurosci. 2012 doi: 10.1159/000346156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Chae SW, Kang BY, Hwang O, Choi HJ. Cyclooxygenase-2 is involved in oxidative damage and alpha-synuclein accumulation in dopaminergic cells. Neurosci. Lett. 2008;436:205–209. doi: 10.1016/j.neulet.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Champy P, Höglinger GU, Félger J, Gleye C, Hocquemiller R, Laurens A, Guérineau V, Laprévote O, Medja F, Lombès A, Michel PP, Lannuzel A, Hirsch EC, Ruberg M. Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: possible relevance for atypical parkinsonism in Guadeloupe. J. Neurochem. 2004;88:63–69. doi: 10.1046/j.1471-4159.2003.02138.x. [DOI] [PubMed] [Google Scholar]
- Chen YR, Chen CL, Zhang L, Green-Church KB, Zweier JL. Superoxide generation from mitochondrial NADH dehydrogenase induces self-inactivation with specific protein radical formation. J. Biol. Chem. 2005;280:37339–37348. doi: 10.1074/jbc.M503936200. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial α-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci. Lett. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming P, Borghammer P. Molecular imaging and the neuropathologies of Parkinson's disease. Curr. Top Behav. Neurosci. 2012;11:117–148. doi: 10.1007/7854_2011_165. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of á-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ryck M, Van Reempts J, Duytschaever H, Van Deuren B, Clincke G. Neocortical localization of tactile/proprioceptive limb placing reactions in the rat. Brain Res. 1992;573:44–60. doi: 10.1016/0006-8993(92)90112-m. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of á-synuclein. Proc. Natl. Acad. Sci. USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S, Cookson MR, Hardy J, Allsop D. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB. J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Fan LW, Chen RF, Mitchell HJ, Lin RCS, Simpson KL, Rhodes PG, Cai Z. α-Phenyl-n-tert-butyl-nitrone attenuates lipopolysaccharide-induced brain injury and improves neurological reflexes and early sensorimotor behavioral performance in juvenile rats. J. Neurosci. Res. 2008a;86:3536–3547. doi: 10.1002/jnr.21812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Mirchell HJ, Rhodes PG, Cai Z. Alpha-phenyl-n-tert-butyl-nitrone attenuates lipopolysaccharide-induced neuronal injury in the neonatal rat brain. Neuroscience. 2008b;151:737–744. doi: 10.1016/j.neuroscience.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Mirchell HJ, Tien LT, Zheng B, Pang Y, Rhodes PG, Cai Z. Alphaphenyl-n-tert-butyl-nitrone reduces lipopolysaccharide-induced white matter injury in the neonatal rat brain. Dev. Neurol. 2008c;68:365–378. doi: 10.1002/dneu.20591. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005a;133:159–168. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Tien LT, Ma T, Rhodes PG, Cai Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J. Neurosci. Res. 2005b;82:71–82. doi: 10.1002/jnr.20623. [DOI] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Mitchell HJ, Rhodes PG, Cai Z. α-Phenyl-n-tert-butylnitrone ameliorates hippocampal injury and improves learning and memory in juvenile rats following neonatal exposure to lipopolysaccharide. Eup. J. Neurosci. >2008d;27:1475–1484. doi: 10.1111/j.1460-9568.2008.06121.x. [DOI] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Lin RCS, Simpson KL, Rhodes PG, Cai Z. Neonatal exposure to lipopolysaccharide enhances vulnerability of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Neurobiol. Dis. 2011a;44:304–316. doi: 10.1016/j.nbd.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Zheng B, Pang Y, Lin RCS, Simpson KL, Ma T, Rhodes PG, Cai Z. Dopaminergic neuronal injury in the adult rat brain following neonatal exposure to lipopolysaccharide and the silent neurotoxicity. Brain Behev. Immunity. 2011b;25:286–297. doi: 10.1016/j.bbi.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleder C, Tseng KY, Calhoon GG, O'Donnell P. Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biol. Psychiatry. 2010;67:386–392. doi: 10.1016/j.biopsych.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Zhu C, Fernagut PO, Mehta A, Dicarlo CD, Seaman RL, Xhesselet MF. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp. Neurol. 2004;187:418–429. doi: 10.1016/j.expneurol.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS. Neuroinflammation and á-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson's disease. Environ. Health Perspect. 2011;119:807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Baucum AJ, 2nd, King JL, Horner KA, Cook GA, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. Mechanisms underlying methamphetamine-induced dopamine transporter complex formation. J. Pharmacol. Exp. Ther. 2009;329:169–174. doi: 10.1124/jpet.108.145631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger GU, Lannuzel A, Khondiker ME, Michel PP, Duyckaerts C, Feger J, Champy P, Prigent A, Medja F, Lombes A, Oertel WH, Ruberg M, Hirsch EC. The mitochondrial complex I inhibitor rotenone triggers a cerebral tauopathy. J. Neurochem. 2005;95:930–939. doi: 10.1111/j.1471-4159.2005.03493.x. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler RN, Transande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environ. Health Perspect. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee SJ. Clearance and deposition of extracellular alphasynuclein aggregates in microglia. Biochem. Biophys. Res. Commun. 2008;372:423–428. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Lin CH, Huang JY, Ching CH, Chuang JI. Melatonin reduces the neuronal loss, downregulation of dopamine transporter, and upregulation of D2 receptor in rotenone-induced parkinsonian rats. J. Pineal. Res. 2008;44:205–213. doi: 10.1111/j.1600-079X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Ling Z, Chang QA, Tong CW, Leurgans SE, Lipton JW, Carvey PM. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Exp. Neurol. 2004;190:373–383. doi: 10.1016/j.expneurol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, Hong JS, Carvey PM. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov. Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp. Neurol. 2006;199:499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Zhu J, Chesnut MD, Dwoskin LP. Mitochondrial toxin inhibition of [3H]dopamine uptake into rat striatal synaptosomes. Biochem. Pharmacol. 2002;63:1499–1505. doi: 10.1016/s0006-2952(02)00910-3. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Do early-life insults contribute to the late-life development of Parkinson and Alzheimer diseases? Metabolism. 2008;57(Suppl 2):S44–S49. doi: 10.1016/j.metabol.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Nickell JR, Krishnamurthy S, Norrholm S, Deaciuc G, Siripurapu KB, Zheng G, Crooks PA, Dwoskin LP. Lobelane inhibits methamphetamine-evoked dopamine release via inhibition of the vesicular monoamine transporter-2. J. Pharmacol. Exp. Ther. 2010;332:612–621. doi: 10.1124/jpet.109.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco C, Aguayo LG, Opazo C. An extracellular mechanism that can explain the neurotoxic effects of á-synuclein aggregates in the brain. Front Physiol. 2012;3:297. doi: 10.3389/fphys.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res. Dev. Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Sai Y, Wu Q, Le W, Ye F, Li Y, Dong Z. Rotenone-induced PC12 cell toxicity is caused by oxidative stress resulting from altered dopamine metabolism. Toxicol. In Vitro. 2008;22:1461–1468. doi: 10.1016/j.tiv.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of a-synuclein neuropathology in a rAAV based model of Parkinson's disease. PLoS One. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Woodlee MT. Motor systems: Orienting and Placing. In: Whishaw IQ, Kolb B, editors. The Behaviour of the Laboratory Rat: A Handbook with Tests. New York: Oxford University Press; 2005. pp. 129–140. [Google Scholar]
- Schapira AH. Mitochondrial pathology in Parkinson's disease. Mt. Sinai J. Med. 2011;78:872–881. doi: 10.1002/msj.20303. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Wersinger C, Vernier P. Alpha-Synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease. FEBS Lett. 2004a;565:1–5. doi: 10.1016/j.febslet.2004.03.063. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB. J. 2004b;18:637–647. doi: 10.1096/fj.03-1112rev. [DOI] [PubMed] [Google Scholar]
- Sierks MR, Chatterjee G, McGraw C, Kasturirangan S, Schulz P, Prasad S. CSF levels of oligomeric alpha-synuclein and beta-amyloid as biomarkers for neurodegenerative disease. Integr. Biol. (Camb) 2011;3:1188–1196. doi: 10.1039/c1ib00018g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J. Neurosci. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN. Neurotoxicant-induced animal models of Parkinson's disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Asseo-garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing "across the midline" reveals distinct, lesion-dependent patterns of recovery in rats. Exp. Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB. J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]