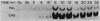Abstract
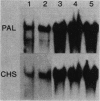
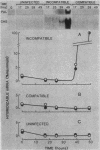
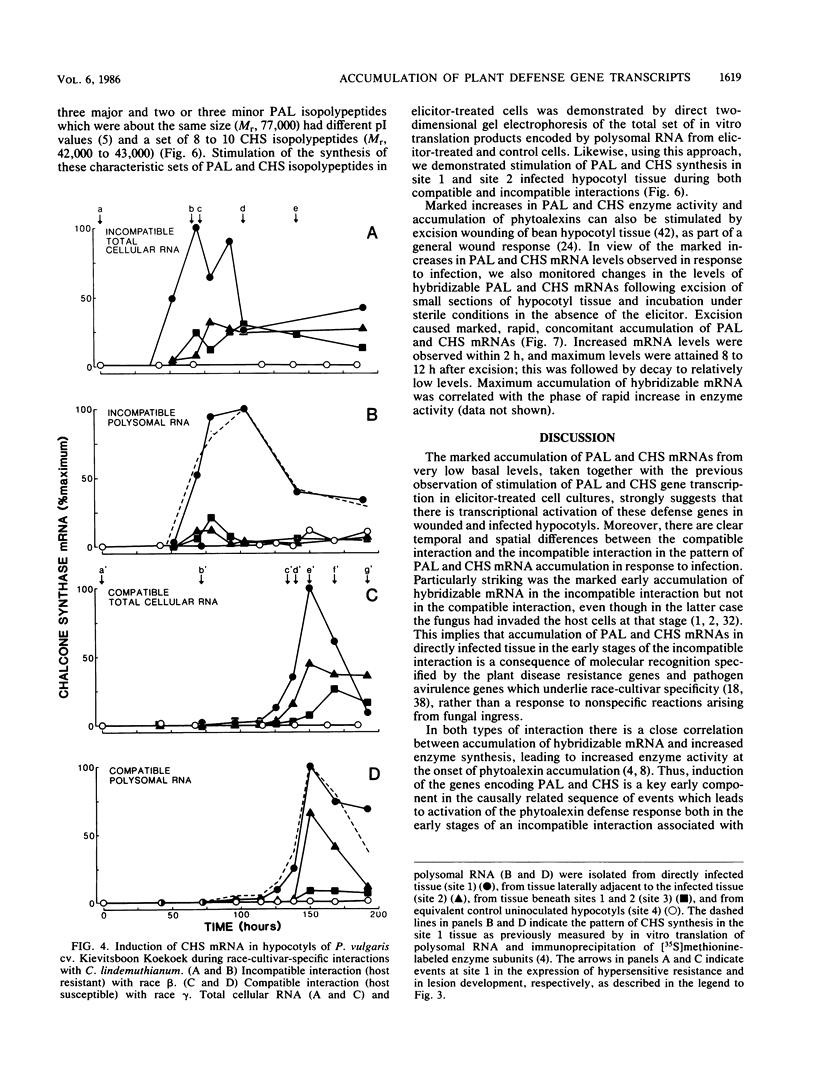
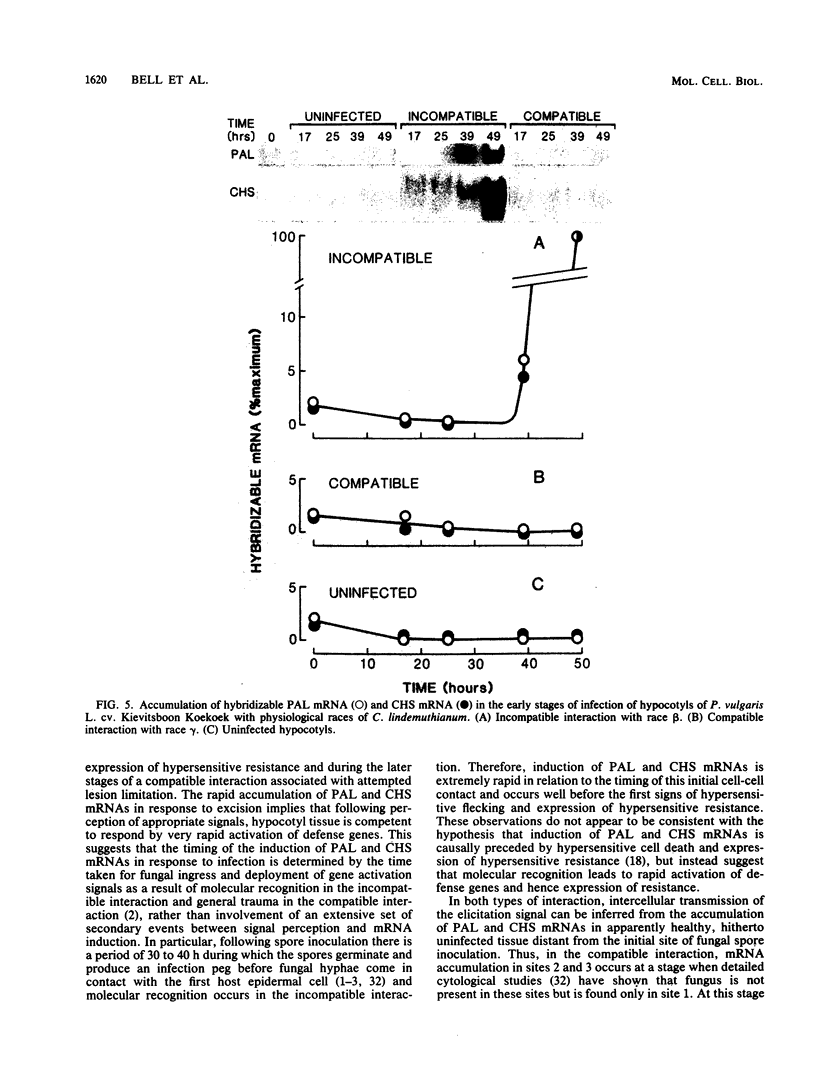
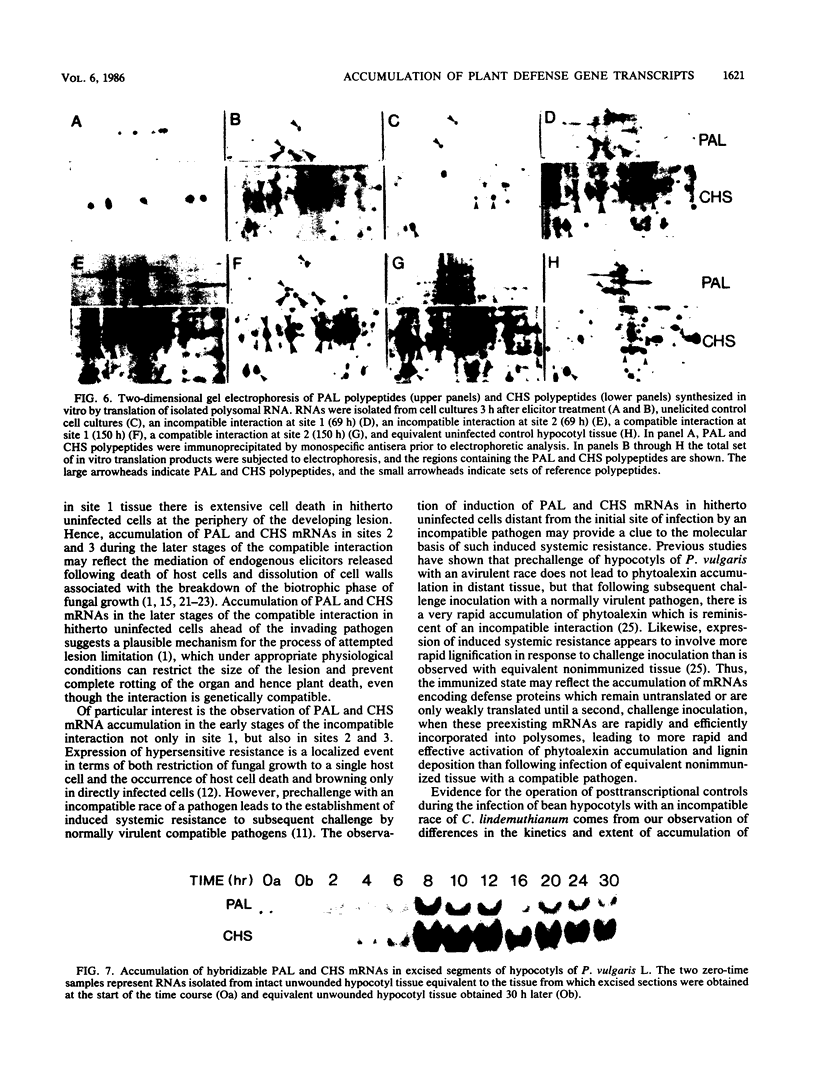
Phenylalanine ammonia-lyase and chalcone synthase catalyze the first reaction of phenylpropanoid biosynthesis and the first reaction of a branch pathway specific for flavonoid-isoflavonoid biosynthesis, respectively. These enzymes are key control elements in the synthesis of kievitone, phaseollin, and related isoflavonoid-derived phytoalexins. RNA blot hybridization with 32P-labeled cDNA sequences was used to demonstrate marked accumulation of phenylalanine ammonia-lyase and chalcone synthase mRNAs in excision-wounded hypocotyls of Phaseolus vulgaris L. (dwarf French bean) and during race-cultivar-specific interactions between hypocotyls of P. vulgaris and the partially biotrophic fungus Colletotrichum lindemuthianum, the causal agent of anthracnose. In an incompatible interaction (host resistant), early concomitant accumulation of phenylalanine ammonia-lyase and chalcone synthase mRNAs, localized mainly but not entirely in tissue adjacent to the site of infection, was observed prior to the onset of phytoalexin accumulation and expression of localized, hypersensitive resistance. In contrast, in a compatible interaction (host susceptible) there was no early accumulation of these transcripts; instead, there was a delayed widespread response associated with phytoalexin accumulation during attempted lesion limitation. Two-dimensional gel electrophoresis of [35S]methionine-labeled polypeptides synthesized in vitro by translation of isolated polysomal RNA demonstrated stimulation of the synthesis of characteristic sets of phenylalanine ammonia-lyase and chalcone synthase isopolypeptides in directly infected tissue and distant, hitherto uninfected tissue in both compatible and incompatible interactions. Our data show that specific accumulation of plant defense gene transcripts is a key early component in the sequence of events leading to expression of defense responses in wounded tissue and in infected tissue during race-cultivar-specific interactions and that an elicitation signal is transmitted intercellularly in response to infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. N., Dixon R. A., Bailey J. A., Rowell P. M., Lamb C. J. Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and incompatible plant-pathogen interactions. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3384–3388. doi: 10.1073/pnas.81.11.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Bell J. N., Cramer C. L., Schuch W., Lamb C. J., Dixon R. A. L-Phenylalanine ammonia-lyase from Phaseolus vulgaris. Characterisation and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem. 1985 Jun 3;149(2):411–419. doi: 10.1111/j.1432-1033.1985.tb08941.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cramer C. L., Bell J. N., Ryder T. B., Bailey J. A., Schuch W., Bolwell G. P., Robbins M. P., Dixon R. A., Lamb C. J. Co-ordinated synthesis of phytoalexin biosynthetic enzymes in biologically-stressed cells of bean (Phaseolus vulgaris L.). EMBO J. 1985 Feb;4(2):285–289. doi: 10.1002/j.1460-2075.1985.tb03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lamb C. J. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 1983;55:1–136. doi: 10.1002/9780470123010.ch1. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lawton M. A., Lamb C. J. Phytoalexin Induction in French Bean : Intercellular Transmission of Elicitation in Cell Suspension Cultures and Hypocotyl Sections of Phaseolus vulgaris. Plant Physiol. 1983 Feb;71(2):251–256. doi: 10.1104/pp.71.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Haffner M. H., Chin M. B., Lane B. G. Wheat embryo ribonucleates. XII. Formal characterization of terminal and penultimate nucleoside residues at the 5'-ends of "capped" RNA from imbibing wheat embryos. Can J Biochem. 1978 Jul;56(7):729–733. doi: 10.1139/o78-109. [DOI] [PubMed] [Google Scholar]
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. J. Elicitor induction of mRNA activity. Rapid effects of elicitor on phenylalanine ammonia-lyase and chalcone synthase mRNA activities in bean cells. Eur J Biochem. 1983 Jan 17;130(1):131–139. [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. Rapid induction of the synthesis of phenylalanine ammonia-lyase and of chalcone synthase in elicitor-treated plant cells. Eur J Biochem. 1983 Jan 1;129(3):593–601. doi: 10.1111/j.1432-1033.1983.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Lamb C. J. Elicitor modulation of the turnover of L-phenylalanine ammonia-lyase in French bean cell suspension cultures. Biochim Biophys Acta. 1980 Dec 1;633(2):162–175. doi: 10.1016/0304-4165(80)90402-x. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rapid switching of plant gene expression induced by fungal elicitor. Science. 1985 Mar 8;227(4691):1240–1243. doi: 10.1126/science.227.4691.1240. [DOI] [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Betz B., Hahlbrock K. Light-induced enzyme synthesis in cell suspension cultures of Petroselinum hortense. Demonstration in a heterologous cell-free system of rapid changes in the rate of phenylalanine ammonia-lyase synthesis. Eur J Biochem. 1976 Aug 16;67(2):527–541. doi: 10.1111/j.1432-1033.1976.tb10719.x. [DOI] [PubMed] [Google Scholar]
- Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- Shields S. E., Wingate V. P., Lamb C. J. Dual control of phenylalanine ammonia-lyase production and removal by its product cinnamic acid. Eur J Biochem. 1982 Apr 1;123(2):389–395. doi: 10.1111/j.1432-1033.1982.tb19781.x. [DOI] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]