Abstract
Objective. To determine the effects of the moxa smoke on human heart rate (HR) and heart rate variability (HRV). Methods. Fifty-five healthy young adults were randomly divided into experimental (n = 28) and control (n = 27) groups. Experimental subjects were exposed to moxa smoke (2.5 ± 0.5 mg/m3) twice for 25 minutes in one week. ECG monitoring was performed before, during, and after exposure. Control subjects were exposed to normal indoor air in a similar environment and similarly monitored. Followup was performed the following week. Short-term (5 min) HRV parameters were analyzed with HRV analysis software. SPSS software was used for statistical analysis. Results. During and after the first exposure, comparison of percentage changes or changes in all parameters between groups showed no significant differences. During the second exposure, percentage decrease in HR, percentage increases in lnTP, lnHF, lnLF, and RMSSD, and increase in PNN50 were significantly greater in the experimental group than in control. Conclusion. No significant adverse HRV effects were associated with this clinically routine 25-minute exposure to moxa smoke, and the data suggests that short-term exposure to moxa smoke might have positive regulating effects on human autonomic function. Further studies are warranted to confirm these findings.
1. Introduction
Moxibustion, one of the classical therapies of Traditional Chinese Medicine (TCM), uses the heat generated by burning moxa floss (usually made by Folium Artemisiae argyi) to stimulate acupuncture points [1]. It is used widely in acupuncture clinics in China and other Asian countries to treat various diseases, especially chronic conditions such as osteoarthritis, asthma, gastrointestinal disorders, and insomnia [2–5]. Smoke is an unavoidable aspect of the therapy. The aim of this study was to evaluate the effects of moxa smoke exposure on human heart rate variability (HRV) parameters.
A moxibustion session typically lasts 20–30 minutes, and patients are often treated several times a week for several weeks. Patients are exposed to the smoke during treatment, while acupuncturists are commonly exposed for prolonged periods during clinical practice. Because of recent concerns as to the safety of the therapy, specifically the potential toxicity of the smoke, many clinics no longer use moxibustion, thus depriving patients of the benefits of this unique treatment. Evaluation of the safety and the effects of moxa smoke is imperative.
Concerns about moxa smoke are similar to those regarding tobacco smoke and air pollutants. Many studies show that exposure to tobacco smoke and air pollutants is positively associated with adverse effects in the respiratory, immune, nervous, and cardiovascular systems [6–11]. Active and passive exposures to tobacco smoke have been found to increase sympathetic nervous system activity and reduce parasympathetic nervous system modulation and HRV [12–14]. Particulate air pollutants affect heart rate (HR), blood pressure, vascular tone, blood coagulation, the progression of atherosclerosis [15], and HRV [16–19]. To our knowledge, the influence of moxa smoke on HRV in the human body has not been sufficiently investigated.
HRV refers to the time variation coefficient between successive heart beat cycles. It is one of the most promising quantitative markers of autonomic nerve system activity [20]. There is a growing recognition of the role of HRV abnormalities in cardiovascular disease [21–23], and HRV decrease is a strong predictor of mortality [24]. HRV measurement in time and frequency domains is a convenient, noninvasive tool for autonomic nervous physiology evaluation, and short-term (5-minute) recording gives reliable measurements [25]. The purpose of this study was to determine whether exposure to moxa smoke influences HR and HRV in healthy subjects.
2. Methods
2.1. Subjects
Participants, most of them students of Beijing University of Chinese Medicine or other nearby universities plus some residents of the area around the University, were recruited between March 2012 and July 2012. The study protocol was approved by the Human Medical Ethics Committee of Beijing University of Chinese Medicine and was registered in the Chinese Clinical Trial Registry (ChiCTR-TRC-12002445). Written informed consent was secured from all participants.
Inclusion criteria required that subjects be normal and healthy according to the American Society of Anesthesiologists Physical Status Classification System, that is, that they have no organic, physiologic, biochemical, or psychiatric disorders, smoke <5 cigarettes per day [26], and are between the ages of 18 and 50.
Individuals were excluded if they (1) had a history of addiction to alcohol or drugs, (2) had had contact with moxa smoke within one month of the test, (3) had used medications within two weeks of the test, (4) had had a cold or other illnesses within one week of the test, (5) had ingested food or drink containing caffeine or alcohol, smoked, or done strenuous exercise within four hours of the test, and (6) were pregnant or lactating.
Participants were instructed to refrain from tobacco, alcohol, medications, and strenuous exercise and to avoid contacting moxa smoke or any other abnormal gas during the two-week test.
2.2. Study Protocol
2.2.1. Equipment and Setting
The trial was performed at the Beijing University of Chinese Medicine in two adjacent, bright, quiet, and similarly laid-out rooms equipped with beds. Ambient temperature and humidity were kept between 24°C∼26°C and 40%∼50% and monitored by a meteorological parameter recorder (Kestrel NK3000, USA).
Room 1 had normal indoor air. In Room 2, moxa smoke was generated by burning moxa sticks (three-year-old pure moxa, 1.8 cm × 20 cm, Nanyang Hanyi Moxa Co., Ltd., China). A digital dust indicator (P5L2C, Binta Green Technology Co., Ltd., Beijing, China) that detects particulate matter <10 μm in diameter (PM10) levels and a volatile organic compound (VOC) detector (model no. PGM-7320, kit MiniRAE3000, Rae Systems, Inc., USA) were set beside the participant to monitor the air. In the moxa room, PM10 and total VOC levels were kept between 2.5 ± 0.5 mg/m3 and 4.2 ± 1.3 mg/m3, respectively, which accord with average moxa smoke levels in acupuncture clinics [27]. In Room 1, PM10 and total VOC levels detected in this trial were lower than 0.01 and 0.2 mg/m3, respectively.
This was a two-arm, open, and randomized study (N = 55). After reading and signing the consent form, the participant was assigned to the experiment or control group by computer-generated random allocation. Group assignments were performed by a statistician blinded to the study design. Each assignment was sealed in an opaque envelope that was opened for the respective participant by the investigator prior to treatment.
2.2.2. Experimental Group
Testing consisted of three phases, one immediately after the other. In phase 1, subjects entered Room 1 and were encouraged to relax in a supine position. After 5–10 minutes of rest, ECG monitoring was performed for 5 minutes. In phase 2, they entered Room 2. After a 5-minute rest in a supine position, ECG monitoring was performed for 20 minutes. In phase 3, they returned to Room 1 for another 5-minute ECG recording (see Figure 1). After each ECG, subjects recorded their subjective sensations and emotions on a questionnaire. These questions were about whether they have experienced drowsiness, shortness of breath, cough, choking, irritation in nose, pharynx, and eyes, body temperature changes, or any other discomfort that might be associated with moxa smoking exposure.
Figure 1.

Experimental procedures for tests 1 and 2.
2.2.3. Control Group
Control subjects were similarly monitored but remained in Room 1 during phase 2 (see Figure 1).
The test was performed on each subject twice in a single week to accord with routine clinic practice. One week later, subjects in both groups returned for another 5-minute ECG (see Figure 2).
Figure 2.
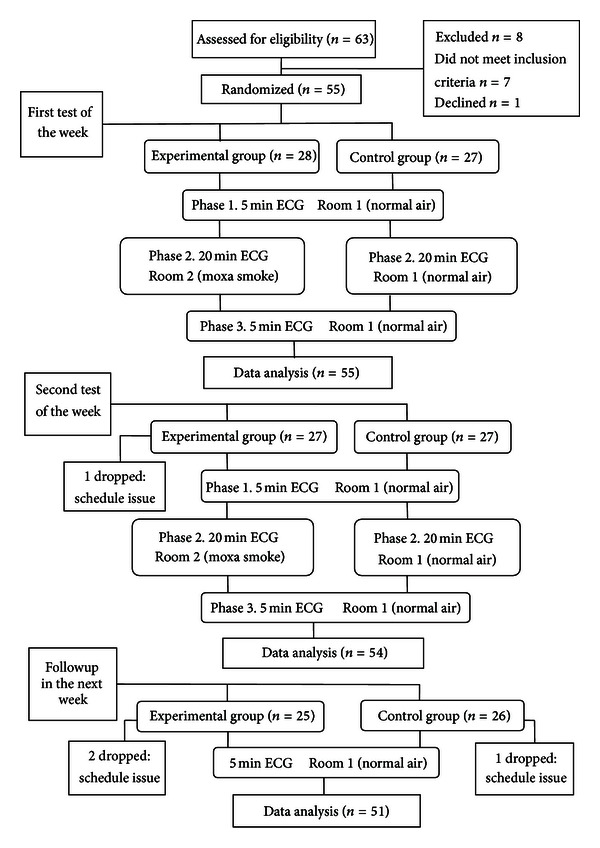
Flow of participants through the whole trial.
2.3. ECG Monitoring and Short-Term (5 min) HRV Data Analysis
With the subjects supine, three ECG electrodes were placed on their right subclavian and double costal arch regions. A data acquisition instrument (DATAQ Instrument Inc., MODEL:DI-720-USB, USA) was connected to the electrodes and a computer. To allow the heart beat to become steady, ECG recording was started 5–10 minutes after they lay down.
ECGs were analyzed by a specialist blinded to group assignment. After removal of extraneous noise, normal-to-normal beat intervals were analyzed for time- and frequency-domain parameters in 5-minute epochs using standard algorithms and HRV analysis software (Catholic University of Leuven). Time-domain analysis estimates the variation of differences between successive RR intervals through statistically developed indices. Frequency-domain analysis estimates respiratory-dependent, high- and low-frequency power through spectral analysis. Widely used HRV parameters [25, 28, 29] were employed in this study (Table 1).
Table 1.
HRV parameters used in this trial.
| Variable | Units | Description |
|---|---|---|
| Time-domain parameters |
||
|
| ||
| SDNN | msec | the standard deviation of all NN intervals, an estimate of overall variability |
| rMSSD | msec | the square root of the mean of the squared differences between adjacent NN intervals, an estimate of the short-term components of variability |
| pNN50 | % | the proportion derived by dividing NN50 (the number of interval differences of successive normal-to-normal intervals greater than 50 ms) count by the total number of normal-to-normal intervals |
|
| ||
| Frequency- domain parameters |
||
|
| ||
| TP | msec2 | total power, frequency range <0.4 Hz |
| HF | msec2 | power in the high-frequency range (0.15–0.4 Hz), considered to be mediated mainly by vagal activity |
| LF | msec2 | power in the low-frequency range (0.04–0.15 Hz), suggested to be mediated by both sympathetic and parasympathetic activities |
| LF/HF | Ratio | an indicator of the balance of the sympathetic and parasympathetic systems |
2.4. Statistical Analysis
SPSS17.0 statistical software was used for data analysis. The paired t-test was used to compare HR data of different phases in the same group; the independent sample t-test was used to compare data from different groups. A related sample nonparametric test (Wilcoxon) was used to compare all HRV parameter data of different phases in the same group, and an independent sample nonparametric test (Mann-Whitney U Test) was used to compare data from different groups.
TP, HF, and LF data were transformed into natural logarithms (ln) for better analysis. The four-segment data of phase 2 were calculated into one mean value for comparison with data from the other two phases. Percentage changes ((mean value in phase 2/value in phase 3 − value in phase 1)/value in phase 1 × 100%) or changes (mean value in phase 2/value in phase 3 − value in phase 1) of all data were used for comparisons between the groups.
3. Results
3.1. Baseline Characteristic of Study Subjects
There were no statistically significant baseline differences between the groups (Table 2).
Table 2.
Baseline characteristics of study subjects.
| Variables | Experimental group (N = 28) | Control group (N = 27) | |
|---|---|---|---|
| Gendera | Male/female | 12/16 | 12/15 |
| Age b | Years | 24.5 ± 2.5 | 25 ± 2.3 |
| BMIb | kg/m2 | 21.2 ± 2.9 | 21.1 ± 2.3 |
| Ethnic groupa | Han/others | 27/1 | 26/1 |
| Nationalitya | China/Singapore | 26/2 | 26/1 |
| Smoking historya | Yes/no | 0/28 | 0/27 |
| Regular exercisea | Yes/no | 11/17 | 7/20 |
| Emotional conditiona | Good/ok/bad | 11/17/0 | 13/14/0 |
| Mean HRb | bpm | 67.57 ± 8.95 | 70.02 ± 7.31 |
| SDNNc | ms | 40.43 (12.89) | 43.02 (26.26) |
| RMSSDc | ms | 35.08 (24.80) | 34.02 (13.8) |
| PNN50c | % | 14.93 (32.12) | 14.02 (17.21) |
| lnTPc | 7.17 (0.77) | 7.17 (1.18) | |
| lnHFc | 6.31 (1.21) | 6.02 (1.36) | |
| lnLFc | 5.77 (0.8) | 5.99 (0.96) | |
| LF/HFc | 0.76 (0.82) | 0.83 (0.72) |
bMean ± standard deviation; cmedian (interquartile range). No significant differences were found between the groups based on aChi-square test, bindependent two-sample t-test, or cMann-Whitney U Test.
3.2. Comparisons: First Test
In phases 2 and 3, during and after exposure, HR (P < 0.05) was significantly reduced, and SDNN (P < 0.05), RMSSD (P < 0.05), PNN50 (P < 0.05), lnTP (P < 0.05), lnLF (P < 0.05), and lnHF (P < 0.05) were significantly increased in both groups (Figures 3(a)–3(g)). No significant change was found in LF/HF in either group (Figure 3(h)).
Figure 3.

Changes in all indicators in the first test of the week. Values are expressed as (a) mean and (b)–(h) median. * indicates a significant difference (P < 0.05) between during/after periods and before periods in the same group using (a) paired t-test and (b)–(h) Wilcoxon test.
Comparison of the percentage changes/changes in HR and HRV parameters showed no significant differences between groups (Table 3).
Table 3.
Comparison of the percentage changes/changes in HR and HRV parameters between the groups in the first test of the week.
| Experimental group | Control group | P value | |
|---|---|---|---|
| HR1,a | |||
| Before-during | −0.04 ± 0.03 | −0.04 ± 0.04 | 0.75 |
| Before-after | −0.06 ± 0.05 | −0.06 ± 0.04 | 0.92 |
| SDNN1,b | |||
| Before-during | 0.19 (0.35) | 0.20 (0.29) | 0.74 |
| Before-after | 0.22 (0.42) | 0.14 (0.33) | 0.62 |
| RMSSD1,b | |||
| Before-during | 0.18 (0.38) | 0.09 (0.35) | 0.78 |
| Before-after | 0.18 (0.36) | 0.15 (0.42) | 0.89 |
| PNN502,b | |||
| Before-during | 4.03 (10.34) | 4.88 (11.44) | 0.56 |
| Before-after | 4.32 (8.46) | 5.26 (15.3) | 0.72 |
| lnTP1,b | |||
| Before-during | 0.03 (0.09) | 0.04 (0.09) | 0.58 |
| Before-after | 0.05 (0.08) | 0.04 (0.07) | 0.60 |
| lnHF1,b | |||
| Before-during | 0.05 (0.1) | 0.04 (0.11) | 0.46 |
| Before-after | 0.05 (0.09) | 0.04 (0.11) | 0.45 |
| lnLF1,b | |||
| Before-during | 0.03 (0.15) | 0.04 (0.09) | 0.57 |
| Before-after | 0.03 (0.17) | 0.04 (0.18) | 0.82 |
| LF/HF2,b | |||
| Before-during | 0.11 (0.51) | 0.09 (0.34) | 0.89 |
| Before-after | −0.03 (0.64) | −0.15 (0.85) | 0.79 |
Number 1 indicates percentage changes; number 2 indicates data changes. Values are expressed as amean ± standard deviation and bmedian (interquartile range). No significant differences were found between the groups using aindependent two-sample t-test and bMann-Whitney U test.
3.3. Comparisons: Second Test
In phases 2 and 3, during and after exposure, each group had significant reductions in HR (P < 0.05) and significant increases in all HRV parameters (P < 0.05; Figures 4(a)–4(h)) except for LF/HF ratio in phase 3 of the experimental group (Figure 4(h)).
Figure 4.

Changes in all indicators in the second test of the week. Values are expressed as (a) mean and (b)–(h) median. *indicates a significant difference (P < 0.05) between the during/after phases and phase 1 in each group using (a) paired t-test and (b)–(h) Wilcoxon test.
In phase 2, the experimental group's percentage decrease in HR (P < 0.001), increases in RMSSD (P < 0.001), lnTP (P < 0.001), lnHF (P < 0.001), and lnLF (P < 0.001), and increase in PNN50 (P = 0.02) were significantly greater than those in control. In phase 3, LF/HF ratio increase was significantly lower in the experimental group than in control (P = 0.005; see Table 4).
Table 4.
Test 2: percentage changes/changes in HR and HRV parameters.
| Experimental group | Control group | P value | |
|---|---|---|---|
| HR1,a | |||
| Before-during | −0.23 ± 0.02* | −0.03 ± 0.09 | <0.001 |
| Before-after | −0.06 ± 0.04 | −0.06 ± 0.09 | 0.63 |
| SDNN1,b | |||
| Before-during | 0.21 (0.32) | 0.15 (0.41) | 0.76 |
| Before-after | 0.16 (0.36) | 0.28 (0.57) | 0.56 |
| RMSSD1,b | |||
| Before-during | 0.42 (0.29)* | 0.19 (0.30) | <0.001 |
| Before-after | 0.12 (0.37) | 0.24 (0.57) | 0.47 |
| PNN502,b | |||
| Before-during | 9.78 (17.32)△ | 5.02 (7.81) | 0.02 |
| Before-after | 4.26 (13.59) | 10.59 (17.26) | 0.34 |
| lnTP1,b | |||
| Before-during | 0.3 (0.09)* | 0.05 (0.08) | <0.001 |
| Before-after | 0.04 (0.09) | 0.09 (0.13) | 0.18 |
| lnHF1,b | |||
| Before-during | 0.29 (0.07)* | 0.03 (0.09) | <0.001 |
| Before-after | 0.06 (0.1) | 0.06 (0.19) | 0.71 |
| lnLF1,b | |||
| Before-during | 0.31 (0.21)* | 0.07 (0.15) | <0.001 |
| Before-after | 0.10 (0.19) | 0.15 (0.23) | 0.15 |
| LF/HF2,b | |||
| Before-during | 0.18 (0.34) | 0.32 (0.84) | 0.30 |
| Before-after | 0.13 (0.69)△ | 0.44 (1.19) | 0.005 |
Number 1 indicates percentage changes; number 2 indicates data changes. Values are expressed as amean ± standard deviation and bmedian (interquartile range). The symbols * and △ indicate significant differences (P < 0.001 and P < 0.05, resp.) between the groups using aindependent two-sample t-Test and bMann-Whitney U Test.
3.4. Comparisons: Follow-Up Test
Mean HR (P = 0.039) in the experimental group was significantly lower thanthatin control; other indicators were not significantly different in the two groups (Table 5).
Table 5.
HR and HRV parameters, follow-up test.
| HRa | SDNNb | RMSSDb | PNN50b | lnTPb | lnHFb | lnLFb | LF/HFb | |
|---|---|---|---|---|---|---|---|---|
| Control group | 72.92 ± 7.83 | 44.07 (22.26) | 34.01 (21.12) | 12.37 (21.9) | 7.22 (1.04) | 5.94 (1.62) | 5.83 (1.04) | 0.93 (1.17) |
| Experimental group | 67.68 ± 9.74* | 52.72 (26.91) | 46.16 (38.15) | 29.75 (45.16) | 7.56 (1.07) | 6.73 (1.54) | 6.19 (1.35) | 1.01 (1.45) |
Values are expressed as amean ± standard deviation and bmedian (interquartile range). The symbol *indicates a significant difference (P < 0.05) between the two groups according to the independent two- sample t-test.
3.5. Participants' Sensations
During moxa smoke exposure, seventeen experimental group subjects felt sleepy and relaxed. One felt refreshed; stomach and bowel movement improved in another. Ten complained of choking and irritation in nose, pharynx, and eyes. One had difficulty in breathing. Eight had no unusual sensations.
In the control group, two subjects felt sleepy; one had neck discomfort; one had numbness in the hand. Twenty-three felt nothing unusual.
4. Discussion
No harmful HR and HRV effects were observed during exposure to clinical levels of moxa smoke. Evidence for this is that there were no differences in HR and HRV, either immediately (after 10 minutes) or at followup a week after exposure, between the experimental group exposed to moxa smoke and the control group without such exposure. These results might explain why reports of adverse reactions associated with smoke produced in this ancient therapy are so rare.
In contrast to retrospective studies based on clinical observation, our present study was a well-controlled, randomized, and prospective study to examine possible adverse effects of moxa smoke. The study is unique in moxa smoke concentration, length of exposure to the smoke, and its carefully controlled and monitored experimental environment, all of which mimic actual clinical moxibustion practice. The sample size is comparable to those reported in similar studies on exposure to other types of potentially hazardous smoke [12–14, 30].
The HRV effects that we observed in this moxa smoke study contrast with findings of air pollution and tobacco smoke studies, which show harmful effects on human health [12–19]. This difference might be the result of the unique constituents of moxa smoke. Moxa floss (burning material of moxibustion) is made from the mugwort leaf (Folium Artemisiae argyi), and its smoke contains multiple essential oils, suspended particulate matters, and products of chemical oxidation [31]. Wheeler et al. [32] tested the chemical products of moxibustion in clinically common dosages and found that neither carbon monoxide nor volatile compounds that present safety hazards are produced under clinical conditions. Air pollution studies show that the suspended particles in polluted air can reduce HRV by affecting the neurological system and consequently affecting the cardiovascular system by increasing HR and blood coagulation and decreasing hemoglobin to cause oxidative stress [33–35]. The respirable particles in moxa smoke mainly consist of unknown, ultrafine particles [27], which might be one reason why we observed no adverse HRV effects from clinical levels of the smoke. However, further investigation is needed to confirm and refine our finding.
Interestingly, in the second test we observed positive HR and HRV parameter changes in the experimental group compared to control. These include decrease in mean HR and increases in both time-domain analysis HRV (RMSSD, PNN50) and frequency-domain (TP, HF, and LF) during the 25-minute moxa smoke exposure (Table 4). HRV has been widely applied as a marker of autonomic nervous activity. Tension of the autonomic nervous system is maintained by opposing actions of the sympathetic and parasympathetic systems. RMSSD, PNN50, and HF are primarily thought to reflect parasympathetic influences. LF has been shown to reflect both sympathetic and parasympathetic influences. The LF/HF ratio is widely used as a relative marker of sympathetic nervous activities or sympathovagal balance [25, 36]. The HRV changes found in this study appear to be linked to the restorative functions of the autonomic system. These include an increase in total variability shown by increased TP and an increase in parasympathetic nervous activity shown by increased RMSSD, PNN50, and HF. LF/HF increase after moxa smoke exposure was significantly lower in experimental subjects than in control, which may indicate that moxa smoke drives autonomic nervous activity toward a balanced state. These findings are consistent with those of our previous pilot study in which 24 healthy volunteers exposed to moxa smoke had significant reduction in HR and increase in total HRV during and after 20 minutes of exposure to moxa smoke [37]. This suggests that moxa smoke has a regulating effect on human autonomic system function and that moxa smoke inhalation might have short-term stress-alleviating effects.
Moxa smoke effects and mechanisms have not been well investigated. We speculate that the effects are similar to those of aromatherapy, as a number of studies [38–42] show that inhalation of certain aromas can induce HRV increase and HR reduction, indicating beneficial autonomic nervous system regulation. Mechanisms of these effects might be pharmacological and/or psychological [43]. The pharmacological hypothesis is that the odor directly interacts with and affects the autonomic nervous system/central nervous system and/or endocrine systems. On the one hand, the pharmacological compound might enter the bloodstream by way of nasal or lung mucosa; on the other, the odor might stimulate the olfactory nerves and the limbic system of the brain. In the clinic, moxibustion is often used to treat insomnia, anxiety, and depression [4, 5]. However, it is unclear whether the treatment effects are induced by heat at the acupuncture point or by the moxa smoke. The present study provides some information for distinguishing the respective roles of heat and smoke. Further studies to elucidate the mechanisms of moxibustion are warranted.
We are aware of the limitations of the present study. Our data only show HRV effects from short-term exposure to moxa smoke; in normal acupuncture practice, patients usually receive multiple moxibustion treatments, and practitioners are usually exposed to the smoke for years. These factors warrant a long-term observational study. Furthermore, because the participants in our study were not blinded, we cannot rule out the possibility of placebo effect. Additionally, our subjects were young and healthy; these results might not reflect how moxa smoke affects the elderly or chronically ill.
Nevertheless, this study is an important step toward understanding the effects of moxa smoke. Our results provide useful information on the feasibility of a future, larger trial and will make it possible to calculate adequate sample sizes for such research.
In conclusion, our data show that short-term moxa smoke exposure at clinical concentrations poses no hazards to patients' HR and HRV and suggest that moxa smoke has a positive regulating effect on human autonomic function. Future studies are needed to further investigate the effects and the safety of moxa smoke.
Conflict of Interests
The authors have no conflict of interests.
Acknowledgments
The research was supported by the National 973 Project (2009CB522906), National Natural Science Fund Project (no. 81072862), and Beijing University of Chinese Medicine Graduate Student Fund Project (JYBZZ-XS043). The authors wish to thank Dr. Lyn Lowry for her editorial assistance.
References
- 1.Organization WHO. WHO international standard terminologies on traditional medicine in the western pacific region. World Health Organization Publications, Genova, Switzerland, 2007.
- 2.Ouyang B, Gao J, Sun G, Pu M, Wu Y, Fan C. Impact of acupoint heat-sensitive moxibustion on lung function and life quality of patients with chronic persistent bronchial asthma: a randomized controlled study. Chinese Acupuncture & Moxibustion. 2011;31(11, article 965) [PubMed] [Google Scholar]
- 3.Son HM, Kim DH, Kim E, Jung SY, Kim AR, Kim TH. A qualitative study of the experiences of patients with knee osteoarthritis undergoing moxibustion. Acupuncture in Medicine. 2013;31(1):39–44. doi: 10.1136/acupmed-2012-010224. [DOI] [PubMed] [Google Scholar]
- 4.Ju YL, Chi X, Liu JX. Forty cases of insomnia treated by suspended moxibustion at Baihui (GV 20) Journal of Traditional Chinese Medicine. 2009;29(2):95–96. doi: 10.1016/s0254-6272(09)60040-6. [DOI] [PubMed] [Google Scholar]
- 5.Lü P. Moxibustion yintang (EX-HN 3) acupoint for insomnia of sleep difficulty. Chinese Acupuncture & Moxibustion. 2011;31(9, article 786) [PubMed] [Google Scholar]
- 6.Stayner L, Bena J, Sasco AJ, et al. Lung cancer risk and workplace exposure to environmental tobacco smoke. American Journal of Public Health. 2007;97(3):545–551. doi: 10.2105/AJPH.2004.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall GH. Effects of nicotine and tobacco smoke on the electrical activity of the cerebral cortex and olfactory bulb. British Journal of Pharmacology. 1970;38(2):271–286. doi: 10.1111/j.1476-5381.1970.tb08516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma: a paradigm for the role of oxidative stress in PM-induced adverse health effects. Clinical Immunology. 2003;109(3):250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Pope CA, III, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 10.Sopori M. Effects of cigarette smoke on the immune system. Nature Reviews Immunology. 2002;2(5):372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 11.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Journal of the American Medical Association. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope CA, Eatough DJ, Gold DR, et al. Acute exposure to environmental tobacco smoke and heart rate variability. Environmental Health Perspectives. 2001;109(7):711–716. doi: 10.1289/ehp.01109711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi F, Watanabe T, Akamatsu Y, et al. Acute effects of cigarrete smoking on the heart rate variability of taxi drivers during work. Scandinavian Journal of Work, Environment and Health. 2005;31(5):360–366. doi: 10.5271/sjweh.919. [DOI] [PubMed] [Google Scholar]
- 14.Karakaya O, Barutcu I, Kaya D, et al. Acute effect of cigarette smoking on heart rate variability. Angiology. 2007;58(5):620–624. doi: 10.1177/0003319706294555. [DOI] [PubMed] [Google Scholar]
- 15.Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury. Epidemiology, toxicology, and mechanisms. Journal of the American College of Cardiology. 2008;52(9):719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. European Respiratory Journal. 2003;21(40, supplement):76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- 17.Pope CA, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environmental Health Perspectives. 2004;112(3):339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold DR, Litonjua A, Schwartz J, et al. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 19.Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007;18(1):95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- 20.Dreifus LS, Agarwal JB, Botvinick EH, et al. Heart rate variability for risk stratification of life-threatening arrhythmias. Journal of the American College of Cardiology. 1993;22(3):948–950. doi: 10.1016/0735-1097(93)90217-o. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 22.Camm AJ, Pratt CM, Schwartz PJ, et al. Mortality in patients after a recent myocardial infarction. Circulation. 2004;109(8):990–996. doi: 10.1161/01.CIR.0000117090.01718.2A. [DOI] [PubMed] [Google Scholar]
- 23.Bilchick KC, Fetics B, Djoukeng R, et al. Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs’ Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure) American Journal of Cardiology. 2002;90(1):24–28. doi: 10.1016/s0002-9149(02)02380-9. [DOI] [PubMed] [Google Scholar]
- 24.La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cadiac death in chronic heart failure patients. Circulation. 2003;107(4):565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 25.Rawenwaaij-Arts C, Kallee L, Hopman J, Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability. Standards of measurement, physiologic interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 26.Ringdal KG, Skaga NO, Steen PA, Hestnes M, Laake P, Jones JM. Classification of comorbidity in trauma: The reliability of pre-injury ASA physical status classification. Injury. 2012;44(1):29–35. doi: 10.1016/j.injury.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Zhao B, Liu P, Shao L. Mass concentration and morphological characteristics of PM10 in moxibustion rooms in Beijing and Tianjin during summer. Chinese Journal of Traditional Chinese Medicine. 2012;(12):3104–3108. [Google Scholar]
- 28.Akselrod S, Gordon D, Ubel FA. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 29.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. The American Journal of Physiology. 1985;248(1):H151–153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 30.Lykke F, Peter M, Ingunn SR, Jakob B, Andreas M, Torben S. Controlled human wood smoke exposure:oxidative stress, inflammation and microvascular function. Particle and Fibre Toxicology. 2012;9, article 7 doi: 10.1186/1743-8977-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng H, Wei D, Huang B, Xin H, Qin L. Advances in ethnobotanic and modern pharmaceutic studies on Folium Artemisiae Argyi. The Chinese Academic Medical Magazine of Organisms. 2003;2:35–39. [Google Scholar]
- 32.Wheeler J, Coppock B, Chen C. Does the burning of moxa (Artemisia vulgaris) in traditional Chinese medicine constitute a health hazard? Acupuncture in Medicine. 2009;27(1):16–20. doi: 10.1136/aim.2009.000422. [DOI] [PubMed] [Google Scholar]
- 33.Seaton A, Soutar A, Crawford V, et al. Particulate air pollution and the blood. Thorax. 1999;54(11):1027–1032. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KW, Lip GYH. Effects of lifestyle on hemostasis, fibrinolysis, and platelet reactivity: a systematic review. Archives of Internal Medicine. 2003;163(19):2368–2392. doi: 10.1001/archinte.163.19.2368. [DOI] [PubMed] [Google Scholar]
- 35.Nemmar A, Hoet PHM, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 36.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology. 2010;141(2):122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 37.Rhoden CR, Wellenius GA, Ghelfi E, Lawrence J, González-Flecha B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochimica et Biophysica Acta. 2005;1725(3):305–313. doi: 10.1016/j.bbagen.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Zhao B, Litscher G, Li J, Wang L, Cui Y, Huang C. Effects of moxa (Artemisia vulgaris) smoke inhalation on heart rate and its variability. Chinese Medicine. 2011;2(2):53–57. [Google Scholar]
- 39.Chien LW, Cheng SL, Liu CF. The effect of lavender aromatherapy on autonomic nervous system in midlife women with insomnia. Evidence-Based Complementary and Alternative Medicine. 2012;2012:8 pages. doi: 10.1155/2012/740813.740813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang KM, Shen CW. Aromatherapy benefits autonomic nervous system regulation for elementary school faculty in Taiwan. Evidence-Based Complementary and Alternative Medicine. 2011;2011:7 pages. doi: 10.1155/2011/946537.946537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehrner J, Eckersberger C, Walla P, Pötsch G, Deecke L. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiology and Behavior. 2000;71(1-2):83–86. doi: 10.1016/s0031-9384(00)00308-5. [DOI] [PubMed] [Google Scholar]
- 42.Itai T, Amayasu H, Kuribayashi M, et al. Psychological effects of aromatherapy on chronic hemodialysis patients. Psychiatry and Clinical Neurosciences. 2000;54(4):393–397. doi: 10.1046/j.1440-1819.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- 43.Herz RS. Aromatherapy facts and fictions: a scientific analysis of olfactory effects on mood, physiology and behavior. International Journal of Neuroscience. 2009;119(2):263–290. doi: 10.1080/00207450802333953. [DOI] [PubMed] [Google Scholar]


