Abstract
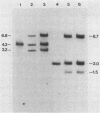
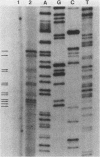
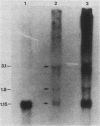
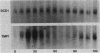
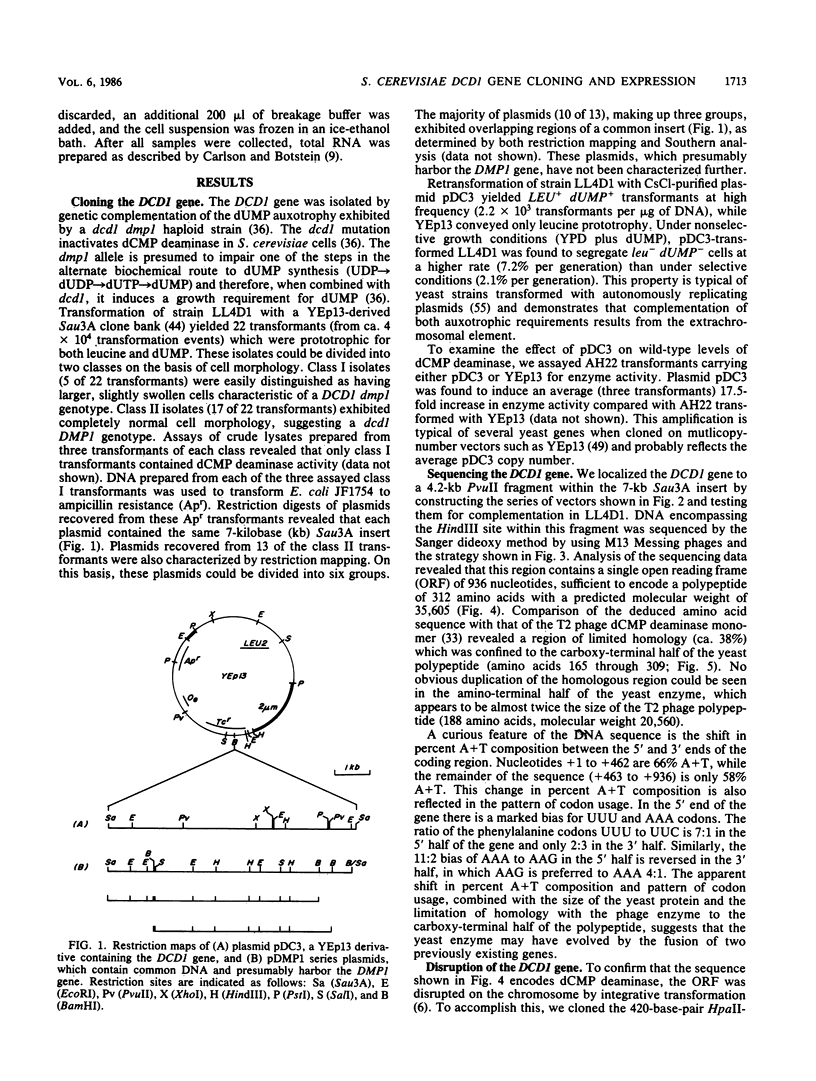
The dCMP deaminase gene (DCD1) of Saccharomyces cerevisiae has been isolated by screening a Sau3A clone bank for complementation of the dUMP auxotrophy exhibited by dcd1 dmp1 haploids. Plasmid pDC3, containing a 7-kilobase (kb) Sau3A insert, restores dCMP deaminase activity to dcd1 mutants and leads to an average 17.5-fold overproduction of the enzyme in wild-type cells. The complementing activity of the plasmid was localized to a 4.2-kb PvuII restriction fragment within the Sau3A insert. Subcloning experiments demonstrated that a single HindIII restriction site within this fragment lies within the DCD1 gene. Subsequent DNA sequence analysis revealed a 936-nucleotide open reading frame encompassing this HindIII site. Disruption of the open reading frame by integrative transformation led to a loss of enzyme activity and confirmed that this region constitutes the dCMP deaminase gene. Northern analysis indicated that the DCD1 mRNA is a 1.15-kb poly(A)+ transcript. The 5' end of the transcript was mapped by primer extension and appears to exhibit heterogeneous termini. Comparison of the amino acid sequence of the T2 bacteriophage dCMP deaminase with that deduced for the yeast enzyme revealed a limited degree of homology which extends over the entire length of the phage polypeptide (188 amino acids) but is confined to the carboxy-terminal half of the yeast protein (312 amino acids). A potential dTTP-binding site in the yeast and phage enzymes was identified by comparison of homologous regions with the amino acid sequences of a variety of other dTTP-binding enzymes. Despite the role of dCMP deaminase in dTTP biosynthesis, Northern analysis revealed that the DCD1 gene is not subject to the same cell cycle-dependent pattern of transcription recently found for the yeast thymidylate synthetase gene (TMP1).
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adzuma K., Ogawa T., Ogawa H. Primary structure of the RAD52 gene in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2735–2744. doi: 10.1128/mcb.4.12.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannwarth H., Ikehara N., Schweiger H. G. Deoxycytidine monophosphate deaminase in Acetabularia: properties and regulation in the early generative phase. Eur J Cell Biol. 1982 Jun;27(2):200–205. [PubMed] [Google Scholar]
- Beacham I. R., Schweitzer B. W., Warrick H. M., Carbon J. The nucleotide sequence of the yeast ARG4 gene. Gene. 1984 Sep;29(3):271–279. doi: 10.1016/0378-1119(84)90056-8. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Levinson B. B., Fabry M., Williams S. R., Martin D. W., Jr Cloned mouse ribonucleotide reductase subunit M1 cDNA reveals amino acid sequence homology with Escherichia coli and herpesvirus ribonucleotide reductases. J Biol Chem. 1985 Jun 10;260(11):7015–7022. [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Clare J., Farabaugh P. Nucleotide sequence of a yeast Ty element: evidence for an unusual mechanism of gene expression. Proc Natl Acad Sci U S A. 1985 May;82(9):2829–2833. doi: 10.1073/pnas.82.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins R. R., Balinsky D. Activities of some enzymes of pyrimidine and DNA synthesis in a rat transplantable hepatoma and human primary hepatomas, in cell lines derived from these tissues, and in human fetal liver. Cancer Res. 1980 Apr;40(4):1235–1239. [PubMed] [Google Scholar]
- Dobson M. J., Tuite M. F., Roberts N. A., Kingsman A. J., Kingsman S. M., Perkins R. E., Conroy S. C., Fothergill L. A. Conservation of high efficiency promoter sequences in Saccharomyces cerevisiae. Nucleic Acids Res. 1982 Apr 24;10(8):2625–2637. doi: 10.1093/nar/10.8.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duker N. J., Grant C. L. Alterations in the levels of deoxyuridine triphosphatase, uracil-DNA glycosylase and AP endonuclease during the cell cycle. Exp Cell Res. 1980 Feb;125(2):493–497. doi: 10.1016/0014-4827(80)90145-7. [DOI] [PubMed] [Google Scholar]
- Duncan B. K., Diamond G. R., Bessman M. J. Regulation of enzymatic activity through subunit interaction. A possible example. J Biol Chem. 1972 Dec 25;247(24):8136–8138. [PubMed] [Google Scholar]
- Ellims P. H., Kao A. Y., Chabner B. A. Deoxycytidylate deaminase. Purification and some properties of the enzyme isolated from human spleen. J Biol Chem. 1981 Jun 25;256(12):6335–6340. [PubMed] [Google Scholar]
- Eriksson S., Martin D. W., Jr Ribonucleotide reductase in cultured mouse lymphoma cells. Cell cycle-dependent variation in the activity of subunit protein M2. J Biol Chem. 1981 Sep 25;256(18):9436–9440. [PubMed] [Google Scholar]
- Fink K. Thymidine phosphorylation in synchronous cultures of Tetrahymena pyriformis GL. Exp Cell Res. 1980 Jun;127(2):438–441. doi: 10.1016/0014-4827(80)90449-8. [DOI] [PubMed] [Google Scholar]
- Gelbard A. S., Kim J. H., Perez A. G. Fluctuations in deoxycytidine monophosphate deaminase activity during the cell cycle in synchronous populations of HeLa cells. Biochim Biophys Acta. 1969 Jun 17;182(2):564–566. doi: 10.1016/0005-2787(69)90209-3. [DOI] [PubMed] [Google Scholar]
- Gordon C. N., Elliott S. C. Fractionation of Saccharomyces cerevisiae cell populations by centrifugal elutriation. J Bacteriol. 1977 Jan;129(1):97–100. doi: 10.1128/jb.129.1.97-100.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A. Y., Kuo C. L., Campbell J. L. The CDC8 gene of yeast encodes thymidylate kinase. J Biol Chem. 1984 Sep 10;259(17):11052–11059. [PubMed] [Google Scholar]
- Kit S., Jorgensen G. N. Formation of thymidine kinase and deoxycytidylate deaminase in synchronized cultures of chinese hamster cells temperature-sensitive for DNA synthesis. J Cell Physiol. 1976 May;88(1):57–64. doi: 10.1002/jcp.1040880108. [DOI] [PubMed] [Google Scholar]
- Krzywicki K. A., Brandriss M. C. Primary structure of the nuclear PUT2 gene involved in the mitochondrial pathway for proline utilization in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2837–2842. doi: 10.1128/mcb.4.12.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowdon M., Vitols E. Ribonucleotide reductase activity during the cell cycle of Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):177–184. doi: 10.1016/0003-9861(73)90611-5. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Widgren E. E., Broglie K. E., Nyunoya H. Yeast carbamyl phosphate synthetase. Structure of the yeast gene and homology to Escherichia coli carbamyl phosphate synthetase. J Biol Chem. 1983 Dec 10;258(23):14466–14477. [PubMed] [Google Scholar]
- MALEY F., MALEY G. F. Nucleotide interconversions. II. Elevation of deoxycytidylate deaminase and thymidylate synthetase in regenerating rat liver. J Biol Chem. 1960 Oct;235:2968–2970. [PubMed] [Google Scholar]
- MALEY G. F., MALEY F. Nucleotide interconversions in embryonic and neoplastic tissues. I. The conversion of deoxycytidylic acid to deoxyuridylic acid and thymidylic acid. J Biol Chem. 1959 Nov;234:2975–2980. [PubMed] [Google Scholar]
- Maehara Y., Nakamura H., Nakane Y., Kawai K., Okamoto M., Nagayama S., Shirasaka T., Fujii S. Activities of various enzymes of pyrimidine nucleotide and DNA syntheses in normal and neoplastic human tissues. Gan. 1982 Apr;73(2):289–298. [PubMed] [Google Scholar]
- Mahagaokar S., Orengo A., Rao P. N. The turnover of deoxyuridine triphosphate during the HeLa cell cycle. Exp Cell Res. 1980 Jan;125(1):86–94. doi: 10.1016/0014-4827(80)90192-5. [DOI] [PubMed] [Google Scholar]
- Maley F., Maley G. F. Studies on identifying the allosteric binding sites of deoxycytidylate deaminase. J Biol Chem. 1982 Oct 25;257(20):11876–11878. [PubMed] [Google Scholar]
- Maley G. F., Guarino D. U., Maley F. Complete amino acid sequence of an allosteric enzyme, T2 bacteriophage deoxycytidylate deaminase. J Biol Chem. 1983 Jul 10;258(13):8290–8297. [PubMed] [Google Scholar]
- Maley G. F., Guarino D. U., Maley F. T2r + bacteriophage-induced enzymes. I. The purification and properties of deoxycytidylate deaminase. J Biol Chem. 1972 Feb 10;247(3):931–939. [PubMed] [Google Scholar]
- McIntosh E. M., Haynes R. H. Isolation of a Saccharomyces cerevisiae mutant strain deficient in deoxycytidylate deaminase activity and partial characterization of the enzyme. J Bacteriol. 1984 May;158(2):644–649. doi: 10.1128/jb.158.2.644-649.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil J. B., Storms R. K., Friesen J. D., Smith M. Efficient expression of the Escherichia coli leuB gene in yeast. Curr Genet. 1985;9(8):653–660. doi: 10.1007/BF00449818. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison J. M., Carter B. L. Cell cycle analysis. Methods Cell Biol. 1975;11:201–219. [PubMed] [Google Scholar]
- Mittermayer C., Bosselmann R., Bremerskov V. Initiation of DNA synthesis in a system of synchronized L-cells: rhythmicity of thymidine kinase activity. Eur J Biochem. 1968 May;4(4):487–489. doi: 10.1111/j.1432-1033.1968.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Møllgaard H., Neuhard J. Deoxycytidylate deaminase from Bacillus subtilis. Purification, characterization, and physiological function. J Biol Chem. 1978 May 25;253(10):3536–3542. [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K. The structure of transposable yeast mating type loci. Cell. 1980 Mar;19(3):753–764. doi: 10.1016/s0092-8674(80)80051-1. [DOI] [PubMed] [Google Scholar]
- Navalgund L. G., Rossana C., Muench A. J., Johnson L. F. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem. 1980 Aug 10;255(15):7386–7390. [PubMed] [Google Scholar]
- Nyunoya H., Lusty C. J. Sequence of the small subunit of yeast carbamyl phosphate synthetase and identification of its catalytic domain. J Biol Chem. 1984 Aug 10;259(15):9790–9798. [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarca R., Haseltine W. A. Sequence similarity among retroviruses--erratum. Nature. 1984 Jun 21;309(5970):728–728. doi: 10.1038/309728b0. [DOI] [PubMed] [Google Scholar]
- Rine J., Hansen W., Hardeman E., Davis R. W. Targeted selection of recombinant clones through gene dosage effects. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6750–6754. doi: 10.1073/pnas.80.22.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz L. D., Friesen J. D. Nucleotide sequence of the tcml gene (ribosomal protein L3) of Saccharomyces cerevisiae. J Bacteriol. 1983 Jul;155(1):8–14. doi: 10.1128/jb.155.1.8-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. R., Schmidt R. R. Enzymic control of nucleic acid synthesis during synchronous growth of Chlorella pyrenoidosa. II. Deoxycytidine monophosphate deaminase. Arch Biochem Biophys. 1966 Jul;115(1):13–20. doi: 10.1016/s0003-9861(66)81031-7. [DOI] [PubMed] [Google Scholar]
- Storms R. K., Ord R. W., Greenwood M. T., Mirdamadi B., Chu F. K., Belfort M. Cell cycle-dependent expression of thymidylate synthase in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2858–2864. doi: 10.1128/mcb.4.12.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. R., Barclay B. J., Storms R. K., Friesen J. D., Haynes R. H. Isolation of the thymidylate synthetase gene (TMP1) by complementation in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Apr;2(4):437–442. doi: 10.1128/mcb.2.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao S. G., Brunk C. F., Pearlman R. E. Hybridization of nucleic acids directly in agarose gels. Anal Biochem. 1983 Jun;131(2):365–372. doi: 10.1016/0003-2697(83)90185-9. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]