Abstract
The purpose of this study was to evaluate the preliminary efficacy and satisfaction/acceptability of training in memory or speed of processing versus wait-list control for improving cognitive function in breast cancer survivors. 82 breast cancer survivors completed a three-group randomized, controlled trial. Primary outcomes were objective neuropsychological tests of memory and speed of processing. Secondary outcomes were perceived cognitive functioning, symptom distress (mood disturbance, anxiety, and fatigue), quality of life, and intervention satisfaction/acceptability. Data were collected at baseline, post-intervention, and 2-month follow-up. Using repeated-measures mixed-linear ANCOVA models, each intervention was compared to wait-list control while adjusting for age, education, and baseline measures. The effect sizes for differences in means and the reliable improvement percentage were reported. The results show that domain-specific effects were seen for both interventions: memory training improved memory performance at 2-month follow-up (p = 0.036, d = 0.59); speed of processing training improved processing speed post-intervention (p = 0.040, d = 0.55) and 2-month follow-up (p = 0.016; d = 0.67). Transfer effects to non-trained domains were seen for speed of processing training with improved memory post-intervention (p = 0.007, d = 0.75) and 2-month follow-up (p = 0.004, d = 0.82). Both interventions were associated with improvements in perceived cognitive functioning, symptom distress, and quality of life. Ratings of satisfaction/acceptability were high for both interventions. It was concluded that while both interventions appeared promising, speed of processing training resulted in immediate and durable improvements in objective measures of processing speed and verbal memory. Speed of processing training may have broader benefits in this clinical population.
Keywords: Memory, Speed of processing, Breast cancer survivors, Symptom distress, Quality of life
Introduction
Breast cancer survivors often report problems with their memory or feelings of mental slowness [1]. Deficits in memory and processing speed have been verified through objective neuropsychological assessments [2–5]. Although subtle, such deficits may have a significant impact on quality of life [6, 7], yet there are very few treatment options for this problem [8, 9].
Behaviorally based cognitive training interventions may be a viable treatment option but, have not been widely tested in individuals with cancer. While memory and speed of processing training have been shown to be effective in improving memory performance and processing speed in older persons without cancer (≥age 65), [10–13] research in cancer patients has been limited [8, 9]. In long-term breast cancer survivors, only one other small controlled cognitive training trial has been conducted [14] with some positive, but mixed results; compelling the need for further research [8, 9].
The purpose of this pilot study was to evaluate preliminary efficacy and satisfaction/acceptability of memory and speed of processing training in improving cognitive functioning in breast cancer survivors compared to wait-list control group. Primary outcomes were performance on objective neuropsychological tests of memory and speed of processing. Secondary outcomes were perceived cognitive function, symptom distress (mood disturbance, anxiety and fatigue), quality of life, and satisfaction/acceptability. Findings from this study will ultimately lead to a full-scale efficacy trial and our overarching goal of identifying an effective treatment for cognitive impairment in breast cancer survivors.
Patients and methods
Study design
This three-group single-blind, randomized controlled trial compared training in memory and speed of processing to wait-list control among long-term breast cancer survivors. Outcomes were assessed at baseline (prior to randomization), post-intervention, and at 2-month follow-up. The study protocol was approved by the Institutional Review Board.
Recruitment of breast cancer survivors occurred from January 1, 2009 to June 1, 2011 at a Midwestern cancer center and affiliated clinics. Participants were recruited sequentially from clinics and advertisements were mailed to research registry participants (tumor registries, Susan Love/Avon Army of Women). Eligible participants were breast cancer survivors who (1) reported concerns regarding their cognitive functioning (poor memory, feelings of mental slowness, etc.), (2) identified that cognitive concerns negatively impacted their self-esteem and/or interfered with their daily life, and (3) reported that they were interested in and seeking treatment to address their cognitive concerns. Other eligibility criteria included breast cancer survivors who were also post-menopausal, 40 years of age, and older, ≥1-year post-treatment which included chemotherapy for primary non-metastatic breast cancer, disease-free, and able to understand, speak, read, and write English.
Exclusion criteria included substantial cognitive impairment (score < 24 Mini-Mental State Examination, MMSE [15]); history of stroke, encephalitis, traumatic brain injury, brain surgery, dementia, Alzheimer’s disease, or Parkinson’s disease; history of cranial radiation therapy or intrathecal therapy; current active major depression or substance abuse or history of bipolar disorder, psychosis, schizophrenia, or learning disability; history of or current other cancer except for basal cell skin cancer; history of other cognitive training; or uncorrected vision problems (worse than 20/70).
We planned a priori to enroll 30 per group to achieve 26 per group after attrition to provide 80 % power for two-sided parametric tests to detect large (0.80) effect sizes between each intervention and control group.
Procedure and methods
Eligibility was determined via telephone review of demographic, health, and breast cancer diagnosis and treatment-related information followed by an in-person assessment of cognition (MMSE). If eligible, project staff conducted the baseline neuropsychological assessment and administered the baseline survey questionnaires.
Telephone reminders were made to all participants in advance of their follow-up assessments which occurred post-intervention and 2-month follow-up. All assessments were conducted in the same manner with repeated neuropsychological testing and questionnaires collected by a trained and blinded staff member. Participants received $25 at each data collection visit.
Randomization and interventions
Subjects were randomized using non-stratified blocks of 9. Biostatisticians provided a password protected randomization list to the non-blinded project manager who had primary responsibility for randomization. Participants were notified by telephone of group assignment and intervention dates. Each intervention included ten 1-hour training sessions done in small groups of 3–5 breast cancer survivors over 6–8 weeks and delivered by a separate trained and certified interventionists to avoid diffusion of treatments.
Memory training was adapted from the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial [16]. Memory training involved teaching participants strategies for remembering word lists, sequences, and text material by learning how to apply principles of meaningfulness, organization, visualization, and association [16]. Strategies included multiple mnemonic techniques including visual memory support, story mnemonic, and method of loci. Sessions 1–5 focused on strategy instruction and exercises to practice the strategy and Sessions 6–10 provided additional practice exercises to promote self-efficacy with regard to performance.
Speed of processing training utilized the commercially available Insight program (Posit Science®), which was originally developed as part of the ACTIVE trial and then refined overtime [16]. This program systematically reduces the stimulus duration during a series of progressively more difficult information-processing tasks presented via computer. The exercises automatically adjust to user performance to maintain an 85 % correct rate. The exercises included time-order judgment, discrimination, spatial-match, forward-span, instruction-following, and narrative-memory tasks [17].
The wait-list control group received a letter explaining that they were not selected to receive any study materials but that one of the training programs would be mailed to them at the end of their study participation. All wait-list group participants received the Insight (Posit Science®) program and written instructions after they completed participating in the study.
Outcome measures
Primary outcomes
Objective memory (immediate and delayed) was assessed by composite scores derived from equally weighted average scores from the Rey Auditory Verbal Learning Test (AVLT) a 15 item list learning task including the Sum Recall (trials 1–5), short delay, and recognition score [18] as well as the immediate recall from the Rivermead Behavioral Paragraph Recall Test [19]. Delayed memory was derived from the long-term delay score from the Rey AVLT and long-term delay score from the Rivermead Behavioral Paragraph Recall Test. As in the ACTIVE trial [10, 16], composite scores were used because they measure ability rather than performance on a specific test, are more reliable and reduce the number of outcome analyses needed, thereby reducing inflation of the overall type I error probability [16]. Alternate forms given in fixed order were used to reduce practice effects [16].
Objective speed of processing was measured with the Useful Field of View (UFOV) [20–22], a computer-administered and computer-scored test of visual attention. The assessment requires participants to identify and localize information, with 75 % accuracy, under varying levels of cognitive demand. The results from three subtests measuring divided attention and two levels of selective attention (parts 2–4) were used in combination to determine the composite speed of processing score, with lower scores indicating better speed.
Secondary outcomes
Perceived cognitive functioning was measured with the 48-item Functional Assessment of Cancer Therapy-Cognitive (FACT-Cog) [23] and 18-item Squire Subjective Memory Questionnaire (SSMQ) [24]. Higher scores on both denote better cognitive functioning. Symptom distress was measured by three separate measures including the 20-item Center for Epidemiologic Studies Depression Scale (CES-D), the 20-item Spielberger State-Trait Anxiety Inventory-State Subscale (STAI-S) [25] and the 13-item Functional Assessment of Cancer Therapy-Fatigue (FACT-F) [26]. Higher scores on the CES-D and STAI-S indicate worse symptom-specific distress, whereas higher scores on the FACT-F indicate lower symptom-specific distress. Quality of Life was measured with the 41-item Quality of Life-Cancer Survivors (QOL-CS) [27] the 66-item quality of life index-cancer version [28] and the 36-Item Short-Form Health Survey (SF-36) [29]. Higher scores on each indicated greater overall life satisfaction. Satisfaction/acceptability were assessed post-intervention (3–7 days) with the 8-item, Likert-based Client Satisfaction Questionnaire [30] and the 10-item, Likert-based Acceptability Scale [31]. Higher scores on both scales indicate more positive response.
Demographics and breast cancer disease and treatment variables were assessed to describe the sample. Self-reported disease information was verified with medical records review. There were no adverse events reported.
Statistical analysis
Group equivalence on baseline characteristics was tested using ANOVA and Chi-square tests or the Kruskal–Wallis and two-sided Fisher exact tests when assumptions were violated.
As in ACTIVE [10], neuropsychological tests were standardized by pooling scores at all time points for all subjects using the Blom (rank-based) transformation, producing more normally distributed scores [32]. Standard z scores were computed (person’s transformed score minus baseline mean divided by baseline standard deviation) at each time point.
Separate general linear mixed models were used to test memory and speed of processing treatment effects compared to wait-list control on each outcome. Models included between-subjects treatment and within-subjects time effects along with age and education (known confounding covariates) and the baseline value for the outcome variable. The treatment effect size was computed as the difference between model-based adjusted means at post-intervention or 2-month follow-up divided by the pooled baseline standard deviation.
Reliable improvement was calculated as improvement in performance on a measure by at least 1 standard error of measurement (SEM). The SEM described generally in the study of Dudek [33] was computed as the standard deviation of difference scores (from baseline to either post-intervention or 2-month follow-up) for the wait-list control group multiplied by the square root of [1 minus test–retest (baseline to immediate post-intervention) reliability] for the wait-list control group.
There was no missing neuropsychological data and less than 0.05 % of questionnaire data. For questionnaires, scale- and person-specific means were computed and substituted for missing items if at least 70 % of items were not missing. Analyses were conducted using SAS 9.3 (SAS Institute Inc, Cary, NC). The significance level was not adjusted for multiple comparisons because this was a pilot study.
Results
Participants
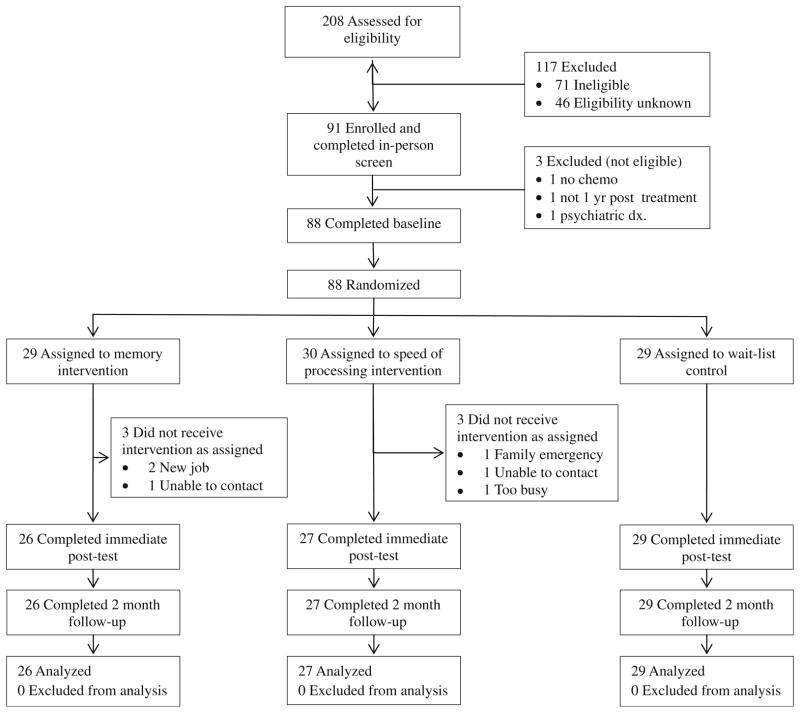
A total of 88 breast cancer survivors consented and were randomized to one of the three groups. Figure 1 shows the accrual flow and reasons for attrition. 208 women were screened for initial eligibility. A total of 91 (43.8 %) participants were eligible upon initial screen, 71 (34.1 %) were ineligible, and 46 (22.1 %) refused (either directly or passively by not responding to follow-up). The top reasons for ineligibility were: no chemotherapy (32.4 %), other cancer (15.5 %), psychiatric diagnosis (14.1 %), and metastatic breast cancer (11.3 %). A total of 91 participants consented and completed the in-person screen; with three breast cancer survivors determined to be ineligible due to no chemotherapy, not 1 year post-adjuvant therapy, or psychiatric diagnosis. Study completion rates by group were 90 % memory training, 90 % speed training, and 100 % wait-list control. Participants that dropped out of the study did so before the start of intervention and they did not differ significantly on demographic, breast cancer variables, or measures of symptom distress than those that completed the study. Because sample sizes slightly exceeded 26 per group, observed power was 81 %.
Fig 1.
Consort diagram. Participant flow diagram
The overall sample (collapsed across treatment groups) was middle aged (average 56.5 ± 8.5 years old), had early-stage breast cancer (89 % stage II or lower), and were long-term survivors (average of 5.5 years post-treatment (SD = 4.2). All subjects had received surgery (100 %) and chemotherapy (100 %) and 74 % also had radiation therapy. Nearly half the subjects (46 %) were receiving adjuvant endocrine therapy at the time of this study. There were no significant group differences at baseline in age, race, education, cancer severity, cancer treatment (including the use of tamoxifen or aromatase inhibitors), current depressive, anxiety and fatigue symptoms, and cognitive abilities (immediate and delayed memory and processing speed) (see Table 1). In addition, based on published norms of the Rey AVLT [34], clinically significant impairment (defined as 1.0 standard deviations below the norm-based test) was noted for subscales used in the immediate memory composite (the rate ranging from 13 % impaired on the Rey AVLT recognition to 20 % impaired on the Rey AVLT short delay), as well as, measures of delayed memory (23 % impaired on the Rey AVLT delayed recall). These findings are similar to our previous work which found 17 % of breast cancer survivors had clinically significant immediate and delayed memory impairment compared to healthy age- and education-matched controls [35].
Table 1.
Description of the sample and equivalence across groups
| Memory training (n = 26) Mean (SD) |
Speed of processing (n = 27) Mean (SD) |
Wait-list control (n = 29) Mean (SD) |
p | |
|---|---|---|---|---|
| Age (years) | 55.19 (7.58) | 56.93 (7.83) | 57.21 (9.80) | 0.645 |
| Education (years) | 15.96 (1.87) | 15.63 (2.50) | 15.43 (2.27) | 0.678 |
| Months post-treatment | 59.50 (46.12) | 78.00 (60.53) | 59.00 (41.42) | 0.665 |
| Cognitive status (MMSE) | 29.15 (1.16) | 29.33 (0.78) | 29.00 (1.13) | 0.553 |
| Depressive symptoms (CES-D) | 8.98 (5.17) | 13.04 (11.03) | 13.69 (10.05) | 0.374 |
| Anxiety (STAI-state score) | 32.87 (7.26) | 36.15 (9.02) | 36.48 (10.13) | 0.269 |
| Fatigue (FACT-F) | 39.15 (10.34) | 35.91 (11.11) | 36.62 (10.88) | 0.314 |
| Immediate memory | ||||
| Rey AVLT (sum recall) | 50.65 (8.28) | 51.70 (7.57) | 48.34 (5.83) | 0.270 |
| Rey AVLT (short delay) | 11.00 (2.70) | 11.19 (2.45) | 10.55 (2.50) | 0.633 |
| Rey AVLT (recognition) | 14.00 (1.60) | 13.67 (1.44) | 13.93 (1.85) | 0.737 |
| Rivermead | 11.29 (2.87) | 11.50 (2.09) | 10.62 (2.70) | 0.413 |
| Delayed memory | ||||
| Rey AVLT (delay) | 10.62 (2.99) | 11.37 (2.73) | 10.24 (3.01) | 0.345 |
| Rivermead (delay) | 10.54 (3.44) | 11.11 (2.03) | 9.81 (2.74) | 0.223 |
| Information-processing speed | ||||
| Divided attention | 52.81 (94.43) | 31.63 (30.52) | 49.71 (28.56) | 0.409 |
| Selective attention 1 | 132.50 (93.59) | 113.26 (51.87) | 140.68 (73.77) | 0.384 |
| Selective attention 2 | 281.46 (113.55) | 246.15 (107.55) | 267.21 (158.77) | 0.607 |
|
| ||||
| n (%) | n (%) | n (%) | ||
|
| ||||
| Race | 0.198 | |||
| White, non-hispanic | 21 (81) | 26 (96) | 26 (90) | |
| Non-white, non-hispanic | 5 (19) | 1 (4) | 3 (10) | |
| Marital status | 0.463 | |||
| Married/partnered | 14 (54) | 19 (70) | 18 (62) | |
| Single/divorced/widow | 12 (46) | 8 (30) | 11 (38) | |
| Tamoxifen user | 0.273 | |||
| No, never used | 12 (46) | 6 (23) | 10 (37) | |
| Yes, but not in the last month | 4 (15) | 5 (19) | 8 (30) | |
| Yes, used in the last month | 10 (38) | 15 (58) | 9 (33) | |
| Aromatase inhibitor user | 0.827 | |||
| No, never used | 19 (73) | 21 (78) | 18 (62) | |
| Yes, but not in the last month | 5 (19) | 5 (18) | 7 (24) | |
| Yes, used in the last month | 2 (8) | 1 (4) | 4 (14) | |
MMSE Mini-Mental State Examination, CES-D Center for Epidemiological Studies Scale, STAI-S State-Trait Anxiety Inventory-State Subscale, FACT-F Functional Assessment of Cancer Therapy-Fatigue, Rey AVLT Rey Auditory Verbal Learning Test, Rivermead Rivermead Behavioral Paragraph Recall test
Effects on primary outcomes: objective memory and speed of processing performance
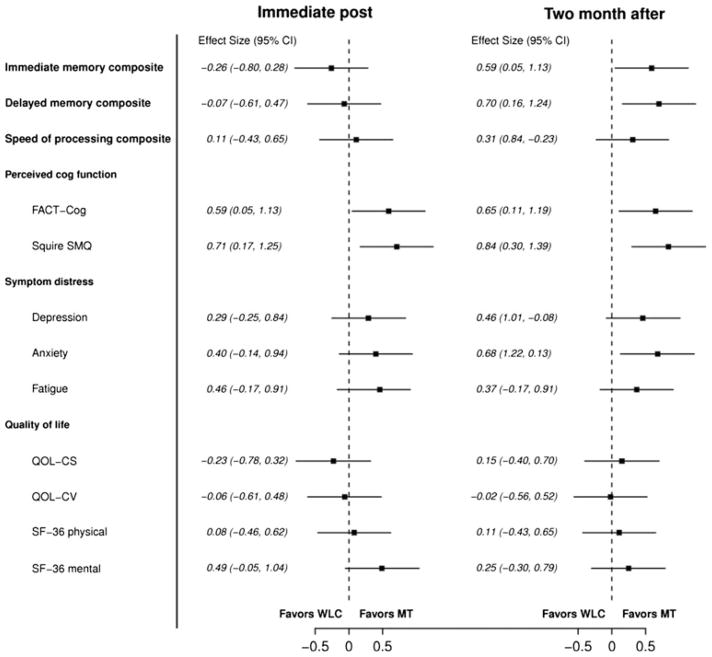
Results of the primary outcome measures of objective neuropsychological performance are detailed in Table 2 and Figs. 2, 3. Compared to the wait-list control, the memory training group demonstrated better immediate (p = 0.036, d = 0.59) and delayed memory performance (p = 0.013, d = 0.70) at the 2-month follow-up (Table 2; Fig. 2). Differences in post-intervention were not significant. At the 2-month follow-up, the percentage of breast cancer survivors who demonstrated reliable improvement was as follows: Immediate memory—39 % memory training group versus 18 % wait-list control; delayed memory—42 % memory training group versus 11 % wait-list control (see Table 2).
Table 2.
Training effects on primary outcomes post and 2-month follow-up (n = 82)
| Measure | Memory training (n = 26)
|
Speed of processing training (n = 27)
|
Wait-list control (n = 29) Reliable improvement (%)‡ |
||
|---|---|---|---|---|---|
| Net effect size (p value)* | Reliable improvement (%)‡ | Net effect size (p value)* | Reliable improvement (%)‡ | ||
| Immediate memory | |||||
| emsp;Post | −0.26 | 23 | 0.75 (p = 0.007) | 41 | 10 |
| emsp;2-month | 0.59 (p = 0.036) | 39 | 0.82 (p = 0.004) | 30 | 18 |
| Delayed memory | |||||
| emsp;Post | −0.07 | 19 | 0.19 | 30 | 24 |
| emsp;2-month | 0.70 (p = 0.013) | 42 | 0.72 (p = 0.010) | 33 | 11 |
| Speed of processing | |||||
| emsp;Post | 0.11 | 65 | 0.55 (p = 0.040) | 68 | 43 |
| 2-month | 0.31 | 73 | 0.67 (p = 0.016) | 67 | 61 |
Post represents immediate post-intervention, 2-month represents 2-month follow-up
Only significant p values reported
Calculated as the percentage of participants in each group who were ≥ 1 SEM above baseline
Fig 2.
Forest plot of effect sizes and confidence intervals for memory training compared to wait-list control at both time points
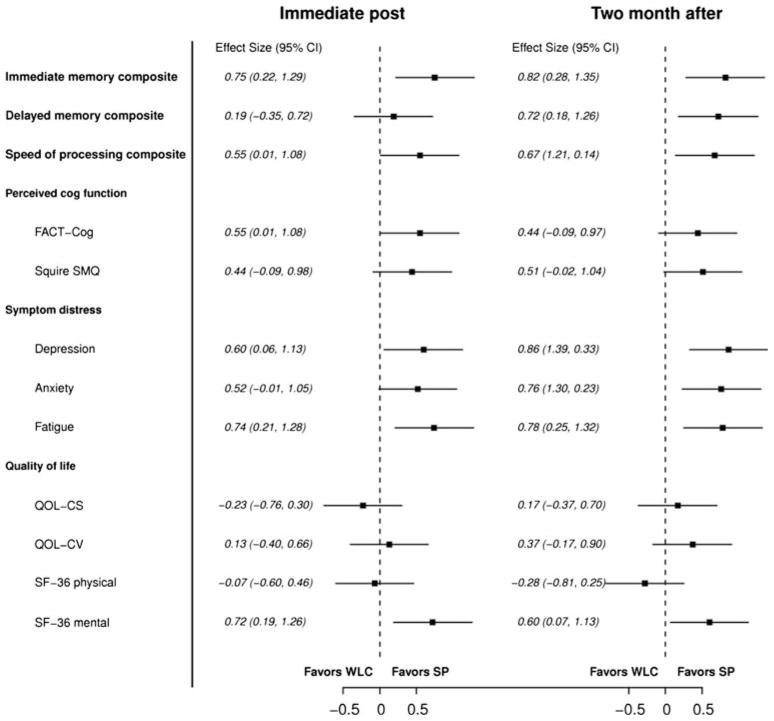
Fig 3.
Forest plot of effect sizes and confidence intervals for speed of processing training compared to wait-list control at both time points
The speed of processing group demonstrated better processing speed compared to the wait-list control group post-intervention (p = 0.040, d = 0.55) and at the 2-month follow-up (p = 0.016, d = 0.67) (Table 3; Fig. 3). Post-intervention, the percentage of breast cancer survivors who demonstrated reliable improvement was 68 % for the speed of processing group and 43 % for the wait-list control group. At the 2-month follow-up, the percentage demonstrating reliable improvement was 67 % for the speed of processing compared to 61 % for wait-list control group.
Table 3.
Training effects on secondary outcomes post and 2-month follow-up
| Measure | Memory training
|
Speed of processing training
|
Wait-list control Mean (SD) |
||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Net effect size (p value)* | 95 % Confidence interval | Mean (SD) | Net effect size (p value)* | 95 % Confidence interval | ||
| FACT-COG | |||||||
| Post | 93.86 (15.54) | 0.59 (p = 0.036) | 0.05, 1.13 | 93.21 (15.50) | 0.55 (p = 0.042) | 0.01, 1.08 | 84.82 (15.80) |
| 2-month | 98.17 (15.34) | 0.65 (p = 0.021) | 0.11, 1.19 | 94.98 (15.29) | 0.44 | −0.09, 0.97 | 88.32 (15.59) |
| Squire | |||||||
| Post | 83.58 (15.59) | 0.71 (p = 0.012) | 0.17, 1.25 | 79.51 (15.54) | 0.44 | −0.09, 0.98 | 72.69 (15.83) |
| 2-month | 86.47 (13.54) | 0.84 (p = 0.003) | 0.30, 1.39 | 81.95 (13.48) | 0.51(p = 0.065) | −0.02, 1.04 | 75.15 (13.74) |
| CES-D | |||||||
| Post | 9.69 (7.47) | −0.29 | −0.25, 0.84 | 10.39 (8.56) | −0.60 (p = 0.031) | 0.06, 1.13 | 15.00 (12.37) |
| 2-month | 7.88 (5.40) | −0.46 | 1.01,−0.08 | 7.85 (7.16) | −0.86 (p = 0.002) | 1.39, 0.33 | 13.79 (11.60) |
| STAI-S | |||||||
| Post | 31.96 (8.10) | −0.40 | −0.14, 0.94 | 33.78 (8.32) | −0.52 (p = 0.059) | −0.01, 1.05 | 37.07 (11.09) |
| 2-month | 30.15 (6.97) | −0.68 (p = 0.017) | 1.22, 0.13 | 32.15 (7.46) | −0.76 (p = 0.006) | 1.30, 0.23 | 36.97 (11.01) |
| FACT-F | |||||||
| Post | 40.20 (9.22) | 0.46 | −0.17, 0.91 | 39.26 (8.90) | 0.74 (p = 0.008) | 0.21, 1.26 | 35.07 (12.07) |
| 2-month | 40.06 (9.93) | 0.37 | −0.17, 0.91 | 39.56 (8.96) | 0.78 (p = 0.005) | 0.25, 1.32 | 35.55 (12.56) |
| QOL-CS | |||||||
| Post | 6.54 (1.29) | −0.23 | −0.78, 0.32 | 6.55 (0.61) | −0.23 | −0.76, 0.30 | 6.73 (0.59) |
| 2-month | 6.02 (1.32) | 0.15 | −0.40, 0.70 | 6.69 (0.63) | 0.17 | −0.37, 0.70 | 6.88 (1.12) |
| QOL-CV | |||||||
| Post | 22.06 (3.44) | −0.06 | −0.61, 0.48 | 22.98 (2.08) | 0.13 | −0.40, 0.66 | 23.38 (2.24) |
| 2-month | 22.15 (4.45) | −0.02 | −0.56, 0.52 | 22.71 (2.08) | 0.37 | −0.17, 0.90 | 22.58 (2.24) |
| SF-36-physical | |||||||
| Post | 45.71 (5.98) | 0.08 | −0.46, 0.62 | 44.69 (4.40) | −0.07 | −0.60, 0.46 | 43.18 (4.72) |
| 2-month | 45.03 (6.17) | 0.11 | −0.43, 0.65 | 45.00 (4.48) | −0.28 | −0.81, 0.25 | 44.49 (4.81) |
| SF-36-Mental | |||||||
| Post | 45.33 (5.98) | 0.49 (p = 0.078) | −0.05, 1.04 | 47.33 (5.10) | 0.72 (p = 0.010) | 0.19, 1.26 | 48.09 (5.03) |
| 2-month | 44.91 (6.82) | 0.25 | −0.30, 0.79 | 43.67 (5.21) | 0.60 (p = 0.031) | 0.07, 1.13 | 45.11 (5.13) |
Post-test represents immediate post-intervention, 2-month represents 2-month follow-up
FACT-C Functional Assessment of Cancer Therapy-Cognitive, SSMQ Squire Subjective Memory Questionnaire, CES-D Center for Epidemiological Studies Scale, STAI-S State-Trait Anxiety Inventory-State Subscale, FACT-F Functional Assessment of Cancer Therapy-Fatigue, QOL-CS Quality of Life-Cancer Survivors, QOL-CV Quality of Life Index-Cancer Version, SF-36 Short-Form Health Survey
Only p values <0.08 reported
Speed of processing training also improved immediate memory at both post-intervention time points (p = 0.007 and p = 0.004) and delayed memory at the 2-month follow-up (p = 0.010). These effect sizes were moderate to large for immediate memory improvement post-intervention and 2-month follow-up (d = 0.75 and d = 0.82, respectively) and delayed memory at the 2-month follow-up (d = 0.72). For the speed of processing training group, the reliable improvement for immediate memory was 41 and 30 % compared to 10 and 18 % for the wait-list control groups, respectively. For the speed of processing training group, the reliable improvement for delayed memory was 30 and 33 % compared to 24 and 11 % for the wait-list control.
Secondary outcomes: perceived cognitive function, symptom distress, and quality of life
Table 3 and Figs. 2, 3 display the effects of memory and speed of processing training on secondary outcomes. Memory training had a positive effect on perceived cognitive functioning on both the FACT-Cog (p = 0.036 and p = 0.021) and SSMQ (p = 0.012 and p = 0.003) at both post-intervention time points. In addition, memory training had a positive effect on one measure of symptom distress (STAI-S) at the 2-month follow-up (p = 0.017) and a marginally significant effect on the SF-36-mental health outcome scale (p = 0.078).
Compared to wait-list control, speed of processing training improved perceived cognitive functioning on the FACT-Cog post-intervention (p = 0.042) and had marginal significant effect on the SSQM at the 2-month follow-up (p = 0.065). Compared to controls, breast cancer survivors who received the speed of processing training also had significantly lower symptom distress on the CES-D and FACT-F at both post-intervention time points and lower symptom distress on the STAI-S at the 2-month follow-up. In addition, compared to the wait-list control, the speed of processing training group had better mental health outcomes (SF-36) post (p = 0.010) and at the 2-month follow-up (p = 0.031).
Acceptability/satisfaction
There were no differences in satisfaction/acceptability between the memory and speed of processing groups. The majority in both the memory and speed of processing groups found the training to be highly satisfactory at 73 and 89 %, respectively. Similarly, participants in both intervention groups (memory, speed) agreed or strongly agreed that the program was understandable (96, 89 %) and enjoyable (81, 73 %). Most disagreed or strongly disagreed that they would have preferred something else (80, 81 %), wanted a different format (100, 96 %), was too difficult (77, 89 %), took too much time (92, 100 %), or that the training was boring (96, 100 %).
Discussion
To our knowledge, this was the largest cognitive training study in long-term breast cancer survivors to date. The main study findings were that both memory and speed of processing training improved objective measures of cognitive performance. Importantly, we also noted significant improvements in perceived cognitive function, symptom distress (mood disturbance, anxiety, and fatigue) and quality of life of breast cancer survivors in the cognitive training groups compared to wait-list control. Similar findings were noted by Ferguson et al. [14], who tested the efficacy of an attention and memory program (n = 19) against wait-list control (n = 21) in long-term breast cancer survivors, and found statistically significant improvements in memory and some quality of life indicators (spirituality). Taken together, findings suggest that cognitive training may be a promising intervention for treating cognitive deficits in breast cancer survivors.
As predicted, we noted cognitive domain-specific intervention effects; that is memory training improved memory performance and speed of processing improved processing speed. There was significant improvement in immediate and delayed memory in the memory training group at the 2-month follow-up. Unlike other cognitive studies [10, 11], the memory training intervention did not demonstrate significant effects post-intervention. However, the percentage of participants demonstrating reliable improvement in immediate memory was comparable to the ACTIVE trial (23 vs. 26 %) [10].
Speed of processing training had significant positive effects on processing speed at both post-intervention time points. In addition, the speed of processing training improved immediate memory performance at both time points and delayed memory at the 2-month follow-up. The InSight program (Posit Science®), originally developed as part of the ACTIVE trial, was revised to include tasks which appear to have resulted in benefits in memory performance. The revised program includes enhanced gaming elements and four additional programs designed to not only improve visual processing speed but also improve attention, learning and memory. In addition, this program now includes game elements that are specifically designed to enhance the level of enjoyment and maximize usage and engagement of the program. Based on the results from the ACTIVE trial, we predicted that this program would significantly improve processing speed and are now encouraged by the significant improvement noted in memory performance. These findings suggest that the InSight program may have broader cognitive benefits in this clinical population.
Notably, for both intervention groups, training effects on objective tests did not wane between the immediate and 2-month post-intervention testing. In fact, training effects actually improved in the memory training group, with increases in number of participants demonstrating reliable improvement (23–39 %). One explanation may be that the participants continued to engage in study-related training and improved their skills over time. For the speed of processing group, who did not have access to the training materials, this finding indicates that the training effect was durable over this period, negating the need for booster training prior to 2-month follow-up. Similar findings were noted in a recent meta-analysis of cognitive training interventions, which found that 4 of 7 studies demonstrated significant positive training effects with follow-up periods ranging from 3 months to 5 years [36].
Importantly, intervention effects transferred to clinically significant improvements in perceived cognitive function, symptom distress (mood disturbance, anxiety, and fatigue), and quality of life. Transfer effects to measures of improved perceived cognitive performance and health is of great importance in this younger, active population of breast cancer survivor. Findings from our previous work and others indicate the detrimental impact of perceived cognitive impairment on quality of life [6, 7] and work ability [37–39]; thus, development and validation of effective interventions are paramount.
Methodological strengths of the study include the blinding of participants and cognitive testers, use of alternate forms, and composite test scores to measure overall ability versus scores on individual tests [16] and examination of those demonstrating reliable improvement. The attrition rate was equivalent across intervention groups and comparable to other cognitive training studies in breast cancer survivor [14]. Both interventions were also rated as highly satisfactory/acceptable.
Limitations of the study include lack of a demographically more diverse population for generalizability and lack of a longer follow-up period to determine the need for or possible timing of booster training. In addition, positive outcomes may be due in part to social contact contributions such as support received within the training groups from the interventionist and/or other breast cancer survivors. While the threat of social contact contributions on objectively measured cognitive abilities is unlikely and was not demonstrated in the original ACTIVE trial [10, 11], future planned research will include an active attention control condition to address this concern.
Conclusion
Memory training and speed of processing training are promising treatment options for breast cancer survivors with self-reported cognitive concerns. The interventions tested here showed preliminary efficacy on primary domain-specific tests. Speed of processing training also had positive effects on memory performance which warrant further study. Importantly, both interventions also had transfer effects on specific self-reported measures of cognitive function, symptom distress, and quality of life which impact individual functioning and well-being. In addition, both interventions were highly satisfactory/acceptable to breast cancer survivors. These pilot study findings point to the importance of full-scale efficacy testing of these interventions in a larger, more diverse sample of breast cancer survivors, and possibly other cancer survivors.
Supplementary Material
Acknowledgments
This study was supported by Robert Wood Johnson Foundation Nurse Faculty Scholar Program (#64194), American Cancer Society Institutional Research Grant (#84-002-25), Indiana University School of Nursing Center for Enhancing Quality of Life in Chronic Illness and the Mary Margaret Walther Program of the Walther Cancer Institute (#0097.01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Robert Wood Johnson Foundation, which funded this trial. The funding agency had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Patrick Monahan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Ethical approval
This study was conducted in accordance with all laws of the United States and the study was approved by the Indiana University Simon Cancer Center Scientific Review Group and Institutional Review Board in which was conducted.
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-012-2210-6) contains supplementary material, which is available to authorized users.
Conflict of interest
The authors declare that they have no conflict of interest. Posit Science Corporation is the developer of the speed of processing (Insight®) program used in this study. Posit Science Corporation holds the patent for and a proprietary interest in this software. The software was provided at cost of the CD by Posit Science. Dr. Karlene Ball is on the Board of Directors of Posit Science and has stock in the company. Dr. Unverzagt has received support for training for an investigator initiated research from Posit Science.
Contributor Information
Diane Von Ah, Email: dvonah@iupui.edu, School of Nursing, Indiana University, 1111 Middle Drive NU431, Indianapolis, IN 46202, USA.
Janet S. Carpenter, School of Nursing, Indiana University, 1111 Middle Drive NU431, Indianapolis, IN 46202, USA
Andrew Saykin, School of Medicine, Indiana University, Indianapolis, IN, USA.
Patrick Monahan, School of Medicine, Indiana University, Indianapolis, IN, USA.
Jingwei Wu, School of Medicine, Indiana University, Indianapolis, IN, USA.
Menggang Yu, University of Wisconsin, Madison, WI, USA.
George Rebok, John Hopkins University, Baltimore, MD, USA.
Karlene Ball, University of Alabama at Birmingham, Birmingham, AL, USA.
Bryan Schneider, School of Medicine, Indiana University, Indianapolis, IN, USA.
Michael Weaver, School of Nursing, Indiana University, 1111 Middle Drive NU431, Indianapolis, IN 46202, USA.
Eileen Tallman, School of Nursing, Indiana University, 1111 Middle Drive NU431, Indianapolis, IN 46202, USA.
Fred Unverzagt, School of Medicine, Indiana University, Indianapolis, IN, USA.
References
- 1.Hess LM, Insel KC. Chemotherapy-related change in cognitive function: a conceptual model. Oncol Nurs Forum. 2007;34(5):981–994. doi: 10.1188/07.ONF.981-994. [DOI] [PubMed] [Google Scholar]
- 2.Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104(10):2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 3.Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006;20(1):76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- 4.Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips KA. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain Cogn. 2005;59(1):60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Anderson-hanley C, Sherman M, Riggs R, Agocha V, Compas B. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9(7):967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 6.Von Ah D, Russell KM, Storniolo AM, Carpenter JS. Cognitive dysfunction and its relationship to quality of life in breast cancer survivors. Oncol Nurs Forum. 2009;36(3):326–336. doi: 10.1188/09.ONF.326-334. [DOI] [PubMed] [Google Scholar]
- 7.Mehnert A, Scherwath A, Schirmer L, Schleimer B, Petersen C, Schultz-Kindermann F, Zander AR, Koch U. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue, and quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ Couns. 2007;66(1):108–118. doi: 10.1016/j.pec.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Von Ah D, Jansen C, Allen DH, Schiavone RM, Wulff J. Putting evidence into practice: evidence-based interventions for cancer and cancer treatment-related cognitive impairment. Clin J Oncol Nurs. 2011;15(6):607–615. doi: 10.1188/11.CJON.607-615. [DOI] [PubMed] [Google Scholar]
- 9.Fardell JE, Vardy J, Johnston IN, Winocur G. Chemotherapy and cognitive impairment: treatment options. Clin Pharmacol Ther. 2011;90(3):366–376. doi: 10.1038/clpt.2011.112. [DOI] [PubMed] [Google Scholar]
- 10.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J Am Geriatr Soc. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zelinski EM, Spina LM, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Smith GE. Improvement in memory with plasticity-based adaptive cognitive training: results of the 3-month follow-up. J Am Geriatr Soc. 2011;59(2):258–265. doi: 10.1111/j.1532-5415.2010.03277.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson RJ, McDonald BC, Rocque MA, Furstenberg CT, Horrigan S, Ahles TA, Saykin AJ. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology. 2012;21(2):176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, Rebok GW, Morris JN, Helmers KF, Leveck MD, et al. Active: a cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;22(4):453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahncke HW, Conner BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci USA. 2006;103(33):12523–12538. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- 19.Wilson B, Cockburn J, Baddeley A. The rivermead behavioral memory test. Thames Valley Test Co., Reading and National Rehabilitation Services; Gaylord: 1985. [Google Scholar]
- 20.Owsley C, Ball K, McGwin G, Jr, Sloane ME, Roenker DE, White MF, Overley ET. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279(14):1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 21.Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1993;64:71–79. [PubMed] [Google Scholar]
- 22.Edwards JD, Ross LA, Wadley VG, Clay OJ, Crowe M, Roenker DL, Ball KK. The useful field of view test: normative data for older adults. Arch Clin Neuropsychol. 2006;21(4):275–286. doi: 10.1016/j.acn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs SR, Jacobsen PB, Booth-Jones M, Wagner LI, Anasetti C. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage. 2007;33(1):13–23. doi: 10.1016/j.jpainsymman.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Squire LR, Wetzel CD, Slater PC. Memory complaint after electroconvulsive therapy: assessment with a new self-rating instrument. Biol Psychiatry. 1979;14:791–801. [PubMed] [Google Scholar]
- 25.Spielberger CD, Gorsuch RI, Lushene RG. Manual for the state-trait anxiety inventory. Consulting Press; Palo Alto: 1971. [Google Scholar]
- 26.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 27.Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P. Quality of life in long-term cancer survivors. Oncol Nurs Forum. 1995;22(6):915–922. [PubMed] [Google Scholar]
- 28.Ferrans CE, Powers MJ. Quality of life index: development and psychometric properties. ANS Adv Nurs Sci. 1985;8(1):15–24. doi: 10.1097/00012272-198510000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 health survey manual and interpretation guide. The Health Institute, The New England Medical Center; Boston: 1993. [Google Scholar]
- 30.Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plan. 1979;2(3):197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter JS, Neal JG, Payne J, Kimmick G, Storniolo AM. Cognitive-behavioral intervention for hot flashes. Oncol Nurs Forum. 2007;34(1):E1–E8. doi: 10.1188/07.ONF.E1-E8. [DOI] [PubMed] [Google Scholar]
- 32.Blom G. Statistical estimates and transformed beta variables. Wiley; New York: 1958. [Google Scholar]
- 33.Dudek FJ. The continuing misinterpretation of the standard error of measurement. Psychol Bull. 1979;86(2):335–337. [Google Scholar]
- 34.Uchiyama CL, D’Elia LF, Dellinger AM, Becker JT, Seines DA, Wescln JE, Chen BB, Satz P, van Gorp WG, Miller EN. Alternate forms of the auditory-verbal learning test: issues of test comparability, longitudinal reliability and moderating demographic variables. Arch Clin Neuropsychol. 1995;10(2):133–145. [PubMed] [Google Scholar]
- 35.Von Ah D, Harvison K, Monahan P, Moser L, Zhao Q, Carpenter J, Sledge G, Jr, Champion V, Unverzagt F. Cognitive function in breast cancer survivors compared to healthy age- and education-matched women. Clin Neuropsychol. 2009;23(4):661–674. doi: 10.1080/13854040802541439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry. 2009;17(3):179–187. doi: 10.1097/JGP.0b013e3181953b57. [DOI] [PubMed] [Google Scholar]
- 37.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3(4):223–232. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calvio L, Peugeot M, Bruns GL, Todd BL, Feuerstein M. Measures of cognitive function and work in occupationally active breast cancer survivors. J Occup Environ Med. 2010;52(2):219–227. doi: 10.1097/JOM.0b013e3181d0bef7. [DOI] [PubMed] [Google Scholar]
- 39.Munir F, Burrows J, Yarker J, Kalawsky K, Bains M. Women’s perceptions of chemotherapy-induced cognitive side affects on work ability: a focus group. J Clin Nurs. 2010;19(9–10):1362–1370. doi: 10.1111/j.1365-2702.2009.03006.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.