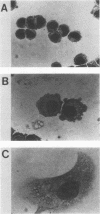
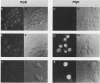
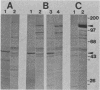
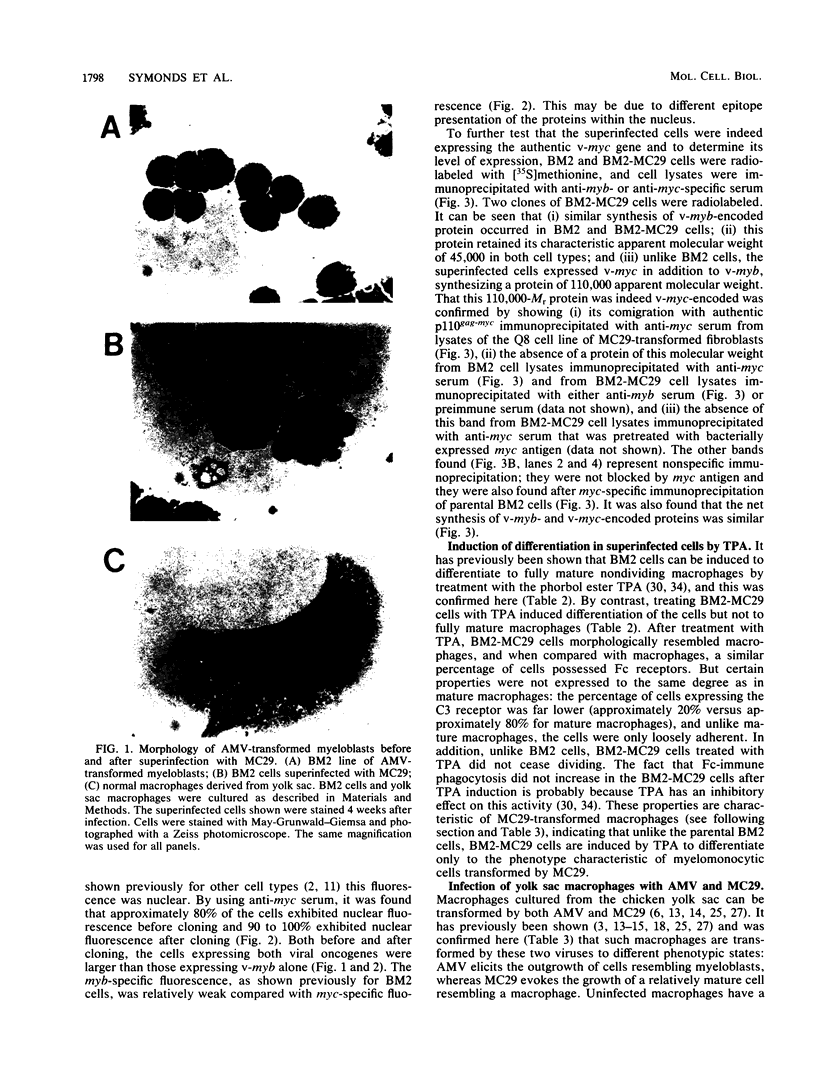
Abstract
Both avian myeloblastosis virus (by the action of v-myb) and avian myelocytomatosis virus MC29 (by the action of v-myc) transform cells of the myelomonocytic lineage. Whereas avian myeloblastosis virus elicits a relatively immature phenotype, cells transformed by MC29 resemble mature macrophages. When cells previously transformed by v-myb were superinfected with MC29, their phenotype was rapidly altered to that of a more mature cell. These superinfected cells expressed both v-myb (at a level similar to that found before superinfection) and v-myc. It therefore appears that the expression of v-myc can elicit certain properties of a more differentiated phenotype. In addition, unlike cells transformed by v-myb alone, the cells expressing both v-myb and v-myc could not be induced by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate to differentiate to fully mature macrophages. Cells with a morphology similar to that of the superinfected cells were elicited by simultaneously infecting yolk sac macrophages with avian myeloblastosis virus and MC29. Such cells expressed both v-myb and v-myc. These results indicate that expression of v-myb and v-myc in infected cells coordinately regulates myelomonocytic phenotype and that the two viral oncogenes vary in their ability to interfere with tumor promoter-induced differentiation. Our findings also sustain previous suggestions that the oncogenes v-myb and v-myc may not transform target cells by simply blocking differentiation.
Full text
PDF

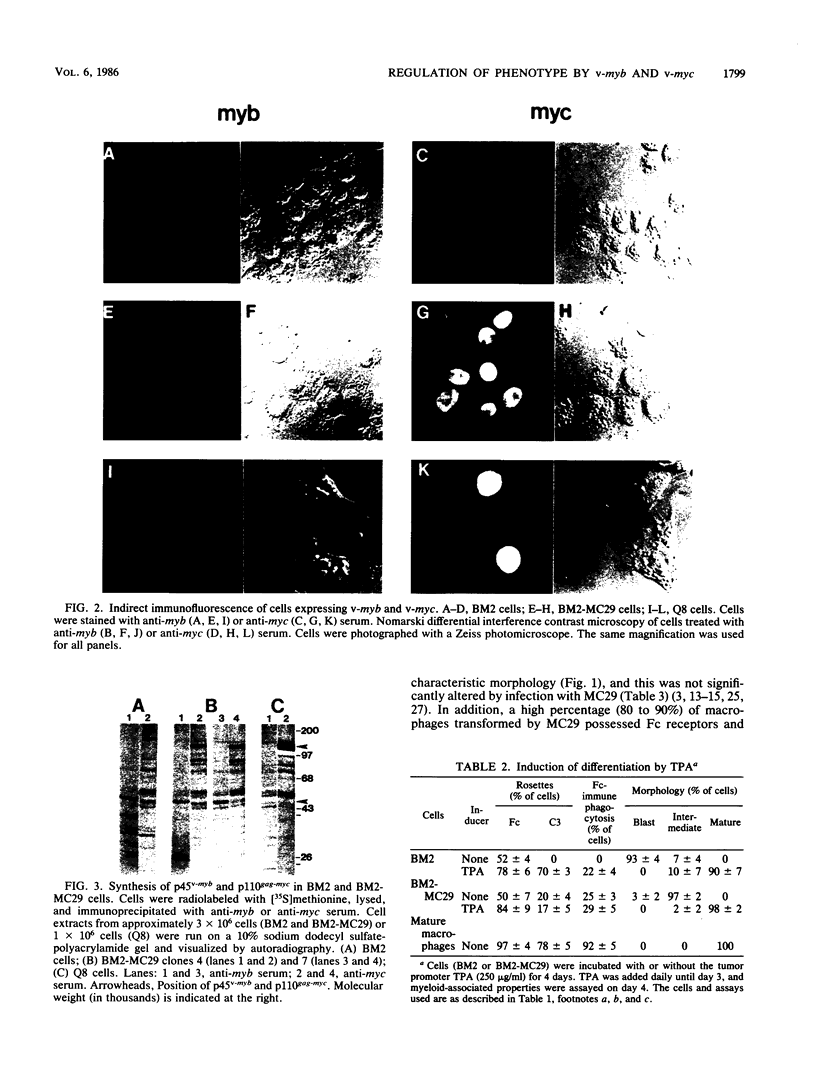
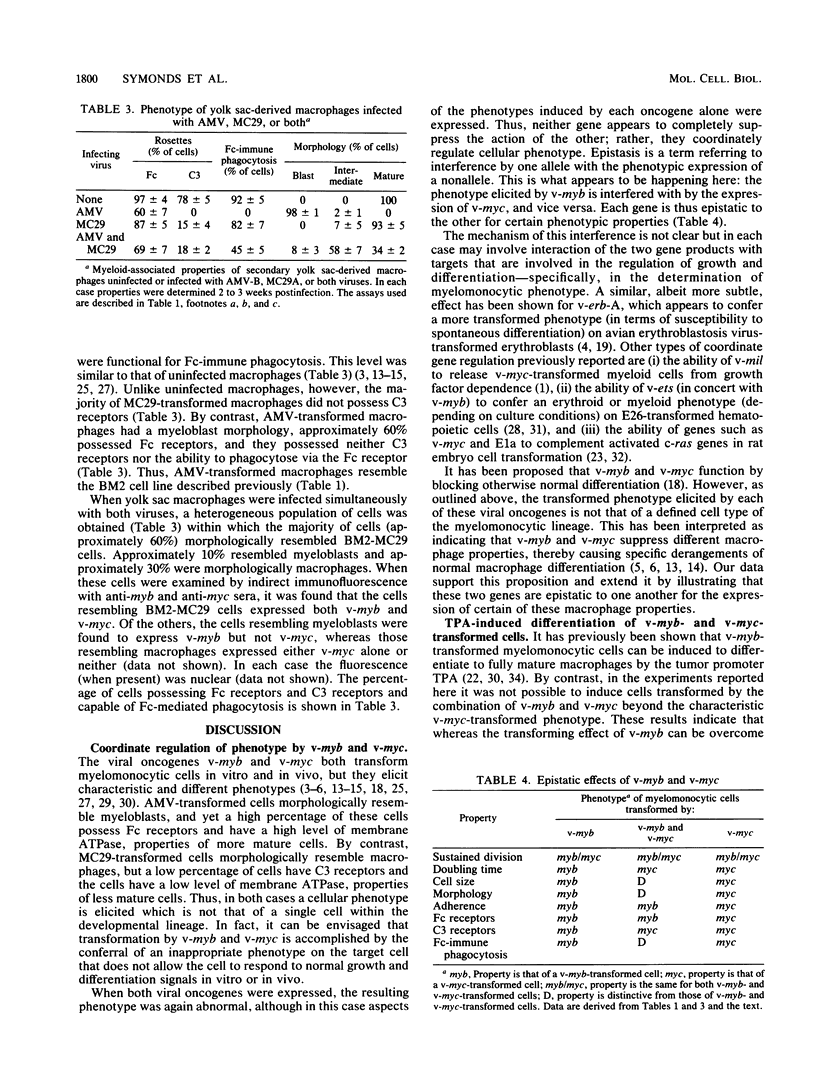


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Ramsay G., Bishop J. M., Pfeifer S. O., Colby W. W., Levinson A. D. Identification of nuclear proteins encoded by viral and cellular myc oncogenes. Nature. 1983 Nov 17;306(5940):274–277. doi: 10.1038/306274a0. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Durban E. M. Progenitor-cell populations can be infected by RNA tumor viruses, but transformation is dependent on the expression of specific differentiated functions. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1249–1254. doi: 10.1101/sqb.1980.044.01.135. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Durban E. Target cells for avian myeloblastosis virus in embryonic yolk sac and relationship of cell differentiation to cell transformation. J Virol. 1984 Mar;49(3):841–847. doi: 10.1128/jvi.49.3.841-847.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Lampert M. A., Lipsick J. S., Baluda M. A. Avian myeloblastosis virus and E26 virus oncogene products are nuclear proteins. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4265–4269. doi: 10.1073/pnas.81.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Lipsick J. S., Reddy E. P., Baluda M. A. Identification of the leukemogenic protein of avian myeloblastosis virus and of its normal cellular homologue. Proc Natl Acad Sci U S A. 1983 May;80(10):2834–2838. doi: 10.1073/pnas.80.10.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Varmus H. E., Bishop J. M., Essex M., Hardy W. D., Jr, Martin G. S., Rosenberg N. E., Scolnick E. M., Weinberg R. A., Vogt P. K. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981 Dec;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. W., Bloch A. Early decline in c-myb oncogene expression in the differentiation of human myeloblastic leukemia (ML-1) cells induced with 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1984 Feb;44(2):442–446. [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Duprey S. P., Boettiger D. Developmental regulation of c-myb in normal myeloid progenitor cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6937–6941. doi: 10.1073/pnas.82.20.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durban E. M., Boettiger D. Differential effects of transforming avian RNA tumor viruses on avian macrophages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3600–3604. doi: 10.1073/pnas.78.6.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durban E. M., Boettiger D. Replicating, differentiated macrophages can serve as in vitro targets for transformation by avian myeloblastosis virus. J Virol. 1981 Jan;37(1):488–492. doi: 10.1128/jvi.37.1.488-492.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzolo L., Moscovici C., Moscovici M. G., Samarut J. Response of hemopoietic cells to avian acute leukemia viruses: effects on the differentiation of the target cells. Cell. 1979 Mar;16(3):627–638. doi: 10.1016/0092-8674(79)90036-9. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Role of the v-erbA and v-erbB oncogenes of avian erythroblastosis virus in erythroid cell transformation. Cell. 1983 Aug;34(1):7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Abrams H. D., Rohrschneider L. R., Eisenman R. N. Proteins encoded by v-myc and c-myc oncogenes: identification and localization in acute leukemia virus transformants and bursal lymphoma cell lines. Cell. 1983 Oct;34(3):789–798. doi: 10.1016/0092-8674(83)90535-4. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Ramsay G., Bishop J. M., Moscovici M. G., Moscovici C., McGrath J. P., Levinson A. D. The product of the retroviral transforming gene v-myb is a truncated version of the protein encoded by the cellular oncogene c-myb. Cell. 1983 Jun;33(2):345–355. doi: 10.1016/0092-8674(83)90416-6. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Symonds G., Evan G. I., Bishop J. M. Subcellular localization of proteins encoded by oncogenes of avian myeloblastosis virus and avian leukemia virus E26 and by chicken c-myb gene. Cell. 1984 Jun;37(2):537–547. doi: 10.1016/0092-8674(84)90384-2. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G. Tissue culture of avian hematopoietic cells. Methods Cell Biol. 1973;7:313–328. doi: 10.1016/s0091-679x(08)61784-7. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Jurdic P., Samarut J., Gazzolo L., Mura C. V., Moscovici C. Characterization of the hemopoietic target cells for the avian leukemia virus E26. Virology. 1983 Aug;129(1):65–78. doi: 10.1016/0042-6822(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sealy L., Privalsky M. L., Moscovici G., Moscovici C., Bishop J. M. Site-specific mutagenesis of avian erythroblastosis virus: erb-B is required for oncogenicity. Virology. 1983 Oct 15;130(1):155–178. doi: 10.1016/0042-6822(83)90125-3. [DOI] [PubMed] [Google Scholar]
- Symonds G., Klempnauer K. H., Evan G. I., Bishop J. M. Induced differentiation of avian myeloblastosis virus-transformed myeloblasts: phenotypic alteration without altered expression of the viral oncogene. Mol Cell Biol. 1984 Dec;4(12):2587–2593. doi: 10.1128/mcb.4.12.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]