Abstract
Increasing evidence has shown that mild hypothermia is neuroprotective for comatose patients resuscitated from cardiac arrest, but the mechanism of this protection is not fully understood. The aim of this study was to determine whether prolonged whole-body mild hypothermia inhibits mitochondrial membrane permeability (MMP) in the cerebral cortex after return of spontaneous circulation (ROSC). Thirty-seven inbred Chinese Wuzhishan minipigs were successfully resuscitated after 8 minutes of untreated ventricular fibrillation (VF) and underwent recovery under normothermic (NT) or prolonged whole-body mild hypothermic (HT; 33°C) conditions for 24 or 72 hours. Cerebral samples from the frontal cortex were collected at 24 and 72 hours after ROSC. Mitochondria were isolated by differential centrifugation. At 24 hours, relative to NT, HT was associated with reductions in opening of the mitochondrial permeability transition pore, release of pro-apoptotic substances from mitochondria, caspase 3 cleavage, apoptosis, and neurologic deficit scores, as well as increases in mitochondrial membrane potential and mitochondrial respiration. Together, these findings suggest that mild hypothermia inhibits ischemia-induced increases in MMP, which may provide neuroprotection against cerebral injury after cardiac arrest.
Keywords: cardiac arrest, mitochondria, mild hypothermia, neuroprotection, pig, reperfusion
Introduction
After cardiac arrest, return of spontaneous circulation (ROSC) can induce brain reperfusion injury, which has been associated with adverse neurologic outcomes and death.1 A complex cascade of processes begins minutes to hours after reperfusion and may continue for 72 hours or longer.2, 3 This cascade includes oxidative stress, disrupted calcium homeostasis, excitotoxicity, pathologic protease cascades, and activation of cell death signaling pathways. Mitochondria are at the center of these processes and have a pivotal role as targets and effectors of injury.4
Cell survival or death depends on critical functions of mitochondrial membranes,5 especially mitochondrial membrane permeability (MMP),6 which includes the permeability of the mitochondrial outer membrane (MOM) and the mitochondrial inner membrane (MIM). The MOM is normally permeable to small molecular metabolites, but not to proteins.5 The MOM permeabilization allows translocation of cytochrome c and other mitochondrial proapoptotic proteins from the intermembrane space to the extramitochondrial compartment, which is a decisive event in many forms of apoptotic cell death. The MIM is usually impermeable to protons, other ions, and water.5 The MIM permeabilization dissipates mitochondrial membrane potential (ΔΨm) and thereby uncouples the process of respiration from adenosine triphosphase (ATP) synthase, halting mitochondrial ATP generation.5
One of the principal mechanisms underlying MIM permeabilization is the so-called ‘permeability transition'.6 Permeability transition is a sudden increase in MIM permeability to solutes with molecular mass up to 1.5 kDa,6, 7 caused by the opening of a voltage-dependent, high-conductance channel located in the MIM, termed as the mitochondrial permeability transition pore (mPTP).6 Long-lasting mPTP opening has been postulated as the event that leads to irreversible changes in cellular function and cell death.8, 9, 10
To date, mild hypothermia is the only treatment that has been clinically confirmed to improve neurologic outcomes.3, 11, 12, 13 The protective effects of mild hypothermia against brain injury may be due to a reduction in brain metabolism, inhibition of excitatory amino-acid release and oxidative stress, attenuation of the immune response, modification of cell death signaling pathways, and other factors.2, 3, 13, 14, 15, 16, 17 However, the effects of mild hypothermia on MMP are unknown. Therefore, the main goal of the present study was to investigate whether whole-body mild hypothermia inhibits MMP after ROSC in a swine model of cardiac arrest.
Materials and methods
Animals
This study used 37 (21 males; 16 females) inbred Chinese Wuzhishan minipigs (Permit Number: SYXK (Beijing) 2008-0007, the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, China), aged 4 to 6 months, weighing 24.5±1.7 kg. This study was conducted in strict accordance with the Guidelines for Animal Care and Use established by the Capital Medical University Institutional Animal Care and Use Committee. The protocol was approved by the Committee on the Ethics of Animal Experiments of Capital Medical University (Permit Number: 2010-D-013).
Animal Preparation
Pigs were fasted overnight with free access to water, then were sedated with ketamine (20 mg/kg, intramuscularly), followed by propofol (2 mg/kg, intravenously). After endotracheal intubation, anesthesia and analgesia were maintained intravenously with sodium pentobarbital (continuous infusion of 8 mg/kg per hour) and fentanyl (continuous infusion of 5 μg/kg per hour). Pigs were ventilated using a volume-controlled ventilator (Servo 900c, Siemens, Munich, Germany) with a tidal volume of 15 mL/kg, FiO2 of 0.21, and ventilation rate of 12 to 20 breaths/minute Tidal volume and ventilation rate were adjusted to maintain normocapnia (end-tidal PCO2 of 35 to 45 mm Hg), as monitored continuously with an in-line infrared capnograph placed in the airway. Arterial blood gases were analyzed (ABL80, Radiometer, Copenhagen, Denmark) to confirm adequate baseline ventilation. For the duration of the experiment, pigs were secured in a supine position on the operating table and were transfused with normal saline (10 mL/kg per hour, intravenously) to maintain a central venous pressure of 5 to 12 mm Hg.
Thorax skin was shaved to secure standard lead II electrocardiogram surface electrodes. To measure aortic pressure, a fluid-filled catheter was inserted from the left femoral artery into the thoracic aorta. To measure cardiac output, a Swan-Ganz catheter (7-Fr, Edwards Life Sciences, Irvine, CA, USA) was inserted from the left femoral vein and flow directed into the pulmonary artery. Electrocardiograph, aortic pressure, and cardiac output were monitored continuously (Vigilance II, Edwards Life Sciences). To induce ventricular fibrillation (VF), a 5-Fr pacing catheter was advanced from the right femoral vein into the right ventricle. To induce hypothermia, a central venous catheter (Icy, Alsius Corp., Irvine, CA, USA) was inserted from the right external jugular vein into the superior vena cava. Also, a temperature-sensing Foley catheter (Integral Medical Products Shaoxing, China) was inserted into the bladder after fistulation. All operations were performed using aseptic surgical techniques.
Programmed Electrical Stimulation-Induced Cardiac Arrest Model
After instrumentation, 30 minutes were allowed for hemodynamic stabilization. During this period, intrabladder temperature was adjusted to 37°C using a heating lamp and warm packs, or an electrical fan and ice bags. The programmed electrical stimulation-induced cardiac arrest model was induced as described in our previous report.3 Once VF occurred, mechanical ventilation was discontinued. After 8 minutes of untreated VF, cardiopulmonary resuscitation (CPR) with manual external chest compressions was performed by an experienced CPR technician. The same technician performed all CPR in the study. Meanwhile, pigs were ventilated with room air using a bag respirator attached to the endotracheal tube and a compression-to-ventilation ratio of 30:2. After 2 minutes of CPR, a single 150 J biphasic electrical shock was attempted with a Smart Biphasic defibrillator (Philips Medical Systems, Andover, MA, USA). If VF persisted, then CPR was resumed for 2 minutes, followed by a bolus of epinephrine (30 μg/kg) via the femoral vein. Additional doses of epinephrine were administered, if needed, every 3 minutes until ROSC was achieved. Two hundred J was used for the second and all subsequent defibrillation attempts. Return of spontaneous circulation was defined as an organized cardiac rhythm with a mean aortic pressure of >60 mm Hg, sustained continuously for at least 10 minutes. Resuscitation procedures were terminated if pigs had no ROSC after 20 minutes of CPR. Immediately after ROSC, mechanical ventilation was resumed using the same settings as before induction of VF.
Treatment Groups
After ROSC, pigs were randomized to receive mild hypothermia (HT, n=16) or normothermia (NT, n=16). An additional five pigs served as surgery controls (SC, n=5), undergoing surgery without VF or hypothermia. Pigs were randomly assigned to be euthanized at 24 or 72 hours after ROSC, creating the following four groups (n=8/group): NT24h, HT24h, NT72h, and HT72h.
Mild Hypothermia Procedure
Immediately after ROSC, according to the landmark study,12 pigs were actively cooled to a target body temperature of 33°C (1.0°C/hour), maintained at this temperature for 12 hours, and then actively rewarmed (0.5°C/hour) to 37°C using a CoolGard 3000 system (Alsius Corp., Irvine, CA, USA). During the induction and maintenance of mild hypothermia, pigs received pancuronium bromide (0.1 mg/kg, intravenously) to prevent shivering and muscle movement. Administration was repeated if needed. After ROSC, pigs received standardized postresuscitative intensive care until the end of the rewarming phase. After the cooling procedure was complete, intravascular catheters were removed, incisions were sutured, and pigs were returned to their cages. At 70 hours after ROSC, pigs were instrumented once again using aseptic surgical techniques, and hemodynamic data were collected at 72 hours after ROSC. Room temperature was maintained between 20° and 24°C.
Neurologic Deficit Scores
We adopted neurologic deficit scores (NDS) to evaluate the outcome of neurologic function in pigs at 24 and 72 hours after ROSC as described in detail in our previous report.3 Investigators were blinded to the pigs' respective treatments.
Brain Tissue Sampling
At 24 or 72 hours after ROSC, pigs were euthanized with propofol (3 mg/kg, intravenously), followed by 10 mL of potassium chloride (10 mol/L, intravenously). The brain was immediately removed by craniotomy and divided by a midsagittal cut. The right hemisphere was dissected, and the hippocampus and a portion of the precentral gyrus of the frontal lobe were fixed in 4% buffered formalin for hematoxylin-eosin staining. Another portion of the precentral gyrus of the frontal lobe was immediately collected on ice for morphologic examination by electron microscopy. From the left hemisphere, frontal cortex samples (1 to 2 g) were collected and mitochondria were rapidly isolated. Samples of mitochondria and frontal cortex were snap frozen in liquid nitrogen and stored at −80°C until analysis. The frontal cortex was selected for analysis based on prior data showing its role in neurologic outcomes in animal and human studies of ischemia.18, 19
Isolation of Mitochondria
Mitochondria were isolated according to a protocol that has been described in detail in our earlier paper.3 The final pellet was resuspended in 300 μL of ice-cold isolation medium (20 mg protein/mL) and kept on ice for further assays. The isolation of mitochondria was validated by western blotting (Figure 4C). Mitochondrial protein concentration was detected using the Bradford method.
Determination of Mitochondrial Permeability Transition Pore Opening
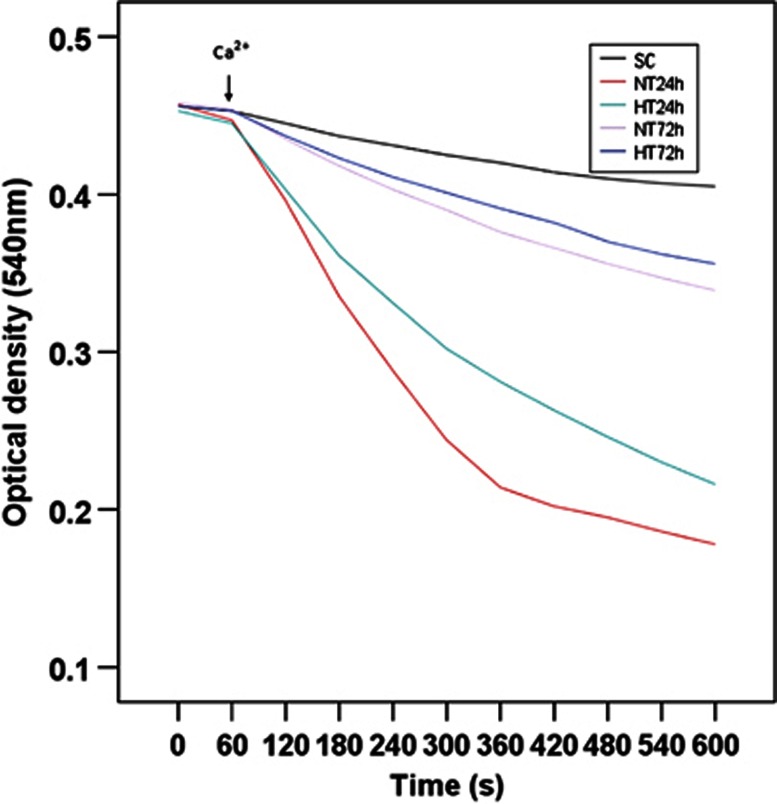
Changes in the status of mPTP were assessed using a spectrophotometer (Infinite M200; Tecan, Männedorf, Switzerland), as previously described.20 Opening of mPTP in isolated mitochondria leads to mitochondrial swelling, which is commonly used to assess the status of mPTP21 via a decrease in light scattering, and thus absorbance, of a mitochondrial suspension.6 Mitochondria (1 mg protein/mL) were suspended in incubation buffer (20 mmol/L MOPS, pH 7.5, 110 mmol/L KCl, 10 mmol/L ATP, 10 mmol/L MgCl2, 10 mmol/L sodium succinate, 1 mmol/L EGTA) at 33°C in a water-jacketed cuvette holder. After a 1-minute equilibration period, mitochondrial swelling was assessed in a spectrophotometer via the decrease in absorbance at 540 nm. Mitochondrial swelling was triggered by the addition of CaCl2 (10 μmol/L; see arrow in Figure 1). Measurements were repeated every 30 seconds for 10 minutes. In another experiment, 6 μmol cyclosporine A (mPTP inhibitor) was also added to test whether permeability transition was involved in mitochondrial swelling.
Figure 1.
Mitochondrial permeability transition at 24 and 72 hours after ROSC. Data represent the mean±s.d. of five to seven independent experiments. HT, mild hypothermia; NT, normothermia; SC, surgery control; ROSC, restoration of spontaneous circulation.
Detection of Mitochondrial Membrane Potential (ΔΨm)
The fluorescent JC-1 dye (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine iodide; Sigma-Aldrich, St Louis, MO, USA) is a lipophilic cation that accumulates in intact mitochondria, emits a green fluorescence at low ΔΨm, and forms red fluorescent ‘J-aggregates' at higher ΔΨm.22 To assess ΔΨm, mitochondria (40 μg) were added to 2 mL incubation buffer (described above). The reaction was initiated by addition of JC-1 to a final concentration of 10 μg/mL, and the mitochondria were then left to incubate for 7 minutes at room temperature in the dark. After incubation, the fluorescence of the sample was immediately analyzed with a spectrofluorimeter (Infinite M200; Tecan) at an excitation wavelength of 490 nm and emission wavelength of 590 nm.23, 24
Measurement of Mitochondrial Oxygen Consumption
Mitochondria (0.25 mg) were incubated in 2 mL respiration buffer (125 mmol/L KCl, 2 mmol/L K2HPO4, 1 mmol/L MgCl2, 10 μmol/L EGTA, 20 mmol/L HEPES, pH 7.0, 37°C). After 1 minute of equilibration, oxygen uptake by mitochondria was measured with a Clark-type oxygen electrode using a Hansatech Oxygraph Measurement System (Hansatech, Norfolk, UK).25 State 3 respiration was initiated by addition of 1 mmol/L ADP to respiration buffer containing glutamate (5 mmol/L) and malate (5 mmol/L). On depletion of ADP, state 4 respiration was monitored. State 4 respiration was initiated by oligomycin (2.5 μg/mL). The respiratory control ratio (RCR) was defined as ADP-stimulated respiration (state 3) divided by resting respiration (state 4). The ADP/O ratio is defined as moles of ADP phosphorylated per moles of oxygen consumed.
Detection of Apoptosis
Tissue blocks (0.5 cm thick) from the frontal cortex of pigs euthanized at 24 hours after ROSC were embedded in paraffin and sliced into 6 mm coronal thick sections. To detect apoptotic cells, sections were stained using a deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay kit (Wako Pure Chemical Industries, Osaka, Japan), according to manufacturer's instructions. An experienced pathologist, blinded to group assignment, counted TUNEL-positive cells under a light microscope (Shanghai Zousun Optical Instrument Co., Ltd, Shanghai, China). The apoptotic index was calculated as a ratio of the apoptotic cell number to the total cell number from four randomly selected high-power fields ( × 400 magnification).
Western Blotting Analysis
Cytoplasmic and nuclear protein fractions were prepared, and western blotting analysis was performed as previously described.3 Primary antibodies included rabbit polyclonal anti-cytochrome c (1:300; Abcam, Cambridge, MA, USA), rabbit polyclonal anti-Smac (1:1,000; Abcam), mouse polyclonal anti-apoptosis inducing factor (AIF) (1:400; Bioss, Beijing, China), rabbit polyclonal anti-cleaved caspase 3 (1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), mouse polyclonal anti-glyceraldehyde-3-phosphatedehydrogenase) (GAPDH; 1:1,000; Abcam), and mouse monoclonal anti-lamin B2 (1:300; Pierce Biotechnology, Rockford, IL, USA). We measured optical densities of the western blots using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Cytoplasmic cytochrome c, Smac, and cleaved caspase 3 protein levels were normalized to GAPDH, and nuclear AIF protein levels were normalized to lamin B2.
Statistical Analysis
Data are presented as the mean±standard deviation (s.d.). Data were analyzed using three-way ANOVA (factors: temperature, time, and gender), followed by Bonferroni test for multiple comparisons. Levene's test was used to assess homogeneity of variance, and Greenhouse-Geisser corrections were used when indicated. Statistical significance was defined as P<0.05. Statistics were performed using the software package SPSS 16.0 (SPSS, Chicago, IL, USA).
Results
Physiologic, Hemodynamic, and Resuscitation Data
At 24 and 72 hours after ROSC, no group differences were observed for gender, weight, core body temperature, Pa𝒪2, mean arterial pressure, or cardiac output, as described in detail in our previous report.3 Cardiopulmonary resuscitation time was similar in NT and HT pigs (3.3±1.2 versus 3.4±1.3 minutes, respectively, P>0.05).
Mild Hypothermia Inhibited Mitochondrial Permeability Transition Pore Opening
As shown in Figure 1, mitochondrial swelling in frontal cortex was triggered by the addition of 10 μmol/L Ca2+ (see arrow). At 24 hours after ROSC, NT pigs showed an enhanced mitochondrial swelling compared with SC pigs (P<0.05), and HT showed a reduced mitochondrial swelling compared with NT pigs (P<0.05). However, at 72 hours after ROSC, mitochondrial swelling was similar across all groups (P>0.05). Mitochondrial swelling in all groups was blocked by cyclosporine A, suggesting the participation of permeability transition (data not shown).
Mild Hypothermia Improved Mitochondrial Membrane Potential
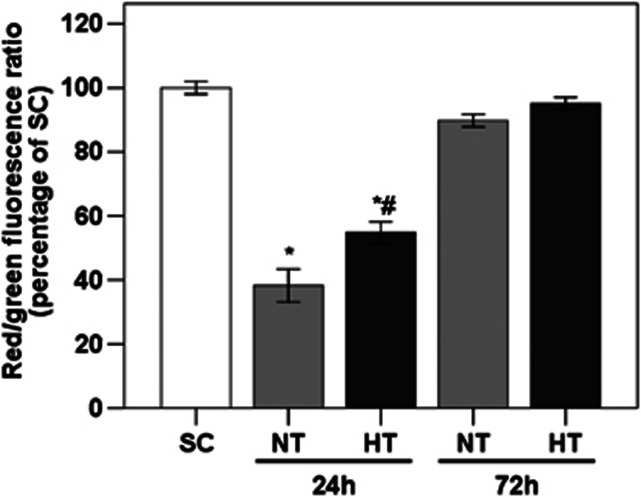
The effect of mild hypothermia on ΔΨm in frontal cortex was assessed using the ratio of red/green JC-1 fluorescence, as shown in Figure 2. At 24 hours after ROSC, HT pigs showed an increased ΔΨm relative to NT pigs (P<0.05). At 72 hours after ROSC, ΔΨm was similar across all groups (all P>0.05).
Figure 2.
Effect of mild hypothermia on mitochondrial membrane potential in frontal cortex of pigs, assessed by the red/green JC-1 fluorescence in NT and HT as a percentage of SC. *P<0.05 compared with SC; #P<0.05 compared with NT24h. HT, mild hypothermia; NT, normothermia; SC, surgery control.
Mild Hypothermia Improved Mitochondrial Respiration
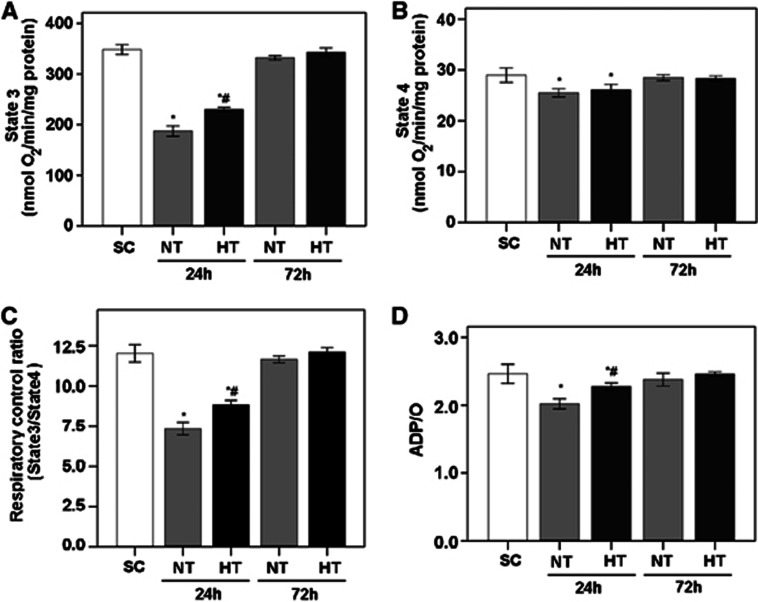
To determine the effect of mild hypothermia on the mitochondrial respiration in frontal cortex after cardiac arrest and resuscitation, oxygen consumption was measured in the presence of the substrates glutamate and malate using a Clark-type oxygen electrode. As shown in Figure 3, at 24 hours after ROSC, NT animals exhibited lower levels of mitochondrial respiration than did SC animals, as indicated by lower values of state 3 and state 4 respiration, RCR (state 3/state 4), and ADP/O ratios (all P<0.05). Moreover, HT animals showed improved mitochondrial respiration compared with NT animals, as indicated by higher state 3 and state 4 respiration, RCR, and ADP/O ratio (all P<0.05). At 72 hours after ROSC, state 3 and state 4 respiration, RCR, and ADP/O ratio in NT and HT animals had recovered to be similar to SC values (all P>0.05).
Figure 3.
Parameters of mitochondrial respiration in frontal cortex of pigs, including state 3 respiration (A), state 4 respiration (B), respiratory control ratio (RCR; state 3/state 4 respiration) (C), and ADP/O ratio (D). Glutamate (5 mmol/L) and malate (5 mmol/L) were used as respiration substrates. Oxygen consumption is expressed in nmol O2/min per mg protein (mean±s.d.). *P<0.05 compared with SC; #P<0.01 compared with NT24h. HT, mild hypothermia; NT, normothermia; SC, surgery control.
Mild Hypothermia Inhibited Mitochondrial Release of Pro-apoptotic Substances and Caspase 3 Cleavage
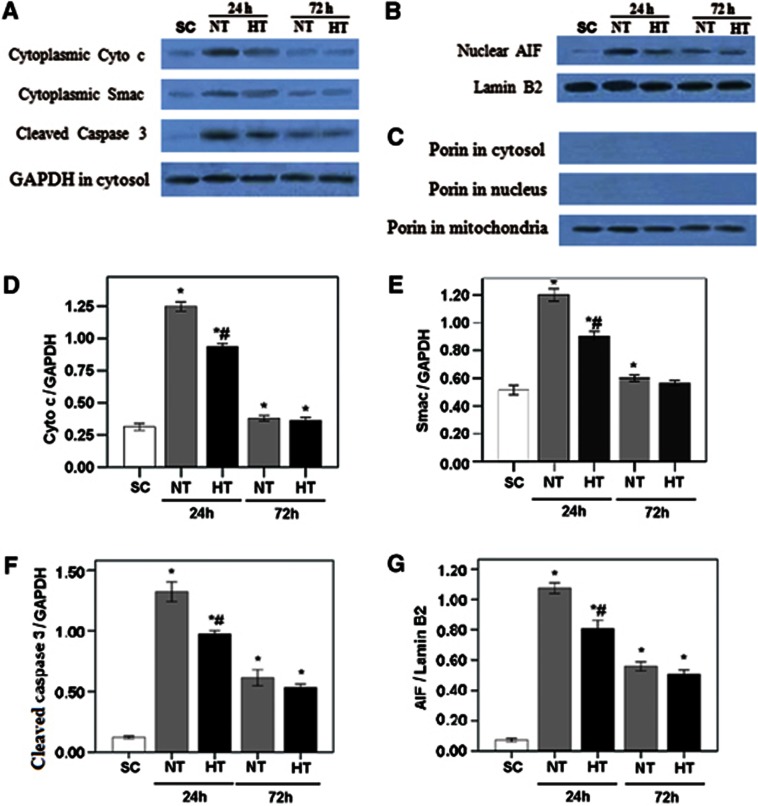
As shown in Figure 4, at 24 hours after ROSC, western blotting analysis showed significant increases in the frontal cortex of NT pigs compared with SC pigs in cytoplasmic cytochrome c, Smac, cleaved caspase 3, and nuclear AIF (all P<0.05). However, HT pigs had a lower expression of these proteins than did NT pigs (all P<0.05). At 72 hours after ROSC, proteins expression in NT and HT animals was similar, but significantly lower than at 24 hours (P<0.05). No gender differences were observed in protein expression (all P>0.05).
Figure 4.
Expression of cytoplasmic cytochrome c, Smac, cleaved caspase 3, and nuclear AIF in frontal cortex of pigs. Western blotting was used to measure cytoplasmic cytochrome c, Smac, and cleaved caspase 3 (A), and nuclear AIF (B). Western blots of Porin (C) showed bands only within the mitochondrial fractions, confirming successful separation of mitochondria. The optical density (OD) of each band was measured using Image J software. Values of cytoplasmic cytochrome c (Cyto c) (D), Smac (E), and cleaved caspase 3 (F) were normalized to GAPDH. Nuclear AIF (G) was normalized to lamin B2. Data are expressed as the mean±s.d. Group sizes were SC, n=5; NT24h, n=6; HT24h, n=7; NT72h, n=5; and HT72h, n=7. *P<0.05 compared with SC; #P<0.01 compared with NT24h. AIF, apoptosis inducing factor; HT, mild hypothermia; NT, normothermia; SC, surgery control.
Mild Hypothermia Reduced Apoptosis
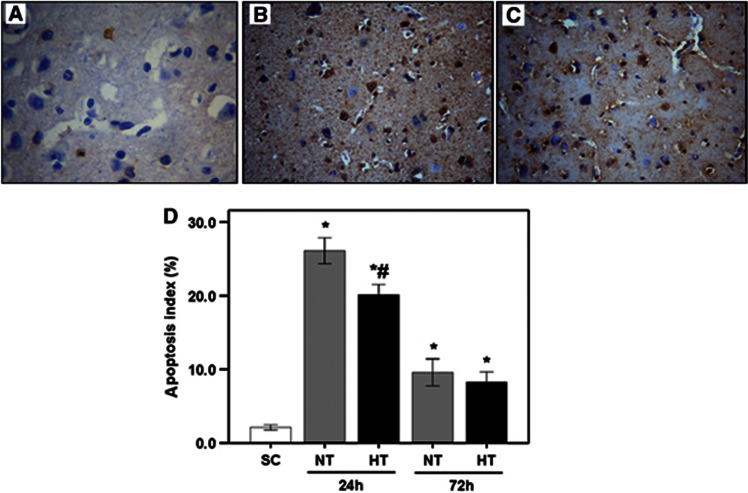
As shown in Figures 5A to 5C, TUNEL-positive cells in frontal cortex had broken nuclei that stained brown/yellow. Figure 5D shows that, at 24 hours after ROSC, the number of TUNEL-positive cells was increased in the NT group (26.1±1.7) compared with SC (2.1±0.3), but was lower in HT (20.1±1.5) than in NT pigs (all P<0.05). At 72 hours after ROSC, TUNEL-positive cells in the NT group were decreased compared with 24 hours (P<0.05), but were similar to HT pigs (P>0.05). No gender differences were observed in the number of TUNEL-positive cells (all P>0.05).
Figure 5.
TUNEL (TUNEL, deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling) analysis of apoptosis in frontal cortex of pigs in SC (A), NT (B), and HT (C) groups and apoptosis index (D). Magnification, × 200. *P<0.05 compared with SC; #P<0.01 compared with NT24h. HT, mild hypothermia; NT, normothermia; SC, surgery control.
Mild Hypothermia Was Associated with Improved Neurologic Function but Not with Survival
Survival rates and NDS were described in detail in our previous report.3 Briefly, 32 pigs were successfully resuscitated from cardiac arrest. After ROSC, survival rates (n=8/group) were 6 (75%) in NT24h, 7 (87.5%) in HT24h, 5 (62.5%) in NT72h, and 7 (87.5%) in HT72h. A total of 7 pigs (4 males, 3 females) died from hemodynamic instability during the postresuscitation period. Compared with NT pigs, HT pigs showed no significant differences in survival rates at 24 and 72 hours (P=0.60 and 0.32, respectively). In the NT and HT groups, survival rates were similar in males and females (P=0.68 and 0.48 at 24 and 72 hours, respectively).
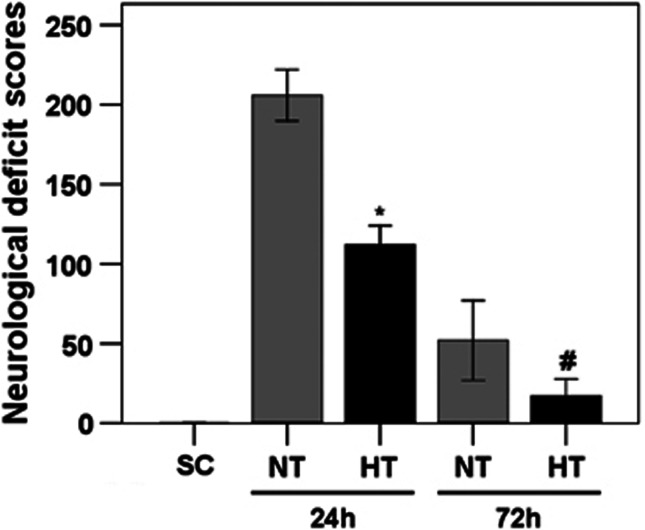
As shown in Figure 6, HT pigs showed significantly lower NDS compared with NT pigs at 24 hours (112.1±12.9 versus 205.8±15.3) and 72 hours (17.1±11.5 versus 52.2±20.2) after ROSC (all P<0.01). Moreover, females in the NT and HT groups showed slightly lower NDS than males at 24 and 72 hours after ROSC, but this difference was not statistically significant (all P>0.05).
Figure 6.
Neurologic deficit scores. Scale range is from 0 (normal) to 400 (brain death). Values are given as median±interquartile range. *P<0.01 compared with NT24h; #P<0.01 compared with NT72h. HT, mild hypothermia group; NT, normothermia group; SC, surgery control.
Discussion
The present study shows that whole-body mild hypothermia after cardiac arrest is associated with positive changes in brain frontal cortex at 24 hours after ROSC, including: inhibition of mPTP opening, mitochondrial release of pro-apoptotic substances, caspase 3 cleavage, and apoptosis, as well as enhanced ΔΨm and mitochondrial respiration. These results indicate that whole-body mild hypothermia reduced the MMP of brain cortex in the well-established VF-induced cardiac arrest model in swine.3, 26, 27 Furthermore, at 24 hours after ROSC, whole-body mild hypothermia improved neurologic outcomes and alleviated microscopic and ultrastructural changes in the cerebral cortex caused by global ischemia-reperfusion, as described in our earlier paper.3 However, at 72 hours after ROSC, NT and HT groups had similar histopathology3 and cellular findings in the present experiment. Thus, we speculate that the neuroprotective effects of mild hypothermia on cerebral injury caused by cardiac arrest might be associated with reduced MMP, and the processes underlying neuronal death and the neuroprotective effects of mild hypothermia might occur predominantly within the first 24 hours (or perhaps 48 hours).
The HT reduced morbidity in our study by improving neurologic outcomes, but it did not reduce mortality, consistent with an Australian study.12 However, an European study showed HT reduced mortality and improved neurologic outcomes.11 Failure to reduce mortality by HT may be due to insufficient sample size. In the European study, a total of 275 patients with out-hospital cardiac arrest were enrolled, and there were a total of 56/137 deaths (41%) in the hypothermia group versus 76/138 (55%) in the NT group (P=0.02). However, in the Australian study, a total of 77 patients with out-hospital cardiac arrest were enrolled, and mortality at discharge was 22/43 (51%) in the hypothermia group and 23/34 (68%) in the NT group (P=0.145).
The MIM permeabilization involves formation of mPTPs that disrupt the ΔΨm across the MIM.6 Thus, MIM permeabilization is generally detected by mPTP opening and altered ΔΨm.6 In the current study, at 24 hours after ROSC, calcium-induced mPTP opening was increased in brain frontal cortex neurons in NT pigs and was inhibited by whole-body mild hypothermia in HT pigs. Beneficial effects of hypothermia on ΔΨm were also evident at 24 hours after ROSC. These data indicate that, even at 24 hours after postischemic normothermic recovery, MIM permeabilization was increased and could be attenuated by mild hypothermia.
The mPTP is a large conductance channel found in the MIM and MOM, which is composed of mitochondrial proteins. When opened, the mPTP allows matrix solutes <1.5 kDa, including protons, to be released from the mitochondria,28 which dissipates ΔΨm and thus decreases ATP production. In the current study, arrested-resuscitated pigs subjected to postischemic normothermic recovery for 24 hours had reduced mitochondrial respiration in brain frontal cortex, indicating decreased ATP production. Moreover, release of mitochondrial matrix solutes increases colloidal osmotic pressure in the mitochondrial matrix, causing matrix swelling, rupture of the MOM, and nonspecific release of intermembrane space proteins into the cytosol.6, 29
Mitochondrial outer membrane normally retains pro-apoptotic proteins in the intermembrane space, including cytochrome c, Smac/DIABLO, and AIF.6 Thus, MOM permeabilization is detected by measuring translocation of these proteins from the intermembrane space.6 In this study, mild hypothermia reduced the release of these pro-apoptotic substances from the mitochondria at 24 hours after ROSC, indicating that mild hypothermia attenuated MOM permeabilization. In general, MOM permeabilization is a point-of-no-return in apoptosis.5 In this study, mild hypothermia was associated with decreased expression of cleaved caspase 3, a final caspase responsible for carrying out apoptosis, and a reduced apoptotic index in the brain frontal cortex neurons at 24 hours after ROSC.
The mechanisms underlying reduction in MMP by mild hypothermia are complex. Mild hypothermia could impact the complex balance between inducers and antagonists of mPTP opening, which determines whether the duration of mPTP opening is transient or intermediate/long lasting.30 Ischemia-reperfusion injury is associated with increases in mPTP activators (Ca2+, ROS, Pi) and reductions in mPTP inhibitors (ATP/ADP).31 The mPTP is inhibited by low pH and is therefore believed to be quiescent during ischemia.31 However, restoration of pH coupled with rapid elevation of mitochondrial Ca2+ and ROS on reperfusion would lead to rapid opening of the mPTP.7, 31 Such a scenario would initiate a vicious cycle, as the subsequent mPTP opening would further dysregulate Ca2+ homeostasis and inhibit the respiratory chain, resulting in further ROS production and mPTP opening. Mild hypothermia has been closely associated with reduced ROS generation, as described in our previous report.3 Thus, we speculate that inhibition of mPTP opening by mild hypothermia might be associated, at least in part, with reduced ROS generation. Another mechanism underlying reduction of MMP by mild hypothermia might be related to direct effects on the molecular composition of the mPTP. The precise molecular architecture of the mPTP is controversial,21, 32, 33 although an emerging consensus believes that a multicomponent protein complex rather than a single protein is responsible for mPTP opening. Elucidating mPTP architecture would greatly advance our understanding of how mild hyperthermia might inhibit mPTP opening.
The current study has some limitations. First, to mimic the process of whole-body mild hypothermia applied to cardiac arrest patients,12 we assessed effects after rewarming and not during the 12-hour period of mild hypothermia. Indeed, the impact of mild hypothermia on the MMP might be most potent during the 12-hour period of mild hypothermia, whereas more recent rodent studies showed that a prolonged reduction in temperature (12 to 48 hours) of only a few degrees provided sustained behavioral and histologic neuroprotection as long as 6 months postischemia onset in rodents.34, 35, 36 Second, our study focused on the frontal cortex, as the cortex is known to have a vital role in recovery of function after cardiac arrest. However, the hippocampus is most vulnerable to global ischemic injury and may therefore have shown greater effects. Finally, due to the limited supply of inbred Chinese Wuzhishan minipigs, pigs of both sexes were used. Hormonal differences due to sexual dimorphism may affect the outcome of cerebral ischemia.37, 38, 39, 40 However, no significant sex differences in postresuscitative brain damage or in the effects of mild hypothermia were found in the current study. The lack of sex differences may be due to the sexual immaturity of the majority of the pigs (31/37 were ∼4 months old) and the small sample size of male and female pigs in each group.
In conclusion, prolonged whole-body mild hypothermia ameliorates the effects of cardiac arrest on MMP and cerebral injury, which may explain its neuroprotective effects.
Acknowledgments
The authors deeply appreciate Jie Xiong, Xuefeng Xu, Xianfei Ji, Zhiyu Su, Junyuan Wu, and Shuo Wang for their technical assistance. This work was supported by the Liaoning Province Nature Science Foundation.
The authors declare no conflict of interest.
References
- Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Gong P, Li CS, Hua R, Zhao H, Tang ZR, Mei X, et al. Mild hypothermia attenuates mitochondrial oxidative stress by protecting respiratory enzymes and upregulating MnSOD in a pig model of cardiac arrest. PLoS One. 2012;7:e35313. doi: 10.1371/journal.pone.0035313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub IM, Radhakrishnan J, Gazmuri RJ. Targeting mitochondria for resuscitation from cardiac arrest. Crit Care Med. 2008;36:S440–S446. doi: 10.1097/ccm.0b013e31818a89f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnareva Y, Newmeyer DD. Bioenergetics and cell death. Ann NY Acad Sci. 2010;1201:50–57. doi: 10.1111/j.1749-6632.2010.05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Rasola A, Sciacovelli M, Pantic B, Bernardi P. Signal transduction to the permeability transition pore. FEBS Lett. 2010;584:1989–1996. doi: 10.1016/j.febslet.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem. 2004;279:17197–17204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Marion D, Bullock MR. Current and future role of therapeutic hypothermia. J Neurotrauma. 2009;26:455–467. doi: 10.1089/neu.2008.0582. [DOI] [PubMed] [Google Scholar]
- Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab. 2007;27:1879–1894. doi: 10.1038/sj.jcbfm.9600540. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Biphasic cytochrome c release after transient global ischemia and its inhibition by hypothermia. J Cereb Blood Flow Metab. 2005;25:1119–1129. doi: 10.1038/sj.jcbfm.9600111. [DOI] [PubMed] [Google Scholar]
- Xu L, Yenari MA, Steinberg GK, Giffard RG. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J Cereb Blood Flow Metab. 2002;22:21–28. doi: 10.1097/00004647-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Meybohm P, Gruenewald M, Albrecht M, Zacharowski KD, Lucius R, Zitta K, et al. Hypothermia and postconditioning after cardiopulmonary resuscitation reduce cardiac dysfunction by modulating inflammation, apoptosis and remodeling. PLoS One. 2009;4:e7588. doi: 10.1371/journal.pone.0007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Puttgen HA, Geocadin R. Predicting neurological outcome following cardiac arrest. J Neurol Sci. 2007;261:108–117. doi: 10.1016/j.jns.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Kristal BS, Park BK, Yu BP. 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. J Biol Chem. 1996;271:6033–6038. doi: 10.1074/jbc.271.11.6033. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. What makes the mitochondria a killer? Can we condition them to be less destructive. Biochim Biophys Acta. 2011;1813:1302–1308. doi: 10.1016/j.bbamcr.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Tretter L, Mayer-Takacs D, Adam-Vizi V. The effect of bovine serum albumin on the membrane potential and reactive oxygen species generation in succinate-supported isolated brain mitochondria. Neurochem Int. 2007;50:139–147. doi: 10.1016/j.neuint.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li CS, Gong P, Tang ZR, Hua R, Mei X, et al. Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation. 2012;83:913–920. doi: 10.1016/j.resuscitation.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Wang S, Li CS, Ji XF, Yang L, Su ZY, Wu JY. Effect of continuous compressions and 30:2 cardiopulmonary resuscitation on global ventilation/perfusion values during resuscitation in a porcine model. Crit Care Med. 2010;38:2024–2030. doi: 10.1097/CCM.0b013e3181eed90a. [DOI] [PubMed] [Google Scholar]
- Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosc. 2009;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both. Biochim Biophys Acta. 2011;1813:616–622. doi: 10.1016/j.bbamcr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F, Corbett D, Zhao Z, Yang J, Buchan AM. Prolonged but delayed postischemic hypothermia: a long-term outcome study in the rat middle cerebral artery occlusion model. J Cereb Blood Flow Metab. 2000;20:1702–1708. doi: 10.1097/00004647-200012000-00009. [DOI] [PubMed] [Google Scholar]
- Corbett D, Hamilton M, Colbourne F. Persistent neuroprotection with prolonged postischemic hypothermia in adult rats subjected to transient middle cerebral artery occlusion. Exp Neurol. 2000;163:200–206. doi: 10.1006/exnr.2000.7369. [DOI] [PubMed] [Google Scholar]
- Arnold S, Beyer C. Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J Neurochem. 2009;110:1–11. doi: 10.1111/j.1471-4159.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, et al. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp M, Berger K, Clarner T, Dang J, Beyer C. Sex steroids control neuroinflammatory processes in the brain: relevance for acute ischaemia and degenerative demyelination. J Neuroendocrinol. 2012;24:62–70. doi: 10.1111/j.1365-2826.2011.02163.x. [DOI] [PubMed] [Google Scholar]
- Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin Reprod Med. 2009;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]