Abstract
LMO1 is a transcriptional regulator and a T-acute lymphoblastic leukaemia (T-ALL) oncogene. Although first identified in association with a chromosomal translocation in T-ALL, the ectopic expression of LMO1 occurs far more frequently in the absence of any known mutation involving its locus. Given that LMO1 is barely expressed in any haematopoietic lineage, and activation of transcriptional drivers in leukaemic cells is not well described, we investigated the regulation of this gene in normal haematopoietic and leukaemic cells. We show that LMO1 has two promoters that drive reporter gene expression in transgenic mice to neural tissues known to express endogenous LMO1. The LMO1 promoters display bivalent histone marks in multiple blood lineages including T-cells, and a 3' flanking region at LMO1 +57 contains a transcriptional enhancer that is active in developing blood cells in transgenic mouse embryos. The LMO1 promoters become activated in T-ALL together with the 3' enhancer, which is bound in primary T-ALL cells by SCL/TAL1 and GATA3. Taken together, our results show that LMO1 is poised for expression in normal progenitors, where activation of SCL/TAL1 together with a breakdown of epigenetic repression of LMO1 regulatory elements induces ectopic LMO1 expression that contributes to the development and maintenance of T-ALL.
Keywords: LMO1, transcriptional regulation, T-ALL, bivalent chromatin
Introduction
Lim-only 1 (LMO1) also known as T-cell translocation gene 1 (TTG-1) and Rhombotin 1 (RBTN-1) encodes a LIM domain transcriptional cofactor, and was originally identified at a chromosomal breakpoint in a T-acute lymphoblastic leukaemia (T-ALL) cell line bearing the t(11;14)(p15;q11) translocation.1, 2 LMO1 is not normally expressed in T-cells, but the t(11;14)(p15;q11) juxtaposes LMO1 to gene regulatory elements within the T-cell receptor α/δ loci leading to ectopic expression in a T-lymphoid environment. Even though <1% of T-ALL patients carry translocations involving LMO1, ∼50% express significant levels of LMO1,3 thus suggesting that aberrant activation of regulatory elements within the LMO1 gene locus represents a common feature of dysregulated transcriptional programs in T-ALL. Given the unequivocal demonstration through transgenic mouse experiments that ectopic LMO1 expression in T-cells causes T-ALL,4 a detailed understanding of transcriptional control mechanisms operating at the LMO1 gene locus would appear vital to elucidate the mechanisms responsible for ectopic expression in T-ALL patients without LMO1 translocations.
Despite its long-established role as a T-ALL oncogene, relatively little is known about the normal function of LMO1. Lmo1-homozygous null mice are viable with no discernible phenotype.5 As knockout mice for the highly related Lmo3 gene were similarly viable5 and because the expression domains of Lmo1 and Lmo3 overlap,6 Lmo1 and Lmo3 double knockout mice were also generated, which lead to perinatal death, the cause of which was not determined.5 When expressed ectopically in T-cells in transgenic mice together with SCL/TAL1, double-transgenic mice display abnormalities of thymocyte development with altered proliferation, apoptosis and immunophenotype prior to the onset of a frank T-cell malignancy.7 LMO1 forms multiprotein complexes with transcriptional regulators such as SCL,8, 9 LDB1,10 GATA3.11 LMO1 has also been proposed to participate in a Hox-dependent regulatory network in the developing hindbrain.12 However, no significance during normal LMO1 function has as yet been ascribed to the formation of such protein complexes. Finally, there has been considerable recent interest in the role of LMO1 in neurological malignancies including work to suggest that levels of LMO1 may have a role in increased susceptibility to and increased aggressiveness of neuroblastoma.13, 14
In the developing mouse embryo, Lmo1 is expressed in forebrain, hindbrain, the developing eye, developing olfactory system and spinal cord (EurEXPRESS15). LMO1 expression in adult mouse tissues is most prominent in the bladder and a subset of neural tissues such as the retina and hippocampus (BIOGPS16). Cis-regulatory control mechanisms responsible for directing the expression of the LMO1 paralogue LMO2, which also functions as a T-ALL oncogene, have been investigated in significant detail.17, 18, 19 However, our current understanding of the cis-regulatory control of LMO1 expression is markedly lacking by comparison. Studies published to date have reported two alternative transcripts in cell lines by northern blotting,2, 20 which are the result of transcriptional initiation from two alternative promoters,21 one of which was shown to drive expression to the developing hindbrain.22 However, no distal regulatory elements nor upstream regulators have been identified so far. Moreover, the possible involvement of LMO1 regulatory elements in mediating ectopic expression in T-ALL had not been explored.
Here, we used a combination of transgenic and transcriptional assays that allowed us to show that the LMO1 promoters are primed for ectopic expression in T-cell leukaemias due to the presence of bivalent promoter histone marks and a latent haematopoietic enhancer. Following activation of the LMO1 promoters and enhancer in T-ALL, the enhancer is bound in primary T-ALL cells by SCL and GATA3, thus suggesting that breakdown of epigenetic repression of LMO1 represents a key step in the activation of a reinforcing loop of T-ALL oncogenes.
Materials and methods
Transgenic mouse analysis
Promoter and enhancer regions were PCR-amplified from human genomic DNA (promoter 1: hg19 chr11; 8290024-8290905, promoter 2: hg19 chr11;8284924-8285968), subcloned into a lacZ reporter vector and transgenic mice were generated and analysed as previously described17 A total of 35 transient transgenic mouse embryos were analysed. Selected embryos were cleared, sectioned, stained and photographed as previously described.17 All animal experiments were performed in accordance with UK Home Office rules and were approved by Home Office inspectors.
Epigenomic data repository
NIH Roadmap Epigenomics data were accessed via www.roadmapepigenomics.org, including histone modification data for human ES cells, CD3 T-cells, CD109 B-cells and CD15 monocytes as well as DNaseI hypersensitivity in CD34 cells. ENCODE Project data were accessed via genome.ucsc.edu for transcription factor-binding sites and CTCF boundaries in K562 cells.
Cell preparation and culture
Human T-ALL peripheral blood and bone marrow aspirate samples were obtained following informed consent at diagnosis from children and adults with T-ALL via a study protocol approved by the Research Ethics Committee of Addenbrooke's Hospital and the University of Cambridge. Banked T-ALL samples were recovered for 12 h in RPMI1640 supplemented with 20% FCS. Fresh T-ALL samples, X-ALLs CD3 and CD19 lymphocytes, CD34 cells, HUVECs and cultured megakaryocytes were prepared as described previously.19
Real-time PCR estimation of total LMO1 expression
RNA was prepared from patient samples and cell lines with Trizol reagent (Invitrogen, Paisley, UK). cDNA was prepared using random hexamers and TaqMan reverse transcriptase reagents kit (Applied Biosystems, Paisley, UK). Quantitative PCRs (qPCR) were run twice in triplicate using Stratagene Brilliant Sybr Green QPCR Master Mix (Agilent Technologies, Wokingham, UK) (Primers listed in Supplementary information). Standard curves for LMO2 and β-actin were created by dilutions of CD4 cDNA. The data are reported as expression (normalised to β-actin housekeeper) relative to CD4. Error bars represent s.d.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was carried out as described previously23 using 1 × 107 cells per condition using commercially available antibodies (Supplementary Information). The relative enrichment of immunoprecipitated DNA was estimated using real-time PCR (Primers listed in Supplementary Information). Enrichment at each region was calculated relative to ChIP IgG pulldown and to the negative control region. Each reaction was undertaken twice and the experiment was undertaken on two occasions. The means of the data points are plotted with error bars representing s.d.
Luciferase reporter assay
Plasmid DNA was prepared and the cells were transfected, subjected to antibiotic selection at an appropriate dose (as determined by kill-curve), lysate prepared and assayed as described previously.17 The mutant enhancer construct was generated by long PCR (using Del_LMO1enh forward and reverse primers; see Supplementary Materials for primer sequences). The PCR primers were designed to replace the conserved core region of the enhancer with an NheI restriction site. Data from four technical replicates of experiments designed with four biological replicates of each construct were normalised against pGL2 basic vector and background was set as 1. The mean of these data points is plotted with error bars representing s.d. Three biological replicates, each assayed in four technical replicates, were analysed for the enhancer deletion construct. Paired t-tests were used to determine the significance of loss of enhancer activity with the enhancer mutation, and made use of all 12 raw datapoints for each construct.
Results
The human LMO1 gene locus contains two alternative promoters that recapitulate endogenous LMO1 expression in transgenic mouse assays
The transcriptional control of LMO1 has not been reported in any detail. We began our investigations by reviewing the RIKEN transcript database and database of transcriptional start sites (DBTSS) as well as ENSEMBL and UCSC genome annotations, and noted two alternative LMO1 transcripts (Figure 1a). These alternative 5′ start sites coincide with peaks of non-coding sequence conservation suggesting possible roles as alternative promoters for LMO1. This is compatible with the work done previously,20, 24 where two alternative promoters of LMO1 were proposed following northern hybridisation in the LMO1-translocated T-ALL cell line RPMI8402 and the human neuroectodermal small cell lung cancer-derived cell line N417. Following the mapping of the sequence data reported by Boehm et al.21 in 1991 to the human reference genome, we confirmed that the limited sequence data reported >20 years ago indeed corresponds to the two evolutionarily conserved start sites indicated in current annotations of the human genome.
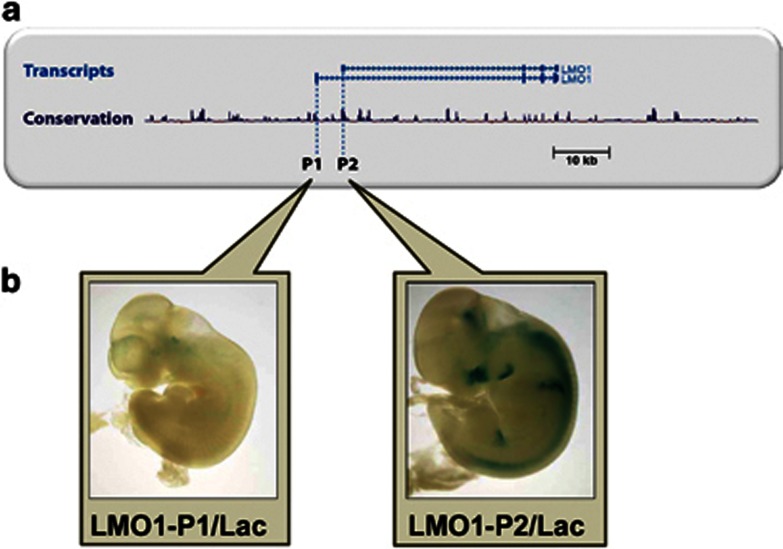
Figure 1.
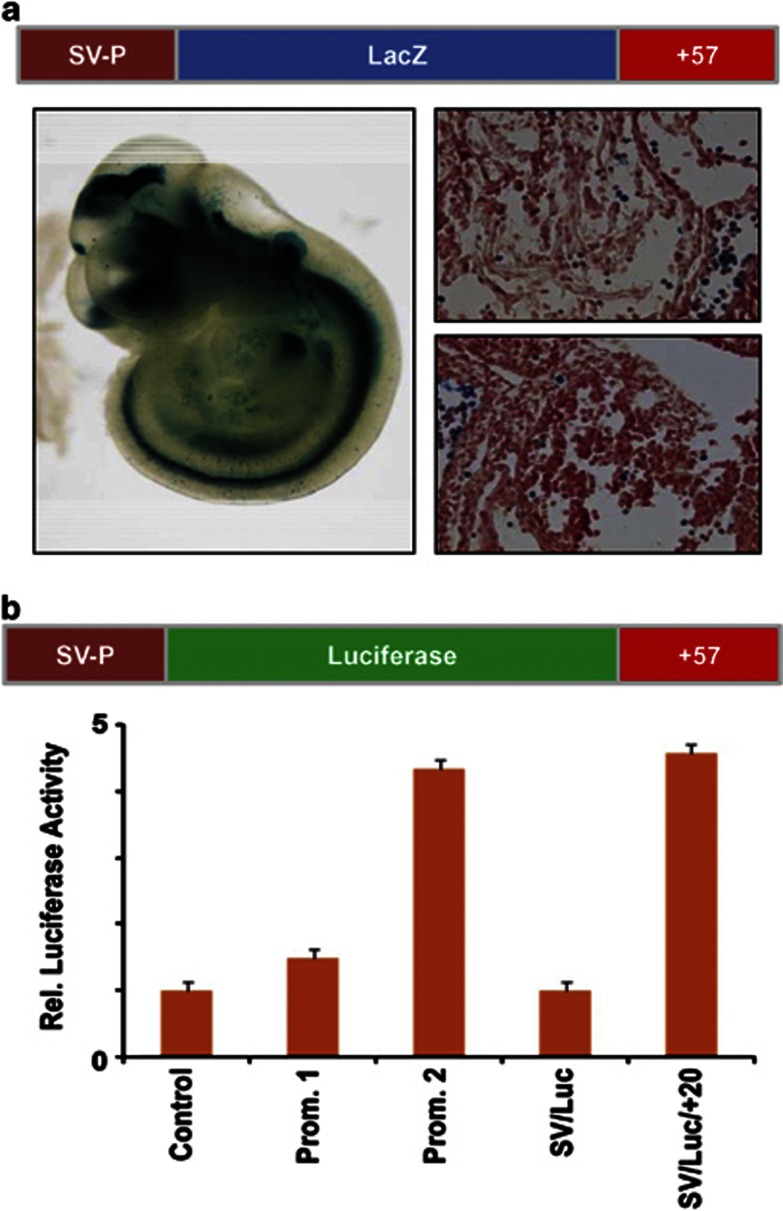
The human LMO1 gene locus contains two alternative promoters that recapitulate endogenous LMO1 expression in transgenic mouse assays. (a) Two alternative LMO1-validated transcripts are evident on review of the UCSC gene transcript database. Each coincides with a peak of non-coding sequence conservation suggesting possible roles as alternative promoters for LMO1. (b) Transgenic analysis of reporter constructs with the two respective promoter sequences upstream of a lacZ reporter gene. Representative embryos demonstrate that the promoter1/lacZ construct generated faint staining compatible with activity in neurological structures in the brainstem and first and second branchial arches. Transgenic embryos carrying the promoter2/lacZ construct showed expression in hindbrain, spinal cord, intersomitic mesoderm and olfactory epithelium thereby recapitulating much of the expression pattern found for endogenous LMO1. No LMO1 expression is seen in tissues of the developing haematopoietic system in embryos generated by either construct.
To investigate the in vivo function of the two LMO1 promoters, reporter constructs were generated (see Materials and Methods) with the two respective promoter sequences upstream of a lacZ reporter gene. Following pronuclear microinjection, F0 transgenic mice were generated, permitting the assessment of promoter activity by wholemount staining of E11.5 embryos. Representative embryos (Figure 1b) demonstrated that the promoter1/lacZ construct generated faint staining compatible with activity in neurological structures in the brainstem and first and second branchial arches. Transgenic embryos carrying the promoter2/lacZ construct showed expression in hindbrain, spinal cord, inter-somitic mesoderm and olfactory epithelium thereby recapitulating much of the expression pattern found for endogenous LMO1 (Supplementary Figure S1). Taken together, these in vivo transgenic data allowed us to validate the two putative LMO1 promoters as bona fide regulatory elements. The observed expression patterns correlated well with the known LMO1 expression domain, and were also consistent with the lack of notable LMO1 expression in tissues of the developing haematopoietic system.
LMO1 promoters display bivalent chromatin modification marks in a range of haematopoietic cells
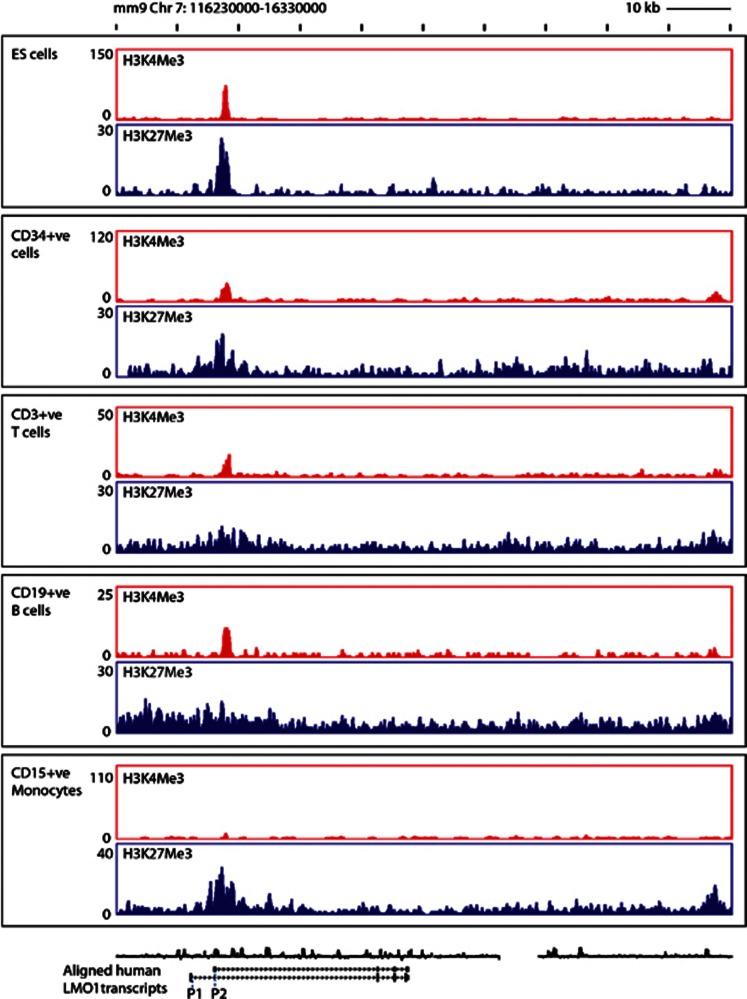
Despite a clear role in leukaemia, there is no significant LMO1 expression at major stages of normal haematopoietic differentiation (Supplementary Figure S2). To investigate whether lack of expression in the haematopoietic system may be due to active repression of the two LMO1 promoters, we analysed ChIP-Sequencing data released to the public domain by the NIH Roadmap Epigenomics Mapping Project25 (http://www.roadmapepigenomics.org/). Remarkably, enrichment peaks for both the activating H3K4Me3 and repressive H3K27Me3 histone mark were present in the 5' region of LMO1 in ES cells, CD34 cells, CD3 T cells and CD19 B cells (Figure 2). Peak regions for both the activating and repressive histone marks coincided and were situated at the promoter 2 (P2) region, thus marking this promoter as a bivalent promoter in these cell types. Of note, the positive H3K4me3 mark was absent in CD15 monocytes with only the repressive mark remaining. Lack of substantial expression was confirmed for all cell types by the absence of H3K36Me3 enrichment, (Supplementary Figure S3) in line with previously reported expression data.6, 16
Figure 2.
LMO1 promoters display bivalent chromatin modification marks in a range of haematopoietic cells. Enrichment peaks are seen for both the activating H3K4Me3 and repressive H3K27Me3 histone mark in the 5' region of LMO1 in ES cells, CD34 cells, CD3 T-cells and CD19 B–cells, with peak regions for both the activating and repressive histone marks over the promoter 2 (P2) region in these cell types. The positive H3K4me3 mark was absent in CD15 monocytes with only the repressive mark remaining.
Bivalent promoter marks in the absence of gene expression have traditionally been associated with genes being poised for expression.26 Given the potentially fatal consequences of expressing LMO1 within the T-cell lineage, the poised status of LMO1 promoters was unexpected. Importantly, inspection of the neighbouring genes confirmed that the absence of H3K36Me3 signal over the LMO1 locus was not subject to technical failure (Supplementary Figure S4). Of note, bivalent promoter marks were also seen in primary mouse megakaryocytes in datasets generated as part of the mouse ENCODE consortium (see Supplementary Figure S5A). Taken together, this analysis suggests that, despite no known role for LMO1 in normal haematopoietic cells, the gene is poised for expression in several haematopoietic lineages including T-lymphoid cells, which given its known function as a T-ALL oncogene is highly significant.
High LMO1 expression is evident in a significant proportion of T-ALL cell lines and patient samples
We next wanted to identify cellular models that would allow us to assess a potential contribution of LMO1 regulatory elements to ectopic expression in T-ALL. To directly quantify LMO1 transcripts in a range of malignant and non-malignant cell types, real-time PCR was undertaken on four normal haematopoietic cell types, cultured endothelial cells (HUVECs), seven T-ALL cell lines and 21 primary T-ALL samples. Individual results are presented relative to the very low signal obtained with the CD4 cell sample, (Figure 3) as described by Asnafi et al.3 As noted previously, LMO1 is not noted to be expressed in blood cells when analysed by expression microarray technology but, by using this more sensitive method, we were able to detect low-level expression that would likely be below the detection threshold of microarray technology. Of note, this low-level expression was significantly below the levels seen in an LMO1-expressing neuroblastoma cell line (Supplementary Figure S6A), and would be consistent with our finding of bivalent promoter marks over the LMO1 promoter in several haematopoietic lineages.
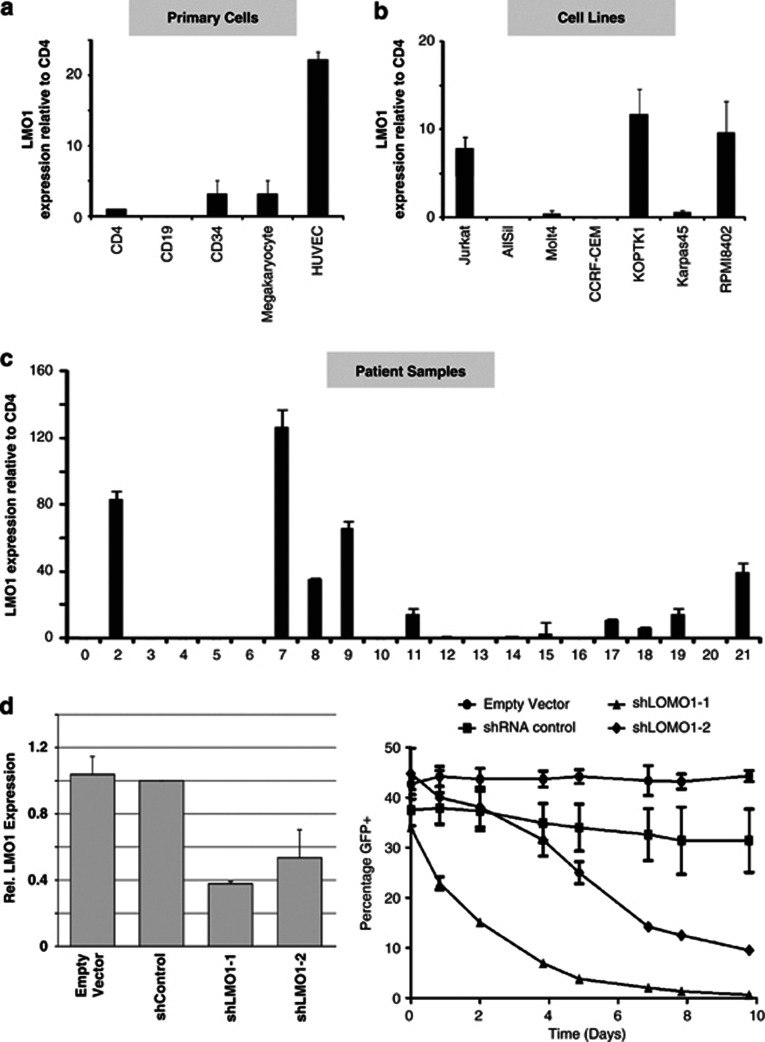
Figure 3.
High LMO1 expression is evident in a significant proportion of T-ALL cell lines and patient samples. (a) Very low level LMO1 expression was observed in CD34 cells and in megakaryocytes at levels not significantly above those seen in CD4 T-cells. By contrast, markedly higher levels of expression were detected in cultured endothelial cells. (b) Three of the seven T-ALL cell lines (Jurkat, KOPTK1 and RPMI8402) had LMO1 expression levels significantly above those in CD4 T-cells, two cell lines had similar LMO1 expression levels to those seen in CD4 T-cells (Molt4 and Karpas45) with the remainder having undetectable levels of expression (AllSill and CCRF-CEM). (c) Nine of twenty-one primary T-ALL samples assayed showed high levels of LMO1 expression with three (patients 2, 7 and 9) showing very high levels of LMO1 expression. (d) LMO1 expression is reduced by shRNA knockdown, leading to lower cell proliferation. Two shRNA LMO1 knockdown vectors were designed using the RNAi Central webtool (http://katahdin.mssm.edu/siRNA/RNAi.cgi?type=shRNA) and cloned into the GFP-encoding pLL3.7 plasmid. Jurkat cells were transduced and both shRNA constructs (shLMO1-1 and shLMO1-2) showed marked reduction in LMO1 expression compared with the negative control shRNA (shControl) and empty vector. Moreover, growth of shRNA-transduced cells was markedly reduced for cells transduced with shLMO1-1 and shLMO-2, compared with empty vector and the negative control. Data shown are from a representative biological replicate experiment performed in duplicate.
As seen in Figure 3a, very low level LMO1 expression was observed in CD34 cells and in megakaryocytes at levels not significantly above those seen in CD4 T-cells. By contrast, markedly higher levels of expression were detected in cultured endothelial cells. Three of the seven T-ALL cell lines (Jurkat, KOPTK1 and RPMI8402) had LMO1 expression levels significantly above those in CD4 T cells with the remainder having undetectable levels of expression (AllSill and CCRF-CEM) or similar levels to those seen in CD4 T cells (Molt4 and Karpas45) (Figure 3b). Of the 21T-ALL primary samples analysed, nine showed high levels of LMO1 expression. Patients 2, 7 and 9 (Figure 3c) showed very high levels of LMO1 expression. This proportion of LMO1-positive T-ALL samples is similar to that previously reported where a significant subset of patients were found to express high levels of LMO1, often with no evidence of a chromosomal translocation involving the LMO1 locus.3 Moreover, LMO1 knockdown in Jurkat cells severely compromises their proliferative capacity (Figure 3d) pointing to a non-redundant role for LMO1 in leukaemia maintenance. The expression survey carried out here therefore provided both cell line and primary patient cellular models to further investigate the potential contribution of LMO1 regulatory elements to ectopic expression in T-ALL.
A conserved non-coding region 3' of LMO1 displays active chromatin marks and transcription factor binding in haematopoietic cells
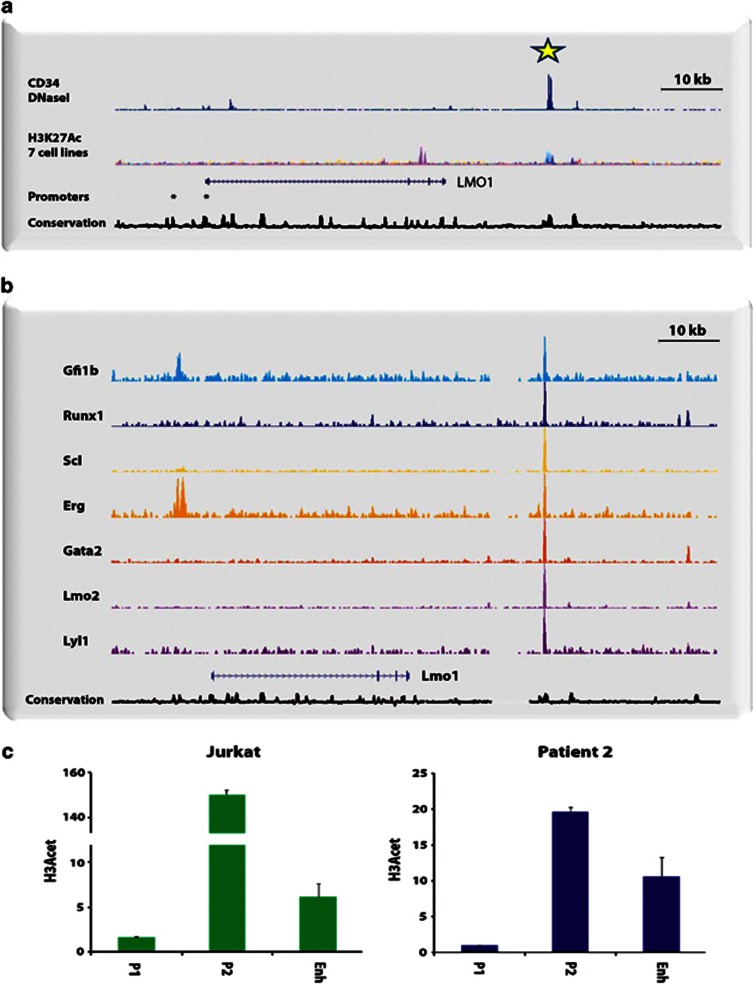
Utilising publicly available datasets from the ENCODE project and Roadmap Epigenomics project, we noted a highly conserved region ∼20 kb 3' to the final exon of human LMO1 (+57 kb from the ATG immediately adjacent to the second exon). This +57 region displayed marked DNaseI hypersensitivity, had histone H3 acetylation peaks in HUVEC cells as well as in the myeloid leukaemia cell line K562, and was bound by multiple transcription factors in a range of cell types (Figure 4a). Of note, this region was situated within regions bound constitutively by the genomic architectural protein CTCF either side of the LMO1 gene locus, thus indicating that the +57 region lies within the likely boundaries of the LMO1 regulatory domain (data not shown). To confirm transcription factor binding to this region in haematopoietic cells, we next investigated a previously published 10 transcription factor dataset for the HPC7 murine blood progenitor cell line.27 Again, we observed binding by multiple transcription factors including Gfi1b, Runx1, Gata2, Erg, Scl/Tal1, Lmo2 and Lyl1 to the mouse equivalent of the human LMO1 +57 region (Figure 4b). The presence of active epigenetic marks (DNaseI and histone acetylation) together with the binding of multiple transcription factors therefore identified the +57 region as a candidate enhancer element. Moreover, as SCL, LMO2 and LYL1 have all been identified as T-ALL oncogenes in human patients28, 29, 30 and Erg has been shown to cause T-ALL in mouse models,31 binding of these factors in a myeloid progenitor cell line suggested that some of these factors may also act on the LMO1 +57 enhancer in LMO1-expressing T-ALL cells.
Figure 4.
A conserved non-coding region 3' of LMO1 displays active chromatin marks and transcription factor binding in haematopoietic cells: (a) A highly conserved region ∼20 kb 3' to the final exon of the human LMO1 gene (+57 kb from the ATG immediately adjacent to the second exon) was identified in publicly available datasets from the ENCODE project, Roadmap Epigenomics project and UCSC goldenpath. This +57 region displayed marked DNaseI hypersensitivity, had histone H3 acetylation peaks in human umbilical vein endothelial cells (HUVEC) as well as the myeloid leukaemia cell line K562, and was bound by multiple transcription factors in a range of cell types. (b) Binding by multiple transcription factors including Gfi1b, Runx1, Gata2, Erg, Scl/Tal1, Lmo2 and Lyl1 to the mouse equivalent of the human LMO1 +57 region in the HPC7 murine blood progenitor cell line. (c) Real-time PCR analysis of H3 Acetylation ChIP material demonstrated minimal enrichment at promoter 1, but significant enrichment at both promoter 2 and the +57 region, in a nontranslocated LMO1-expressing T-ALL (Jurkat) cell line and a non-translocated T-ALL patient sample.
We next undertook chromatin immunoprecipitation assays in nuclei generated from Jurkat, a nontranslocated LMO1-expressing T-ALL cell line as well as a nontranslocated LMO1 expressing T-ALL patient sample using an antibody to acetylated histone H3, as this histone modification marks active promoters and enhancers (Figure 4c). Real-time PCR analysis demonstrated minimal enrichment at promoter 1, but significant enrichment was evident at both promoter 2 and the +57 region. Of note, enrichment levels were significantly lower in two primagrafts samples that did not express LMO1 (see Supplementary Figure S5B). Taken together, these observations suggest that the LMO1 +57 region functions as a transcriptional enhancer element in T-ALL cells, and may utilise known T-ALL oncogenic transcription factors such as SCL to mediate its activity.
The LMO1 +57 kb element directs reporter gene expression to haematopoietic tissues in F0 transgenic mice
Having established the LMO1 +57 region as a DNA sequence carrying active epigenetic marks and bound by multiple transcription factors, we next addressed whether it can indeed function as a transcriptional enhancer. To this end, lacZ reporter constructs were generated by inserting the human +57 region downstream of a minimal promoter/lacZ reporter cassette, which would permit assessing the ability of the LMO1 +57 kb region to direct expression in vivo in transgenic mice. Following pronuclear microinjection, F0 transgenic mice were generated and enhancer activity assessed by wholemount staining of E11.5 embryos. Seven out of eight transgenic embryos with lacZ staining showed activity in several neural tissues as well as in the heart and liver.
As evidenced by the representative embryo shown in Figure 5a, the LMO1 +57 region indeed functions as a transcriptional enhancer element in transgenic mice, directing staining to multiple tissues including the eye, olfactory placodes, apical ectodermal ridge, intersomitic mesoderm, liver and heart. Importantly, LMO1 +57 also directed expression to haematopoietic cells in the foetal liver (see histological sections in Figure 5a). Transgenic mouse assays therefore allowed us to validate the LMO1 +57 region as a bona fide enhancer element with tissue-specific activity in transgenic mice that included developing haematopoietic cells within the midgestation embryo.
Figure 5.
The LMO1 +57 kb element functions as a transcriptional enhancer in blood cells. (a) F0 transgenic mouse analysis of the LMO1 +57 region. Shown is a representative wholemount E11.5 embryo stained with X-Gal to determine the β-galactosidase expression pattern driven by the putative +57 enhancer. Histological sections of the same embryo confirm LacZ staining in circulating blood cells within the heart as well as the foetal liver. Wholemount images were taken at 2 × and tissue section images at 40 × magnification. (b) The promoter and enhancer elements are active in T-ALL cell lines. Luciferase reporter constructs were generated for the P1 and P2 promoter regions and the +57 putative enhancer region (LMO1 P1/luc, P2/luc and SV/luc/+57). These were stably transfected into LMO1-expressing, non-LMO1-translocated Jurkat cells alongside empty vector counterparts. All constructs showed greater activity than the empty vector (pGL2basic). Data shown are from three independent experiments, each performed in triplicate.
The LMO1 promoter and enhancer elements are active in T-ALL cell lines
Given that LMO1 expression is minimal in normal blood cells but can be substantial in a subset of T-ALL leukaemia patients, we next investigated whether the LMO1 promoters and +57 enhancer element showed activity in T-ALL cells. To this end, luciferase reporter constructs were generated using the same fragments of genomic DNA from the human LMO1 locus that were used for the lacZ reporter constructs described in the previous sections. LMO1 P1/luc, P2/luc and SV/luc/ +57 were then stably transfected into LMO1-expressing, non-LMO1-translocated Jurkat cells alongside their empty vector counterparts. Following antibiotic selection, four independent pools of cells were assayed for each construct, and the whole experiment was repeated four times.
As shown in Figure 5b, both promoter elements showed greater activity than the control vector with promoter 1 demonstrating 1.5-fold higher activity than baseline and promoter 2 having 4.5-fold higher activity. Both these differences were statistically significant (P-values<0.01). Similarly, the enhancer construct SV/luc/+57 showed greater activity than the control vector (SV/luc), when assayed by stable transfection in Jurkat (P-values<0.01). Taken together, these results confirm that the +57 region not only functions as a haematopoietic enhancer in developing mouse embryos, but also displays transcriptional enhancer activity in a T-ALL transcriptional context.
SCL/TAL1 and GATA3 bind to the LMO1 +57 enhancer in T-ALL patient cells
Having demonstrated that the LMO1 +57 enhancer is active in a LMO1-expressing T-ALL cell line, we next wanted to assess how its activating function may be mediated in LMO1-expressing T-ALL cells. To this end, we measured LMO1 expression in six primary T-ALL xenografts32 passaged in immunodeficient mice (so called primagrafts). One sample (primagraft X31) showed high LMO1 expression without any evidence of translocations involving the LMO1 gene locus, and was therefore chosen for further analysis. Chromatin immunoprecipitation assays using an antibody against acetylated histone H3 (H3K9/K14) revealed elevated levels of histone acetylation at both LMO1 promoters as well as the +57 enhancer region (Figure 6c). Given that histone acetylation is indicative of transcriptionally active promoters and enhancers, this observation suggested that the +57 enhancer is involved in mediating LMO1 expression in T-ALL cells from primagraft X31.
Figure 6.
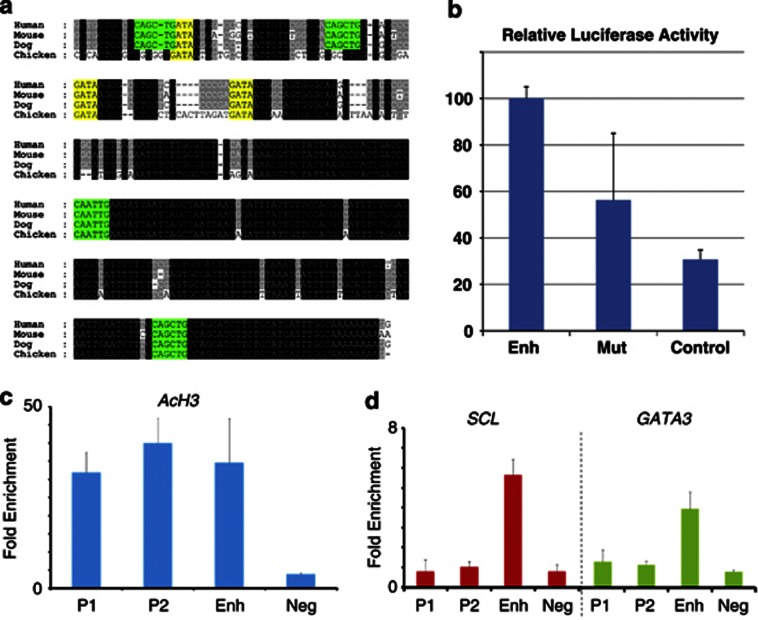
SCL/TAL1 and GATA3 bind to the LMO1 +57 enhancer in T-ALL patient cells. (a) Nucleotide sequence alignment of the LMO1 gene (+57 kb) enhancer region. Alignment of human, mouse, dog and chicken sequences extracted from the UCSC genome browser with conserved GATA (yellow) and E-Box (green) motifs coloured for clarity. Black boxes indicate 100% cross-species sequence conservation, grey boxes show less conserved sequences. (b) Stable transfection assays in Jurkat cells show that deletion of the region containing conserved E-Box and GATA motifs causes a significant reduction in activity of the +57 enhancer. Data from four technical replicates each of three biological replicates were normalised against the wild-type enhancer. The results show the mean of these data points with error bars representing s.d. Paired t-test analysis using all 12 raw data-points for each construct confirmed that the reduction in enhancer activity seen with the mutant enhancer was highly significant (P<0.01). (c) ChIP assays using an antibody against acetylated histone H3 (H3K9/K14) reveal elevated levels of histone acetylation at both LMO1 promoters as well as the +57 enhancer region in an LMO1-expressing primagraft primary patient sample. (d) ChIP assays on T-ALL cells from the same primagraft (X31) using antibodies against SCL/TAL1and GATA3. Analysis by quantitative PCR demonstrates significant binding of both SCL/TAL1 and GATA3 to the LMO1 +57 enhancer, with no binding to either of the two LMO1 promoters.
As shown in Figure 6a, the central core sequence of the LMO1 +57 enhancer is highly conserved between mammals, including consensus binding sites for transcription factors from the bHLH and GATA family (E-box and GATA motifs respectively). Moreover, deletion of the region containing conserved E-box and GATA motifs caused a significant reduction in enhancer activity (Figure 6b). Given that the leukaemogenic function of LMO1 is thought to require collaboration with bHLH factors such as SCL/TAL1 or LYL1.8, 9, 33, 34 The presence of the E-box motif within the +57 enhancer suggested the possibility of crosstalk between SCL/TAL1 and LMO1 through enhancing the activity of the LMO1 +57 element. Moreover, Scl/Tal1 has previously been shown to interact with the T-lymphoid GATA factor GATA3 within T-ALL cells,11, 35 thus suggesting possible joint regulation of LMO1 by SCL/TAL1 and GATA3 acting through the LMO1 +57 enhancer. To assess possible involvement of SCL/TAL1 and GATA3, we performed ChIP assays using T-ALL cells from primagraft X31 using previously validated antibodies against SCL/TAL127 and GATA3.36 (Figure 6d) Analysis of ChIP material by quantitative PCR demonstrated significant binding of both SCL/TAL1 and GATA3 to the LMO1 +57 enhancer, but not to either of the two LMO1 promoters. Moreover, Jurkat cells as well as our LMO1-expressing T-ALL primagraft sample showed high expression of GATA3 and SCL/TAL1, and bioinformatic analysis of expression data for 92 T-ALL patient samples demonstrated that the TAL1 subcategory of patients showed the highest levels of LMO1 expression (P=0.02; Mann–Whitney test) with GATA3 expression high across all T-ALL subtypes (see Supplementary Figure S6B). Taken together therefore, these observations are consistent with a model whereby LMO1 forms part of a core T-ALL regulatory circuit that involves other T-ALL oncogenes such as SCL/TAL1 and within which, the LMO1 +57 enhancer has a central role integrating inputs from upstream regulators to mediate ectopic LMO1 expression.
Discussion
Dysregulation of transcription represents a common theme in human acute leukaemias including T-ALL.37 Molecular characterisation of translocation breakpoints demonstrated that in addition to the generation of fusion proteins with altered functionality, an alternative pathogenic event following translocation can be the juxtaposition of genes ordinarily not expressed into the vicinity of powerful regulatory elements, thus causing ectopic expression. The early paradigm for this mode of leukaemia oncogene function was provided by analysis of the t(8;14) translocation in Burkitt's lymphoma that results in the ectopic expression of C-MYC.38 Within T-ALL, the lim-only proteins LMO1 and LMO2 are similarly expressed ectopically as a consequence of translocations involving T-cell receptor gene loci.24 Importantly, mouse models have shown that ectopic expression of either LMO1 or LMO2 is sufficient to cause the development of T-ALL,4, 39 thus proving that out-of-context expression of native LMO proteins represents a leukaemogenic event during T-ALL development.
Recurrent translocations provide powerful proof of the oncogenic function of a particular gene product, as they would not be observed within a given disease unless they provide a clonal advantage. However, with a few exceptions, translocations involving an individual gene are only present in a minority of patients with a given type of leukaemia. Researchers have long argued that translocations involving a given gene may be suggestive of a wider role for this gene within a disease, extending to patients that do not carry the particular translocation. Particularly in the case of ectopic expression of the native protein, a multitude of mechanisms other than translocations could be considered as relevant underlying causes. Indeed, extensive surveys of T-ALL oncogene expression in patient samples demonstrated that for both LMO1 and LMO2, the proportion of patients that express the oncogene vastly exceed the frequency of translocations.3, 40 In the case of LMO2, at least some of this excess expression has been explained by the demonstration of interstitial deletions removing a repressor element,41 and also by the realisation that a previously unrecognised 3rd LMO2 promoter is active in T-ALL cells and bound by the ERG transcription factor, the ectopic expression of which can also cause T-ALL.19 By contrast however, potential mechanisms underlying ectopic expression of LMO1 in the absence of translocations had not been explored.
Here, we have shown that the LMO1 gene locus is characterised by bivalent histone marks in multiple haematopoietic lineages including T-cells. Bivalent chromatin marks had originally been identified in ES cells, where simultaneous presence of the activating H3K4 and repressing H3K27 marks had been associated with promoters of many important lineage regulators, not expressed in pluripotent ES cells, but important for their differentiation into a range of different tissue types.26 Although the extent and biological implications of bivalency in ES cell chromatin biology have been challenged recently,42 the simultaneous observation of both activating and repressing histone marks within a population of cells not expressing the gene of interest is still likely to be indicative of a transcriptional status poised for expression. Given the potentially disastrous consequences of ectopic LMO1 expression in the T-cell lineage, our observation of bivalent promoter marks in normal T-cells was particularly surprising.
With the somewhat paradoxical observation that a gene locus such as LMO1 should be poised for expression in haematopoietic cells, the question arises as to whether there could be any biological reason for this potentially hazardous arrangement. Of interest in this context may be the recent report that single nucleotide polymorphisms within the LMO1 locus carry significant associations with the risk of developing precursor B-cell leukaemia.43 Although the underlying mechanisms remain to be determined, it is possible that the increased risk of developing B-ALL is related to the expression levels of LMO1, particularly as none of the SNPs were coding and because SNPs affecting expression levels are thought to underlie the association of LMO1 with neuroblastoma.14 Any model implicating expression levels would however posit that LMO1 can be expressed in blood cells, in contrast to the current thinking based largely on northern blot and microarray analysis. Our more sensitive Q-reverse transcriptase-PCR analysis was able to detect low level expression of LMO1 in several haematopoietic lineages. These observations agree with recent comprehensive microarray surveys of both the mouse and human haematopoietic hierarchy, where low level LMO1 expression can be observed in a small subset of haematopoietic cell types (see Supplementary Figure S7). Investigating any possible functions of LMO1 within normal haematopoietic cells should be the subject of future studies, and is likely to involve investigations into possible redundancy with other LMO proteins, in particular LMO2.
Ectopic expression accompanied by the resolution of bivalent promoter marks into activating marks only is consistent with biallelic expression of LMO1 in nontranslocated T-ALL patients, in line with what has been shown before for LMO2.40 Biallelic expression not only focuses attention on the alterations within the leukaemic transcriptional environment that may be responsible for ectopically activating LMO1, but also the regulatory sequences within the gene locus through which such ectopic activation may occur. The presence of a highly conserved region downstream of LMO1 that was bound by multiple transcription factors in haematopoietic cells prompted us to investigate the possible involvement of this genomic region in ectopic expression, particularly since several of the transcription factors bound to this region in stem/progenitor cells were known T-ALL leukaemia oncogenes (for example, SCL/TAL1 and LYL1). Indeed, we were able to show that this LMO1 +57 region displays histone marks characteristic of transcriptional enhancers both in T-ALL cell lines and patient samples, and was bound by Scl/Tal1 and GATA3 in a primagraft T-ALL patient sample. Together with our demonstration that this region has haematopoietic activity when assayed in transgenic mice, this observation suggests that the LMO1 +57 region may be involved in the low level LMO1 expression observed in normal blood cells, and also become responsive to the altered transcriptional environment in a subset of T-ALL patients with the consequence of enhancing LMO1 expression.
SCL/TAL1 and GATA3 had previously been shown to collaborate in mediating retinaldehyde dehydrogenase 2 expression in T-ALL cells,11 thus establishing this complex as a component of dysregulated transcriptional programmes in T-ALL. Our observation of SCL/TAL1 binding to the LMO1 +57 enhancer now provides for the first time, evidence for a direct cross-regulatory link between SCL/TAL1 and LMO1, two leukaemia oncogenes known to collaborate during T-ALL development in mouse models.7, 9 A model is therefore emerging whereby high levels of SCL/TAL1 might contribute to the ectopic expression of LMO1, which in turn would serve to enhance the leukaemogenic function of SCL/TAL1 and thus provide a clonal advantage to SCL/TAL1-expressing preleukaemic T-ALL cells that activate expression of LMO1. Such a model then also focuses attention onto the molecular events that cause breakdown of the epigenetic processes that keep the LMO1 locus repressed in normal haematopoietic cells, a better understanding of which would not only provide new insights into the pathogenesis of T-ALL, but may also open up new treatment opportunities, especially in light of the major current investment into the development of small molecule drugs targeting the epigenetic machinery.44
Acknowledgments
This work was supported by Leukaemia and Lymphoma Research UK, The Leukaemia and Lymphoma Society, the Kay Kendall Leukaemia Fund, Cancer Research UK, The Raymond and Beverly Sackler Foundation, the National Health and Medical Research Council of Australia, the Cancer Institute of New South Wales and Wellcome Trust infrastructure and core funding to the Cambridge Institute for Medical Research and Wellcome Trust and MRC Cambridge Stem Cell Institute.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Boehm T, Baer R, Lavenir I, Forster A, Waters JJ, Nacheva E, et al. The mechanism of chromosomal translocation t(11;14) involving the T-cell receptor C delta locus on human chromosome 14q11 and a transcribed region of chromosome 11p15. EMBO J. 1988;7:385–394. doi: 10.1002/j.1460-2075.1988.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire EA, Hockett RD, Pollock KM, Bartholdi MF, O'Brien SJ, Korsmeyer SJ. The t(11;14)(p15;q11) in a T-cell acute lymphoblastic leukemia cell line activates multiple transcripts, including Ttg-1, a gene encoding a potential zinc finger protein. Mol Cell Biol. 1989;9:2124–2132. doi: 10.1128/mcb.9.5.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnafi V, Beldjord K, Libura M, Villarese P, Millien C, Ballerini P, et al. Age-related phenotypic and oncogenic differences in T-cell acute lymphoblastic leukemias may reflect thymic atrophy. Blood. 2004;104:4173–4180. doi: 10.1182/blood-2003-11-3944. [DOI] [PubMed] [Google Scholar]
- McGuire EA, Rintoul CE, Sclar GM, Korsmeyer SJ. Thymic overexpression of Ttg-1 in transgenic mice results in T-cell acute lymphoblastic leukemia/lymphoma. Mol Cell Biol. 1992;12:4186–4196. doi: 10.1128/mcb.12.9.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse E, Smith AJ, Hunt S, Lavenir I, Forster A, Warren AJ, et al. Null mutation of the Lmo4 gene or a combined null mutation of the Lmo1/Lmo3 genes causes perinatal lethality, and Lmo4 controls neural tube development in mice. Mol Cell Biol. 2004;24:2063–2073. doi: 10.1128/MCB.24.5.2063-2073.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroni L, Boehm T, White L, Forster A, Sherrington P, Liao XB, et al. The rhombotin gene family encode related LIM-domain proteins whose differing expression suggests multiple roles in mouse development. J Mol Biol. 1992;226:747–761. doi: 10.1016/0022-2836(92)90630-3. [DOI] [PubMed] [Google Scholar]
- Chervinsky DS, Zhao XF, Lam DH, Ellsworth M, Gross KW, Aplan PD. Disordered T-cell development and T-cell malignancies in SCL LMO1 double-transgenic mice: parallels with E2A-deficient mice. Mol Cell Biol. 1999;19:5025–5035. doi: 10.1128/mcb.19.7.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplan PD, Jones CA, Chervinsky DS, Zhao X, Ellsworth M, Wu C, et al. An scl gene product lacking the transactivation domain induces bony abnormalities and cooperates with LMO1 to generate T-cell malignancies in transgenic mice. EMBO J. 1997;16:2408–2419. doi: 10.1093/emboj/16.9.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Tremblay CS, Herblot S, Aplan PD, Hebert J, Perreault C, et al. Modeling T-cell acute lymphoblastic leukemia induced by the SCL and LMO1 oncogenes. Genes Dev. 2010;24:1093–1105. doi: 10.1101/gad.1897910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valge-Archer V, Forster A, Rabbitts TH. The LMO1 and LDB1 proteins interact in human T cell acute leukaemia with the chromosomal translocation t(11;14)(p15;q11) Oncogene. 1998;17:3199–3202. doi: 10.1038/sj.onc.1202353. [DOI] [PubMed] [Google Scholar]
- Ono Y, Fukuhara N, Yoshie O. TAL1 and LIM-only proteins synergistically induce retinaldehyde dehydrogenase 2 expression in T-cell acute lymphoblastic leukemia by acting as cofactors for GATA3. Mol Cell Biol. 1998;18:6939–6950. doi: 10.1128/mcb.18.12.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis C, Oury F, Remacle S, Lampe X, Gofflot F, Picard JJ, et al. Identification of Lmo1 as part of a Hox-dependent regulatory network for hindbrain patterning. Dev Dyn. 2007;236:2675–2684. doi: 10.1002/dvdy.21266. [DOI] [PubMed] [Google Scholar]
- Nguyen le B, Diskin SJ, Capasso M, Wang K, Diamond MA, Glessner J, et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility Loci. Plos Genet. 2011;7:e1002026. doi: 10.1371/journal.pgen.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Diskin SJ, Zhang H, Attiyeh EF, Winter C, Hou C, et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011;469:216–220. doi: 10.1038/nature09609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry JR, Bonadies N, Kinston S, Knezevic K, Wilson NK, Oram SH, et al. Expression of the leukemia oncogene Lmo2 is controlled by an array of tissue-specific elements dispersed over 100 kb and bound by Tal1/Lmo2, Ets, and Gata factors. Blood. 2009;113:5783–5792. doi: 10.1182/blood-2008-11-187757. [DOI] [PubMed] [Google Scholar]
- Landry JR, Kinston S, Knezevic K, Donaldson IJ, Green AR, Fli1 GottgensB. Elf1, and Ets1 regulate the proximal promoter of the LMO2 gene in endothelial cells. Blood. 2005;106:2680–2687. doi: 10.1182/blood-2004-12-4755. [DOI] [PubMed] [Google Scholar]
- Oram SH, Thoms JA, Pridans C, Janes ME, Kinston SJ, Anand S, et al. A previously unrecognized promoter of LMO2 forms part of a transcriptional regulatory circuit mediating LMO2 expression in a subset of T-acute lymphoblastic leukaemia patients. Oncogene. 2010;29:5796–5808. doi: 10.1038/onc.2010.320. [DOI] [PubMed] [Google Scholar]
- Boehm T, Greenberg JM, Buluwela L, Lavenir I, Forster A, Rabbitts TH. An unusual structure of a putative T cell oncogene which allows production of similar proteins from distinct mRNAs. EMBO J. 1990;9:857–868. doi: 10.1002/j.1460-2075.1990.tb08183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T, Spillantini MG, Sofroniew MV, Surani MA, Rabbitts TH. Developmentally regulated and tissue specific expression of mRNAs encoding the two alternative forms of the LIM domain oncogene rhombotin: evidence for thymus expression. Oncogene. 1991;6:695–703. [PubMed] [Google Scholar]
- Greenberg JM, Boehm T, Sofroniew MV, Keynes RJ, Barton SC, Norris ML, et al. Segmental and developmental regulation of a presumptive T-cell oncogene in the central nervous system. Nature. 1990;344:158–160. doi: 10.1038/344158a0. [DOI] [PubMed] [Google Scholar]
- Wilson NK, Miranda-Saavedra D, Kinston S, Bonadies N, Foster SD, Calero-Nieto F, et al. The transcriptional program controlled by the stem cell leukemia gene Scl/Tal1 during early embryonic hematopoietic development. Blood. 2009;113:5456–5465. doi: 10.1182/blood-2009-01-200048. [DOI] [PubMed] [Google Scholar]
- Boehm T, Foroni L, Kaneko Y, Perutz MF, Rabbitts TH. The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc Natl Acad Sci USA. 1991;88:4367–4371. doi: 10.1073/pnas.88.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Stamatoyannopoulos JA, Costello JF, Ren B, Milosavljevic A, Meissner A, et al. The NIH Roadmap Epigenomics Mapping Consortium. Nat Biotechnol. 2010;28:1045–1048. doi: 10.1038/nbt1010-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Wilson NK, Foster SD, Wang X, Knezevic K, Schutte J, Kaimakis P, et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–544. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Elwood NJ, Cook WD, Metcalf D, Begley CG. SCL, the gene implicated in human T-cell leukaemia, is oncogenic in a murine T-lymphocyte cell line. Oncogene. 1993;8:3093–3101. [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Mellentin JD, Smith SD, Cleary ML. lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix-loop-helix DNA binding motif. Cell. 1989;58:77–83. doi: 10.1016/0092-8674(89)90404-2. [DOI] [PubMed] [Google Scholar]
- Thoms JA, Birger Y, Foster S, Knezevic K, Kirschenbaum Y, Chandrakanthan V, et al. ERG promotes T-acute lymphoblastic leukemia and is transcriptionally regulated in leukemic cells by a stem cell enhancer. Blood. 2011;117:7079–7089. doi: 10.1182/blood-2010-12-317990. [DOI] [PubMed] [Google Scholar]
- Liem NL, Papa RA, Milross CG, Schmid MA, Tajbakhsh M, Choi S, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103:3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Look AT. Gene expression profiling in T-cell acute lymphoblastic leukemia. Semin Hematol. 2003;40:274–280. doi: 10.1016/s0037-1963(03)00195-1. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- Ono Y, Fukuhara N, Yoshie O. Transcriptional activity of TAL1 in T cell acute lymphoblastic leukemia (T-ALL) requires RBTN1 or -2 and induces TALLA1, a highly specific tumor marker of T-ALL. J Biol Chem. 1997;272:4576–4581. doi: 10.1074/jbc.272.7.4576. [DOI] [PubMed] [Google Scholar]
- Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, et al. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci USA. 2009;106:17876–17881. doi: 10.1073/pnas.0909357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci USA. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack MP, Young LF, Vasudevan S, de Graaf CA, Codrington R, Rabbitts TH, et al. The Lmo2 oncogene initiates leukemia in mice by inducing thymocyte self-renewal. Science. 2010;327:879–883. doi: 10.1126/science.1182378. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Herblot S, Palomero T, Hansen M, Hoang T, Fox EA, et al. Biallelic transcriptional activation of oncogenic transcription factors in T-cell acute lymphoblastic leukemia. Blood. 2004;103:1909–1911. doi: 10.1182/blood-2003-07-2577. [DOI] [PubMed] [Google Scholar]
- Van Vlierberghe P, van Grotel M, Beverloo HB, Lee C, Helgason T, Buijs-Gladdines J, et al. The cryptic chromosomal deletion del(11)(p12p13) as a new activation mechanism of LMO2 in pediatric T-cell acute lymphoblastic leukemia. Blood. 2006;108:3520–3529. doi: 10.1182/blood-2006-04-019927. [DOI] [PubMed] [Google Scholar]
- Marks H, Kalkan T, Menafra R, Denissov S, Jones K, Hofemeister H, et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell. 2012;149:590–604. doi: 10.1016/j.cell.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuten J, Gelfond JA, Piwkham D, Pollock BH, Winick NJ, Collier AB, et al. Candidate gene association analysis of acute lymphoblastic leukemia identifies new susceptibility locus at 11p15 (LMO1) Carcinogenesis. 2011;32:1349–1353. doi: 10.1093/carcin/bgr091. [DOI] [PubMed] [Google Scholar]
- McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.