Gliomas are a common malignancy of the central nervous system with poor prognosis. In this paper the authors identified miR-181b as a tumor suppressor in glioma that acts by targeting Insulin-like growth factor 1 receptor (IGF-1R) and inhibiting its downstream signaling. The levels of miR-181b were found reduced in human glioma tissues, which inversely correlated with IGF-1R levels. The authors show that miR-181b overexpression regulates IGF-1R signaling and its overexpression suppresses tumor growth in vivo.
Keywords: glioma, miR-181b, IGF-1R, carcinogenesis
Abstract
MicroRNAs (miRNAs) are single-stranded, 18- to 23-nt RNA molecules that function as regulators of gene expression. Previous studies have shown that microRNAs play important roles in human cancers, including gliomas. Here, we found that expression levels of miR-181b were decreased in gliomas, and we identified IGF-1R as a novel direct target of miR-181b. MiR-181b overexpression inhibited cell proliferation, migration, invasion, and tumorigenesis by targeting IGF-1R and its downstream signaling pathways, PI3K/AKT and MAPK/ERK1/2. Overexpression of IGF-1R rescued the inhibitory effects of miR-181b. In clinical specimens, IGF-1R was overexpressed, and its protein levels were inversely correlated with miR-181b expression. Taken together, our results indicate that miR-181b functions in gliomas to suppress growth by targeting the IGF-1R oncogene and that miR-181b may serve as a novel therapeutic target for gliomas.
INTRODUCTION
Gliomas are the most common malignancies of the central nervous system in humans. Prognosis is highly dependent on the histological grade of a tumor, with the poorest survival rates associated with the most malignant grades (Vredenburgh et al. 2009). New molecular targets and treatment strategies are urgently needed to combat this disease. MicroRNAs (miRNAs) are small, endogenous noncoding RNAs composed of 18–23 nucleotides (nt) that post-transcriptionally regulate gene expression by targeting the 3′-untranslated regions of mRNAs (Bartel 2004). Many miRNAs are proto-oncogenes or tumor suppressors, and their functions have been extensively studied in various cancer types, including glioma (Esquela-Kerscher and Slack 2006; Sana et al. 2011). Recent studies using genome-wide approaches have revealed that miRNAs, such as miR-7, miR-124, miR-128, and miR-21, are globally dysregulated in glioma (Godlewski et al. 2008; Kefas et al. 2008; Kwak et al. 2011; Xia et al. 2012). Our previous studies have indicated that miR-181a and miR-181b are down-regulated in glioma, which contributes to apoptosis and reduced rates of proliferation and invasion (Shi et al. 2008). This tumor-suppressive effect of miR-181b in glioma cells was more apparent than that of miR-181a. Sun et al. (2012) confirmed that miR-181b is also a potent regulator of downstream NF-κB signaling in the vascular endothelium. However, the potential role of miR-181b in the carcinogenesis and tumor development of glioma is unknown.
The Insulin-like growth factor I receptor (IGF-1R) is a tyrosine kinase receptor that is mainly activated by IGF1 and IGF2 in autocrine and paracrine manners (Zhao et al. 2012). IGF-1R activates multiple downstream signaling cascades, including PI3K/AKT and MAPK/ERK signaling pathways, which regulate cell proliferation, differentiation, and survival (Baserga et al. 2003; Cao et al. 2007). More recently, several miRNAs, such as miR-7, miR-145, miR-223, and miR-375, have been reported to negatively regulate IGF-1R (Shi et al. 2007; Jia et al. 2011; Kong et al. 2012; Zhao et al. 2012), suggesting that miRNAs targeting IGF-1R have an important role in carcinogenesis and are potential therapeutic agents for human cancer.
However, previous reports have not clearly determined the role of miR-181b in glioma progression. Here, we report that miR-181b acts as a tumor suppressor by directly targeting IGF-1R, resulting in the inhibition of IGF-1R signaling pathways. In addition, miR-181b overexpression suppressed cell proliferation, migration, and invasion, and attenuated tumor growth in vivo. Our findings not only provide new insights into the mechanisms of tumorigenesis in glioma, but also reveal a novel regulatory mechanism mediated by receptor signaling. These results may lead the way to novel strategies for glioma therapy.
RESULTS
Aberrant expression of miR-181b in human gliomas
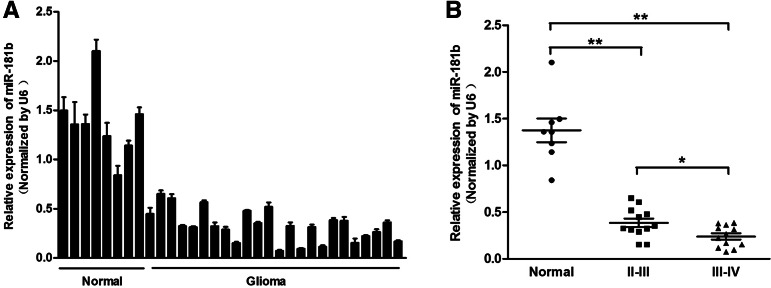
To assess the expression of miR-181b in gliomas, qRT-PCR analysis was conducted on eight normal brain tissues and 24 glioma tissue samples. The results showed that the expression of miR-181b was consistently lower in the glioma tissues compared with the normal brain tissues (Fig. 1A). We then divided the glioma samples into two groups according to their pathological diagnosis. We found that miR-181b levels were down-regulated in both groups relative to the normal brain group (P < 0.01). Moreover, the levels of miR-181b expression in WHO clinical stage III–IV cases were much lower than those in stage II–III cases, indicating that miR-181b expression correlated with glioma malignancy (Fig. 1B, P < 0.05). These data support the notion that miR-181b may act as a tumor suppressor in glioma.
FIGURE 1.
MiR-181b is down-regulated in glioma tissue samples. (A) Expression levels of miR-181b in normal brain tissues and glioma tissues were analyzed by stem–loop qRT-PCR and normalized to the levels of U6. (B) Relative expression levels of miR-181b in different stages of cancer tissues. Twenty-four glioma samples were divided into two groups according to the pathological classification. A Student’s t-test was used to analyze the significant differences among the groups. (**) Significant difference when compared with normal brain tissues (P < 0.01).
MiR-181b overexpression inhibits cell proliferation, migration, and invasion
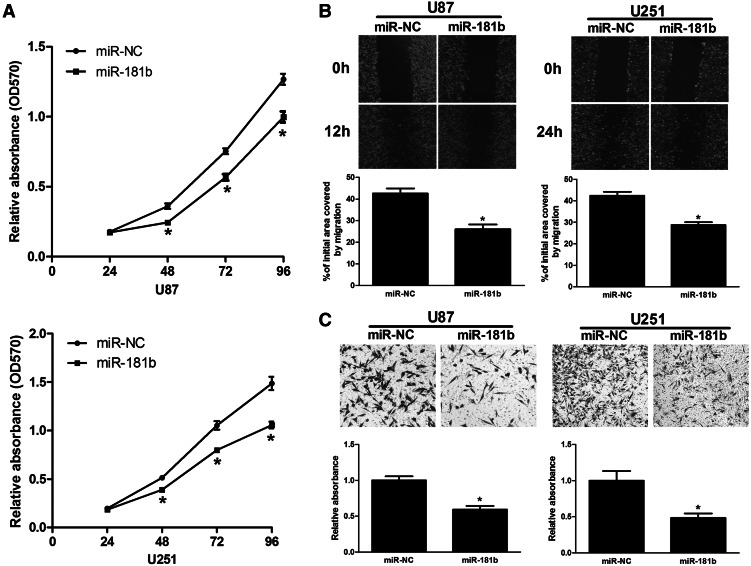
To investigate the biological functions of miR-181b in glioma cells, U87 and U251 cells were transfected with miR-181b or miR-NC and analyzed for cell growth. Proliferation assays showed that cell growth was reduced in miR-181b-transfected U87 and U251 cells compared with miR-NC-transfected control cells (Fig. 2A). To further detect whether miR-181b is associated with progression of glioma, we analyzed the effect of miR-181b expression on the migratory and invasive behavior of U87 and U251 cells. We found that overexpression of miR-181b decreased the migration capacity of glioma cells (Fig. 2B). In addition, miR-181b overexpression dramatically inhibited the normally strong invasive capacity of U87 and U251 cells (Fig. 2C). These results showed that miR-181b overexpression contributes to regulation of glioma cell motility and progression in vitro.
FIGURE 2.
MiR-181b overexpression inhibited cell proliferation, migration, and invasion in vitro. (A) Overexpression of miR-181b decreased U87 and U251 cell growth. (*) Significant difference when compared with the miR-NC group (P < 0.05). (B) Wound-healing assay in U87 and U251 cells overexpressing miR-181b. The wound gaps were photographed and measured. (*) Significant difference when compared with the miR-NC group (P < 0.05). (C) Transwell invasion assay of U87 and U251 cells overexpressing miR-181b. Cells in the bottom of the invasion chamber were fixed, stained, and photographed, and the absorbance at OD570 was read. (*) Significant difference when compared with the miR-NC group (P < 0.05).
IGF-1R is a direct target of miR-181b, and IGF-1R levels are inversely correlated with miR-181b levels in glioma tissues
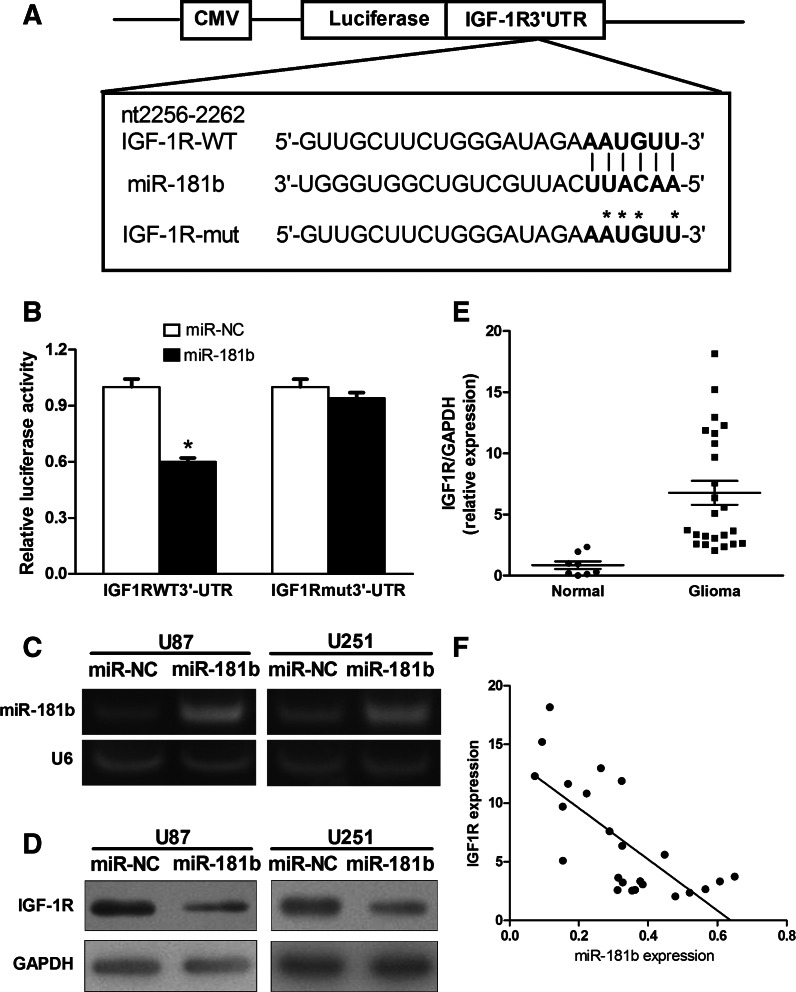
To fully understand the mechanisms of miR-181b action in glioma, we performed a bioinformatics search for potential targets of miR-181b and IGF-1R was selected for further analysis. Luciferase assays were performed using the pMIR-REPORT miRNA reporter vector containing the putative miR-181b-binding site in the IGF-1R 3′ UTR (WT), or a mutated version of the same binding site (Mut) (Fig. 3A). Overexpression of miR-181b in HEK293 cells inhibited wild-type IGF-1R reporter activity but not mutant reporter activity, demonstrating that miR-181b can specifically target the IGF-1R 3′ UTR by binding to the seed sequence (Fig. 3B). Next, we established cell lines stably expressing miR-181b and miR-NC using human glioma U87 and U251 cells and lentiviral transduction. RT-PCR assays confirmed that expression of mature miR-181b was increased in pLe-miR-181b-expressing U87 and U251 cells, indicating that these stable cell lines successfully expressed miR-181b (Fig. 3C). Cells overexpressing miR-181b showed low levels of IGF-1R protein when compared with those of negative control cells (Fig. 3D).
FIGURE 3.
IGF-1R is a target of miR-181b, and IGF-1R levels are inversely correlated with miR-181b levels in glioma tissues. (A) Sequence of the miR-181b-binding site within the human IGF-1R 3′ UTR and a schematic diagram of the reporter constructs showing the entire IGF-1R 3′-UTR sequence and the mutated IGF-1R 3′-UTR sequence. The mutated nucleotides of the IGF-1R 3′ UTR are labeled by an asterisk. (B) Relative luciferase activities of IGF-1R-WT and IGF-1R-Mut reporters were obtained by cotransfection of scrambled control miRNA or miR-181b and pRL-TK plasmid and calculated as the ratio of firefly/Renilla activities and normalized to those of the control. (*) Significant difference when compared with control (P < 0.05). (C) miR-181b and U6 expression levels in U87 and U251 cells stably expressing miR-NC and miR-181b were determined by RT-PCR analysis. (D) Overexpression of miR-181b inhibited IGF-1R expression at the protein level. (E) The expression of IGF-1R in normal human brain tissues and glioma specimens was determined by Western blot analysis, and fold changes were obtained from the ratio of IGF-1R to GAPDH levels. (F) Spearman’s correlation analysis was used to determine the correlation between the expression levels of IGF-1R and miR-181b in human glioma specimens (Spearman’s correlation analysis, r = −0.7252; P < 0.01).
To determine whether reduced miR-181b expression correlates with levels of IGF-1R expression in tumor tissues, protein levels of IGF-1R in glioma and normal brain tissues were analyzed by immunoblotting. The results showed that the average expression level of IGF-1R was significantly higher in glioma specimens than in normal brain tissues (Fig. 3E). Furthermore, Spearman’s correlation analysis showed an inverse correlation between the expression levels of IGF-1R and miR-181b (Spearman, r = −0.7252, P < 0.01) in human glioma specimens (Fig. 3F).
MiR-181b overexpression inhibits AKT and ERK signaling pathways
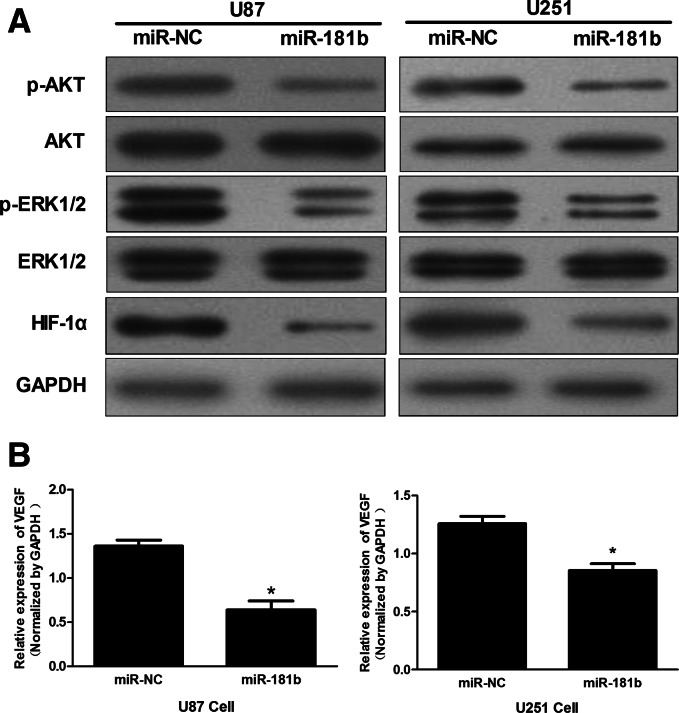
PI3K/AKT and MAPK/ERK signaling pathways play key roles in controlling cell proliferation, survival, and motility (De Luca et al. 2012). IGF-1R can activate downstream tyrosine kinase cascades, including the PI3K/AKT and ERK pathways. Therefore, we investigated whether miR-181b can suppress PI3K/AKT and ERK pathways in glioma cells. In U87 and U251 cells stably expressing miR-181b, the levels of p-AKT and p-ERK1/2 were reduced compared with cells stably expressing miR-NC, while no significant reduction in levels of AKT or ERK was detected (Fig. 4A). The expression of Hypoxia-inducible factor-1α (HIF-1α) and Vascular endothelial growth factor (VEGF), which play important roles in tumor angiogenesis, are mediated by PI3K/AKT and MAPK/ERK signaling pathways (Fang et al. 2005). Here, we observed that HIF-1α and VEGF levels in miR-181b-expressing U87 and U251 cells were down-regulated (Fig. 4B). These data suggest that the tumor-suppressing activity of miR-181b in glioma cells may be regulated by the PI3K/AKT and MAPK/ERK pathways.
FIGURE 4.
MiR-181b overexpression regulates IGF-1R signaling. (A) Levels of p-Akt, Akt, p-ERK1/2, ERK, HIF-1α, and GAPDH protein were detected by Western blot analysis. (B) VEGF mRNA levels were determined by qRT-PCR and normalized to those of GAPDH. (*) Significant difference when compared with control (P < 0.05).
MiR-181b inhibits angiogenesis in nude mice
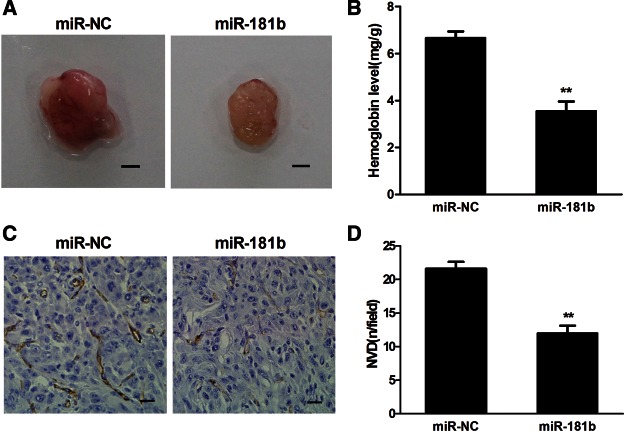
Angiogenesis is a key process for tumorigenesis and tumor development. We have previously demonstrated that miR-181b down-regulates HIF-1α and VEGF expression by targeting IGF-1R. Given the importance of HIF-1α and VEGF in promoting angiogenesis, we tested whether miR-181b can inhibit tumor angiogenesis. U87 cells stably expressing miR-181b or miR-NC were mixed with Matrigel and injected into both flanks of nude mice. Matrigel plugs from the mice are shown in Figure 5A, and the relative angiogenesis responses were assayed by measuring hemoglobin levels. When compared with the miR-NC group, the angiogenesis response in the miR-181b group was decreased by 50% (Fig. 5B). In addition, the number of CD31-positive microvessels was much lower in sections from xenografts of miR-181b-expressing U87 cells (Fig. 5C,D), indicating that miR-181b attenuated glioma cell-induced angiogenesis.
FIGURE 5.
MiR-181b overexpression suppressed tumor angiogenesis in vivo. (A) Representative Matrigel plugs are shown. Scale bar, 2 mm. (B) The hemoglobin levels in eight tumors from the miR-181b group were decreased 50% compared with the miR-NC group. (**) Significant difference when compared with the miR-NC group (P < 0.01). (C) Representative tumor sections stained with antibody against human CD31. Magnification, 200×. (D) CD31-positive microvessels were counted from eight tumors in three different fields per section at 400× magnification. (**) Significant difference compared with the miR-NC group (P < 0.01).
MiR-181b suppresses tumor growth of glioma cells in nude mice
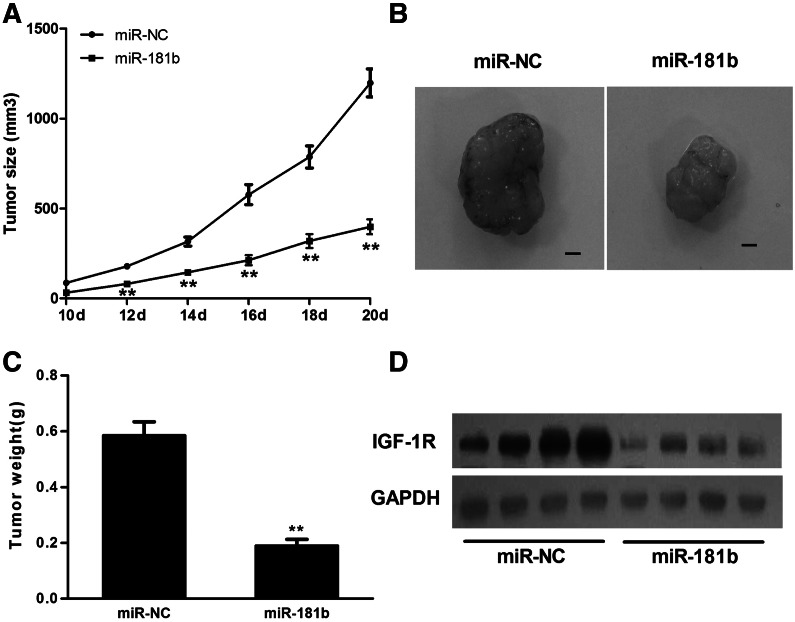
To further investigate the relationship between miR-181b and tumorigenesis, an in vivo model was employed. U87 cells stably expressing miR-181b or miR-NC were injected subcutaneously into both flanks of nude mice. The lengths and widths of tumors were measured when the xenografts were visible at 10 d after injection, and the volumes of tumors were calculated. As shown in Figure 6, A and B, the size of xenografts in the miR-181b group was smaller than that in the miR-NC control group after days of implantation. After 20 d, the xenografts were removed, and tumor weight was measured. Overexpression of miR-181b attenuated tumor growth by 65% when compared with the miR-NC control group (Fig. 6C). Furthermore, the protein levels of IGF-1R in xenografts from the miR-181b group were much lower than those from the miR-NC control group (Fig. 6D), confirming that miR-181b overexpression suppressed IGF-1R expression in vivo.
FIGURE 6.
MiR-181b overexpression suppressed tumor growth in vivo. (A) Nude mice were injected subcutaneously with 5 × 106 U87 cells stably expressing miR-181b or miR-NC. Each treatment group contained 10 tumors. When the xenografts were visible, the width and length of tumors were measured. (**) Significant difference when compared with the miR-NC group (P < 0.01). (B) The mice were euthanized on Day 20 and the xenografts were removed. Representative tumors from each group are shown. Scale bar, 2 mm. (C) The tumor weight was measured for each xenograft. (**) Significant difference when compared with the miR-NC group (P < 0.01). (D) Western blot analysis shows that the levels of IGF-1R from the tumor tissues of the miR-181b-expressing group were much lower than those of the miR-NC group.
Overexpression of IGF-1R reverses the inhibitory effects of miR-181b
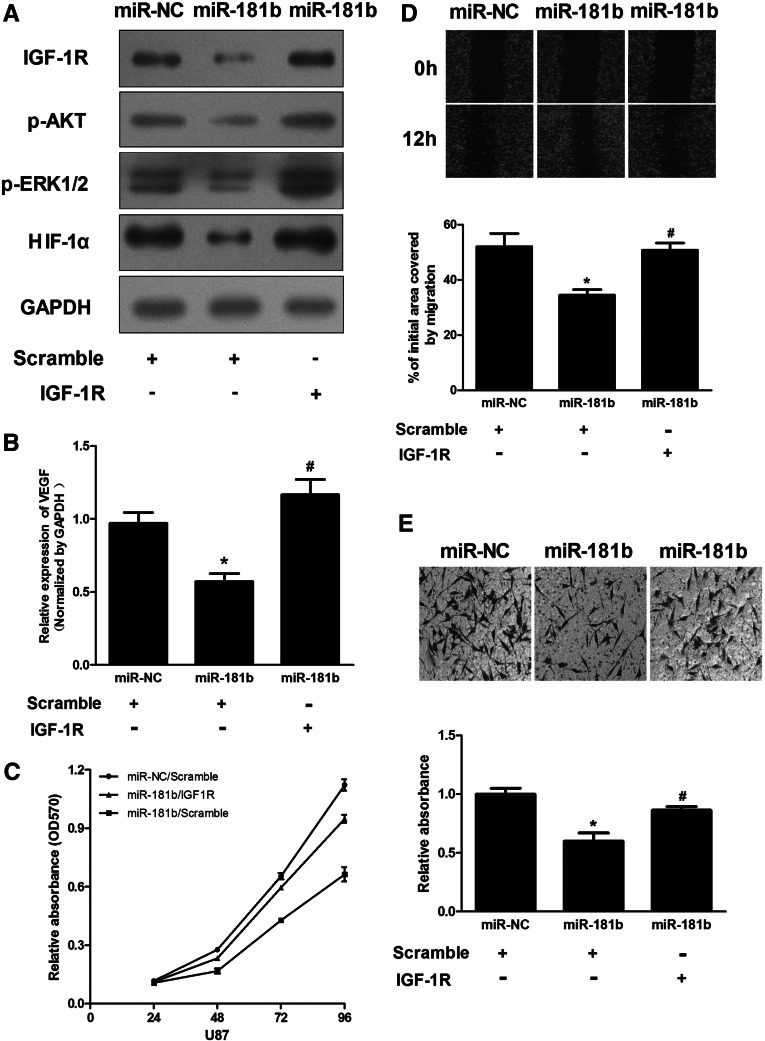
As shown above, overexpression of miR-181b inhibited proliferation, migration, and invasion of glioma cells. We also validated IGF-1R as a direct target of miR-181b. Therefore, we addressed whether changes in cell phenotypes after miR-181b overexpression resulted directly from the down-regulation of IGF-1R and its downstream pathways. pReceiver-Lv105-IGF1R was transfected into U87 cells stably expressing miR-181b or miR-NC. As shown in Figure 7A, the decreased level of IGF-1R due to miR-181b overexpression was rescued by overexpression of IGF-1R. Interestingly, the phosphorylation levels of AKT and ERK1/2 were altered in a similar way to the expression level of IGF-1R; that is, decreased levels of p-Akt and p-ERK1/2 due to miR-181b overexpression could be rescued by up-regulation of IGF-1R. Similarly, overexpression of IGF-1R in miR-181b-treated cells rescued the expression of HIF-1α and VEGF (Fig. 7A,B).
FIGURE 7.
IGF-1R overexpression rescued the miR-181b overexpression phenotype. (A) Overexpression of IGF-1R increased levels of p-AKT, p-ERK1/2, and HIF-1α protein. (B) Overexpression of IGF-1R rescued VEGF mRNA expression inhibited by miR-181b. The VEGF mRNA level was normalized to that of GAPDH. (C,E) pReceiver-Lv105-IGF1R or pReceiver-Lv105-Negative Control transduced U87 cells stably expressing miR-NC or miR-181b. Forty-eight hours after transduction, cells were trypsinzed and seeded into 96-well plates or into the upper well of the invasion chamber. Cell proliferation and invasion assays were performed as described in Materials and Methods. (D) U87 cells stably expressing miR-NC or miR-181b were treated as above, and wounds were made. (*) Significant differences in VEGF levels between miR-NC and miR-181b treatment (P < 0.05). Hashes indicate significant difference in VEGF levels in U87/miR-181b cells with or without IGF-1R overexpression (P < 0.05).
To further confirm whether IGF-1R is an important target of miR-181b in cell proliferation, migration, and invasion, we measured cell growth and found an increase in cell growth in the U87/miR-181b IGF-1R-transduced group compared with the U87/miR-181b scramble group (Fig. 7C). Furthermore, the capacity of cell migration in U87/miR-181b cells was rescued by overexpression of IGF-1R when compared with control cells (Fig. 7D). Similarly, the invasive capacity of U87/miR-181b cells was also rescued by overexpression of IGF-1R (Fig. 7E). Based on these findings, we concluded that miR-181b could regulate glioma cell proliferation, migration, and invasion by targeting the IGF-1R pathway.
DISCUSSION
Dysregulation of microRNAs (miRNAs) is a common feature in human cancers, including glioma. miRNAs can function as tumor suppressors or oncogenes, and the expression of more than one-third of the protein-coding genes in the human genome is thought to be controlled by miRNAs (Lewis et al. 2003, 2005). Here, consistent with previous studies, we found that miR-181b was down-regulated in human glioma tissues. On the basis of bioinformatic analysis, we further predicted IGF-1R as a target of miR-181b. Moreover, for the first time, we showed that IGF-1R was up-regulated in glioma specimens and was inversely correlated with miR-181b levels. Thus, this study may provide new therapeutic strategies for glioma prevention and treatment.
IGF-1R belongs to a family of tyrosine kinase receptors that plays important roles in signal transduction pathways. In recent years, mounting evidence indicates that IGF-1R and its ligands may be involved in human cancer progression (Tognon and Sorensen 2012). Aberrant expression of IGF-IR, via interactions with the adaptor protein IRS, can activate multiple downstream signaling cascades, including PI3K/AKT and MAPK/ERK signaling pathways (Cao et al. 2007; Pollak 2008), which mediate key mechanisms underlying tumor growth and progression. In this study, we identified IGF-1R as a direct and functional target of miR-181b. We confirmed that overexpression of miR-181b in glioma cells inhibited cell proliferation, migration, and invasion, and decreased levels of both p-AKT and p-ERK1/2, which play vital functions in regulating tumorigenesis and angiogenesis. Furthermore, overexpression of IGF-1R in stable miR-181b-expressing cell lines can restore the inhibitory effect on p-AKT and p-ERK1/2, as well as restore HIF-1α and VEGF expression. Meanwhile, restoring expression of IGF-1R can partially, or even totally, restore the miR-181b-induced inhibition of cell proliferation, migration, and invasion. These results show that miR-181b is a tumor suppressor that inhibits tumor growth and angiogenesis through targeting IGF-1R.
In summary, our results indicate that miR-181b regulates IGF-1R signaling at multiple levels. We confirmed that miR-181b acts as a tumor suppressor gene through various mechanisms, including inhibition of tumor cell growth, migration, and invasion, and by direct targeting of the Akt and ERK signaling pathways. Although miRNA-based therapeutics are still in the initial stages of development, our findings are encouraging and suggest that miR-181b could be a potential target for the treatment of glioma.
MATERIALS AND METHODS
Human tissue samples
Human glioma tissue samples were obtained from patients undergoing surgery for glioma in the Department of Neurosurgery, The First Affiliated Hospital of Nanjing Medical University. Normal brain tissues were collected as negative controls from patients undergoing decompressive craniectomy for traumatic brain injury. All study procedures were approved by the Institutional Review Board of the hospital. Informed consent was given by all participants. Tissues were immediately snap-frozen in liquid nitrogen after surgery. All samples were histologically classified and graded according to WHO guidelines by a clinical pathologist.
Cell culture
Human U87 and U251 glioma cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 ng/mL streptomycin. HEK-293 and HEK-293T cells were cultured in DMEM supplemented with 10% FBS, 100 units/mL penicillin, 100 ng/mL streptomycin, and 2 mmol/mL glutamine. All cells were incubated at 37°C in an atmosphere of 5% CO2.
Oligonucleotides and cell transfection
Oligonucleotides were chemically synthesized by GenePharma. Cells at 50%–70% confluence were transfected using lipofectamine reagent (Invitrogen). Oligonucleotides were transfected into U87 and U251 glioma cells at a final concentration of 50 nmol/L according to the manufacturer’s instructions.
Lentivirus packaging and establishment of stable cell lines
A lentiviral packaging kit was purchased from Open Biosystems. Lentivirus carrying hsa-miR-181b or hsa-miR-negative control (miR-NC) was packaged following the manufacturer’s manual. Lentivirus was packaged in HEK-293T cells and collected from the medium supernatant. Stable cell lines were established by infecting lentivirus into U87 and U251 cells, followed by puromycin selection. pReceiver-Lv105-IGF-1R and pReceiver-Lv105-Negative Control were purchased from Gene Copoeia. The packaging steps were performed according to the manufacturer’s instructions, and the lentiviral supernatant obtained from HEK-293T cells was used to infect U87 cells stably expressing miR-181b or miR-NC.
RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
Total RNAs were extracted from cultured cells or human tissue specimens using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Real-time PCR was performed to detect VEGF mRNA levels. The primers used were as follows: VEGF forward primer, 5′-TCGGGCCTCCGAAACCATGA-3′; VEGF reverse primer, 5′-CCTGGTGAGAGATCTGGTTC-3′; GAPDH forward primer, 5′-CCACCCATGGCAAATTCCATGGCA-3′; and GAPDH reverse primer, 5′-TCTAGACGGCAGGTCAGGTCCACC-3′. The reaction program was 30 sec at 95°C, followed by 40 cycles of 5 sec at 95°C, 30 sec at 55°C, and 31 sec at 72°C, and the melting curve was determined. To measure miR-181b expression levels, RNAs were transcribed by a stem–loop RT primer method using the PrimeScript RT Reagent Kit (Takara) as previously described (Chen et al. 2005; Wang 2009). The qRT-PCR primers were miR-181b RT primer, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAAC CCACC-3′; miR-181b PCR primers, sense 5′-ACACTCCAGCTGGGAACATTCATTGCTGTCGG-3′; anti-sense 5′-TGGTGTCGTGGAGTCG-3′; U6 RT primer, 5′-TGGTGTCGTGGAGTCG-3′; U6 PCR primers, sense 5′-CTCGCTTCGGCAGCACA-3′; anti-sense 5′-AACGCTTCACGAATTTGCGT-3′. qRT- PCR was performed using SYBR Premix DimerEraser (Takara) on a 7900HT system. GAPDH or U6 levels were used as internal controls, and fold changes were calculated by relative quantification (2−ΔΔCt) (Livak and Schmittgen 2001).
Western blotting
Cells or tissues were harvested and lysed on ice for 30 min in radio-immunoprecipitation assay (RIPA) buffer supplemented with protease inhibitors (100 mM Tris-HCl at pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1% deoxycholate acid, 0.1% SDS, 2 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 2 mM DTT, 2 mM leupeptin, 2 mM pepstatin). Lysates were centrifuged at 12,000 rpm for 10 min, and supernatants were collected as total proteins. Protein concentrations were determined by the BCA method (Beyotime), and aliquots of protein lysates were separated by SDS-PAGE and then transferred to a nitrocellulose membrane (Whatman). Membranes were blocked with 5% nonfat dried milk solution for 2 h and then incubated with primary antibodies. The antibodies used were against IGF-1R, phospho-AKT (Ser-473), total AKT, phospho-ERK1/2, total ERK1/2 (Cell Signaling Technology), hypoxia-inducible factor 1α (Bioworld), and GAPDH (Kang Cheng). The ECL Detection System (Thermo Scientific) was used for signal detection.
Luciferase reporter assay
For the luciferase reporter assay, the 3′ UTR of IGF-1R was amplified by PCR from human cDNA using the following primers: IGF-1R forward primer, 5′-GCGAGCTCTCTGGGATAGAAATGTTTAGGAGTA-3′; IGF-1R reverse primer, 5′-GCAAGCTTCAGGTGCTGAGAAAGGTGAGATGT-3′. To mutate the binding site of miR-181b, its complementary sequence in the 3′ UTR of IGF-1R (AATGTT) was replaced by ATACTA. The PCR products were digested using SacI and HindIII and inserted into pMIR- REPORTER. These constructs were validated by sequencing. HEK-293 cells were seeded into 24-well plates and cotransfected with the wild-type or mutated IGF-1R 3′-UTR reporter plasmids and pRL-TK, or transfected with miR-181b and miR-NC. Luciferase assays were performed 24 h after transfection using the Dual Luciferase Reporter Assay System (Promega).
Cell proliferation assay
Cells in the logarithmic phase of growth were seeded at 2000 per well in 96-well plates and cultured. Cell proliferation was assayed at the indicated time points using a CCK8 kit (Dojindo Laboratories) according to the manufacturer’s instructions. Experiments were performed in triplicate.
Wound healing assay
Cells were cultured to 95% confluence in six-well plates. Cell layers were scratched using a 20-µL tip to form wound gaps, washed twice with PBS, and cultured. The wound healing was photographed at different time points, and each wound was analyzed by measuring the distance migrated by cells in three different areas.
Invasion assays
Invasion was determined using 24-well BD Matrigel invasion chambers (BD Biosciences) in accordance with the manufacturer’s instructions. 5 × 104 cells were seeded in the upper well of the invasion chamber in DMEM without serum. The lower chamber well contained DMEM supplemented with 10% FBS to stimulate cell invasion. After incubation for 24 h, noninvading cells were removed from the top well with a cotton swab while the bottom cells were fixed with 3% paraformaldehyde, stained with 0.1% crystal violet, and photographed in three independent 10× magnification fields. The membrane was then air-dried and soaked for 15 min at room temperature with 33% acetic acid (200 µL/well). This destaining solution was then transferred to a 96-well plate, and the absorbance value at OD570 was read. Experiments were performed in triplicate.
Matrigel plug assay
Male BALB/cA-nu nude mice (6 wk old) were purchased from the Shanghai Experimental Animal Center (Chinese Academy of Sciences, Shanghai, China) and maintained in specific pathogen-free conditions. Eight mice were randomly divided into two groups. U87 cells stably expressing miR-181b were harvested and resuspended in serum-free medium. Aliquots of the cells (2 × 106 cells in 100 μL) were mixed with 200 μL of Matrigel. The mixture was immediately injected into both flanks of nude mice. U87 cells stably expressing miR-NC in equal volumes of solvent were used as the control. On Day 11 after implantation, the Matrigel plugs were removed. Half of each Matrigel plug was used to measure hemoglobin content using Drabkin’s Reagent Kit (Sigma-Aldrich) according to the manufacturer’s instructions. The other half of each Matrigel plug was used for immunohistochemical examination.
Immunohistochemical analysis
Matrigel plug samples were formalin-fixed, paraffin-embedded, and sectioned at 5 μm. Microwave antigen retrieval was then performed. After incubation with hydrogen peroxide, sections were washed, blocked for 1 h with 5% bovine serum albumin in PBS buffer, and incubated with a 1:50 dilution of rabbit anti- CD31 (Abcam) in a humidified chamber for 16 h at 4°C. After washing, the slides were incubated with HRP-conjugated goat anti-rabbit IgG for 2 h. The antibody signals were detected using DAB reagent. Sections were prepared from three Matrigel plugs in each group, and microvessels were counted in three different fields per section as follows: Slides were first scanned under low power (×100) to determine three “hotspots” or areas with the maximum number of microvessels, then the positive-stained blood vessels in the selected areas were analyzed at 400× magnification (Liu et al. 2008).
Tumor growth assay in mice
Nude mice (Male BALB/cA-nu, 6 wk old) were purchased from the Shanghai Experimental Animal Center (Chinese Academy of Sciences, Shanghai, China) and maintained in specific pathogen-free conditions. Ten mice were randomly divided into two groups. In one group, U87 cells stably expressing miR-181b were injected subcutaneously into both flanks of each mouse (5 × 106 cells in 100 μL), and in the other group, U87 cells stably expressing miR-NC (as a negative control) were similarly injected. When visible, tumor size was measured using Vernier calipers every 2 d, and tumor volume was calculated according to the formula: volume = 0.5 × length × width2. The mice were euthanized after 20 d, and tumors were weighed.
Statistical analysis
All experiments were performed three times, and data were analyzed with GraphPad Prism 5. Statistical evaluation of data was performed using the t-test. P < 0.05 was considered to be statistically significant. Spearman’s nonparametric correlation test was performed to test the correlation between the expression levels of miR-181b and IGF-1R by SPSS.
ACKNOWLEDGMENTS
This study was supported by the China Natural Science Foundation (81072078, 81101901, 81172389, and 91229121), the Natural Science Foundation of Jiangsu Province (201123808 and 2010580), Jiangsu Province’s Key Provincial Talents Program (RC2011051), Jiangsu Province’s Key Discipline of Medicine (XK201117), the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU, a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and National High Technology Research and Development Program 863 (2012AA02A508). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- Bartel DP 2004. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281–297 [DOI] [PubMed] [Google Scholar]
- Baserga R, Peruzzi F, Reiss K 2003. The IGF-1 receptor in cancer biology. Int J Cancer 107: 873–877 [DOI] [PubMed] [Google Scholar]
- Cao Z, Liu LZ, Dixon DA, Zheng JZ, Chandran B, Jiang BH 2007. Insulin-like growth factor-I induces cyclooxygenase-2 expression via PI3K, MAPK and PKC signaling pathways in human ovarian cancer cells. Cell Signal 19: 1542–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al. 2005. Real-time quantification of microRNAs by stem–loop RT–PCR. Nucleic Acids Res 33: e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N 2012. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 16 Suppl 2: S17–S27 [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ 2006. Oncomirs—microRNAs with a role in cancer. Nat Rev 6: 259–269 [DOI] [PubMed] [Google Scholar]
- Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH 2005. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J 19: 342–353 [DOI] [PubMed] [Google Scholar]
- Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca EA, Lawler S 2008. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res 68: 9125–9130 [DOI] [PubMed] [Google Scholar]
- Jia CY, Li HH, Zhu XC, Dong YW, Fu D, Zhao QL, Wu W, Wu XZ 2011. MiR-223 suppresses cell proliferation by targeting IGF-1R. PloS ONE 6: e27008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefas B, Godlewski J, Comeau L, Li Y, Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S, et al. 2008. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res 68: 3566–3572 [DOI] [PubMed] [Google Scholar]
- Kong KL, Kwong DL, Chan TH, Law SY, Chen L, Li Y, Qin YR, Guan XY 2012. MicroRNA-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptor. Gut 61: 33–42 [DOI] [PubMed] [Google Scholar]
- Kwak HJ, Kim YJ, Chun KR, Woo YM, Park SJ, Jeong JA, Jo SH, Kim TH, Min HS, Chae JS, et al. 2011. Downregulation of Spry2 by miR-21 triggers malignancy in human gliomas. Oncogene 30: 2433–2442 [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB 2003. Prediction of mammalian microRNA targets. Cell 115: 787–798 [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP 2005. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20 [DOI] [PubMed] [Google Scholar]
- Liu LZ, Zheng JZ, Wang XR, Jiang BH 2008. Endothelial p70 S6 kinase 1 in regulating tumor angiogenesis. Cancer Res 68: 8183–8188 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Pollak M 2008. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev 8: 915–928 [DOI] [PubMed] [Google Scholar]
- Sana J, Hajduch M, Michalek J, Vyzula R, Slaby O 2011. MicroRNAs and glioblastoma: Roles in core signalling pathways and potential clinical implications. J Cell Mol Med 15: 1636–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B, Sepp-Lorenzino L, Prisco M, Linsley P, deAngelis T, Baserga R 2007. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem 282: 32582–32590 [DOI] [PubMed] [Google Scholar]
- Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, You Y 2008. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res 1236: 185–193 [DOI] [PubMed] [Google Scholar]
- Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM, et al. 2012. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest 122: 1973–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognon CE, Sorensen PH 2012. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert Opin Ther Targets 16: 33–48 [DOI] [PubMed] [Google Scholar]
- Vredenburgh JJ, Desjardins A, Reardon DA, Friedman HS 2009. Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol 11: 80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X 2009. A PCR-based platform for microRNA expression profiling studies. RNA 15: 716–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Cheung WK, Ng SS, Jiang X, Jiang S, Sze J, Leung GK, Lu G, Chan DT, Bian XW, et al. 2012. Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J Biol Chem 287: 9962–9971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Dou W, He L, Liang S, Tie J, Liu C, Li T, Lu Y, Mo P, Shi Y, et al. 2012. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene 10.1038/onc.2012.156 [DOI] [PubMed] [Google Scholar]