Abstract
Induced pluripotent stem cells (iPSCs) can be generated from patients with specific diseases by the transduction of reprogramming factors and can be useful as a cell source for cell transplantation therapy for various diseases with impaired organs. However, the low efficiency of iPSC derived from somatic cells (0.01–0.1%) is one of the major problems in the field. The phosphoinositide 3-kinase (PI3K) pathway is thought to be important for self-renewal, proliferation, and maintenance of embryonic stem cells (ESCs), but the contribution of this pathway or its well-known negative regulator, phosphatase, and tensin homolog deleted on chromosome ten (Pten), to somatic cell reprogramming remains largely unknown. Here, we show that activation of the PI3K pathway by the Pten inhibitor, dipotassium bisperoxo(5-hydroxypyridine-2-carboxyl)oxovanadate, improves the efficiency of germline-competent iPSC derivation from mouse somatic cells. This simple method provides a new approach for efficient generation of iPSCs.
Introduction
Mammalian somatic cells can be reprogrammed into induced pluripotent stem cells (iPSCs) by ectopic expression of Oct3/4 (also known as Pou5f1), Klf4, and Sox2 with or without c-Myc (hereafter referred to as “OKSM” and “OKS”, respectively).1,2,3,4 iPSCs are similar to embryonic stem cells (ESCs) in their morphology, gene expression, and ability to differentiate into the three germ layers in vitro and in vivo.1,5 Compared with ESCs, iPSCs avoid ethical controversy and immune rejection and have a great potential to be a cell source for personalized stem cell-based transplantation therapies. However, the efficiency of iPSC generation is quite low (0.01–0.1%), which is one of the obstacles to be overcome.1,2,3,4,6,7 This low efficiency of iPSC generation is considered to be partially due to senescence and apoptosis induced by ectopic expression of OKSM,8 although the molecular mechanisms involved in somatic cell reprogramming have not been fully elucidated.1,2,3,7 Thus, understanding of the molecular mechanisms leading to reprogramming of somatic cells is crucial, and development of a new strategy for efficient iPSC generation is strongly desired.8
Numerous studies have shown that the phosphoinositide 3-kinase (PI3K) signaling pathway is required for maintenance of ESC pluripotency by regulating Nanog and Sox2, both of which are transcription factors involved in self-renewal of ESCs.9,10,11,12 In addition, the PI3K pathway is known to be negatively regulated by phosphatase and tensin homolog deleted on chromosome ten (Pten), a well-known tumor suppressor that is deleted or mutated in various types of cancer.13,14,15,16 Recent studies have shown that knockdown of Pten in ESCs promotes self-renewal, as well as cell survival and proliferation,16,17,18 indicating that Pten is also involved in the control of stem cell behavior through PI3K regulation.
Here, we report that transient inhibition of Pten by its inhibitor during the process of reprogramming mouse embryonic fibroblasts (MEFs) promotes their proliferation and enhances the efficiency of germline-competent iPSC generation by ectopic expression of OKSM.
Results
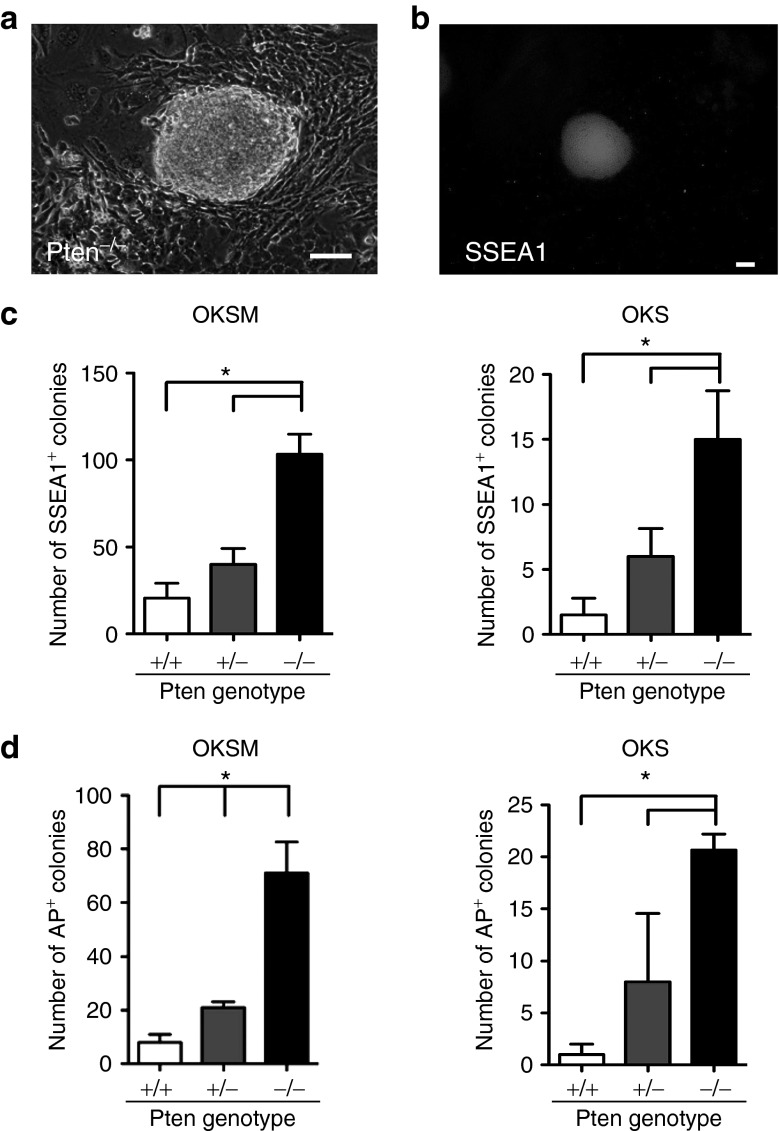
To examine whether inhibition of Pten facilitates the reprogramming process for iPSC generation, we first retrovirally transduced OKSM into MEFs lacking Pten (Pten−/− MEFs)19 and cultured these cells on mitomycin C (MMC)-treated SNL feeders (a SIM mouse embryo-derived thioguanine and ouabain resistant (STO) cell line transformed with neomycin resistance and mouse leukemia inhibitory factor genes) in mouse ESC medium. At about 7 days after OKSM transduction into Pten−/− MEFs, ESC-like round-shaped colonies were observed (Figure 1a). We examined the expression of stage-specific mouse embryonic antigen1 (SSEA1), a marker of mouse ESCs, by immunocytochemistry (Figure 1b,c).20 The number of SSEA1+ colonies induced by OKSM significantly increased in Pten−/− MEF cultures (103 ± 2) compared with that in Pten+/− and wild-type MEF cultures (40 ± 9 and 21 ± 9, respectively; Figure 1c, left panel). Similar results were obtained when OKS were transduced (15 ± 4 for Pten−/− MEFs, 6 ± 2 for Pten+/− MEFs and 2 ± 1 for wild-type MEFs; Figure 1c, right panel).
Figure 1.
Loss of Pten promotes reprogramming of MEFs into iPSCs. (a) Representative image of an iPSC colony derived from Pten−/− MEFs by transduction of mouse OKSM. Scale bar = 100 μm. (b) Immunocytochemical staining of SSEA1 on iPSCs induced by transduction of OKSM into Pten−/− MEFs. Scale bar = 0.2 mm. (c) Counts of SSEA1+ colonies. A total of 5,000 (OKSM) or 50,000 (OKS) retrovirally transduced MEFs with Pten+/+, Pten+/−, or Pten−/− genotypes were transferred onto feeders on day 4 after transduction. SSEA1+ colonies were counted on day 14 (OKSM, left) and day 28 (OKS, right) after transduction. Data are the mean ± SD (n = 3), *P < 0.05 versus wild type. (d) Counts of AP+ colonies derived from single cells. A total of 100 MEFs (OKSM or OKS) with Pten+/+, Pten+/−, or Pten−/− genotypes were transferred onto feeders on day 4 after transduction. AP+ colonies generated from Pten+/+, Pten+/−, or Pten−/− MEFs transduced with OKSM (left) or OKS (right) were counted on day 12 (OKSM) and day 28 (OKS) after transduction. Data are the mean ± SD (n = 3 or more), *P < 0.05 versus wild type. iPSC, induced pluripotent stem cell; MEF, mouse embryonic fibroblast.
To confirm that SSEA1+ colonies were derived from a single cell, and not from cell clusters, we replated fewer OKSM-transduced Pten−/− MEFs onto MMC-treated SNL feeders (100 cells per well in a six-well plate) in ESC medium. At 14 days after retroviral transduction, the number of alkaline phosphatase-positive (AP+) colonies was counted. As a result, 71 ± 12 AP+ colonies were generated from Pten−/− MEFs, whereas only 8 ± 1 colonies were generated from wild-type MEFs (Figure 1d, left panel), indicating that ~70% of OKSM-transduced Pten−/− MEFs had the potential to become iPSCs. Similar results were obtained when OKS were transduced into Pten−/− MEFs (Figure 1d, right panel). These results strongly indicated that the deficiency of Pten significantly increased the number of iPSCs generated by transduction of OKSM or OKS.
Loss of Pten has been shown to activate the PI3K-Akt pathway.13,21 We next activated the PI3K pathway by expression of phosphatase-deficient Pten mutants that contained a Cys-124 to serine substitution at the phosphatase catalytic center (CS-Pten),22 or the active myristoylated form of Akt (myr-Akt),23 and then the efficiency of iPSC generation from wild-type MEFs by OKSM transduction was examined. We observed significantly more AP+ colonies generated from MEFs expressing CS-Pten+OKSM or myr-Akt+OKSM compared with those generated from the control (354 ± 41, 355 ± 10 and 231 ± 25, respectively) (Figure 2a). These results indicated that activation of the PI3K pathway in MEFs enhanced the generation of iPSCs by co-expression of OKSM.
Figure 2.
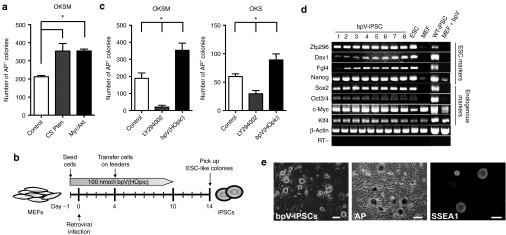
Activation of the PI3K-Akt pathway enhances iPSC generation. (a) Counts of AP+ colonies formed by transduction of OKSM combined with the dominant-negative form of Pten (CS-Pten) or activated form of Akt (myr-Akt) into MEFs. Wild-type MEFs (1 × 105) were transduced with OKSM (control, left), OKSM+CS-Pten (middle), or OKSM+myr-Akt (right). Cells (5,000) were transferred onto SNL feeders on day 4 after transduction and cultured in ESC medium. AP+ colonies were counted on day 14 after transduction. Data are the mean ± SD (n = 3), *P < 0.05 versus control. (b) Experimental scheme for iPSC generation. MEFs (1 × 105) were infected with retroviruses carrying OKSM on day 0. Cells (5,000) were transferred onto SNL feeders on day 4 after transduction and cultured in ESC medium containing 100 nmol/l bpV(HOpic). Colonies were collected based on ESC-like morphology on day 14 after transduction. The Pten inhibitor bpV(HOpic) was added from day –1 to 10. (c) Counts of AP+ colonies formed by transduction of OKSM or OKS into MEFs in the presence of LY294002 or bpV(HOpic). A total of 5,000 (OKSM) or 50,000 (OKS) retrovirally transduced MEFs were transferred onto SNL feeders on day 4 after transduction and then cultured in the presence of 100 nmol/l bpV(HOpic) or 5 μmol/l LY294002 for 10 (OKSM) or 28 days (OKS). AP+ colonies were counted on day 10 (OKSM, left panel) and day 28 (OKS, right panel). AP+ colonies generated without drugs were used as controls. Data are the mean ± SD (n = 3), *P < 0.05 versus controls. (d) Characteristics of bpV-iPSCs in vitro. The expression of ESC marker genes in bpV-iPSCs (OKSM) was examined by RT PCR (bpV-iPSC clones 1–8). Mouse β-actin was used as a loading control. The RT (−) control is shown at the bottom. (e) Representative images of bpV-iPSC colonies (left panel), AP staining (middle panel), and SSEA1 staining (right panel). Scale bars = 100 μm. ESC, embryonic stem cell; iPSC, induced pluripotent stem cell; MEF, mouse embryonic fibroblast; RT, reverse transcription.
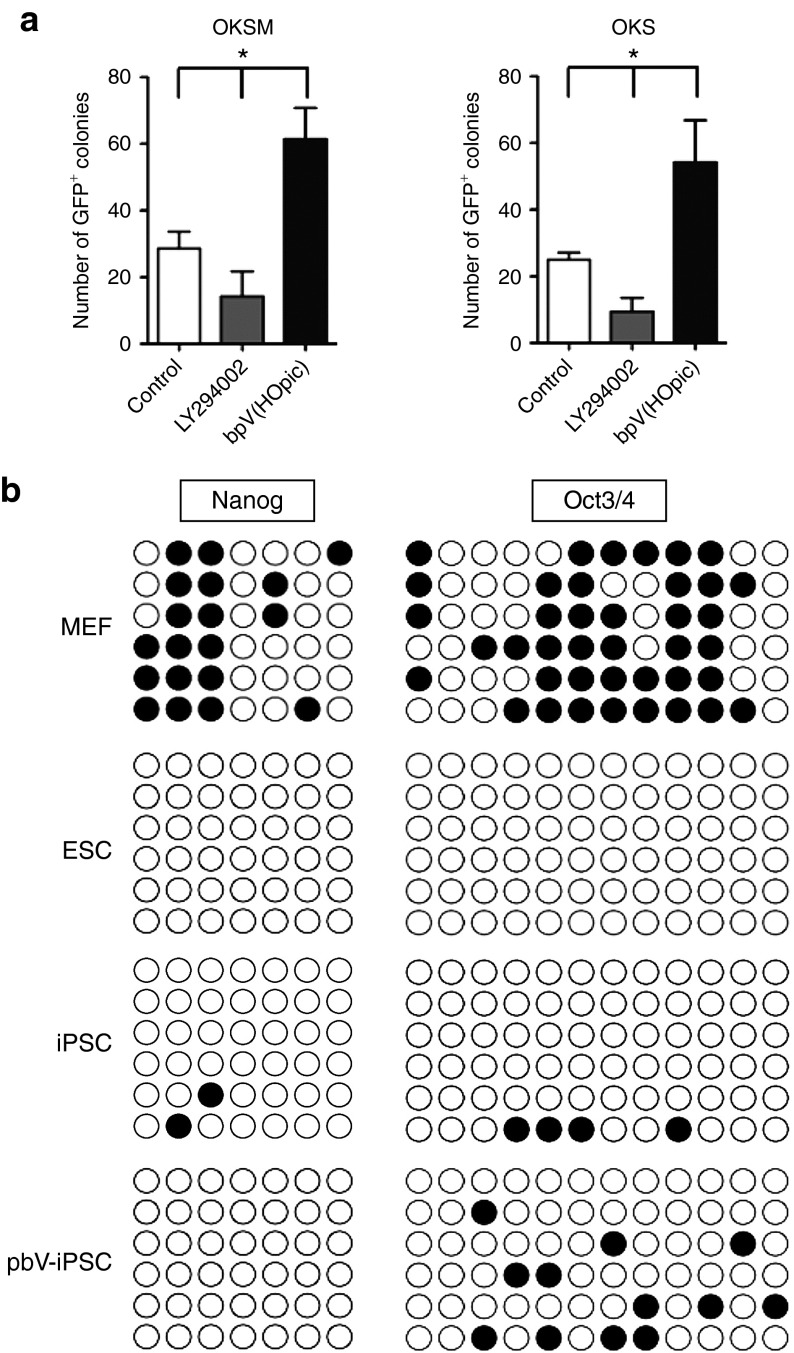
It is thought that continuous activation of the PI3K pathway may cause transformation of cells.24 Therefore, to efficiently and safely generate iPSCs, transient activation of the PI3K pathway combined with transduction of OKSM might be desirable. To establish transient activation of the PI3K pathway, we used a Pten inhibitor, dipotassium bisperoxo(5-hydroxypyridine-2-carboxyl)oxovanadate [bpV(HOpic)],25 during the process of iPSC generation (from day 0 to 10 after transduction) (Figure 2b). The bpV(HOpic) concentration was considered optimal at 100 nmol/l, which was determined by analyzing the activation status of Akt in MEFs by western blotting (Supplementary Figure S1 and Supplementary Materials and Methods). MEFs cultured in medium containing 100 nmol/l bpV(HOpic) were transduced with OKSM, resulting in generation of ESC-like colonies (bpV-iPSCs) on day 14 after transduction (Figure 2b,e, left panel). After isolation of these ESC-like colonies on day 14 after transduction, eight bpV-iPSC lines were established and expanded in conventional ESC medium on SNL feeders and then characterized further (Supplementary Table S1). Reverse transcription PCR showed that all of the bpV-iPSC lines examined (8/8) expressed ESC markers such as endogenous Oct3/4, Sox2, and Nanog (Figure 2d). In addition, bpV-iPSC lines were positive for AP activity and SSEA1 staining (Figure 2e, middle and right panels, respectively). It should be noted that the efficiency of iPSC generation from OKSM-transduced MEFs in the presence of bpV(HOpic) was much higher than that from the untreated control (353 ± 42 versus 189 ± 32; Figure 2c, left panel). Furthermore, inhibition of the PI3K pathway by LY294002,26 a reversible inhibitor of all classes of PI3Ks, resulted in a sharp decrease of the efficiency of iPSC generation (20 ± 11; Figure 2c, left panel). Similar results were obtained when OKS were transduced in the presence of bpV(HOpic) (89 ± 11 versus 60 ± 5; Figure 2c, right panel). Moreover, the emergence of SSEA1+ colonies from MEFs transduced with OKSM was enhanced by bpV(HOpic) treatment (n = 3, P < 0.05; Supplementary Figure S2).
These results were further confirmed by experiments using MEFs carrying the green fluorescent protein (GFP) gene under the control of the Nanog promoter (Nanog-GFP MEFs).12 We found that transient treatment with bpV(HOpic) significantly increased the number of Nanog-GFP+ colonies from MEFs transduced with OKSM or OKS under a feeder-free condition (n > 3, P < 0.05; Figure 3a). These results strongly indicated that inhibition of Pten promoted the efficiency of iPSC generation by transduction of OKSM or OKS.
Figure 3.
Addition of bpV(HOpic) improves the reprogramming of Nanog-GFP MEFs by OKSM or OKS. (a) Counts of Nanog-GFP+ colonies formed by transduction of OKSM or OKS into MEFs in the presence of LY294002 or bpV(HOpic). Nanog-GFP MEFs were retrovirally transduced with OKSM (left panel) or OKS (right panel) and then cultured in the presence of 5 μmol/l LY294002 or 100 nmol/l bpV(HOpic) for 9 days (from day 5 to 14 after transduction, left panel) or 33 days (from day 5 to 38 after transduction, right panel). The number of GFP+ colonies was counted by observation under an immunofluorescence microscope. Data are the mean ± SD (n = 12 for LY294002- or bpV(HOpic)-treated groups and n = 6 for the control group), *P < 0.05. (b) DNA methylation analysis of the promoter regions for endogenous Nanog and Oct3/4 genes. Genomic DNA from wild-type MEFs, mouse ESCs, wild-type iPSCs, and bpV-iPSCs (OKSM) were analyzed by bisulfite sequencing. Open and closed circles indicate unmethylated and methylated CpG islands, respectively. ESC, embryonic stem cell; iPSC, induced pluripotent stem cell; MEF, mouse embryonic fibroblast.
It has been reported that the use of PS48, a small molecule activator of 3′-phosphoinositide–dependent kinase-1 (PDK1) that is involved in the PI3K pathway, can enhance the reprogramming efficiency for iPSC generation from human cells.27 We therefore examined whether bpV(HOpic) or PS48 could further enhance the reprogramming efficiency. We found that a significantly higher number of AP+ colonies were formed from bpV(HOpic)-treated MEFs compared with that from PS48-treated MEFs (n = 3, P < 0.05; Supplementary Figure S3), indicating that inhibition of Pten by bpV(HOpic) might be a better approach than activation of PDK1 by PS48 for the enhancement of reprogramming.
To exclude the possibility that nonspecific effects of bpV(HOpic) had affected the iPSC generation, we also tested other specific Pten inhibitors such as SF1670 and bpV(Phen). As a result, the number of AP+ colonies appeared to significantly increase on day 10 after transduction in the presence of SF1670 or bpV(Phen) (Supplementary Figure S4).
It has been reported that various kinds of chemicals and supplements, such as histone deacetylase inhibitors, valproic acid (VPA), MAPK/ERK kinase inhibitors + glycogen synthase kinase 3β (GSK3β) inhibitors (2i), and vitamin C (Vc), enhance the reprogramming efficiency of somatic cells into iPSCs.6,28,29,30 Therefore, various combinations of bpV(HOpic) were tested with these compounds, such as bpV(HOpic)+Vc, bpV(HOpic)+2i, and bpV(HOpic)+VPA. We found that combined use of bpV(HOpic) with each compound significantly increased the number of AP+ or Nanog-GFP+ colonies generated from OKSM-transduced MEFs (n = 3, P < 0.05; Supplementary Figure S5 and Supplementary Materials and Methods). In particular, bpV(HOpic)+VPA strongly enhanced the reprogramming efficiency by more than fourfold compared with that of bpV(HOpic) alone (12 ± 4 versus 48 ± 7; Supplementary Figure S5).
Next, we examined the characteristics of bpV-iPSCs. Bisulfite genomic sequencing analyses were performed to examine epigenetic modification of pluripotency-associated promoter regions such as Nanog and Oct3/4 genes.1,2,12,31 The promoters of Nanog and Oct3/4 genes in OKSM- and OKS-transduced bpV-iPSCs were less methylated than those in MEFs, which was similar to those in ESCs (Figure 3b and Supplementary Figure S6), suggesting that similar DNA methylation patterns of pluripotency genes, such as Nanog and Oct3/4, stably maintained the undifferentiated state of bpV-iPSCs.
In addition, karyotype analyses showed that the chromosomal status of bpV-iPSCs was normal over 15 passages (Supplementary Figure S7), indicating that transient activation of the PI3K pathway by a Pten inhibitor, bpV(HOpic), did not affect chromosomal stability in the process of reprogramming.
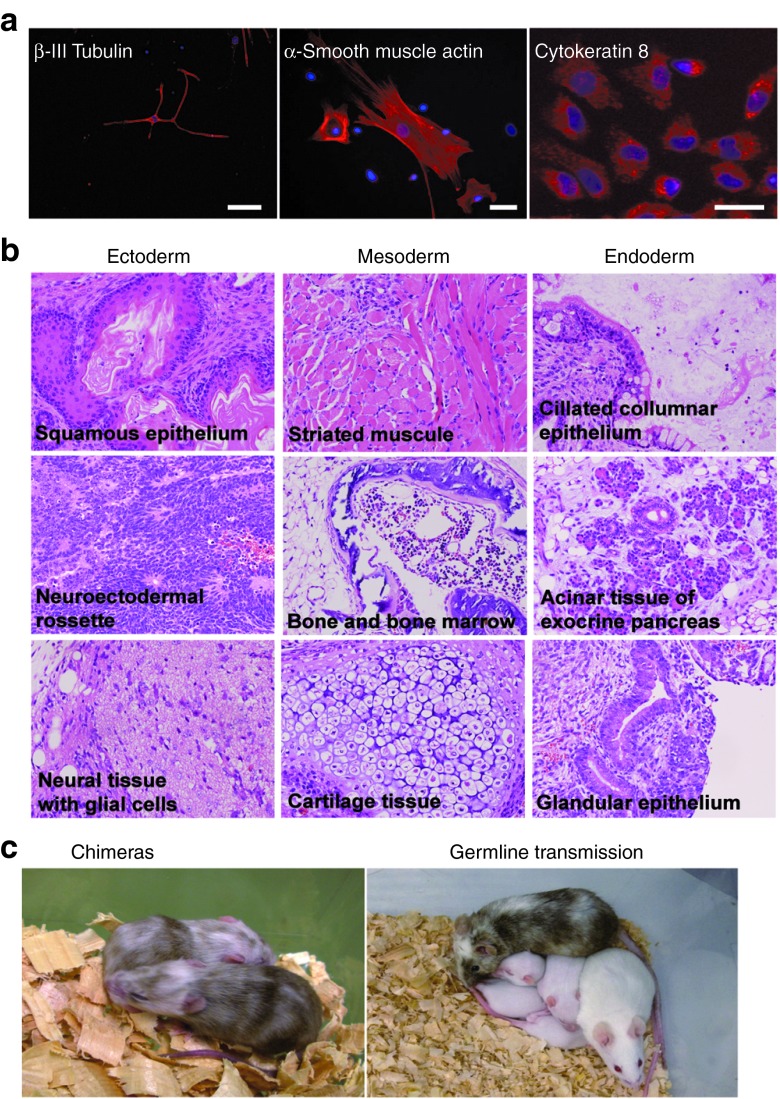
We next examined in vitro and in vivo differentiation potentials of bpV-iPSCs. bpV-iPSC differentiation was induced by embryoid body (EB) formation in vitro. Immunocytochemical analysis revealed that EB-formed cells expressed lineage markers of the ectoderm (β-III tubulin), mesoderm (α-smooth muscle actin), and endoderm (cytokeratin 8) (Figure 4a). We then performed a teratoma formation assay in vivo. One-million bpV-iPSCs were injected into the testes and backs of SCID mice. At 4–5 weeks after injection, we observed tumor formation. Histological analyses by hematoxylin-eosin staining showed that the tumors contained various derivatives of the three germ layers, indicating development of a well-differentiated teratoma (Figure 4b). Moreover, bpV-iPSCs contributed to somatic tissue formation in chimeric mice and showed a germline transmission capability (Figure 4c). These results strongly indicated that bpV-iPSCs possessed pluripotency in vitro and in vivo.
Figure 4.
Pluripotency of bpV-iPSCs. (a) In vitro differentiation ability of bpV-iPSCs. After EB formation for 7 days, EBs were transferred onto 0.1% gelatin-coated dishes, cultured for another 6 days, fixed, and then processed for immunocytochemistry using antibodies against β-III tubulin (ectoderm marker, left panel), α-smooth muscle actin (mesoderm marker, middle panel), and cytokeratin 8 (endoderm marker, right panel). Scale bars = 50 μm. (b) In vivo differentiation ability of bpV-iPSCs. Hematoxylin-eosin staining of tissue sections showing teratomas composed of mature tissues derived from the three embryonic germ layers. Magnification ×200. (c) Contribution of bpV-iPSCs to chimeric mice. Injection of bpV-iPSCs derived from ICR mice into C57BL/6 blastocysts led to the generation of chimeric mice (left panel). Offspring (chimera male × ICR female) were white, indicating germline transmission of bpV-iPSCs (right panel). iPSC, induced pluripotent stem cell.
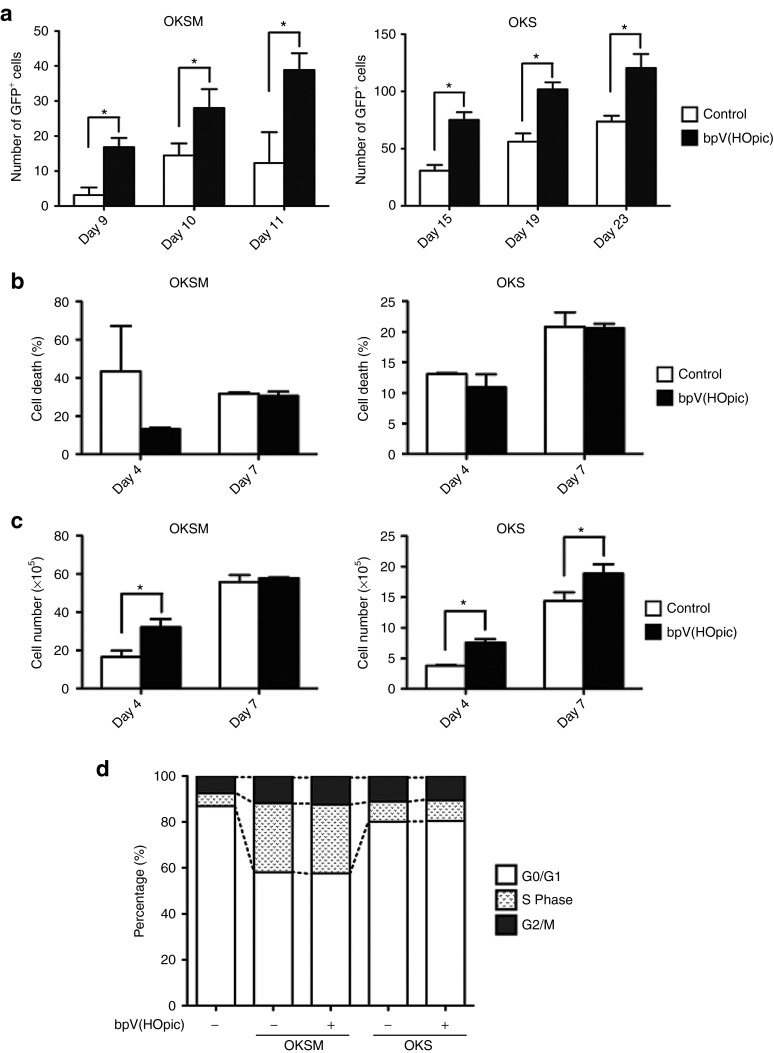
Next, we attempted to elucidate the mechanisms that promoted iPSC generation by the Pten inhibitor. We first examined the effect of bpV(HOpic) on the reprogramming time window by transduction of OKSM or OKS into Nanog-GFP MEFs. We found that the reprogramming time window was not affected by the addition of bpV(HOpic), although the number of GFP+ cells was increased on around day 10 (OKSM) and day 15 (OKS) after transduction (Figure 5a).
Figure 5.
Effects of bpV(HOpic) on survival, proliferation, and reprogramming of MEFs. (a) Promotion of reprogramming by bpV(HOpic). Nanog-GFP MEFs were retrovirally transduced with OKSM (upper panel) or OKS (lower panel) and then cultured in the presence (black bar) or absence (white bar) of bpV(HOpic). The number of GFP+ cells was counted by observation under an immunofluorescence microscope at the indicated time points after transduction. Data are the mean ± SD (n = 3), *P < 0.05. (b) Analysis of cell death. OKSM- (left panel) or OKS- (right panel) transduced MEFs were cultured with or without 100 nmol/l bpV(HOpic) for 4 or 7 days. The annexin V+ cell population was analyzed by flow cytometry. Data are the mean ± SD (n = 3). (c) Effects of bpV(HOpic) on cell proliferation. The number of OKSM- (left panel) or OKS- (right panel) transduced MEFs cultured with or without 100 nmol/l bpV(HOpic) for 4 or 7 days was counted. Data are the mean ± SD (n = 3), *P < 0.05. (d) Cell cycle analysis. OKSM- or OKS-transduced MEFs were cultured in the presence of 100 nmol/l bpV(HOpic) for 4 days, and then a BrdU assay was performed. MEFs and OKSM- or OKS-transduced MEFs without bpV(HOpic) treatment were used as controls. Data are the mean ± SD (n = 3). GFP, green fluorescent protein; MEF, mouse embryonic fibroblast.
To further address how the efficiency of iPSC generation was enhanced by Pten inhibition, we examined cell death, cell cycle distribution, and proliferation affected by bpV(HOpic). Cell survival of OKSM- and OKS-transduced MEFs treated with bpV(HOpic) in the process of reprogramming was assessed using annexin V staining. We did not observe any significant increase or decrease in cell death induced by bpV(HOpic) at the indicated time points (Figure 5b), although cell death on day 4 after transduction tended to be inhibited in the presence of bpV(HOpic). However, we found that the proliferation rate of OKSM- and OKS-transduced MEFs treated with bpV(HOpic) was slightly higher than that of the untreated control (Figure 5c), although the data from OKSM-transduced MEFs on day 7 after transduction showed no effect on proliferation induced by bpV(HOpic). These data indicated that bpV(HOpic) treatment slightly promoted cell proliferation, particularly during the early phase of reprogramming of OKSM- and OKS-transduced MEFs. However, the cell cycle distribution was not changed significantly (Figure 5d). These results indicated that the enhancement of iPSC generation by Pten inhibition was associated with slightly accelerated cell proliferation during the early phase of reprogramming.
Discussion
It is known that the efficiency of iPSC generation by retroviral transfer of reprogramming factors into MEFs is ~0.1–1%.1,2,3,4,6,7 In this study, we have developed a novel method that enhances the reprogramming efficiency of OKSM-transduced mouse somatic cells by transient activation of the PI3K pathway using a Pten inhibitor, bpV(HOpic). The efficiency of AP+ cell generation from OKSM-transduced MEFs by the addition of bpV(HOpic) was ~7% (Figure 2c, left panel). This high proportion of AP+ cells was surprising, but there was a possibility that insufficiently reprogrammed cells were included in the AP+ population. Therefore, we also examined the efficiency of SSEA1+ cell generation from MEFs. The proportion of SSEA1+ cells generated from OKSM-transduced MEFs treated with bpV(HOpic) was ~3% (n = 3, P < 0.05; Supplementary Figure S2), strongly suggesting the ability of bpV(HOpic) to enhance iPSC generation. It should also be noted that combined use of bpV(HOpic) with VPA further improved iPSC generation (Supplementary Figure S5b).
The mechanisms of bpV(HOpic) treatment, which increase the efficiency of iPSC generation, are considered to be crucial. We have shown that treatment with LY294002 from day 0 to 10 after OKSM transduction inhibited the efficiency of iPSC generation (Figure 2c), whereas expression of the activated form of Akt (myr-Akt)-enhanced iPSC generation (Figure 2a). Thus, it is conceivable that activation of the PI3K-Akt pathway due to inhibition of Pten by bpV(HOpic) at least in part plays a role in the reprogramming process.
Several molecules downstream of the PI3K-Akt pathway are responsible for critical biological phenomena such as self-renewal, and cell survival and proliferation. It has been reported that c-Myc and its downstream target, cyclin D, are activated by the PI3K-Akt pathway,32 which leads to acceleration of the cell cycle. Another report has described that Akt inhibits cell cycle inhibitory molecules, p21 and p27.8,33 This evidence indicates that activation of the PI3K-Akt pathway promotes cell proliferation. Indeed, MEFs treated with bpV(HOpic) showed a slightly higher rate of proliferation (Figure 5b). Previous reports have described that promotion of cell proliferation results in the enhancement of iPSC generation.34 Thus, the acceleration of cell proliferation caused by the treatment with bpV(HOpic) may be associated with the activation of cell cycle molecules, which leads to the elevation of the efficiency of iPSC generation from somatic cells.
Several reports have shown that GSK3β is a key regulator of cellular fate and a participant in differentiation events during embryonic development through the Wnt/GSK3β/β-catenin signaling pathway.35 Phosphorylation of GSK3β by Akt allows translocation of β-catenin into the nucleus, which activates transcription factors such as c-Myc and Nanog and promotes reprogramming.29,36 Therefore, the use of bpV(HOpic) might inhibit GSK3β through activation of the PI3K-Akt pathway, resulting in the enhancement of iPSC generation.
Recently, treatment with 0.3 μmol/l LY294002, a PI3K pathway inhibitor, from day 1 to 3 after OKSM transduction was shown to enhance the efficiency of iPSC generation from MEFs.37 However, in our study, treatment with 5 μmol/l LY294002 from day 0 to 10 after OKSM transduction inhibited the efficiency of iPSC generation (Figure 2c). The discrepancy between these data might be due to the difference in drug concentrations and/or the duration of drug treatment used for iPSC generation.
It has been reported that the efficiency of reprogramming is enhanced by increased cell proliferation and survival by inhibition of the p53-p21 pathway and Ink4a/Arf locus.8,33,34,38,39,40,41,42 Indeed, use of the p53 inhibitor pifithrin-α hydrobromide (PFTα) tended to show a higher reprogramming efficiency (Supplementary Figure S8). However, p53 was slightly accumulated in OKSM-transduced MEFs treated with bpV(HOpic) (Supplementary Figure S9), indicating that promotion of iPSC generation by bpV(HOpic) was not due to reduced p53 protein levels. Further investigation is needed to reveal the significance of slight p53 stabilization in OKSM-transduced MEFs treated with bpV(HOpic).
Interestingly, for cell proliferation, previous reports have shown that mitochondrial oxidation is generally used in differentiated somatic cells, whereas glycolysis is mainly used in pluripotent cells.27,43 In addition, it has been shown that modulation of cell metabolism from mitochondrial oxidation to glycolysis plays an important role in the process of iPSC generation.27,43,44 Because PI3K signaling is known to be a potent regulator of cellular metabolism,45 the possibility that inhibition of Pten by bpV(HOpic) induces a change of metabolic pathways from mitochondrial oxidation to glycolysis should also be carefully examined in the future.
Because continuous activation of the PI3K pathway causes transformation of cells,46 bpV(HOpic) treatment may cause the emergence of cancer cells. However, iPSCs generated in the presence of bpV(HOpic) could be readily and stably expanded over a long term under conventional ESC culture conditions (>15 passages) and exhibited a normal karyotype (Supplementary Figure S7). Furthermore, they could directly differentiate into the three germ layers in vitro and formed teratomas in vivo. In mice, injection of these bpV-iPSCs into C57BL/6 host blastocysts resulted in the generation of healthy chimeric mice showing a germline transmission capability (Figure 4). Out of 11 chimeric mice aged over 1 year, nine mice did not survive owing to unknown reasons, and only one mouse formed a teratoma in the left leg (data not shown). Teratoma formation in a bpV-iPSC chimeric mouse might be influenced by reactivation of reprogramming factors or transient activation of the PI3K pathway in the process of reprogramming. In addition, some of the bpV-iPSCs injected into blastocysts might remain in an undifferentiated state. These possibilities need to be carefully considered when iPSC-based cell replacement therapies are conducted for regenerative medicine in the future.
Materials and Methods
Plasmids. pMXs-based retroviral vectors for mouse Oct3/4, Klf4, Sox2, and c-Myc were obtained from Addgene (Cambridge, MA).47 pMXs vectors encoding a mutant derivative of Pten containing a Cys-124 to serine substitution (CS-Pten),22 myr-Akt, or GFP genes were gifts from Akira Suzuki (Kyushu University, Fukuoka, Japan).
Cell culture. PLAT-E cells for production of retroviruses were kindly provided by Dr. Toshio Kitamura (Tokyo University, Tokyo, Japan) and maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Invitrogen), 1 mg/ml puromycin (InvivoGen, San Diego, CA), and 10 μg/ml blasticidin S (InvivoGen).48 MEFs were isolated from pregnant mice at gestational day 12 (Crlj: CD1, Charles River, Japan; Nanog-GFP Mouse, Rikken, Japan) and expanded in fibroblast medium consisting of DMEM/10% FBS with 1% antibiotic-antimycotic mixed stock solution (Nacalai Tesque, Kyoto, Japan). Pten-deficient MEFs were kindly provided from Dr. Akira Suzuki (Kyushu University, Fukuoka, Japan).19 SNL feeders and MEFs were cultured in DMEM/10% FBS with antibiotics on 0.1% gelatin-coated dishes at 37 °C with 5% CO2. SNL feeders were treated with 12 μg/ml MMC (Kyowa Hakko Kirin, Tokyo, Japan) for around 2 hours before coculture with iPSCs. Mouse iPSCs were cultured on gelatin-coated plates with MMC-treated SNLs in mouse ESC medium consisting of knockout DMEM containing 1000 U/ml leukemia inhibitory factor (Wako, Osaka, Japan), 2 mmol/l L-glutamine (Nacalai Tesque), 10% FBS, 0.1 mmol/l 2-mercaptoethanol (Sigma-Aldrich, St Louis, MO), 1% non-essential amino acids (Invitrogen), and 50 IU/ml penicillin and 50 mg/ml streptomycin mixed solution (Nacalai Tesque).
Retrovirus production and iPSC generation. For retroviral production, PLAT-E cells were seeded at 3.3 × 106 cells per 100 mm dish. The following day, 9 μg pMXs-based retroviral vectors for expression of GFP, Oct3/4, Klf4, Sox2, or c-Myc were individually introduced into PLAT-E cells using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN). After 24 hours, the medium was replaced with 10 ml DMEM/10% FBS, and the supernatant was collected on the following day. On day 0, equal volumes of supernatants from PLAT-E cell cultures containing retroviruses carrying each factor were mixed, and then 1 × 105 (OKSM) − 1 × 106 (OKS) ICR-MEFs or 8 × 104 (OKSM) − 1 × 106 (OKS) Nanog-GFP MEFs were incubated with the mixture. At 4 days after infection, the cells were transferred onto MMC-treated SNL feeders and cultured in ESC medium. Using 35-mm dishes, 5,000 OKSM-transduced and 50,000 OKS-transduced cells were transferred onto SNL feeders. AP+ and SSEA1+ colonies were examined on day 14 for OKSM-transduced cells, and on day 28 for OKS-transduced cells. To examine the efficiency of iPSC generation from single cell population, 100 of OKSM-transduced cells or 100 of OKS-transduced cells were transferred onto MMC-treated SNL feeders on day 4 after transduction, and their AP activity was examined on days 14 (OKSM) and 28 (OKS), respectively.
Regulation of the PI3K-Akt pathway. To activate the PI3K-Akt pathway, MEFs were retrovirally transduced with a dominant-negative mutant type of Pten (CS-Pten)22 or activated form of myristoylated Akt (myr-Akt).23 Moreover, to activate the PI3K-Akt pathway with the Pten inhibitor, MEFs were treated with bpV(HOpic) (100 nmol/l; Calbiochem, Darmstadt, Germany), bpV(Phen) (100 nmol/l; Calbiochem), SF1670 (500 nmol/l; Cellagen Technology, San Diego, CA), or PS48 (5 μmol/l; Wako). Then, to inhibit the PI3K-Akt pathway, cells were treated with LY294002 (5 μmol/l; Santa Cruz Biotechnology, Heidelberg, Germany).
AP staining. AP staining was performed using an Alkaline Phosphatase Kit (Sigma-Aldrich). Colonies grown in 35-mm dishes were fixed in a fixative solution for 30 seconds at room temperature and then washed twice with deionized water for 45 seconds. Fixed cells were incubated with the AP staining solution while protected from light for 1 hour at room temperature and then washed twice with deionized water for 2 minutes. The samples were then observed by optical microscopy (Axiovert 135; ZEISS, Deutschland, Germany).
Immunocytochemical staining. Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 minutes and then washed extensively with PBS. The cells were then permeabilized with 0.3% Triton X-100 in PBS for 5 minutes, followed by blocking with 3% bovine albumin serum in PBS. Staining was carried out by incubation with an anti-SSEA1 antibody (Santa Cruz Biotechnology) overnight at 4 °C. After extensive washing, an antimouse Alexa546 antibody (Invitrogen) was applied for 1 hour at room temperature, and then the cells were counterstained with 4,6-diamindino-2-phenylindole-2 (Invitrogen). Images were obtained by immunofluorescence microscopy (BZ-9000, KEYENCE, Osaka, Japan).
Reverse transcription PCR. Total RNA was extracted from cultured cells using an RNeasy kit (Qiagen, Courtaboeuf, France), and cDNA was synthesized using a reverse transcription system (Invitrogen). PCR was performed using KOD FX DNA polymerase (Toyobo, Tokyo, Japan) according to the manufacturer's instructions. Primer sequences are described in Supplementary Tables S2 and S3. PCR products were size fractionated on 1% agarose gels. β-actin was used as a loading control.
Bisulfite sequencing and karyotype analysis. Genomic DNA from mouse ESCs, MEFs, and bpV-iPSCs was extracted with a DNeasy Blood & Tissue Kit (Qiagen) and then treated with sodium bisulfite using a BisulFast DNA Modification Kit (Toyobo). Treated DNA was purified using an EZ kit (Zymo Research, Orange, CA). Then, Nanog and Oct3/4 gene promoter regions49 were amplified by PCR using primers shown in Supplementary Table S4. PCR products were inserted into a pGEM-T easy vector (Promega, Madison, WI) and then sequenced using M13 primers. Karyotype analysis was performed at the ICLAS Monitoring Center, Central Institute for Experimental Animals.
Spontaneous differentiation in vitro and immunocytochemistry. The pluripotency of bpV-iPSCs was assessed by an in vitro differentiation assay. Briefly, single cells were harvested by trypsinization, and 1 × 106 cells were cultured on low adhesion plates in mouse ESC medium without leukemia inhibitory factor. Half medium volumes were exchanged every day, and EBs were allowed to grow for 6–7 days in suspension. Then, EBs were trypsinized and replated onto 0.1% gelatin-coated dishes and cultured for another 7 days (ectoderm) or 14 days (endoderm and mesoderm). Spontaneous differentiation was examined by immunocytochemistry using antibodies against cytokeratin 8 (Invitrogen), α-smooth muscle actin (Sigma, St Louis, MO), and β-III tubulin (Sigma). The samples were observed by immunofluorescence microscopy (BZ-9000).
Teratoma formation assay. The bpV-iPSCs (1 × 106) were resuspended in 50 μl PBS and then injected into the testis or back of SCID mice (Charles River Laboratories). At 4–8 weeks after injection, formed tumors were removed and fixed in 4% paraformaldehyde/PBS overnight at 4 °C. Tumor tissues were embedded in paraffin. Tissue blocks were sectioned at 3 μm and stained with hematoxylin-eosin. All animal experiments were approved by the Animal Committees at Kyushu University and Kumamoto University, Japan.
Generation of chimeric mice. To generate chimeric mice derived from bpV-iPSCs, C57BL/6 host blastocysts injected with bpV-iPSCs were transplanted into the uteri of surrogate ICR mice. Detailed protocols have been described before.50
Proliferation and cell death analyses. OKSM- or OKS-transduced MEFs (1 × 105) were cultured in DMEM/10% FBS with or without bpV(HOpic). On days 4 and 7, cells were harvested and counted with a TC10 automated cell counter (Bio-Rad, Hercules, CA) to determine the cell number. The cells were then stained with annexin V-APC (BD Pharmingen, San Diego, CA), and the population of annexin V+ dead cells was analyzed by flow cytometry (FACS Verse; BD Biosciences, San Jose, CA).
BrdU assay. The BrdU incorporation assay was performed with BrdU FLOW Kits according to the manufacturer's instructions (BD Pharmingen). Briefly, OKSM- or OKS-transduced MEFs were cultured in the presence of 10 μmol/l BrdU-labeling reagent (BD Pharmingen) for 1 hour. Then, the cells were harvested and dissociated into single cells with a trypsin/EDTA solution (Nacalai Tesque). Single cells were fixed, permeabilized, and stained with antibodies against BrdU and 7-AAD, and then samples were analyzed by flow cytometry (FACS Verse).
Statistical analyses. Data were shown as the mean ± SD and analyzed for statistical significance by GraphPad Prism version 5.0d (GraphPad Software, San Diego, CA). For comparisons between groups, the data were analyzed using a t-test or one-way analysis of variance with Tukey's multiple comparison. A value of P < 0.05 was considered as significant.
SUPPLEMENTARY MATERIAL Figure S1. Effect of Pten inhibitor, bpV(HOpic) on Akt activation. Figure S2. Number of SSEA1+ colonies generated from MEFs in the presence of bpV(HOpic). Figure S3. Number of AP+ colonies generated in the presence of PDK1 activator, PS48, or Pten inhibitor, bpV(HOpic). Figure S4. Effects of various Pten inhibitors on iPSC generation. Figure S5. Enhanced efficiency of iPSC generation by combined use of bpV(HOpic) with various compounds. Figure S6. DNA methylation analysis of the promoter regions of endogenous Nanog and Oct3/4 genes in OKS-transduced iPSCs. Figure S7. Karyotype analysis of OKSM-transduced bpV-iPSCs. Figure S8. Effects of p53 and Pten inhibitors on iPSC generation. Figure S9. p53 expression in MEFs treated with bpV(HOpic). Table S1. Summary of established bpV-iPSC cell lines. Table S2. Primers used for RT-PCR. Table S3. Endogenous primers used for RT-PCR. Table S4. Primers used for bisulfite sequencing. Materials and Methods.
Acknowledgments
The authors thank Ken-ichi Yamamura (Scientific Support Programs for Cancer Research Grant-in-Aid for Scientific Research on Innovative Areas, Kumamoto University, Japan), and Masato Tanaka and Kaori Nagatoshi for their help to establish chimeras. They also thank Michiko Ushijima for administrative assistance, Atsushi Takahashi and the members of Kenzaburo Tani's laboratory for providing constructive criticism and technical assistance, and Peng Xiong (Kyushu University) for his help in performing statistical analysis. They also thank Hiroyuki Sasaki (Kyushu University) for helpful discussions and providing bisulfite PCR primers for Nanog. This work was partly performed in the Cooperative Research Project Program of the Medical Institute of Bioregulation, Kyushu University, the Research Support Center (Graduate School of Medical Sciences, Kyushu University) and Laboratory for Technical Support (Medical Institute of Bioregulation, Kyushu University) for technical assistance. This work was supported by grants from the Project for Realization of Regenerative Medicine (K.T., 08008010) and KAKENHI (T.M., 23590465) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan. The authors declare no conflict of interest.
Supplementary Material
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm MP, Bone HK, Beck CG, Bourillot PY, Schreiber V, Damiano T, et al. Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem. 2007;282:6265–6273. doi: 10.1074/jbc.M610906200. [DOI] [PubMed] [Google Scholar]
- Jeong CH, Cho YY, Kim MO, Kim SH, Cho EJ, Lee SY, et al. Phosphorylation of Sox2 cooperates in reprogramming to pluripotent stem cells. Stem Cells. 2010;28:2141–2150. doi: 10.1002/stem.540. [DOI] [PubMed] [Google Scholar]
- Chen L, Khillan JS. A novel signaling by vitamin A/retinol promotes self renewal of mouse embryonic stem cells by activating PI3K/Akt signaling pathway via insulin-like growth factor-1 receptor. Stem Cells. 2010;28:57–63. doi: 10.1002/stem.251. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Alva JA, Lee GE, Escobar EE, Pyle AD. Phosphatase and tensin homolog regulates the pluripotent state and lineage fate choice in human embryonic stem cells. Stem Cells. 2011;29:1952–1962. doi: 10.1002/stem.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Masuyama N, Fukui Y, Suzuki A, Gotoh Y. Akt mediates Rac/Cdc42-regulated cell motility in growth factor-stimulated cells and in invasive PTEN knockout cells. Curr Biol. 2001;11:1958–1962. doi: 10.1016/s0960-9822(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci USA. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–2707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, et al. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130:1691–1700. doi: 10.1242/dev.00392. [DOI] [PubMed] [Google Scholar]
- Posner BI, Faure R, Burgess JW, Bevan AP, Lachance D, Zhang-Sun G, et al. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem. 1994;269:4596–4604. [PubMed] [Google Scholar]
- Salh B, Wagey R, Marotta A, Tao JS, Pelech S. Activation of phosphatidylinositol 3-kinase, protein kinase B, and p70 S6 kinases in lipopolysaccharide-stimulated Raw 264.7 cells: differential effects of rapamycin, Ly294002, and wortmannin on nitric oxide production. J Immunol. 1998;161:6947–6954. [PubMed] [Google Scholar]
- Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci USA. 2008;105:6584–6589. doi: 10.1073/pnas.0802785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, He J, Zhang Y. Kdm2b promotes induced pluripotent stem cell generation by facilitating gene activation early in reprogramming. Nat Cell Biol. 2012;14:457–466. doi: 10.1038/ncb2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio IM, Garcia-Herreros M, Fair T, Lonergan P. Identification and regulation of glycogen synthase kinase-3 during bovine embryo development. Reproduction. 2010;140:83–92. doi: 10.1530/REP-10-0040. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu X, Li J, Liu J, Gu H, Zhang R, et al. Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells. Cell Res. 2011;21:1424–1435. doi: 10.1038/cr.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Shen L, Yu J, Wan H, Guo A, Chen J, et al. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell. 2011;10:908–911. doi: 10.1111/j.1474-9726.2011.00722.x. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Strati K, Ortega S, Cañamero M, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marión RM, Strati K, Li H, Murga M, Blanco R, Ortega S, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, et al. 2013Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Naturedoi:10.1038/nature11799 [DOI] [PMC free article] [PubMed]
- Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. Metabolic Regulation in Pluripotent Stem Cells during Reprogramming and Self-Renewal. Cell Stem Cell. 2012;11:589–595. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T, et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- Blelloch R, Wang Z, Meissner A, Pollard S, Smith A, Jaenisch R. Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells. 2006;24:2007–2013. doi: 10.1634/stemcells.2006-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Wang J, Zhang Y, Kou Z, Gao S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 2009;5:135–138. doi: 10.1016/j.stem.2009.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.