Abstract
The global tuberculosis (TB) control plan has historically emphasized passive case finding (PCF) as the most practical approach for identifying TB suspects in high burden settings. The success of this approach in controlling TB depends on infectious individuals recognizing their symptoms and voluntarily seeking diagnosis rapidly enough to reduce onward transmission. It now appears, at least in some settings, that more intensified case-finding (ICF) approaches may be needed to control TB transmission; these more aggressive approaches for detecting as-yet undiagnosed cases obviously require additional resources to implement. Given that TB control programs are resource constrained and that the incremental yield of ICF is expected to wane over time as the pool of undiagnosed cases is depleted, a tool that can help policymakers to identify when to implement or suspend an ICF intervention would be valuable. In this article, we propose dynamic case-finding policies that allow policymakers to use existing observations about the epidemic and resource availability to determine when to switch between PCF and ICF to efficiently use resources to optimize population health. Using mathematical models of TB/HIV coepidemics, we show that dynamic policies strictly dominate static policies that prespecify a frequency and duration of rounds of ICF. We also find that the use of a diagnostic tool with better sensitivity for detecting smear-negative cases (e.g., Xpert MTB/RIF) further improves the incremental benefit of these dynamic case-finding policies.
Keywords: approximate dynamic programming, dynamic resource allocation, mathematical model, screening, cost-effectiveness
The global tuberculosis (TB) control plan, codified by the World Health Organization (WHO) and the Stop TB Partnership as the Directly Observed Treatment, Short course (DOTS) strategy in the mid-1990s (1), emphasizes passive case finding (PCF) as a central tactic for identifying infectious cases requiring treatment. PCF approaches depend on individuals with symptomatic TB to seek out treatment on their own, a practice that is supported by studies indicating that the most infectious patients are aware of their symptoms and seek care (2, 3). Adoption of PCF strategies has been motivated by practical considerations as well. In most high TB incidence settings, resources are limited and PCF allows diagnostic efforts to be focused within existing health facilities and concentrated among suspects at highest risk of TB.
The DOTS strategy has significantly improved treatment success rates for individual patients (4) and, where studies have been attempted, has been associated with reduced TB-related mortality in populations (5, 6). Despite clear successes of DOTS programs, there are inherent shortcomings of PCF since this approach may result in either delayed or missed opportunities for diagnosis. These limitations may be especially important in settings where HIV has emerged and triggered large and rapid increases in TB incidence (7).
There are many different types of interventions that could be used to increase the vigorousness of TB case detection efforts beyond PCF; in this article, we broadly refer to these alternative approaches as intensified case finding (ICF). ICF approaches are often subclassified as either “enhanced” or “active” case finding and are differentiated by whether emphasis is placed on increasing the probability of symptomatic patients seeking care or on asking providers to proactively seek out undiagnosed cases within the community. In either case, the central goals of ICF are to decrease delays to diagnosis and to increase the overall proportion of cases that are diagnosed. In theory, the direct benefits of ICF would include reduced morbidity and better treatment results for those detected earlier in the course of disease; indirect benefits would accrue to the community if detecting cases more rapidly led to reductions in the incidence of new TB infections.
To date, evidence for these indirect benefits of ICF approaches for TB control remains limited (8, 9). Nonetheless, previous modeling work demonstrating the importance of improved case finding for interrupting epidemic TB (10–13), coupled with growing consensus that PCF will not be sufficient to control TB in some settings, highlights the need for further efforts to understand how ICF might best be used (14, 15). Regardless of the specific ICF approach used, interventions to increase the aggressiveness of case finding will impose additional stresses on TB programs that are already resource-constrained. Since the pool of undiagnosed TB will be depleted during ICF, these approaches will produce diminishing returns over time (16). Accordingly, the optimal use of alternative case-finding practices will require intermittent implementation of more aggressive case-finding efforts. This will ensure that resources are diverted to ICF only at times when the yield will be high enough to justify its use.
Previous efforts to identify efficient use of ICF have focused on identifying policies that specify (1) a frequency of and (2) a duration for which more aggressive forms of case detection should be used (10, 11, 16, 17). These static policies (i.e., the frequency and duration of ICF remain fixed) are relatively easy to evaluate using mathematical or simulation models, and thus, the best choices for the frequency and duration of ICF can be readily identified for different epidemiological scenarios. However, it is reasonable to assume that a policymaker responsible for deciding when to implement ICF (and when to switch back to PCF) will want to use information about the current state of the epidemic and information about local resource availability to decide when more aggressive case-finding practices should be used. For example, a rising trend in case notifications or TB-related mortality during a period where PCF policies are in place might indicate that switching to ICF may be cost-effective and affordable, depending on resource constraints.
Our objective in this article is to identify dynamic policies that make recommendations about when to switch between PCF and ICF based on observed (but likely imperfect) measures of the TB epidemic (e.g., TB case notifications) and information about resource availability (e.g., annual budget). These policies depend on using recent observations to inform decision-making and thus are distinct from previously described policies that specify a fixed periodicity and duration of ICF efforts. In identifying these dynamic ICF policies, we seek to optimize a measure of population’s health (e.g., cumulative TB cases or TB deaths) while satisfying resource constraints (e.g., budget limitations).
Characterizing optimal dynamic policies is generally computationally prohibitive. However, the recent advent of more powerful optimization techniques allows for (approximately) optimal dynamic health policies to be characterized through computationally efficient methods (SI Appendix). Given that infectious diseases spread stochastically, it can also be mathematically proven that the optimal decision rule for implementation of ICF will be a function of the current state or the trajectory of the epidemic (18, 19), and thus, dynamic policies are expected to be more efficient than static policies.
In this article, we consider TB epidemics in settings that differ by (i) the level of HIV prevalence and (ii) the type of TB diagnostic tests in use (i.e., sputum microscopy versus a more sensitive and more expensive rapid diagnostic test). For each scenario, we identify approximately optimal dynamic policies that minimize the expected number of TB cases during the epidemic while satisfying the policymaker’s annual budget. For the TB/HIV epidemics considered here, we also compare the performance of dynamic case-finding policies to static policies that specify fixed periodicity and duration of ICF.
Methods
Model for Examining ICF in the Context of TB/HIV Coepidemics.
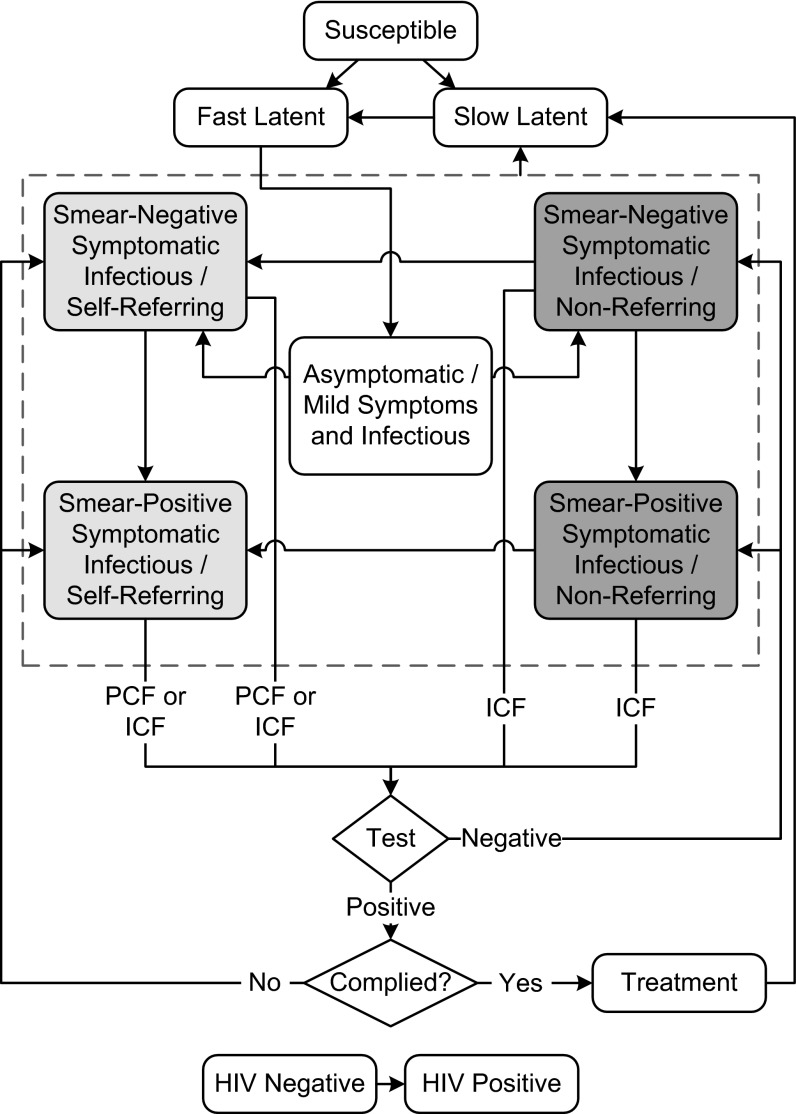
We develop a dynamic compartmental model of TB/HIV epidemic based on earlier models (20–22) with additions necessary to accommodate the evaluation of different case-finding strategies (Fig. 1).
Fig. 1.
TB/HIV model structure. The model states depict stages in the natural history of tuberculosis; individuals in any of the tuberculosis states may also be infected with HIV. States of infectious tuberculosis are shown within the dotted box. Under PCF, only self-referring cases will be eligible for detection (light gray boxes), whereas when ICF is implemented, all sufficiently symptomatic cases can be detected (both light and dark gray boxes). Note that not all model transitions are shown here (additional details and parameter values are provided in the SI Appendix).
Following previous TB modeling conventions, we allow that individuals enter the model susceptible to infection and that newly infected individuals enter into one of two asymptomatic latent states differentiated by slow or fast progression to disease. Individuals with slowly progressing latent infections may transition to the fast latent compartment after reinfection or through natural progression of their initial infection. Individuals in any state may be coinfected by HIV; coinfection alters the natural history of TB in several ways: a higher probability of rapidly progressive TB after initial infection (23, 24), a higher probability of progression from latency to active TB (25), a lower probability of sputum smear-positive disease among infectious individuals (26–28), and higher mortality rates (26, 29, 30). To capture the impact of HIV infection, each compartment in Fig. 1 represents two subcompartments indexed by HIV status (11).
We explicitly include model classes for (i) newly infectious individuals who are asymptomatic or whose symptoms are currently too new or are too minimal to trigger self-presentation or to be picked up by symptom screens (31–34) or (ii) infectious patients with more advanced, symptomatic disease. Those with symptomatic disease are either sputum smear-positive or smear-negative and may or may not self-refer for care. Symptomatic individuals who do not self-refer may currently lack access, knowledge, or willingness to present to diagnostic or treatment facilities. We assume that as disease progresses, individuals are more likely to be smear-positive and also more likely to self-refer for diagnosis, but some individuals may never access diagnosis on their own volition. We assume that those with symptoms are tested upon arrival to a diagnostic center and, if diagnosed with TB, referred for TB treatment.
Modeling Case-Finding Interventions.
In our model, we assume that only symptomatic, self-referring individuals will seek TB diagnosis and receive care when the PCF policy is in place. In comparison with PCF, implementation of the ICF policy increases the effective rate at which symptomatic cases seek and receive care. The degree to which the ICF intervention improves the speed of detection of symptomatic cases and captures cases that would have gone undetected under PCF depends on the operating characteristics of the diagnostic test used as well as the population coverage of ICF.
In most high TB burden settings, TB diagnosis is made based primarily on sputum smear microscopy, which is inexpensive but has limited sensitivity, especially in HIV-infected patients (26–28). Recently, nucleic acid amplification tests, such as the Xpert MTB/RIF assay, have been introduced. While more expensive, these new diagnostics can detect a substantial fraction of sputum smear-negative TB; a single Xpert MTB/RIF test may identify 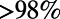 of patients with smear-positive and
of patients with smear-positive and 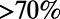 of patients with smear-negative TB (35–37). In our simulations, we examine how the performance of case-finding policies is influenced by the choice of diagnostic tool.
of patients with smear-negative TB (35–37). In our simulations, we examine how the performance of case-finding policies is influenced by the choice of diagnostic tool.
In the base case, we assume that on average 15% of the population will have access to diagnosis through ICF while ICF is used. We have not attempted to do a sophisticated costing of ICF interventions in this analysis, but to demonstrate the ability of the model framework to identify the cost-effectiveness of such policies, we have used a crude costing estimate (SI Appendix). Based on a previous study in Harare (38), we assume that implementation of ICF at this coverage level would cost US$31,500 per day in Zimbabwe and $9,600 per day in the Central African Republic.
Model Calibration.
To model TB/HIV epidemics, we use the modeling framework proposed in ref. 39 to find the probability distribution of events that may occur (e.g., birth, transmission of infection, or recovery) and then use Monte Carlo simulation to generate epidemic trajectories. We calibrated the model to TB/HIV epidemics in Zimbabwe and Central African Republic. Zimbabwe has a relatively high HIV prevalence (14.3% in year 2009), while Central African Republic has a lower HIV prevalence (4.7% in year 2009) (40) (SI Appendix).
Decision Model.
Let 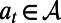 denote the intervention in effect during the decision period
denote the intervention in effect during the decision period 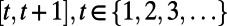 , where
, where 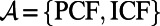 . Let the random variable
. Let the random variable 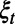 denote the set of events during the decision period
denote the set of events during the decision period 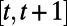 that may trigger an observation, incur costs, or lead to change in the population health status or resource availability. Examples of such events include a new infection, hospitalization, or death of an infective. Clearly, the decisions in effect during period
that may trigger an observation, incur costs, or lead to change in the population health status or resource availability. Examples of such events include a new infection, hospitalization, or death of an infective. Clearly, the decisions in effect during period 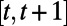 will influence the set of events that may occur over this period. While the evolution of the random variable
will influence the set of events that may occur over this period. While the evolution of the random variable 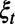 over time is not fully observable by the decision maker, we can use mathematical or simulation models to sample from the stochastic process
over time is not fully observable by the decision maker, we can use mathematical or simulation models to sample from the stochastic process 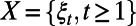 .
.
Our goal is to find a decision rule that for each decision index 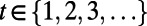 specifies which action to take to minimize the expected total discounted loss in the population’s net monetary benefit (NMB) (41):
specifies which action to take to minimize the expected total discounted loss in the population’s net monetary benefit (NMB) (41):
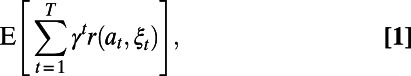 |
where 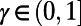 is the discount rate and
is the discount rate and 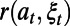 is the loss in the population’s NMB if action
is the loss in the population’s NMB if action 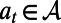 is in effect and the random events
is in effect and the random events 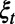 occur during the decision period
occur during the decision period 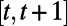 . The decision horizon T can be a constant predetermined by the decision maker (e.g., 10 y) or can be a random variable governed by the stochastic process
. The decision horizon T can be a constant predetermined by the decision maker (e.g., 10 y) or can be a random variable governed by the stochastic process 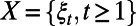 (e.g., time when the disease is eradicated).
(e.g., time when the disease is eradicated).
Our objective is to guide the selection of the intervention to use during the next decision period using statistics that are defined by observable past or current measures from the epidemic (e.g., the TB case notifications during the past month). We refer to these statistics as features, and we use 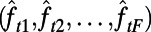 to denote the vector of selected features observed at time index t. Let the function
to denote the vector of selected features observed at time index t. Let the function 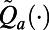 approximate the optimal expected loss in the population’s NMB if the policymaker observes the features
approximate the optimal expected loss in the population’s NMB if the policymaker observes the features 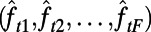 at time t and implements the intervention
at time t and implements the intervention  during the period
during the period 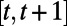 . Given the approximation functions
. Given the approximation functions 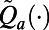 and the observed feature vector
and the observed feature vector 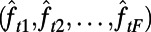 , the approximately optimal decision at time index t can be found according to:
, the approximately optimal decision at time index t can be found according to:
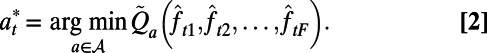 |
See SI Appendix for the detailed description of the decision model and the method to characterize the approximation functions 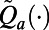 .
.
During an epidemic, TB case notifications and TB-related mortality are potentially observable, and hence statistics defined by these data can be used as features. Our experiments with the TB model depicted in Fig. 1 show that TB deaths and TB case notifications are highly correlated under both PCF and ICF, and so including both in approximation functions 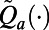 produces unstable regression models. Similarly, our experiments show that distinguishing TB case notifications by the HIV status of patients also leads to correlated regressors in approximation functions
produces unstable regression models. Similarly, our experiments show that distinguishing TB case notifications by the HIV status of patients also leads to correlated regressors in approximation functions 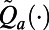 . Although this property restricts options for features, it can be advantageous for generating policies that are convenient to implement since the policymaker needs only to gather data on TB case notifications. We chose the following two features to use to guide decision-making: (i) TB case notifications over the past decision period and (ii) the case finding strategy that was used during this period. This collection of features produces policies that are straightforward to visualize and communicate. If there is a delay in the availability of surveillance data (e.g., significant time lag between when cases are diagnosed and reported), we redefine these features accordingly (SI Appendix, §S5).
. Although this property restricts options for features, it can be advantageous for generating policies that are convenient to implement since the policymaker needs only to gather data on TB case notifications. We chose the following two features to use to guide decision-making: (i) TB case notifications over the past decision period and (ii) the case finding strategy that was used during this period. This collection of features produces policies that are straightforward to visualize and communicate. If there is a delay in the availability of surveillance data (e.g., significant time lag between when cases are diagnosed and reported), we redefine these features accordingly (SI Appendix, §S5).
We characterize dynamic case-finding policies implemented within four epidemiological scenarios: scenarios 1 and 2 reflect a TB epidemic where the current HIV burden is relatively high (Zimbabwe) while scenarios 3 and 4 reflect a TB epidemic where the current HIV burden is lower (Central African Republic). For scenarios 1 and 3 we assume that smear microscopy is the sole diagnostic TB test used, while for scenarios 2 and 4 we assume that 50% of TB suspects will be screened with Xpert MTB/RIF and the rest will be screened with smear microscopy.
To define the loss function 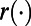 , we use the number of incident TB cases as a measure of health. Therefore, the willingness-to-pay (WTP) is defined as the amount of money a policymaker is willing to spend to avert one additional incident TB case. The loss function
, we use the number of incident TB cases as a measure of health. Therefore, the willingness-to-pay (WTP) is defined as the amount of money a policymaker is willing to spend to avert one additional incident TB case. The loss function 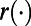 also includes the TB diagnosis and treatment costs as well as the costs incurred during courses of ICF. To characterize dynamic case-finding policies, we used the algorithm proposed in the SI Appendix to find the approximation function
also includes the TB diagnosis and treatment costs as well as the costs incurred during courses of ICF. To characterize dynamic case-finding policies, we used the algorithm proposed in the SI Appendix to find the approximation function 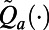 such that decisions made according to Eq. 2 minimize the expected total discounted NMB loss defined in Eq. 1. Here we assume that decision periods are of length 1 mo and the annual interest rate is 3%.
such that decisions made according to Eq. 2 minimize the expected total discounted NMB loss defined in Eq. 1. Here we assume that decision periods are of length 1 mo and the annual interest rate is 3%.
Results
Identifying an (approximately) optimal dynamic case-finding policy for a particular setting requires specifying two figures: an affordability curve and a decision rule. The affordability curve (Fig. 2A) returns the expected annual costs as a function of WTP for health and the decision rule (Fig. 2B) specifies which intervention to use during the next decision period given the value of selected features. In this illustration, we use the number of TB case notifications over the past month and the case finding strategy in effect during this period as the relevant set of features (see SI Appendix for a detailed discussion of feature selection).
Fig. 2.
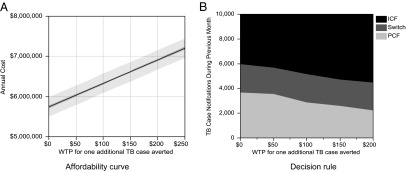
Approximately optimal dynamic case-finding policies for scenario 1 reflecting the TB/HIV epidemic in Zimbabwe where the available TB diagnosis test is assumed to be sputum microscopy. For each value of WTP, A returns the expected annual cost (with 95% confidence interval) that would be incurred by following the corresponding dynamic health policy. A policymaker would use A to first select a level of WTP for health that satisfies the annual budget constraints, and then would consult B to make real-time decisions given this selected WTP value.
To use the policy depicted in Fig. 2, the policymaker must first select a level of WTP for health that satisfies the annual budget constraints. For example, for an annual budget of US$6.5 million under scenario 1, the policymaker may choose the WTP $100 per TB case averted from Fig. 2A. The policymaker can then consult the graph in Fig. 2B to make real-time decisions given this selected WTP value. For example, at the WTP threshold of $50 per TB case averted, the policymaker should use PCF while the TB case notification during the past month is below 3,561 cases; if the TB case notification exceeds this threshold while PCF is in place, the case-finding policy should be switched to ICF. ICF should be sustained if the TB case notification during the past month is greater than 5,706 cases; if the TB case notification falls below this level, ICF should be suspended and replaced by PCF. The existence of two distinct thresholds for switching (PCF to ICF and ICF to PCF) reflects the fact that the fraction of TB cases that is detected and notified is dependent on the case-finding strategy being used. We present the approximately optimal dynamic case-finding policies for scenarios 2–4 in SI Appendix. Policies based on different thresholds for WTP produce different health outcomes. A higher WTP is expected to result in fewer TB case notifications and deaths throughout the epidemic. The expected cumulative TB case notifications and expected deaths over a 10-y horizon for the four scenarios considered here are provided in the SI Appendix, Fig. S11.
Using cost-effectiveness planes (41) (Fig. 3), we compare the performance of dynamic case-finding strategies with that of static policies that only specify the frequency of ICF. In these figures, the incremental costs (displayed on horizontal axes) and additional TB cases averted (displayed on vertical axes) are calculated with respect to the baseline scenario where TB cases are passively identified. Fig. 3 reveals that the cost-effectiveness frontiers corresponding to dynamic case-finding policies strictly dominate the cost-effectiveness frontiers corresponding to static policies for all scenarios. This implies that for any given budget, following the appropriate dynamic case-finding policy results in statistically superior health outcomes in comparison with the static policy that satisfies the same budget limit.
Fig. 3.
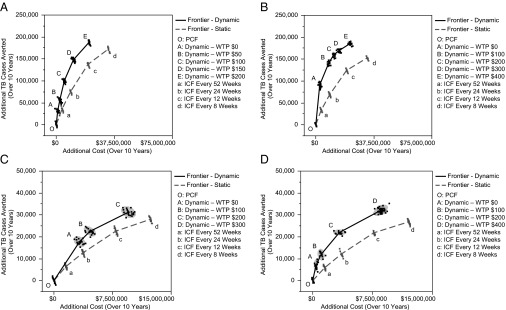
Cost-effectiveness planes comparing the performance of static versus dynamic ICF policies. A–D show the cost-effectiveness planes for scenarios 1–4, respectively. The cost-effectiveness frontiers corresponding to dynamic case-finding policies strictly dominate the cost-effectiveness frontiers corresponding to static policies for all scenarios. A resembles the TB/HIV epidemic in Zimbabwe and sputum microscopy is assumed to be the only available TB diagnosis test. B resembles the TB/HIV epidemic in Zimbabwe and sputum microscopy and Xpert MTB/RIF each have 50% coverage as TB diagnosis test. C resembles the TB/HIV epidemic in Central African Republic and sputum microscopy is assumed to be the only available TB diagnosis test. D resembles the TB/HIV epidemic in Central African Republic and sputum microscopy and Xpert MTB/RIF each have 50% coverage as TB diagnosis test.
We also note that in the two settings we examine, the relative benefit of dynamic policies compared with static policies is amplified when Xpert MTB/RIF is used as the TB diagnostic test (compare the gap between the dynamic and static frontiers in Fig. 3 A and B or the similar gap in Fig. 3 C and D). Furthermore, we observe that line “OA” has a steeper slope than line “Oa” in both Fig. 3 B and D; this implies that when sufficient resources are available to implement Xpert MTB/RIF, the policymaker can choose a dynamic health policy (e.g., the policies associated to point “A” in Fig. 3 B and D) to reach a significantly higher level of health gain with only a small increase in spending. Finally, we note that ICF results in more cost-effective outcomes in settings of higher HIV prevalence. The greater gains associated with ICF are seen in both dynamic and static policies and regardless of which type of diagnostic tool is used (compare Fig. 3A with Fig. 3C and Fig. 3B with Fig. 3D).
Our sensitivity analysis shows that the comparative benefit of dynamic policies are maintained even when we assume different levels of coverage of the ICF intervention and when there is a significant delay between when cases are diagnosed and when they are reported (SI Appendix, §S5). Furthermore, for the scenarios considered here, for any level of additional health that the decision maker intends to achieve, dynamic policies require fewer switches than static policies (SI Appendix, §S7).
Discussion
Previous empirical and theoretical studies (12, 42–45) demonstrate that ICF can be a useful strategy to mitigate TB epidemics. However, continuous ICF may not be sustainable, especially in settings with high TB burdens and limited resources. Faced with limited budgets, policymakers would benefit from access to tools that can help them to identify when they should use or to remove ICF during a TB epidemic.
In this article, we proposed a different type of case finding approaches, referred to as dynamic case-finding policies, which recommend when to implement ICF using available data on TB case notifications and the current case-finding approach. The results we present have several key implications for health policymakers. First, while previous studies of case-finding strategies have identified static policies for the frequency of ICF interventions that are likely to be cost-effective, our dynamic policies strictly dominate these types of static policies. This implies that for a given fixed budget, following a dynamic case-finding policy would produce significantly better health outcomes in comparison with following a static policy. While both dynamic and static ICF policies appear more cost-effective in higher prevalence HIV settings, it is possible that more aggressive case-finding approaches may also meet acceptable cost-effectiveness thresholds in lower prevalence HIV settings.
We also found that for the two settings considered here, a diagnostic tool with better sensitivity for smear-negative cases improves the incremental benefit of using dynamic case-finding policies. This is due to the fact that while static policies only specify the frequency and duration of ICF (which are assumed to be fixed during the epidemic), dynamic case-finding policies adjust recommendations based on the accumulated observations. Hence, dynamic case-finding policies incorporate additional flexibility that allow for adjustment of recommendations based on both epidemic (e.g., trends in case notifications) and health care system characteristics (e.g., the diagnostic tool in use) to produce superior outcomes (SI Appendix, §S4). Our results suggest that when sufficient resources are available to implement Xpert MTB/RIF, a policymaker may achieve substantial health gains for a very small increase in spending by choosing a dynamic case-finding policy instead of a static policy. We also found that our dynamic policies require fewer switches than static policies, which can be advantageous if switching is costly, but this requires the policymaker to be more flexible about the timing of switching (SI Appendix, §S7).
The simple TB/HIV model we describe has important limitations. We have made simplifying assumptions about the natural history and transmission of TB and HIV; these choices have allowed us to maintain focus on how more aggressive case-finding policies can impact onward transmission of disease. Most importantly, we have assumed that early case finding will reduce the expected duration of infectiousness of TB cases and thus reduce onward transmission of disease. While studies (46, 47) and intuition support the idea that early case finding should identify those with less severe forms of disease who would have otherwise progressed and infected additional contacts, there are yet few data that demonstrate that aggressive case finding reduces the incidence of TB. Our model does not differentiate between TB cases detected passively or through ICF. If TB cases detected through ICF were more likely to default from treatment (46), the cost-effectiveness of ICF policies would be eroded. Furthermore, our model does not differentiate between infected children and adults, nor does it distinguish HIV cases receiving antiretroviral therapy; yet relaxing these assumption is not expected to significantly affect the comparative evaluation of static versus dynamic policies (Fig. 3).
We also have made crude assumptions about the costs of interventions and have not incorporated any HIV-related costs. Accordingly, we do not intend for the actual costs of the interventions to reflect reality, but instead have elected to report these estimates to facilitate comparison of the relative cost and cost-effectiveness across interventions (i.e., static versus dynamic ICF policies) and settings (i.e., higher versus lower HIV prevalence). While the case-finding policies presented here are generated using models calibrated to publicly available data describing the TB/HIV epidemics in Zimbabwe and Central African Republic, to use these policies in practice, one would need to develop and calibrate models that adequately capture additional details of the dynamics of TB/HIV epidemics and local costs. Also, if an observed change in TB case notifications is believed to be associated with a change in surveillance (i.e., use of new diagnostic tools/algorithms or fluctuating performance of existing approaches), dynamic case-finding policies should be regenerated using an updated model that accurately captures the new programmatic situation.
In summary, we demonstrate that dynamic ICF policies may be an efficient approach for implementing more aggressive case finding in the context of TB/HIV coepidemics. These dynamic policies, in addition to averting more incident TB cases than static ICF policies for similar costs, are easy to visualize and communicate and allow policymakers to update their decisions about which intervention to use based on recent and current data about the epidemic, the diagnostic tools available, and budget constraints. We believe this type of dynamic decision support tool may be more useful to a policymaker than a tool that requires a commitment to implement multiple rounds of ICF at a prespecified periodicity.
Supplementary Material
Acknowledgments
The authors would like to thank Marc Lipsitch for his comments and suggestions. The project described was supported by Award U54GM088558 and DP2OD006663 from the National Institute of General Medical Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218770110/-/DCSupplemental.
References
- 1. World Health Organization (2012) Global Tuberculosis Report. www.who.int/tb/publications/global_report/en/. Accessed April 28, 2013.
- 2.Banerji D, Andersen S. A sociological study of awareness of symptoms among persons with pulmonary tuberculosis. Bull World Health Organ. 1963;29(5):665–683. [PMC free article] [PubMed] [Google Scholar]
- 3.Nagpaul DR, Vishwanath MK, Dwarakanath G. A socio-epidemiological study of out-patients attending a city tuberculosis clinic in India to judge the place of specialized centres in a tuberculosis control programme. Bull World Health Organ. 1970;43(1):17–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJL, et al. Cost effectiveness of chemotherapy for pulmonary tuberculosis in three sub-Saharan African countries. Lancet. 1991;338(8778):1305–1308. doi: 10.1016/0140-6736(91)92600-7. [DOI] [PubMed] [Google Scholar]
- 5.Suárez PG, et al. The dynamics of tuberculosis in response to 10 years of intensive control effort in Peru. J Infect Dis. 2001;184(4):473–478. doi: 10.1086/322777. [DOI] [PubMed] [Google Scholar]
- 6.Dye C, Fengzeng Z, Scheele S, Williams B. Evaluating the impact of tuberculosis control: Number of deaths prevented by short-course chemotherapy in China. Int J Epidemiol. 2000;29(3):558–564. [PubMed] [Google Scholar]
- 7.De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis. 1999;3(6):457–465. [PubMed] [Google Scholar]
- 8. Godfrey-Faussett P (2011) Do we need to go beyond the clinic to control TB? 42nd Union World Conference on Lung Health, Lille, France. Available at http://uwclh.conference2web.com/content/1060.
- 9. H. Ayles and the ZAMSTAR Study Team (2011) A household-based HIV and TB intervention increases HIV testing in households and reduces prevalence of TB at the community level: The ZAMSTAR community randomized trial. 19th Conference on Retroviruses and Opportunistic Infections, Seattle, WA. Available at www.retroconference.org/2012b/Abstracts/45440.htm.
- 10.Murray CJL, Salomon JA. Expanding the WHO tuberculosis control strategy: Rethinking the role of active case-finding. Int J Tuberc Lung Dis. 1998;2(9, Suppl 1):S9–S15. [PubMed] [Google Scholar]
- 11.Murray CJL, Salomon JA. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci USA. 1998;95(23):13881–13886. doi: 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowdy DW, Chaisson RE. The persistence of tuberculosis in the age of DOTS: Reassessing the effect of case detection. Bull World Health Organ. 2009;87(4):296–304. doi: 10.2471/BLT.08.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currie CSM, Floyd K, Williams BG, Dye C. Cost, affordability and cost-effectiveness of strategies to control tuberculosis in countries with high HIV prevalence. BMC Public Health. 2005;5:130. doi: 10.1186/1471-2458-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lönnroth K, et al. Systematic screening for active tuberculosis: Rationale, definitions and key considerations. Int J Tuberc Lung Dis. 2013;17(3):289–298. doi: 10.5588/ijtld.12.0797. [DOI] [PubMed] [Google Scholar]
- 15.Borgdorff MW, Yew WW, Marks G. Active tuberculosis case finding: Why, when and how? Int J Tuberc Lung Dis. 2013;17(3):285. doi: 10.5588/ijtld.12.0806. [DOI] [PubMed] [Google Scholar]
- 16.Dodd PJ, White RG, Corbett EL. Periodic active case finding for TB: When to look? PLoS ONE. 2011;6(12):e29130. doi: 10.1371/journal.pone.0029130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Churchyard GJ, et al. Twelve-monthly versus six-monthly radiological screening for active case-finding of tuberculosis: a randomised controlled trial. Thorax. 2011;66(2):134–139. doi: 10.1136/thx.2010.139048. [DOI] [PubMed] [Google Scholar]
- 18.Martin L. Puterman. Markov Decision Processes: Discrete Stochastic Dynamic Programming. New York: Wiley; 1994. [Google Scholar]
- 19.White CC, Scherer WT. Finite-memory suboptimal design for partially observed Markov decision processes. Oper Res. 1994;42(3):439–455. [Google Scholar]
- 20.Abu-Raddad LJ, et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci USA. 2009;106(33):13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Currie CSM, Williams BG, Cheng RCH, Dye C. Tuberculosis epidemics driven by HIV: Is prevention better than cure? AIDS. 2003;17(17):2501–2508. doi: 10.1097/01.aids.0000096903.73209.ac. [DOI] [PubMed] [Google Scholar]
- 22.Dowdy DW, Chaisson RE, Moulton LH, Dorman SE. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: A mathematical model. AIDS. 2006;20(5):751–762. doi: 10.1097/01.aids.0000216376.07185.cc. [DOI] [PubMed] [Google Scholar]
- 23.Daley CL, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326(4):231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 24.Shafer RW, Singh SP, Larkin C, Small PM. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in an immunocompetent patient. Tuber Lung Dis. 1995;76(6):575–577. doi: 10.1016/0962-8479(95)90537-5. [DOI] [PubMed] [Google Scholar]
- 25.Bucher HC, et al. Isoniazid prophylaxis for tuberculosis in HIV infection: A meta-analysis of randomized controlled trials. AIDS. 1999;13(4):501–507. doi: 10.1097/00002030-199903110-00009. [DOI] [PubMed] [Google Scholar]
- 26.Corbett EL, et al. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 27.Elliott AM, et al. The impact of human immunodeficiency virus on presentation and diagnosis of tuberculosis in a cohort study in Zambia. J Trop Med Hyg. 1993;96(1):1–11. [PubMed] [Google Scholar]
- 28.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: Informing urgent policy changes. Lancet. 2007;369(9578):2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 29.Manosuthi W, et al. Treatment outcomes of patients co-infected with HIV and tuberculosis who received a nevirapine-based antiretroviral regimen: A four-year prospective study. Int J Infect Dis. 2010;14(11):e1013–e1017. doi: 10.1016/j.ijid.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 30.van der Sande MAB, et al. Incidence of tuberculosis and survival after its diagnosis in patients infected with HIV-1 and HIV-2. AIDS. 2004;18(14):1933–1941. doi: 10.1097/00002030-200409240-00009. [DOI] [PubMed] [Google Scholar]
- 31.Barry CE, 3rd, et al. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack U, et al. C. Lange TBNET LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33(5):956–973. doi: 10.1183/09031936.00120908. [DOI] [PubMed] [Google Scholar]
- 33.Corbett EL, et al. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med. 2007;4(1):e22. doi: 10.1371/journal.pmed.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood R, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: Implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175(1):87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boehme CC, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boehme CC, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theron G, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184(1):132–140. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 38.Corbett EL, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): A cluster-randomised trial. Lancet. 2010;376(9748):1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaesoubi R, Cohen T. Generalized Markov models of infectious disease spread: A novel framework for developing dynamic health policies. Eur J Oper Res. 2011;215(3):679–687. doi: 10.1016/j.ejor.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. UNAIDS AIDSInfo. www.unaids.org/en/dataanalysis/datatools/aidsinfo/ Accessed April 28, 2013.
- 41.Andrew Briggs . Karl Claxton, and Mark Sculpher. Decision Modelling for Health Economic Evaluation. Oxford: Oxford Univ Press; 2006. [Google Scholar]
- 42.Ahmad RA, Mahendradhata Y, Cunningham J, Utarini A, de Vlas SJ. How to optimize tuberculosis case finding: Explorations for Indonesia with a health system model. BMC Infect Dis. 2009;9(1):87. doi: 10.1186/1471-2334-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becerra MC, et al. Expanding tuberculosis case detection by screening household contacts. Public Health Rep. 2005;120(3):271–277. doi: 10.1177/003335490512000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kranzer K, et al. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10(2):93–102. doi: 10.1016/S1473-3099(09)70326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller AC, et al. Controlled trial of active tuberculosis case finding in a Brazilian favela. Int J Tuberc Lung Dis. 2010;14(6):720–726. [PMC free article] [PubMed] [Google Scholar]
- 46.den Boon S, et al. Comparison of symptoms and treatment outcomes between actively and passively detected tuberculosis cases: The additional value of active case finding. Epidemiol Infect. 2008;136(10):1342–1349. doi: 10.1017/S0950268807000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward HA, Marciniuk DD, Pahwa P, Hoeppner VH. Extent of pulmonary tuberculosis in patients diagnosed by active compared to passive case finding. Int J Tuberc Lung Dis. 2004;8(5):593–597. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.