Abstract
Objectives/Hypothesis
To estimate the distribution of head and neck squamous cell carcinoma (HNSCC) recurrence after definitive chemoradiation therapy (CRT) among patients who underwent 18F-fluorodeoxyglucose positron-emission tomography and computed tomography (PET/CT) surveillance.
Study Design
Retrospective review.
Methods
HNSCC patients who underwent definitive CRT from 2001 to 2008 were evaluated for recurrence with serial PET/CT. Patients were excluded if they were previously treated for recurrent disease, were treated with surgery as the primary therapeutic modality, or had inadequate clinical follow-up. Recurrence was defined by histopathologic evidence of tumor.
Results
Three hundred eighty-eight patients were studied. Patients in whom recurrence was not detected were followed clinically and radiographically for a median of 27 months. Tumor recurrence was detected in 110 patients. For 37 patients, recurrence was heralded by clinical signs. Among the 73 asymptomatic patients who had a confirmed recurrence, disease was detected by PET/CT between 2 and 43 months, median of 6 months. Forty-five percent of observed asymptomatic recurrences were detected during the first 6 months of surveillance (95% confidence interval [CI], 34%–57%), 79% within the first 12 months (95% CI, 68%–88%), 95% within the first 24 months (95% CI, 87%–98%), and 100% within the first 48 months (95% CI, 95%–100%).
Conclusions
Among HNSCC patients followed with PET/CT surveillance, 95% of observed asymptomatic recurrences were detected within 24 months after completing CRT. For patients without clinical signs of recurrence, routine PET/CT surveillance beyond the first 24 months may be of limited value and may not be cost effective.
Keywords: Head and neck, cancer, squamous cell carcinoma, positron-emission tomography/computed tomography, surveillance
INTRODUCTION
Each year, almost 50,000 cases of head and neck cancer occur in the United States, and over 500,000 cases occur worldwide.1,2 Head and neck squamous cell carcinoma (HNSCC) can be successfully treated in many cases, and chemoradiation therapy (CRT) is often employed as the first-line treatment to preserve vital structures in the head and neck.3 Even after therapy with curative intent, however, up to 50% of HNSCC patients will experience a recurrence.4–6 Patients therefore undergo post-treatment surveillance in the form of clinical examinations and imaging studies with the aim of identifying asymptomatic recurrences at an earlier stage, when further treatment may be more successful.
Most HNSCCs will recur within the first 3 years after treatment,5,7 and thus the majority of follow-up examinations and imaging studies are performed during this time frame. The National Comprehensive Cancer Network (NCCN) Guidelines recommend clinical head and neck examinations every 1 to 3 months in the first year, tapering to every 6 to 12 months after 5 years.8 New imaging modalities and modifications of existing modalities continue to evolve to supplement physical examination for recurrence detection. Among these, combined 18F-fluorodeoxyglucose (18F-FDG) positron-emission tomography and computed tomography (PET/CT) has been shown to be highly sensitive for detecting HNSCC recurrence,6,9,10 and this technique is widely used for surveillance. However, evidence-based recommendations do not yet exist to guide the interval or duration of PET/CT surveillance in HNSCC patients.6,9
PET/CT is a highly useful technique for detecting recurrent disease,6,9 but it is costly and administers a high dose of radiation.11 Moreover, radiation from computed tomography (CT) scans has been suggested to predispose to the development of new cancers.12,13 Thus, physicians must balance the need to evaluate potential recurrences with the risk of effects from radiation. To date, a PET/CT surveillance schedule that balances the benefits with the disadvantages, while also factoring in costs, has not yet been determined. At our institution, HNSCC patients are typically evaluated for recurrence with serial clinical examinations continued indefinitely and with PET/CT at 2 months, 5 months, 8 months, and 14 months post-treatment. Beyond 14 months, additional PET/CT scans are performed at the discretion of the clinical team. Thus, the aims of this study were to estimate the distribution of HNSCC recurrence after definitive CRT among patients who underwent PET/CT surveillance and to explore if future study in this area is warranted.
MATERIALS AND METHODS
This retrospective review was approved by the institutional review board of the University of Pittsburgh.
Patient Population
Patients with histologically confirmed HNSCC from 2001 to 2008 were identified for analysis. Patients were included if they were treated for primary HNSCC with definitive CRT, were evaluated with serial PET/CT scans, and if adequate follow-up information was available. Patient were excluded if they had received prior treatment for recurrent HNSCC, were treated mainly with surgery, or if adequate follow-up information on disease progression was not available. Paper charts, electronic records, radiology reports, operative notes, and pathology reports were reviewed to determine which patients had clinical signs or symptoms when they recurred. Patients were followed for recurrence until May 2010.
PET/CT Imaging
Combined PET/CT imaging was performed with a Reveal scanner (CTI Medical Systems, Knoxville, TN), which combines a dual-chamber CT scanner with a PET scanner. Patients were instructed to fast for at least 6 hours prior to imaging with the exception of water. An intravenous (IV) catheter was placed for radiopharmaceutical administration, and the patient’s blood glucose level was measured prior to 18F-FDG injection. Patients with blood glucose levels >200 mg/dL were rescheduled. Each patient received an IV injection of 400 to 610 MBq (11–16.5 mCi) of 18F-FDG and then rested on a comfortable chair during tracer uptake. PET/CT was initiated 60 minutes after tracer injection. Patients were asked to maintain shallow respirations during the scan.
Helical CT (pitch of 1.0, 120–140 mAs, 130 kVp) was performed from the top of the skull through the abdomen in one imaging procedure immediately before the acquisition of PET emission data. IV contrast (120 mL ioversol, Optiray-350; Mallinckrodt, St. Louis, MO) was given to all patients unless it was contraindicated due to an allergy.
PET images were acquired in 4 to 5 bed positions (5 minutes per bed position) and were reconstructed with and without attenuation correction. Helical CT scans were reconstructed with a section thickness of 2.4 mm and a matrix of 512 × 512 pixel images to match the PET scan parameters. Images were reconstructed using ordered subset expectation maximization with two full iterations of eight subsets. Rescaled CT images were used to produce attenuation correction values for PET emission reconstruction.
Post-CRT Surveillance
Patients were scheduled to undergo clinical examinations at 1 and 2 months after completing CRT, and a minimum of every 3 months thereafter, as long as they remained free of detectable disease.
Patients up to and including January 2007 were evaluated with serial post-treatment PET/CT at the discretion of the clinical team. A surveillance protocol was instituted in January 2007. All patients undergoing surveillance after January 2007 were evaluated with post-treatment PET/CT at 2 months, 5 months, 8 months, and 14 months, and thereafter at the discretion of the treating physicians. In addition, for all patients, if clinical examination identified possible recurrence, PET/CT was performed to further evaluate the clinical finding.
Image Interpretation
CT images were reviewed on a picture archiving and communication system workstation (Philips iSite; Philips Healthcare, Andover, MA). PET images, fused PET/CT images, and reconstructions of PET, CT, and PET/CT into sagittal and coronal planes were reviewed on a dedicated postprocessing workstation.
All images were interpreted by one of two board-certified neuroradiologists with 12 and 10 years of head and neck PET/CT experience, respectively. Each radiologist had access to all prior clinical and radiological data, including prior PET/CT scans. PET/CT results were considered in combination with other clinical data to determine recurrence. Strict standardized uptake value thresholds were not applied, in keeping with our usual clinical protocol. All recurrences were confirmed histologically. Tumors of the same histologic type that presented in a locoregional recurrence pattern were considered recurrences as opposed to second primary tumors.
Statistical Analysis
Data were entered into an Excel spreadsheet (Microsoft Corp., Redmond, WA). Statistical analysis was performed using SPSS (PASW) Statistics version 18.0 (IBM, Armonk, NY). Cumulative incidence curves were plotted and 95% confidence intervals (CI) were calculated for proportions of patients with disease recurrence at various times. Recurrence time for symptomatic versus asymptomatic patients was tested for equality of location (median) with the Wilcoxon test and for equality of scale (variance) with the Ansari-Bradley test. Differences by site of primary tumor and site of recurrence were tested with an exact χ2 test. Fisher exact test was used to test differences of proportions of recurrence beyond 2 years.
RESULTS
Patient Characteristics
Six hundred forty-six patients were initially identified. Of these, 101 were excluded for having received prior treatment for recurrent HNSCC, and 157 were excluded for being treated surgically. Three hundred and eighty-eight patients who completed CRT from 2001 to 2008 were then included for evaluation. The mean age was 59 years, and the male to female ratio was 3.6:1.0. Patient demographics and tumor characteristics are shown in Table I and Table II. For two patients who presented with simultaneous primaries, the higher stage tumor was used for data analysis. Patients who did not recur were followed clinically and radiographically for a median of 27 months (range, 1–93 months).
TABLE I.
Demographics and Tumor Characteristics of 388 Head and Neck Squamous Cell Carcinoma Patients Who Completed Chemoradiation Therapy and Underwent Positron-Emission Tomography/Computed Tomography Surveillance at a Single Tertiary Care Center, 2001–2010.
| Total patients, no. | 388 |
| Age, mean (range), yr | 59 (20–88) |
| Sex, no. (%) | |
| Male | 303 (78) |
| Female | 85 (22) |
| Location of primary tumor, no. (%) | |
| Oropharynx | 144 (37) |
| Oral cavity | 85 (22) |
| Larynx | 73 (19) |
| Hypopharynx | 24 (6) |
| Nasopharynx | 17 (4) |
| Unknown | 15 (4) |
| Paranasal sinus | 12 (3) |
| Major salivary gland | 8 (2) |
| Skin of head and neck | 6 (2) |
| Other | 4 (1) |
| Nasal cavity | 3 |
| Lacrimal gland | 1 |
| T stage, no. (%) | |
| Tis | 2 (0.5) |
| T1 | 47 (12) |
| T2 | 127 (33) |
| T3 | 77 (20) |
| T4 | 103 (27) |
| Tx | 32 (8) |
| N stage, no. (%) | |
| N0 | 127 (33) |
| N1 | 73 (19) |
| N2 | 150 (39) |
| N3 | 21 (5) |
| Nx | 17 (4) |
| AJCC stage of primary tumor, no. (%) | |
| 0 | 2 (0.5) |
| I | 9 (2) |
| II | 42 (11) |
| III | 78 (20) |
| IV | 236 (61) |
| Unknown | 21 (5) |
| Early stage (I and II) | 53 (14) |
| Late stage (III and IV) | 314 (81) |
| Unknown | 21 (5) |
AJCC = American Joint Committee on Cancer.
TABLE II.
Tumor Staging According to American Joint Committee on Cancer, 7th Edition, Cancer Staging Manual.
| Tis | T1 | T2 | T3 | T4 | Tx | |
|---|---|---|---|---|---|---|
| N0 | 2 | 9 | 42 | 32 | 41 | 1 |
| N1 | 7 | 24 | 15 | 24 | 3 | |
| N2 | 26 | 53 | 27 | 36 | 8 | |
| N3 | 4 | 8 | 3 | 2 | 4 | |
| Nx | 1 | 16 |
Detection of Recurrence
Tumor recurrence was discovered in 110 patients. For the purposes of clinical utility and data analysis, patients who recurred were divided into two groups: patients who were asymptomatic when their recurrence was detected via PET/CT and patients who manifested clinical signs of recurrence before recurrent disease was detected by PET/CT. Sixty-six percent (73/110) of patients who recurred were asymptomatic when their recurrence was detected by PET/CT (95% CI, 57%–75%). Thirty-four percent (37/110) of patients who recurred presented clinically with disease (95% CI, 25%–43%).
Asymptomatic Recurrences
Seventy-three patients recurred asymptomatically with recurrent disease detected from 2 to 43 months, median 6 months. Among the asymptomatic cohort, 45% of all observed recurrences were detected during the first 6 months of surveillance (95% CI, 34%–57%), 79% within the first 12 months (95% CI, 68%–88%), 92% within the first 18 months (95% CI, 83%–97%), 95% within the first 24 months (95% CI, 87%–98%), 99% within the first 36 months (95% CI, 93%–100%), and 100% within the first 48 months (95% CI, 95%–100%).
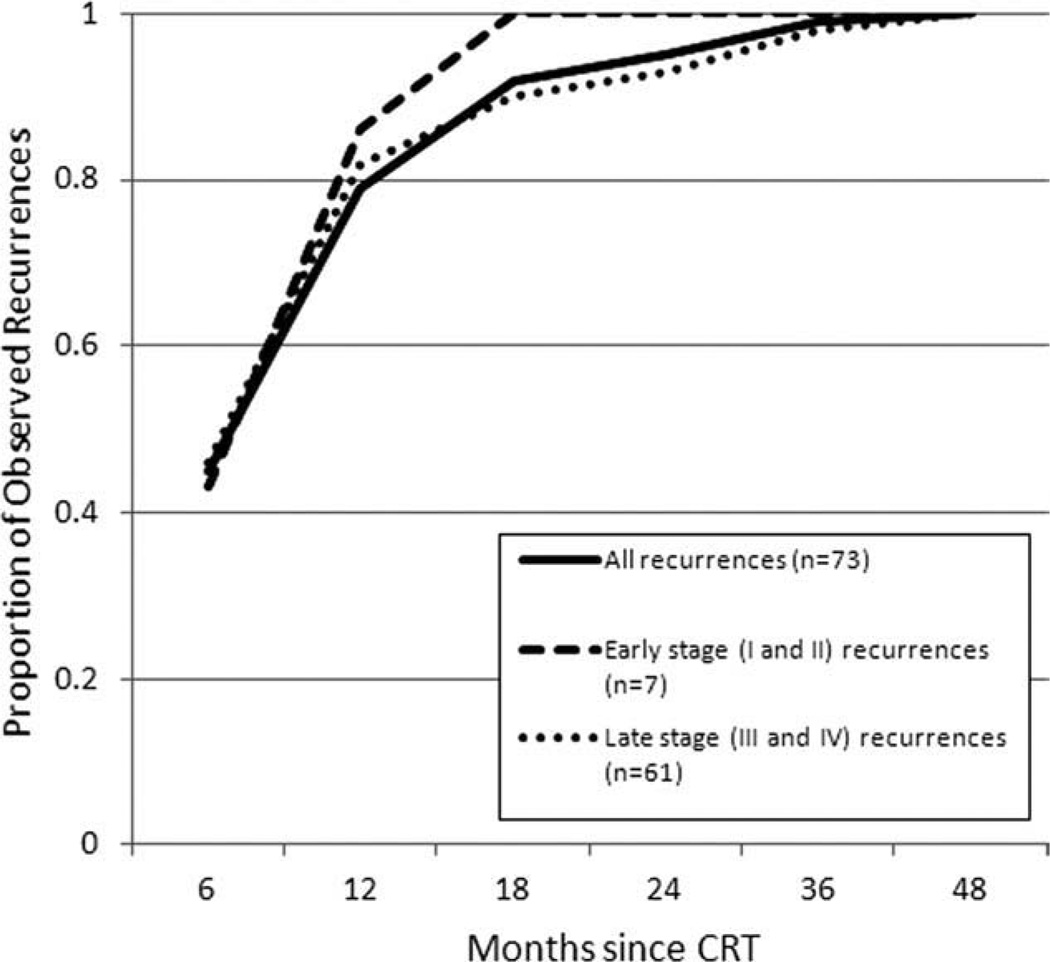
For patients with early-stage disease (stages I and II), all observed asymptomatic recurrences were detected within 18 months (95% CI, 65%–100%). For patients with late-stage disease (stages III and IV), 90% of observed asymptomatic recurrences were detected within 18 months (95% CI, 80%–95%). The recurrence times for asymptomatic patients did not differ by early-stage versus late-stage disease (Wilcoxon test, P = .593). Temporal patterns of HNSCC recurrence detected with PET/CT surveillance are shown in Figure 1. Locoregional and distant disease accounted for 93% (95% CI, 85%–98%) and 7% (95% CI, 2%–15%) of recurrences, respectively, in the asymptomatic cohort (Table III). The percent of asymptomatic recurrences by site of primary tumor paralleled the frequency of all patients included in this study, with oropharyngeal, oral cavity, and laryngeal cancer most commonly represented.
Fig. 1.
Temporal recurrence patterns of 73 head and neck squamous cell carcinoma patients who remained clinically asymptomatic after chemoradiation therapy (CRT) and underwent positronemission tomography/computed tomography surveillance at a single tertiary care center from 2001 to 2010. The recurrence times did not differ by early stage versus late stage (Wilcoxon test, P = .593). Staging information was unavailable for five patients who recurred.
TABLE III.
Tumor Details of 110 Head and Neck Squamous Cell Carcinoma Patients Who Were Followed With Positron Emission Tomography/Computed Tomography Surveillance After Chemoradiation Therapy and Recurred at a Single Tertiary Care Center, 2001–2010.
| Asymptomatic Recurrences, No. (%), n = 73 |
Symptomatic Recurrences, No. (%), n = 37 |
|
|---|---|---|
| Location of primary tumor | ||
| Oropharynx | 20 (27) | 7 (19) |
| Oral cavity | 19 (26) | 13 (35) |
| Larynx | 12 (16) | 6 (16) |
| Hypopharynx | 8 (11) | 2 (5) |
| Nasopharynx | 5 (7) | 1 (3) |
| Skin of head and neck | 3 (4) | 2 (5) |
| Unknown | 3 (4) | 0 (0) |
| Paranasal sinus | 1 (1) | 2 (5) |
| Major salivary gland | 1 (1) | 4 (11) |
| Nasal cavity | 1 (1) | |
| Site of recurrence | ||
| Local | 45 (62) | 22 (59) |
| Regional | 22 (30) | 14 (38) |
| Distant | 5 (7) | 1 (3) |
| Unknown | 1 (1) |
Symptomatic Recurrences
In 35 patients, recurrence was heralded by clinical signs/symptoms before it was detected via PET/CT: 13 patients presented with increasing pain, nine with visible ulcer, four with new mass, two with new dysphagia, two with visual symptoms, one with stridor, one with bleeding into the airway, one with hoarseness, one with hearing loss, and one with evidence of metastatic disease. In addition, two patients who did not have a complete response to CRT presented clinically with disease within 2 months of completing CRT.
Among 37 patients with clinically heralded recurrences, 51% of all observed recurrences presented within the first 6 months of surveillance (95% CI, 34%–68%), 62% within the first 12 months (95% CI, 45%–78%), 76% within the first 24 months (95% CI, 59%–88%), 89% within the first 36 months (95% CI, 75%–97%), and 100% within the first 60 months (95% CI, 91%–100%). Locoregional recurrence and distant metastasis accounted for 97% (95% CI, 86%–100%) and 3% (95% CI, 0%–14%) of recurrences, respectively, in the symptomatic cohort (Table III). The most frequent site of primary tumor among patients who recurred symptomatically was the oral cavity.
Comparison Between Asymptomatic and Symptomatic Recurrences
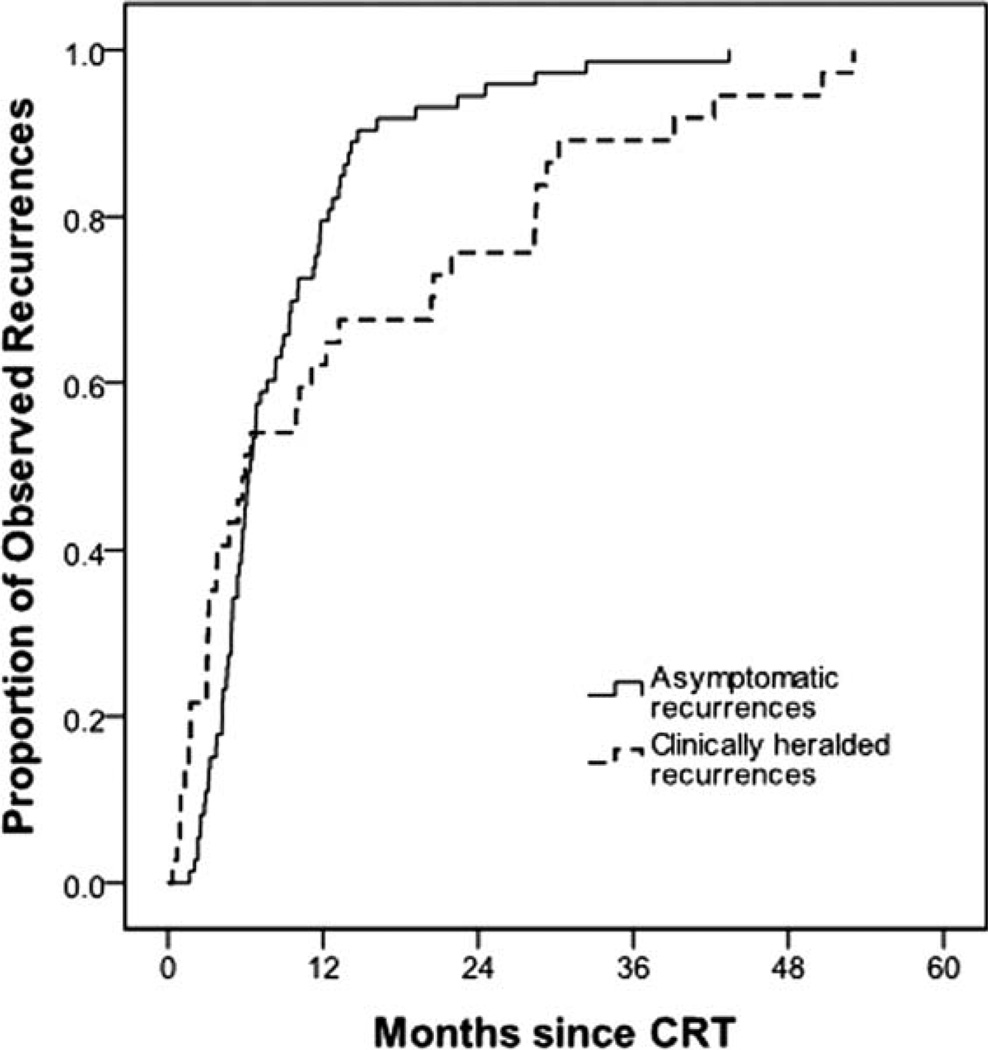
The distribution of recurrence times for asymptomatic versus symptomatic patients differed by scale but not location. Median recurrence times were similar; the median for asymptomatic patients was 6.4 months versus 6.0 months for symptomatic presentation (Wilcoxon test, P = .944). However the interquartile range was substantially larger for symptomatic patients (3–22 months) compared to asymptomatic patients (5–11 months, Ansari-Bradley, P < .001), suggesting that in the absence of PET/CT surveillance, times to disease recurrence exhibit much greater variation than for surveilled patients. A greater proportion of patients presented with symptoms after 2 years when compared to patients whose symptoms were detected by PET/CT (69% vs. 31%, P = .0093). A cumulative incidence curve demonstrating observed recurrences identified via PET/CT surveillance in asymptomatic patients and identified clinically in patients who presented clinically is shown in Figure 2. Patients with symptomatic recurrences did not differ from patients with asymptomatic recurrences with respect to either site of primary disease (P = .446) or site of recurrence (P = .556).
Fig. 2.
Cumulative incidence curve demonstrates observed head and neck squamous cell carcinoma recurrence in patients monitored with routine positron emission tomography/computed tomography (PET/CT) surveillance after chemoradiation therapy (CRT). The solid line shows recurrences observed via PET/CT in asymptomatic patients (n = 73). The dashed line shows recurrences observed in patients who presented clinically (n = 37).
DISCUSSION
This review was performed to determine the temporal patterns of HNSCC recurrence as detected with PET/CT surveillance. To our knowledge, no prior studies have evaluated when PET/CT detects HNSCC recurrence after definitive treatment. We aimed to estimate the distribution of HNSCC recurrence with PET/CT monitoring, such that this information may be built upon in future studies to further explore the utility of PET/CT surveillance in HNSCC patients. Our study was prompted by a desire to optimize use and timing of PET/CT, to avoid overuse, and to allow clinicians a perspective on the distribution of recurrence identified with the aid of PET/CT to improve patient counseling and afford the earliest opportunity for intervention. Ultimately, the incentive for early detection of recurrence is to provide patients with an improved chance of responding to secondary therapy, although it remains to be determined if PET/CT surveillance results in improved survival in HNSCC patients.
PET/CT has been shown to be highly sensitive and specific for detecting HNSCC recurrence and helps detect subclinical recurrences.6,9,10,14 Moreover, PET/CT may help defer the need for neck dissection after initial nonsurgical therapy,15,16 and it has been shown to be a predictor of outcome after curative CRT.17 Recognizing the efficacy of PET/CT, the NCCN recommends that a PET/CT scan be performed in certain head and neck cancer patients after confirming a response to nonsurgical treatment.8 However, little data exist to guide the frequency or duration of PET/CT surveillance.6,9 Due to irradiation and the high cost of these scans, it would be a boon for both patients and the health care system to determine the duration and frequency of surveillance necessary to detect the majority of recurrences.
Our data show that the vast majority (95%) of HNSCC patients who received definitive CRT remained asymptomatic, and those who recurred had their recurrence detected within 2 years of completing therapy by routine PET/CT surveillance. Considering the high cost and radiation burden of PET/CT, we believe that it is reasonable to end routine PET/CT surveillance at a maximum of 2 years after definitive treatment. As with many cancer follow-up programs, a small percentage of patients would recur after this radiographic surveillance period ends. We emphasize that this recommendation is intended for patients who remain asymptomatic.
The data from this study demonstrate that in patients who presented clinically with recurrence, almost one quarter (9 of 37) of observed recurrences (22%) occurred 2 years or more from CRT completion. It is possible that this result is due to a decrease in the frequency of surveillance several years after treatment, but without a better controlled study this cannot be concluded. Thus, although the value of routine PET/CT surveillance continued beyond 2 years post-treatment is uncertain, it is important that patients continue to have clinical screenings for disease recurrence beyond this time frame. For patients who present with clinical signs and/or symptoms of recurrent cancer, PET/CT is valuable for restaging the tumor and planning additional therapy.
The results from this study demonstrate that PET/CT has the ability to detect almost all HNSCC recurrences within 2 years. In the current study, 95% (95% CI, 87%–98%) of observed asymptomatic recurrences were detected within 2 years. The majority, 93% (95% CI, 85%–98%), of all asymptomatic recurrences detected in this series were locoregional. Ritoe et al.5 determined that 78% of locoregional recurrences, second primary tumors, and metastases were detected within 3 years using clinical examinations, laryngoscopy, and pharyngoscopy. Boysen et al.7 found that 76% of recurrences were detected within 2 years using clinical examinations with or without endoscopy and roentograms. Wensing et al.18 determined that 83% of recurrences of oral cavity squamous cell carcinoma presented within 2 years. A larger study is needed to determine if PET/CT has a relative surveillance benefit over CT. Schwartz et al.19 determined that 90% of recurrences were detected within 24 months employing clinical examinations and CT imaging. Schwartz et al.19 also reported that 86% of locoregional HNSCC recurrences were discovered secondary to symptomatic complaints. In the current study, only 34% (95 CI, 25%–43%) of observed recurrences were detected due to a symptomatic complaint or finding on physical examination.
Our study has some limitations. Our pilot study of PET/CT surveillance was a retrospective study of screening for clinical management of disease. We determined recurrence from a combination of radiological and clinical data, not solely on the basis of PET/CT. We cannot compare our results to a similar group of patients screened without PET/CT or with less frequent PET/CT. We acknowledge that PET/CT surveillance was not standardized across all patients. Our current HNSCC PET/CT surveillance schedule was implemented in 2007; thus, post-treatment surveillance was not identical for all patients. Additionally, surveillance after 14 months post-treatment was determined by the clinical team, and there was likely a degree of noncompliance with the surveillance protocol, in as much as physicians outside our institution were involved in patient care and monitoring. Penultimately, we note that CRT is not always the first-line treatment for patients with oral cavity squamous carcinoma, which accounted for about one-fifth of our study population. Finally, all tumors of the same histological type were considered recurrences, and it is possible that some of these tumors were second primary cancers.
CONCLUSION
It is well recognized that PET/CT is more sensitive than other available monitoring modalities for detecting HNSCC recurrence. Data from this study suggest that 90% to 98% of patients who will eventually have recurrent disease can be identified within the first 24 months after CRT with PET/CT surveillance. PET/CT use after this time might be restricted to patients who present with new symptoms or clinical findings. It remains to be determined if early detection of recurrence by PET/CT results in improved survival in the cohort of patients who recur. Further study is warranted to explore the utility of PET/CT in detecting HNSCC recurrence and to ascertain if earlier detection of recurrence improves survival.
Footnotes
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Presented as a poster presentation at the Triological Society Combined Sections Meeting, Scottsdale, Arizona, U.S.A., January 27–29, 2011.
BIBLIOGRAPHY
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 5.Ritoe SC, Krabbe PF, Kaanders JH, van den Hoogen FJ, Verbeek AL, Marres HA. Value of routine follow-up for patients cured of laryngeal carcinoma. Cancer. 2004;101:1382–1389. doi: 10.1002/cncr.20536. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal V, Branstetter BFt, Johnson JT. Indications for PET/CT in the head and neck. Otolaryngol Clin North Am. 2008;41:23–49. v. doi: 10.1016/j.otc.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Boysen M, Lovdal O, Tausjo J, Winther F. The value of follow-up in patients treated for squamous cell carcinoma of the head and neck. Eur J Cancer. 1992;28:426–430. doi: 10.1016/s0959-8049(05)80068-1. [DOI] [PubMed] [Google Scholar]
- 8.NCCN Guidelines. Version 1.2011, Head and Neck Cancers. 2011 Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. [Google Scholar]
- 9.Subramaniam RM, Truong M, Peller P, Sakai O, Mercier G. Fluorodeoxy-glucose-positron-emission tomography imaging of head and neck squamous cell cancer. AJNR Am J Neuroradiol. 2010;31:598–604. doi: 10.3174/ajnr.A1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abgral R, Querellou S, Potard G, et al. Does 18F-FDG PET/CT improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? J Nucl Med. 2009;50:24–29. doi: 10.2967/jnumed.108.055806. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 12.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuryak I, Sachs RK, Brenner DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst. 2010;102:1628–1636. doi: 10.1093/jnci/djq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao J, Vu HL, Genden EM, et al. The diagnostic and prognostic utility of positron emission tomography/computed tomography-based follow-up after radiotherapy for head and neck cancer. Cancer. 2009;115:4586–4594. doi: 10.1002/cncr.24493. [DOI] [PubMed] [Google Scholar]
- 15.Nayak JV, Walvekar RR, Andrade RS, et al. Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET-CT. Laryngoscope. 2007;117:2129–2134. doi: 10.1097/MLG.0b013e318149e6bc. [DOI] [PubMed] [Google Scholar]
- 16.Rabalais AG, Walvekar R, Nuss D, et al. Positron emission tomography-computed tomography surveillance for the node-positive neck after chemoradiotherapy. Laryngoscope. 2009;119:1120–1124. doi: 10.1002/lary.20201. [DOI] [PubMed] [Google Scholar]
- 17.Passero VA, Branstetter BF, Shuai Y, et al. Response assessment by combined PET-CT scan versus CT scan alone using RECIST in patients with locally advanced head and neck cancer treated with chemoradiotherapy. Ann Oncol. 2010;21:2278–2283. doi: 10.1093/annonc/mdq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wensing BM, Merkx MA, Krabbe PF, Marres HA, Van den Hoogen FJ. Oral squamous cell carcinoma and a clinically negative neck: the value of follow-up. Head Neck. 2011;33:1400–1405. doi: 10.1002/hed.21642. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz DL, Barker J, Jr, Chansky K, et al. Postradiotherapy surveillance practice for head and neck squamous cell carcinoma—too much for too little? Head Neck. 2003;25:990–999. doi: 10.1002/hed.10314. [DOI] [PubMed] [Google Scholar]