Abstract
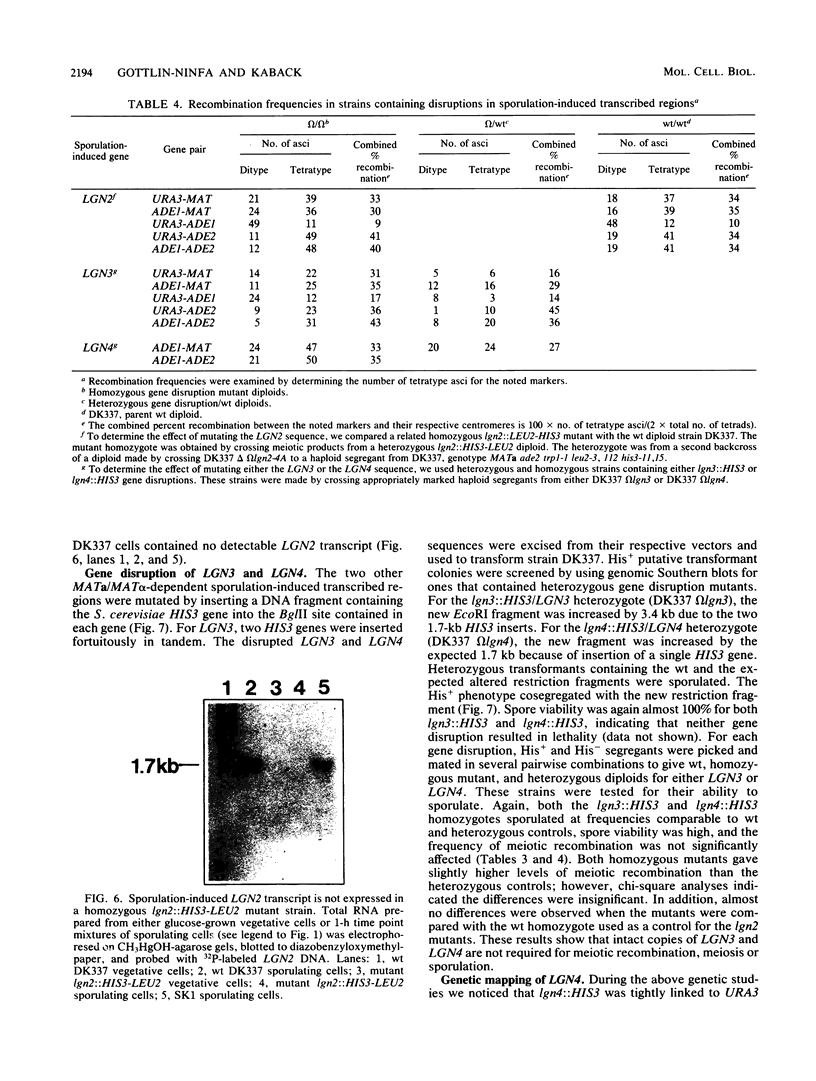
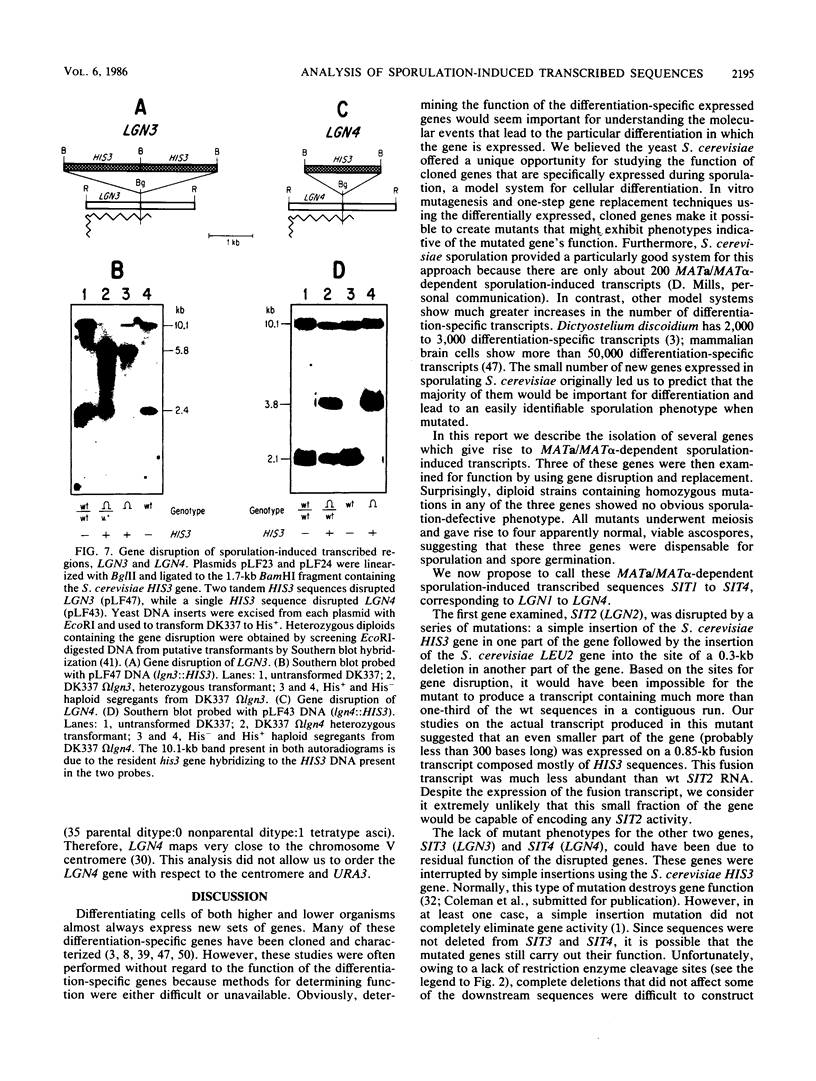
Strains of the yeast Saccharomyces cerevisiae that are heterozygous for the mating-type locus (MATa/MAT alpha) undergo meiosis and spore formation when they are starved for nitrogen and are provided with a nonfermentable carbon source such as potassium acetate. Haploids and diploids homozygous for the mating-type locus (MAT alpha/MAT alpha or MATa/MATa) are asporogenous and undergo neither meiosis nor spore formation when incubated under the same conditions. A small number of genes produce transcripts that appear to be induced specifically in sporulating cells. These transcripts either are not found or are present at much lower levels both in vegetatively growing cells and in cells from asporogenous strains that have been incubated in sporulation medium. Several genes complementary to these MATa/MAT alpha-dependent sporulation-induced transcripts were isolated from a gene-size insert yeast-lambda recombinant DNA library, by differential-plaque filter hybridization. An attempt was made to determine the function of three of these genes by mutating them in the yeast genome with in vitro mutagenesis and one-step gene replacement techniques. One gene was extensively disrupted by both a 0.3-kilobase deletion and the insertion of two large DNA sequences at different sites within the gene. Surprisingly, this compound mutation did not appear to affect meiosis or the production of viable ascospores, indicating that this gene was dispensable for differentiation. The other two genes were disrupted by simple insertion mutations at a site where it was possible that they might still possess some gene activity. These mutations also did not appear to affect sporulation. These results suggest that not all sporulation-induced genes are essential for meiosis and the production of viable ascospores under the conditions examined.
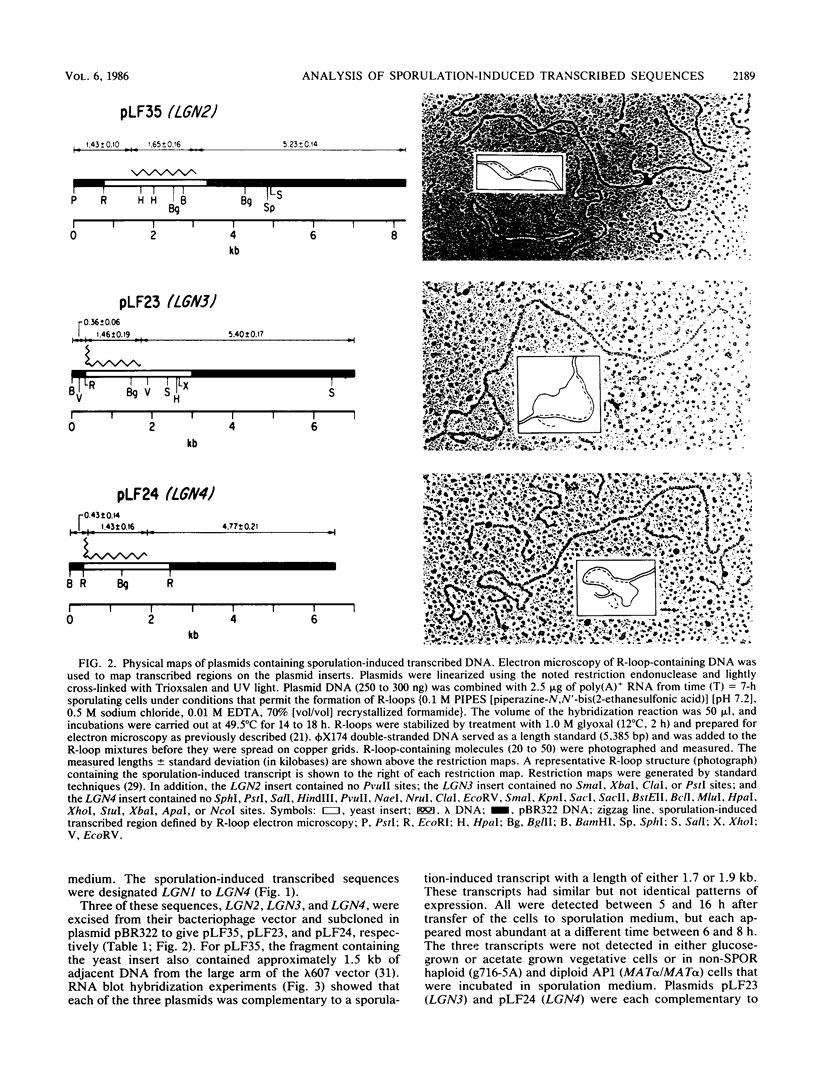
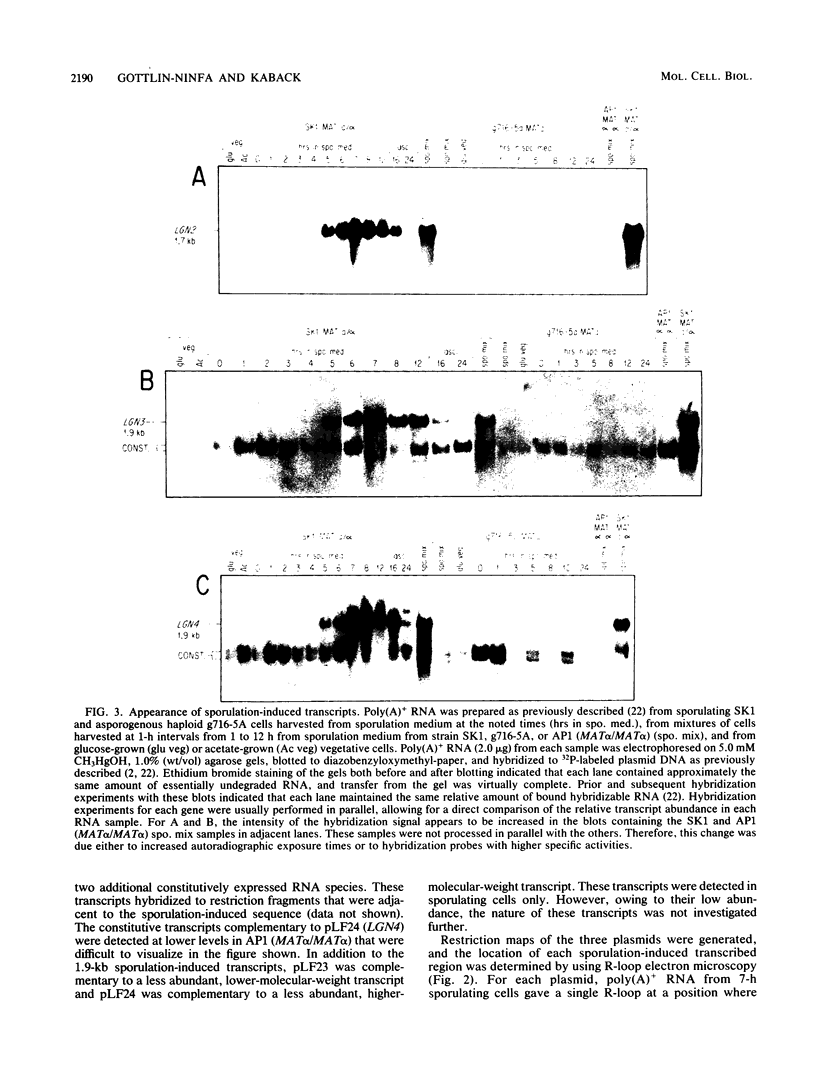
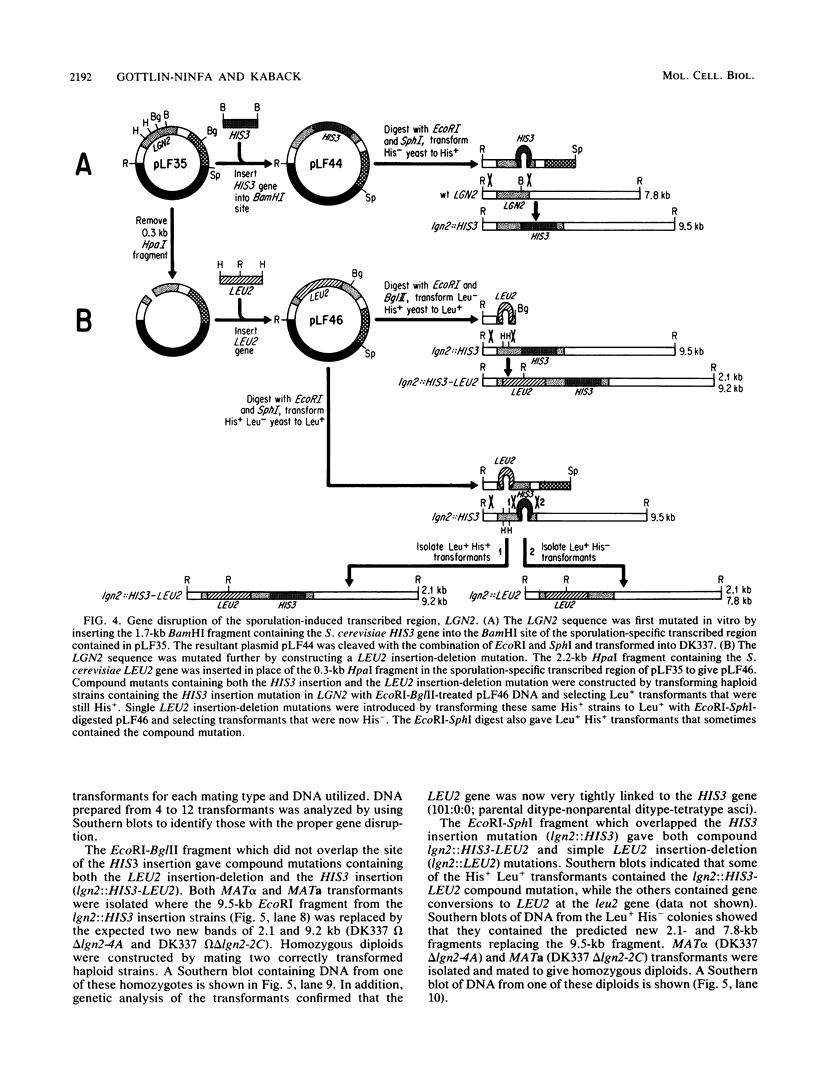
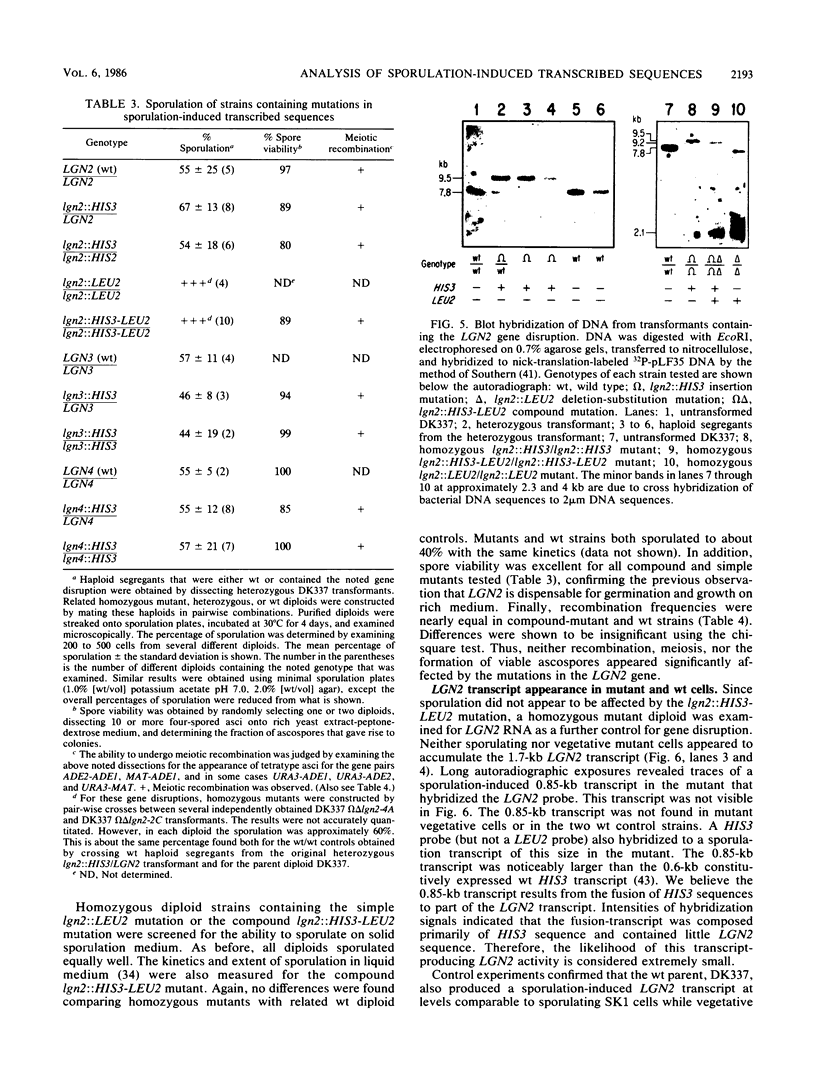
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abovich N., Rosbash M. Two genes for ribosomal protein 51 of Saccharomyces cerevisiae complement and contribute to the ribosomes. Mol Cell Biol. 1984 Sep;4(9):1871–1879. doi: 10.1128/mcb.4.9.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D., Lodish H. F. Changes in the messenger RNA population during differentiation of dictyostelium discoideum. Dev Biol. 1980 Aug;78(2):285–300. doi: 10.1016/0012-1606(80)90337-1. [DOI] [PubMed] [Google Scholar]
- Brandriss M. C. Proline utilization in Saccharomyces cerevisiae: analysis of the cloned PUT2 gene. Mol Cell Biol. 1983 Oct;3(10):1846–1856. doi: 10.1128/mcb.3.10.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Clancy M. J., Buten-Magee B., Straight D. J., Kennedy A. L., Partridge R. M., Magee P. T. Isolation of genes expressed preferentially during sporulation in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 May;80(10):3000–3004. doi: 10.1073/pnas.80.10.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Genomic substitutions of centromeres in Saccharomyces cerevisiae. Nature. 1983 Sep 1;305(5929):23–28. doi: 10.1038/305023a0. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough-Evans B. R., Britten R. J. Molecular biology of the sea urchin embryo. Science. 1982 Jul 2;217(4554):17–26. doi: 10.1126/science.6178156. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Garvik B., Haber J. E. New cytoplasmic genetic element that controls 20S RNA synthesis during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):261–269. doi: 10.1128/jb.134.1.261-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Phillips S. L. Polyadenylate metabolism in the nuclei and cytoplasm of Saccharomyces cerevisiae. J Biol Chem. 1975 Jul 25;250(14):5640–5646. [PubMed] [Google Scholar]
- Hereford L. M., Rosbash M. Number and distribution of polyadenylated RNA sequences in yeast. Cell. 1977 Mar;10(3):453–462. doi: 10.1016/0092-8674(77)90032-0. [DOI] [PubMed] [Google Scholar]
- Heywood P., Magee P. T. Meiosis in protists. Some structural and physiological aspects of meiosis in algae, fungi, and protozoa. Bacteriol Rev. 1976 Mar;40(1):190–240. doi: 10.1128/br.40.1.190-240.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Angerer L. M., Davidson N. Improved methods for the formation and stabilization of R-loops. Nucleic Acids Res. 1979 Jun 11;6(7):2499–2317. doi: 10.1093/nar/6.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Feldberg L. R. Saccharomyces cerevisiae exhibits a sporulation-specific temporal pattern of transcript accumulation. Mol Cell Biol. 1985 Apr;5(4):751–761. doi: 10.1128/mcb.5.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Rosbash M., Davidson N. Determination of cellular RNA concentrations by electron microscopy of R loop-containing DNA. Proc Natl Acad Sci U S A. 1981 May;78(5):2820–2824. doi: 10.1073/pnas.78.5.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Hicks J. B., Strathern J. N. Directionality of yeast mating-type interconversion. Cell. 1982 Mar;28(3):551–561. doi: 10.1016/0092-8674(82)90210-0. [DOI] [PubMed] [Google Scholar]
- Kraig E., Haber J. E. Messenger ribonucleic acid and protein metabolism during sporulation of Saccharomyces cerevisiae. J Bacteriol. 1980 Dec;144(3):1098–1112. doi: 10.1128/jb.144.3.1098-1112.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S., Lindquist S. Changing patterns of gene expression during sporulation in yeast. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7323–7327. doi: 10.1073/pnas.81.23.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küenzi M. T., Tingle M. A., Halvorson H. O. Sporulation of Saccharomyces cerevisiae in the absence of a functional mitochondrial genome. J Bacteriol. 1974 Jan;117(1):80–88. doi: 10.1128/jb.117.1.80-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. A DNA repair gene required for the incision of damaged DNA is essential for viability in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4818–4821. doi: 10.1073/pnas.80.15.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival-Smith A., Segall J. Isolation of DNA sequences preferentially expressed during sporulation in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):142–150. doi: 10.1128/mcb.4.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Kasimos J. N., Game J. C., Braun R. J., Roth R. M. Changes in DNA during meiosis in a repair-deficient mutant (rad 52) of yeast. Science. 1981 May 1;212(4494):543–545. doi: 10.1126/science.7010606. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rowekamp W., Firtel R. A. Isolation of developmentally regulated genes from Dictyostelium. Dev Biol. 1980 Oct;79(2):409–418. doi: 10.1016/0012-1606(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Struhl K., Davis R. W. Transcription of the his3 gene region in Saccharomyces cerevisiae. J Mol Biol. 1981 Nov 5;152(3):535–552. doi: 10.1016/0022-2836(81)90267-9. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey D., Wise J. A., Guthrie C. A U4-like small nuclear RNA is dispensable in yeast. Cell. 1983 Dec;35(3 Pt 2):753–762. doi: 10.1016/0092-8674(83)90108-3. [DOI] [PubMed] [Google Scholar]
- Trew B. J., Friesen J. D., Moens P. B. Two-dimensional protein patterns during growth and sporulation in Saccharomyces cerevisiae. J Bacteriol. 1979 Apr;138(1):60–69. doi: 10.1128/jb.138.1.60-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness J., Maxwell I. H., Hahn W. E. Complex population of nonpolyadenylated messenger RNA in mouse brain. Cell. 1979 Dec;18(4):1341–1349. doi: 10.1016/0092-8674(79)90244-7. [DOI] [PubMed] [Google Scholar]
- Wright J. F., Ajam N., Dawes I. W. Nature and timing of some sporulation-specific protein changes in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Oct;1(10):910–918. doi: 10.1128/mcb.1.10.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita I., Fukui S. Transcriptional control of the sporulation-specific glucoamylase gene in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1985 Nov;5(11):3069–3073. doi: 10.1128/mcb.5.11.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann C. R., Orr W. C., Leclerc R. F., Barnard E. C., Timberlake W. E. Molecular cloning and selection of genes regulated in Aspergillus development. Cell. 1980 Oct;21(3):709–715. doi: 10.1016/0092-8674(80)90434-1. [DOI] [PubMed] [Google Scholar]