Summary
Introduction
The NMDA receptor is a ligand-gated ion channel that plays a critical role in higher level brain processes and has been implicated in a range of neurological and psychiatric conditions. Although initial studies for the use of NMDA receptor antagonists in neuroprotection were unsuccessful, more recently, NMDA receptor antagonists have shown clinical promise in other indications such as Alzheimer’s disease, Parkinson’s disease, pain and depression. Based on the clinical observations and more recent insights into receptor pharmacology, new modulatory approaches are beginning to emerge, with potential therapeutic benefit.
Areas Covered
The article covers the known pharmacology and important features regarding NMDA receptors and their function. A discussion of pre-clinical and clinical relevance is included, as well. The subsequent patent literature review highlights the current state of the art targeting the receptor since the last review in 2010.
Expert Opinion
The complex nature of the NMDA receptor structure and function is becoming better understood. As knowledge about this receptor increases, it opens up new opportunities for targeting the receptor for many therapeutic indications. New strategies and advances in older technologies will need to be further developed before clinical success can be achieved. First-in-class potentiators and subunit-selective agents form the basis for most new strategies, complemented by efforts to limit off-target liability and fine-tune on-target properties.
Keywords: channel blocker, glycine site antagonist, NMDA receptor antagonist, NMDA receptor potentiator, NMDA receptor subunit selectivity, deuterated analogues
1. Introduction
The N-methyl-D-aspartate (NMDA) receptor is a member of the ionotropic glutamate receptor (iGluR) family that mediates a slow, Ca2+ permeable component of excitatory synaptic transmission in the central nervous system (CNS). The iGluR family also comprises the related α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) and kainate receptors, which are distinguished on the basis of sequence identity and pharmacology. NMDA receptors consist of two glycine-binding GluN1 subunits encoded by eight splice variants of a single gene and two glutamate-binding GluN2 (A-D) subunits, arising from four genes [1]. NMDA receptors uniquely require both glutamate and glycine as co-agonists for receptor activation [2]. Following channel opening, membrane depolarization is required to relieve the voltage-dependent Mg2+ block before ions can permeate the channel pore [3,4]. The NMDA receptor subtypes have diverse functional and pharmacological properties, which render them attractive therapeutic targets [1,5-7].
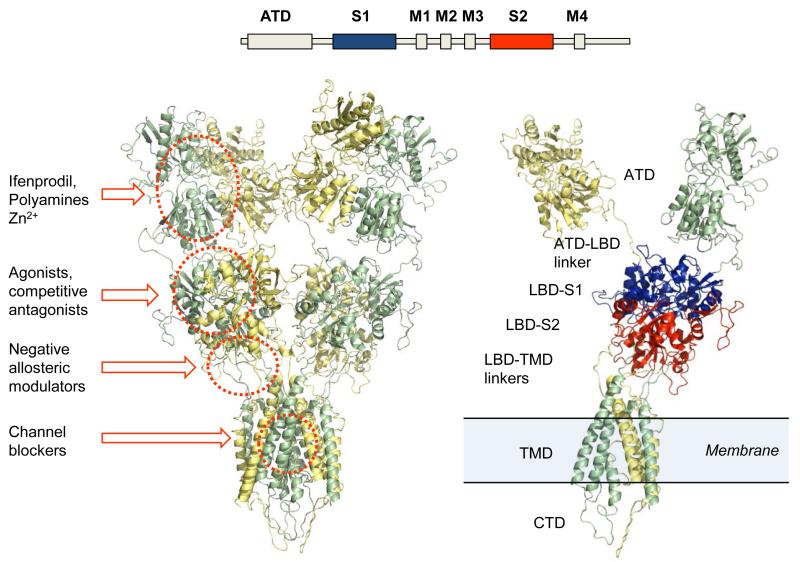
Various ligand binding sites are distributed among semi-autonomous domains that make up each subunit within a functional receptor (Figure 1). The amino terminal domain (ATD), the ligand binding domain (LBD), and the transmembrane domain (TMD) are each thought to have binding sites and structural determinants that are responsible for the actions of endogenous and exogenous modulatory ligands [1,8-10]. The carboxy terminal domain (CTD) binds to intracellular proteins such as calmodulin, calmodulin-dependent protein kinase II (CamKII), and PSD-95, and is subject to post-translational modifications that alter the function of the receptor [1].
Figure 1. Homology model of an NMDA receptor.
(Top) Schematic representation of the linear amino acid sequence that encodes each subunit. ATD is the amino terminal domain. The S1 and S2 domains fold together to form the ligand binding domain (LBD). M1 through M4 form the transmembrane domain (TMD); M1, M3 and M4 are transmembrane helices and M2 is a re-entrant loop. (Left) Ribbon diagram of homology modeled GluN1-GluN2A NMDA receptor (generated using PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC). The GluN1 subunits are depicted in yellow and the GluN2A subunits are in green. Known modulatory regions are circled and labeled. (Right) Single subunits of GluN1-GluN2 receptor describing the domain architecture. The S1 region of the LBD is in blue and the S2 region is in red.
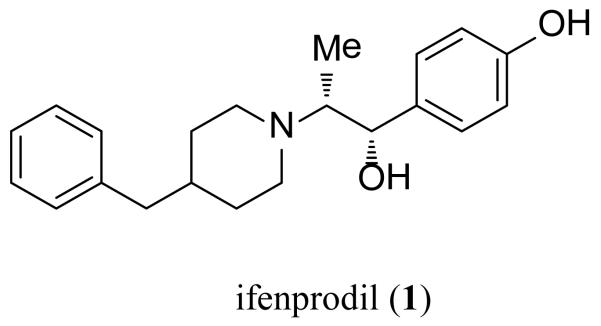
The ATD controls important pharmacological and functional properties (i.e. open probability, deactivation time course, agonist EC50) among the four GluN2 subunits [11,12]. Furthermore, a number of ligands are thought to bind to the extracellular ATD, which exhibits low sequence homology between the GluN2 subunits. For example, the prototypical subunit-selective antagonist ifenprodil (1) (Figure 2) binds selectively to the ATD of GluN2B-containing receptors. The co-crystal structure of this complex shows the ligand binding between the GluN1-GluN2B ATD heterodimer interface [13]. Modulation of NMDA receptors also occurs through the binding of Zn2+ and other transition metals, as well as the endogenous polyamines spermine and spermidine to the ATD [14-18].
Figure 2. Ifenprodil.
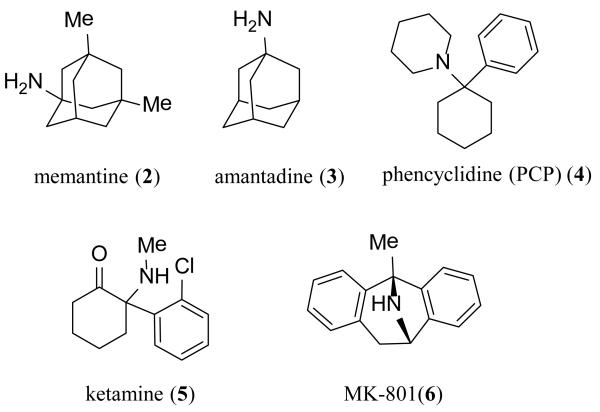
The ligand binding domains of GluN1 and GluN2 form a back-to-back dimer (Figure 1) in a manner similar to that observed for AMPA and kainate receptors [3,19,20]. The conserved sequence in the glutamate binding sites across the GluN2 subunits has impeded the development of subunit-selective competitive antagonists that bind within the LBD of NMDA receptors [20,21]. While modest subunit selectivity has been obtained in some instances, this strategy has not yet successfully translated into positive clinical outcomes [22]. Competitive antagonists exist that act at the glycine binding site on GluN1 with modest preference across receptor subtype, but are still being pursued in the patent literature. Several classes of subunit-selective allosteric modulators interacting with the LBD have been identified and characterized, including the previously identified AMPA receptor antagonizing quinazolin-4-one scaffold, the dihydroquinolone-pyrazoline and the phenanthrene-containing modulators, which share some structural determinants of activity in the S2 region of the LBD [8-10,23]. The unique structural determinants of action of these ligands suggest novel binding sites, which could establish a precedent for therapeutically relevant modulatory sites in regions other than the ATD [8,10,24-26]. The first allosteric modulator of glycine binding has recently been described and has structural determinants of activity at the dimer interface of the GluN1-GluN2 LBD [27-29]. Additionally, the S1-M1, S2-M4 and M3-S2 linker regions are critically involved in receptor gating and could potentially influence the binding of ligands that either positively or negatively modulate receptor function [8,30,31]. These regions have previously been implicated as structural determinants of endogenous modulatory ions, as tonic inhibition of NMDA receptors by protons is influenced by mutations of residues within the linker regions, as well as within the LBD dimer interface [32,33]. The membrane spanning helices plus a re-entrant loop of the AMPA receptor subunits are arranged in a fashion similar to an inverted potassium channel. The high degree of sequence similarity in this region across the glutamate receptor family suggests a similar arrangement for the NMDA receptor channel pore [30]. Mg2+ ions, which block the receptor at resting membrane potential, reside within the permeation pathway and occlude ion flow when present [2,34]. The clinically tolerated organic cationic channel blockers memantine (2) and amantadine (3), as well as other channel blockers such as phencyclidine (PCP) (4), ketamine (5) and MK-801 (6) (Figure 3), are thought to share partially overlapping binding sites within the pore [35-39]. The initial channel blockers caused adverse events and undesirable side effects in humans, thought to reflect on-target actions associated with strong, nonselective inhibition of normal NMDA function [40]. As a result, the early channel blockers were removed from the market, reduced in scope of use, or abandoned for further development. Both of the FDA-approved NMDA channel blockers memantine (2) and amantadine (3) have low potency (high IC50 values), rapid dissociation rates, and are well-tolerated [41,42]. Interestingly, different organic cationic channel blockers show varied levels of trapping block, in which the channel can close and agonist dissociate with the channel blocker still residing within the pore [43,44]. The molecular basis for this is poorly understood.
Figure 3. Organic cationic channel blockers.
Recently, a class of subunit-selective NMDA receptor potentiators was described for which the structural determinants of selectivity reside within the M1 transmembrane helix, a region not previously identified as interacting with other modulatory ligands in the glutamate receptor family [45,46]. This class of compounds appears highly selective for GluN2C- and GluN2D-containing receptors. It remains unclear whether these compounds access a binding site in the lipid bilayer or interact with elements in or near the transmembrane helices from the extracellular side. Another class of potentiators favoring GluN2C- and GluN2D-containing receptors has been described with a complex structure-activity relationship, which may involve the S2 region of the LBD [9,47].
The intracellular C-terminus shows low sequence homology between subunits. This region can bind to a variety of intracellular scaffolding proteins and is a target for post-translational modification such as phosphorylation. Modulation of the protein-protein interactions that occur between the CTD and multiple binding partners can impact cellular localization, thus representing an intriguing new therapeutic strategy for regulating NMDA receptors [1,48-50].
2. Therapeutic Indications for NMDA Modulators
Several neurological diseases and disorders have long been considered to involve NMDA receptors, suggesting potential therapeutic strategies based on NMDA receptor antagonists and allosteric modulators. Decades of work has shown that NMDA receptor antagonists have neuroprotective properties, making them target candidates for the treatment of hypoxic or ischemic conditions that occur during stroke and cardiac arrest [51,52]. An important sequence of events leading to neurodegeneration in these conditions is the elevation of glutamate and the resulting glutamate-induced excitotoxicity, secondary to overactivation of Ca2+ permeable NMDA receptors [53,54]. NMDA receptor antagonists have also been studied extensively pre-clinically and in clinical trials for indications such as Alzheimer’s disease, Parkinson’s disease, traumatic brain injury (TBI), and stroke [55-59]. Most of these trials either failed to obtain statistically significant results or were discontinued due to significant adverse events [52,58-63]. In some cases, reduction of the dose to limit off-target side effects likely contributed to lack of efficacy [58-64]. In addition to these trials, those involving GluN2B-selective inhibitors (i.e.-CP-101,606) and channel blockers (i.e. 5) for treatment-resistant depression have yielded promising results; with acute dosing regimens and long lasting effects [65,66]. Memantine (2) and amantadine (3) are FDA-approved for the symptomatic treatment of Alzheimer’s disease and Parkinson’s disease, respectively (Figure 3)[67,68]. However, neither is thought to alter the course of the disease; their primary utility is to improve quality of life.
Strikingly, some early NMDA receptor antagonists, such as PCP (4, Figure 3), produce symptoms indistinguishable from the acute psychosis observed in schizophrenia [69]. These observations led to the hypothesis that dysfunction of the NMDA receptor may play a role in the etiology of schizophrenia [70-72]. Positive modulators of NMDA function, such as glycine transport inhibitors and agonists acting at the glycine site, have shown some improvement of the negative effects of schizophrenia in a few clinical trials; these symptoms are typically resistant to treatment with available antipsychotics [73-76]. Accordingly, NMDA receptor potentiators have been proposed to have potential therapeutic benefit for patients with schizophrenia.
NMDA receptors play a crucial role in learning and memory, and as such, NMDA receptor potentiators have been considered clinically useful for enhancement of these processes [77-79]. Ablation of GluN2B in knockout mice compromised synaptic plasticity, thought to be a cellular model of learning [80,81]. Moreover, pharmacological blockade of NMDA receptor function can disrupt synaptic plasticity, and some NMDA receptor antagonists impair learning [82-90]. D-cycloserine (DCS) is a partial agonist at GluN2A-, GluN2B-, and GluN2D-containing receptors, but produces a larger response than glycine at GluN2C receptors [91,92]. Interestingly, DCS showed a pronounced effect on facilitation of fear extinction [93-95]. Some clinical data suggest that use of D-cycloserine (clinically approved for use in tuberculosis) may improve the effectiveness of behavioral therapy for anxiety disorders such as post-traumatic stress disorder (PTSD) and phobias by affecting emotional learning; the exact mechanism and extent of the effect is still under investigation [96].
Although significant data suggest that NMDA receptors may be an effective therapeutic target for many CNS-related diseases and possibly cognitive enhancement, a full understanding of the role of the receptor subtypes in the physiology of disease states is still developing. As next generation subunit-selective modulators are developed, they are expected to improve efficacy, while minimizing side effects. Since the last review was published in 2010, a number of NMDA receptor modulators have appeared in the patent literature and are summarized below [97].
3. NMDA Receptor Channel Blockers
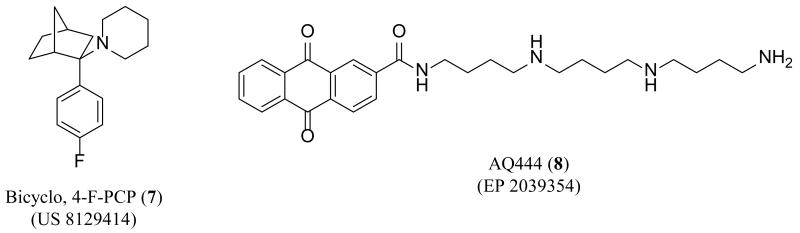
Two new patents have been published since 2010 that describe NMDA receptor channel blockers. A patent issued in 2012 claimed analogues structurally similar to PCP (4) (Figure 3) that contain a bicyclic moiety [98]. The authors claimed that the introduction of constrained alkyl rings and para-substituted phenyl rings decreased acute toxicity, while maintaining efficacy. Substitution of a hydrogen atom with a fluorine atom is a well-known strategy to modify the physicochemical properties of a drug and was often employed by the inventors in other areas of research [99-101]. Acute toxicity was assessed by monitoring animals for signs of impaired neurological or muscular function using the rotorod test. The representative compound, bicyclo-4-F-PCP (7) (Figure 4) competes with the known channel blocker MK-801 (6) (Figure 3) and showed no toxicity at 100 mg/kg with modest non-lethal toxicity at 300 mg/kg.
Figure 4. Novel NMDA receptor channel blockers.
A patent claiming tricyclic compounds as NMDA receptor channel blockers was published by researchers at the National University Corporation Chiba University in Japan for use in the treatment or prevention of diseases characterized by impairment of higher cerebral functions [102]. In a voltage clamp assay using GluN1/GluN2A-expressing Xenopus oocytes, AQ444 (8) (Figure 4) had an IC50 value of 0.47 μM at −70 mV. Investigation of the subunit selectivity of 8 showed approximately a 10-fold lower IC50 value at GluN1/GluN2A-, GluN1/GluN2B-, and GluN1/GluN2D-containing receptors than at GluN1/GluN2C-containing receptors. This observation is consistent with aromatic polyamine derivatives that have also shown some subunit selectivity [17,103].
4. Glycine Site Antagonists
Several lines of evidence suggest that glycine site antagonists might have a better therapeutic index than glutamate site antagonists or channel blockers. For example, competitive glycine site antagonists do not produce side effects typically associated with NMDA receptor blockade, such as neurodegenerative changes in the cingulate/retrosplenial cortex, psychotomimetic effects, and learning impairment [104-110]. Recently, it has also been shown that several glycine site antagonists and partial agonists do not cross discriminate with PCP at therapeutically relevant concentrations [111].
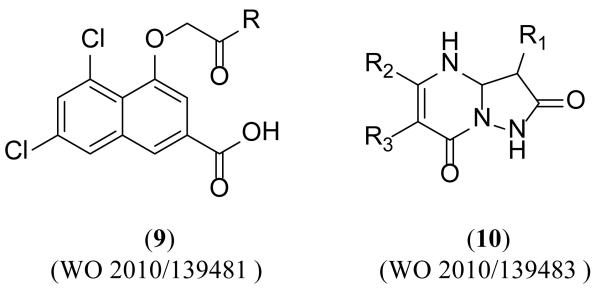
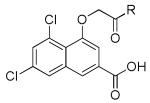
Two patent applications claiming over 200 compounds were disclosed by Merz Pharma [112,113]. A class of naphthalene derivatives (9) and a class of pyrazolopyrimidine derivatives (10) (Figure 5) were characterized in a competitive binding assay against radiolabeled MDL-105,519, a high affinity NMDA receptor glycine site antagonist. Each class was functionally evaluated using electrophysiological whole cell patch-clamp recordings in rat hippocampal neurons (co-applied with the glycine site agonist D-serine and NMDA) and/or fluorometric intracellular Ca2+-flux assays (FLIPR). Biological data was only reported for the naphthalene derivatives (9). Examples from each series are shown in Tables 1-2, along with available binding and functional data.
Figure 5. Glycine-site antagonists recently disclosed by Merz Pharma.
Table 1. Antagonists binding at the GluN1 subunit.
|
||||
|---|---|---|---|---|
| R | [3H]-MDL-105,519 binding IC50 [μM] |
Patch-clamp IC50 [μM] |
Calcium FLIPR IC50 [μM] |
|
| 34 |

|
0.0119 | 0.023 | 0.211 |
| 42 |
|
0.055 | 0.050 | 0.105 |
| 59 |
|
0.083 | 0.059 | 0.88 |
Table 2. Antagonists binding at the GluN1 subunit.
|
|||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | [3H]-MDL-105,519 binding IC50 [μM] |
Patch-clamp IC50 [μM] |
|
| 22 | NHCOPh |
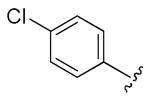
|
H | 0.96 | 0.38 |
| 63 |

|
Ph | H | 1.62 | 0.96 |
5. GluN2B-Selective NMDA Receptor Antagonists
The discovery of an extensive class of GluN2B-subunit-selective antagonists (typified by ifenprodil (1)) has allowed a detailed exploration of the role of the GluN2B subunit in clinical indications ranging from ischemic cell death to depression [58,114,115]. However, the off-target effects associated with the compounds selected for clinical testing have hindered approval for human use [114].
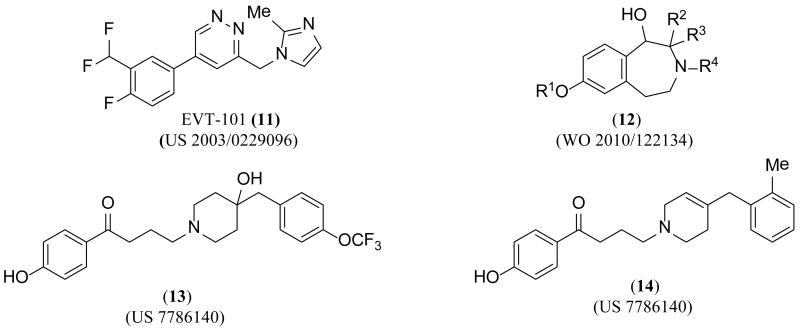
EVT-101 (11) (Figure 6), first claimed by Hoffman-La Roche in 2003, has more recently been claimed by Evotec in a method of use patent for cognitive impairment, neurodegenerative diseases, pain, depression, attention deficit hyperactivity disorder, and addiction [116,117]. The patent described data collected from phase I and phase II clinical trials, some of which is summarized below. The inventors listed various pharmacokinetic (PK) data points collected from cerebral spinal fluid (CSF) after single dose administration for eight consecutive days. The phase II clinical trial in treatment-resistant depression was voluntarily terminated in 2011.
Figure 6. GluN2B-selective antagonists disclosed in the patent literature since 2006.
A PCT (Patent Cooperation Treaty) application from the University of Münster claiming 30 specific structures related to benzo-fused nitrogen heterocycles of generic structure 12 showed competition against radiolabeled ifenprodil with affinities in the nanomolar region (5.1 nM to 586 nM) [118]. The compounds showed no competition with radiolabeled MK-801, but several compounds exhibited comparable binding affinities for the opioid (σ1 and σ2) receptors to that of NMDA receptors; more data is needed to better understand the ramifications of actions on opioid receptors. The inventors claimed indications including pain, ischemic and hemorrhagic stroke, Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, depression, and numerous forms of addiction.
A US patent claiming 24 specific piperidine derivatives, similar to 13 and14, was published in August 2010 from Shionogi & Co., Ltd [119]. The inventors showed competition data using radiolabeled ifenprodil to determine nanomolar affinity for several compounds, but reported only one functional IC50 from an electrophysiological experiment (GluN2B IC50 4.6 μM, compound 14). The authors also claimed efficacy in a two phase formalin-induced pain mouse model using compound 14 at a dose of 1.1 mg/kg for the first phase of the assay and 0.7 mg/kg for the second phase.
6. Antagonists Selective for Receptor Subunits Other than GluN2B
The general lack of subunit-selective antagonists has made it difficult to assess the role the NMDA receptor subtypes play in pathophysiological conditions [1,7,42]. Since global antagonism of the NMDA receptor with competitive antagonists and channel blockers has often shown undesirable clinical effects, the recent discovery of subunit-selective modulators for subunits other than GluN2B could have significant impact on both pre-clinical and clinical research. The temporal and spatial distribution of the various receptor subtypes has led to several hypotheses about the involvement of the individual subtypes in a host of pathophysiological states [1,42,56]. For example, multiple lines of evidence suggest that GluN2D is expressed in the external globus pallidus, subthalamic nuclei, and internal globus pallidus/substantia nigra reticulate, leading to a rationale for how inhibition of GluN2D-containing receptors could impact basal ganglia function in Parkinson’s disease [56,120,121].
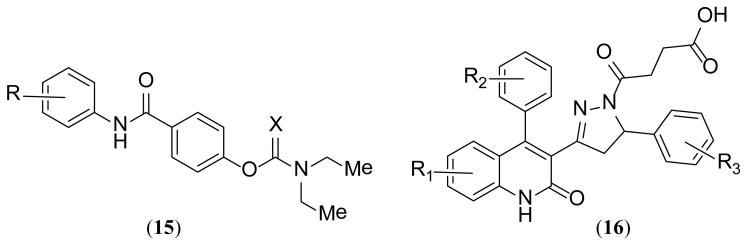
Researchers at Emory University filed a patent that disclosed two classes of compounds typified by 15 and 16 (Figure 7) and provided numerous examples of each [122]. IC50 data was collected using two-electrode voltage clamp recordings from recombinant receptors expressed in Xenopus oocytes. Several compounds claimed had low micromolar IC50 values at GluN2C- and GluN2D-containing receptors with minimal activity at GluN2A- or GluN2B-containing receptors. The compounds act in a glutamate-dependent manner, with structural determinants of selectivity comprised of residues encoded by the S2 region of the polypeptide, chain, which forms a portion of the LBD (Figure 1) [8,24]. Inhibition is non-competitive and voltage independent.
Figure 7. GluN2C/D-selective antagonists disclosed in the patent literature since 2010.
Researchers at the University of Nebraska and the University of Bristol patented compounds that had varied (positive and negative) modulatory activity and selectivity across the NMDA receptor subtypes [47]. The patent expanded on phenanthrene (17-19) and naphthalene (20-22) (Figure 8) derivatives previously claimed in the patent literature and studied in the primary literature [9,123-125]. The authors showed data from two-electrode voltage clamp experiments across all four receptor subtypes. The compounds had potencies in the low to high micromolar range, and some compounds exhibited less than full inhibition. The authors concluded from the experiments that the site of action does not reside in the ATD, and suggest that the structural determinants of selectivity reside in the LBD. A subsequent paper further explaining the structure activity relationship (SAR) around the compounds has been published [126]. A wide range of indications was claimed.
Figure 8. Phenanthrene and naphthalene compound classes claimed by University of Nebraska.
Although not discussed in the patent literature, another important advance has been the description of a family of highly selective NR2A inhibitors by Bettini et al.[27]. This series of compounds, typified by TCN-201, show remarkable selectivity for GluN2A over all other NMDA receptor subunits. TCN-201 has been shown to be an allosteric modulator of glycine binding, in that the TCN-bound molecule shows a decreased glycine affinity [28,29]. Because TCN-201 is an allosteric modulator, glycine reciprocally reduces TCN-201 potency[28]. Site-directed mutagenesis has identified residues at the GluN1 LBD/GluN2 LBD interface that control TCN-201 potency, suggesting that the binding site for this modualtor may exist at this interface[28]. These compounds could become invaluable tools for use in the study of GluN2A function in neuronal circuits.
7. Positive Modulators of NMDA Receptor Function
Enhancement of NMDA receptor activity has been suggested to have therapeutic benefit in a range of neurological conditions, including schizophrenia, anxiety, and bone disorders [75,96,127]. Several endogenous compounds including polyamines and neurosteroids have been reported to positively modulate the NMDA receptor complex [7,25,128,129]. Spermine and spermidine selectively potentiate GluN2B-containing receptors with EC50 values near 100 μM [129-131]. The neurosteroid pregnenolone sulfate (PS) is known to potentiate GluN2A- and GluN2B-containing NMDA receptors, while weakly inhibiting GluN2C- and GluN2D-containing receptors [25,128]. Potentiation by cations and aminoglycosides has also been described [5,131-134]. Until recently, no other compounds capable of allosterically potentiating recombinant NMDA receptors have been reported [7].
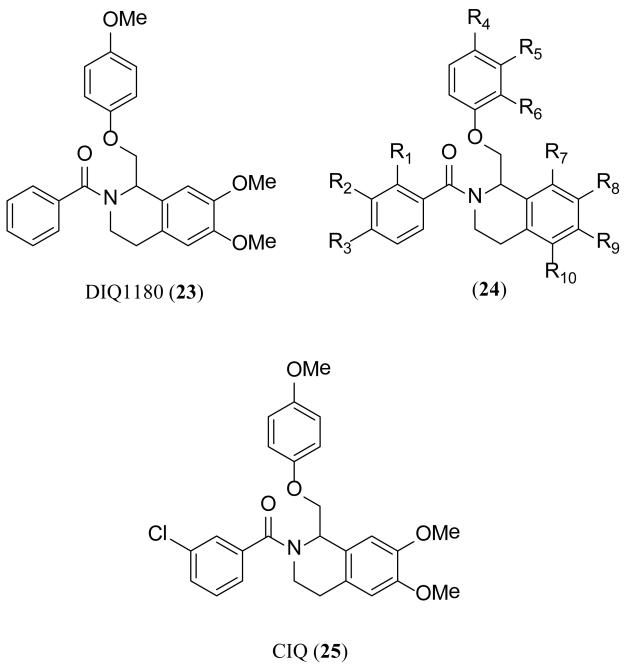
Recently, a series of analogues typified by 23 and 24 (Figure 9) was described that selectively potentiates GluN2C- and GluN2D-containing NMDA receptors over GluN2A- and GluN2B-containing receptors [46]. A detailed SAR was described, listing 119 novel structures, using a two-electrode voltage clamp assay to characterize the compounds. Compound 23 was reported to have an EC50 of 11 μM at GluN2C-containing receptors with a maximum potentiation of 181%, and an EC50 of 13 μM at GluN2D-containing receptors with a maximum potentiation of 162%. No effect was observed at GluN2A- or GluN2B-containing receptors. The inventors claimed additional analogues with a 3-fold increase in potency through the SAR efforts. A large number of CNS-related disorders affecting NMDA receptor activity were claimed as methods of use. In a subsequent manuscript, the inventors showed activity of 25 on native receptors in the subthalamic nucleus [45]. They also identified a single residue within the TMD, Thr592, required for the activity of 25, suggesting that a novel modulatory site might exist in this region of the GluN2C- and GluN2D-containing recombinant receptors [45].
Figure 9. Potentiators of GluN2C/D-containing receptors.
A second series of analogues was recently disclosed by researchers at the University of Nebraska in a patent describing compounds that either positively or negatively modulated the receptor (Figure 8) [47]. A voltage clamp assay illustrated the ability of several compounds to selectively potentiate responses at GluN2A-, GluN2B-, GluN2C- or GluN2D-containing receptors, with no effect or weak inhibition at the other subunits [47]. UBP512 (17) (Figure 8) exhibited modest potentiation (125% of control at 300 μM) at GluN1/GluN2A receptors, inhibition at GluN1/GluN2C and GluN1/GluN2D, and no effect at GluN1/GluN2B [9,77,135]. A second derivative, UBP710 (18) (Figure 8), potentiated GluN2A- and GluN2B-containing receptors while inhibiting GluN2C- and GluN2D-containing receptors. Chimeric receptors suggested that the S2 region of the LBD may be important for the potentiating activity of both 17 and 18. Selective potentiation of GluN2D-containing receptors was demonstrated by UBP551 (20), with inhibition at all other subunits. The ability of 20 to distinguish between the GluN2C- and GluN2D-subunit, despite their high degree of sequence homology, makes this an intriguing analogue [9,77].
8. Indirect Modulators of NMDA Receptor Function
A number of patents have focused on using RNA, small peptides, and peptido-mimetics to indirectly modulate NMDA receptor function [136-143]. Of note are those describing technology developed by NoNo Inc. and Arbor Vita Corporation [137-139,141,142,144]. The inventors have recently published a study which showed promising results using an inhibitor targeting PSD-95 binding in a non-human primate model of stroke; the compounds could be administered more than three hours post occlusion and still maintain efficacy [50].
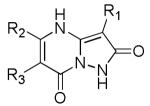
A patent from Aventis Pharmaceuticals Inc. claimed 42 novel dipyrazole-based inhibitors (26) (Figure 10) of spinophilin binding to protein phosphatase 1 (PP-1) which enhance NMDA receptor function [145]. Spinophilin anchors PP-1 in the vicinity of the receptor to facilitate dephosphorylation of specific GluN1 residues, which is thought to reduce the NMDA response [146,147]. Therefore, disruption of the spinophilin-PP-1 complex leads to potentiation of NMDA response [145,146]. An ELISA time-resolved fluorescence 384-well assay was used to evaluate the ability of the compounds to prevent spinophilin-PP-1 interaction. An increase in NMDA-evoked current was observed upon administration of the compounds to the intracellular side in voltage clamp whole-cell recordings (Table 3), and a MK-801(6)-induced psychosis rodent model was used to evaluate the efficacy of these compounds against neuropsychiatric disorders.
Figure 10. Potentiators of NMDA function via spinophilin inhibition.
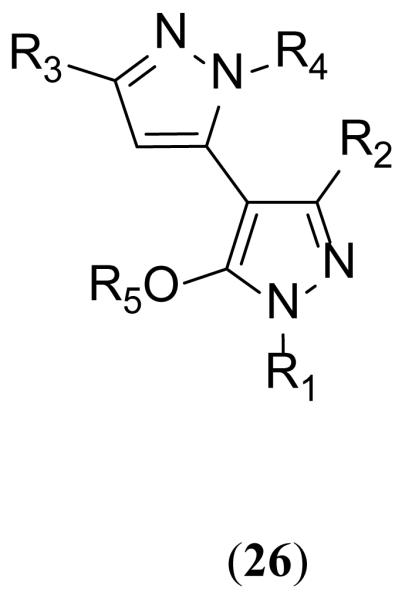
Table 3. Potentiation of NMDA-Evoked Current.
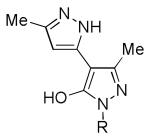
|
|||
|---|---|---|---|
| R | Minimal Effective Concentration (μM) |
Average percent increase in NMDA current (10 μM) |
|
| 8 |
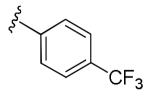
|
1 | 303% (n = 3 neurons) |
| 18 |
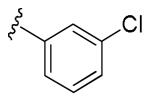
|
5 | 55% (n = 4 neurons) |
Indirect NMDA receptor modulators also include glycine transport (GlyT) inhibitors, metabotropic glutamate receptor (mGluR) modulators, and muscarinic modulators. These strategies represent significant therapeutic potential for this growing area of research and are being studied in both the primary and patent literature [148].
9. Unknown Site Modulators
A patent application describing a class of benzothiadiazepine compounds with modulatory activity on both AMPA and NMDA receptors was published by inventors from Les Laboratoires Servier in France in 2010 [149]. The benzothiadiazine compound IDRA-21 (27) (Figure 11) was reported to reduce AMPA receptor desensitization in vitro, and reduce NMDA receptor whole-cell currents in cerebellar granule cells. Experiments on recombinant NMDA receptors expressed in HEK-293 cells suggested that the compound was more effective on GluN1/GluN2B receptors than on GluN1/GluN2A receptors. Similarly, additional compounds described are claimed as positive allosteric modulators of AMPA and antagonists of NMDA receptor function, however only one compound had data reported; compound (28) had an IC50 of 9 μM in a two-electrode voltage clamp assay recorded from Xenopus oocytes injected with poly(g) mRNA from rat cortex.
Figure 11. Benzothiadiazepines as unknown site NMDA antagonists.
A group at Hong Kong University of Science and Technology protected a series of steroidal compounds which targeted both melanocortin 4 (MC4) and NMDA receptors [150]. Compound DA001 (29) (Figure 12) appears to inhibit NMDA-evoked current in a whole cell patch clamp assay, as well as show concentration-dependent decrease of NMDA-evoked response in rat primary cortical neurons.
Figure 12. Unknown site inhibitor of NMDA function.
10. Deuterated Analogues
Over the past few years, pharmaceutical companies have expressed a growing interest in deuterium-hydrogen substitution in order to improve the pharmacokinetic properties of currently approved drugs [151]. Evidence suggests that selective deuterium incorporation may slow or prevent undesired metabolism, while still retaining the biochemical potency and physiological selectivity of the parent compound [152]. A full discussion of the patents published prior to 2010 based on this strategy was published in an earlier review [97]. An additional patent covering deuterated analogs of known NMDA receptor modulators has since been filed by Auspex Pharmaceuticals, Inc. [153].
11. New Therapeutic Indications for Patented NMDA Receptor Modulators
NMDA receptor modulators have been well studied for use in stroke, Alzheimer’s disease, and Parkinson’s disease [55,56,59,67]. The new appreciation for possible use of NMDA receptor antagonists follows work with ketamine, which has shown tremendous promise for the treatment of depression [66,154,155]. Additional pre-clinical and clinical trials have focused on other indications, including tinnitus and pseudobulbar affect (PBA) in multiple sclerosis (MS) and ALS, broadening the range of diseases for which NMDA receptor modulators may have therapeutic potential. Patents covering the use of these compounds, along with clinical trial data from 2010, support the benefits of NMDA receptor modulators in these disease states [156-159].
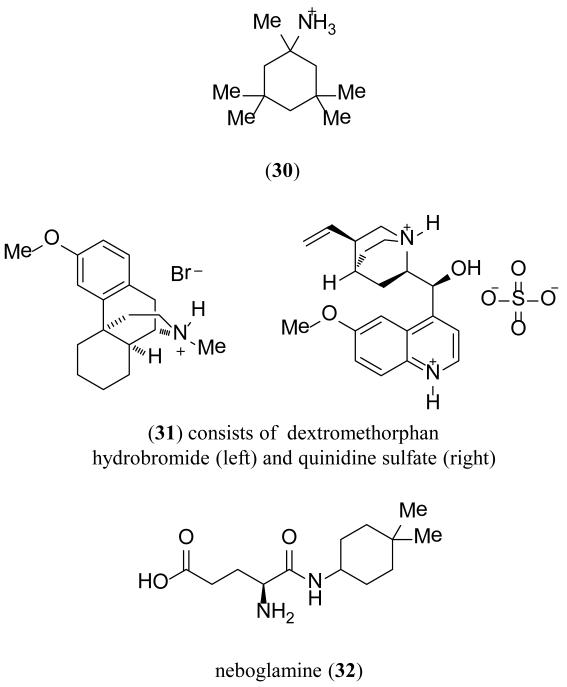
Tinnitus, or “ringing in the ears,” is the perception of noise despite the lack of an external auditory source [160]. It has been proposed that there may be interplay between salicylate-induced tinnitus, which involves the inhibition of the cyclooxygenase and potentiation of NMDA receptors in the cochlea [161]. Neramexane (30) (Figure 13), an NMDA receptor channel blocker, was recently patented by Merz Pharma for use in cochlear tinnitus [156]. A self-reported questionnaire was given at the end of a 16-week double-blind placebo-controlled trial. Patients who had experienced onset of tinnitus three to eight months before the trial were the most responsive to treatment. Between 2010 and 2011, 30 was studied in four separate phase II and III trials [162]. An additional study on the long term safety and tolerability of 30 was terminated in 2012 [162].
Figure 13. Pre-clinical and clinical trial compounds for new indications of NMDA dysfunction.
Although dextromethorphan is a known NMDA receptor antagonist and sigma receptor agonist, a more recent combination therapy directed towards decreasing the metabolism of dextromethorphan met FDA approval in 2010. The combination of dextromethorphan hydrobromide and the supplement quinidine sulfate, also called Nuedexta (35), was approved for the treatment of PBA in MS and ALS patients [163,164]. PBA is an emotional state, characterized by intense involuntary outbursts of laughing and/or crying, associated with approximately 10% of MS patients and 50% of ALS patients [165]. A patent was recently issued to AVANIR Pharmaceuticals Inc. for the use of 31 in the treatment of a variety of neurological disorders, including ALS and associated PBA [157]. Additionally, other uses for 31 are currently being explored including a phase II clinical trial for treating central neuropathic pain in MS patients, which is currently recruiting, and a phase II trial for agitation in Alzheimer’s patients which has been verified, but is not yet recruiting [166].
Also noteworthy is a patent from Rottapharm S.p.A. regarding a new method of use for neboglamine (32), which may act as a positive allosteric modulator of NMDA receptor function [159,167]. A second patent application was published for 32 in the treatment of toxico-dependency against sedatives and stimulants [158].
12. Conclusion
Although many of the NMDA antagonists studied in the clinic for neuroprotection were ultimately unsuccessful, more recent studies have demonstrated important advances in the field with potential to treat other conditions. Of particular significance has been the positive data showing the utility of ketamine for treatment-resistant depression. The patent literature over the past two years highlights several noteworthy advances, many with potential to treat new therapeutic indications. Only a modest number of patents were published describing NMDA receptor channel blockers. Although GluN2B-selective antagonists have been under intense scrutiny for years, advances in these technologies have been slow for this widely studied class. The discovery of antagonists selective for other subunits has been highlighted with the addition of two novel structural classes selective for GluN2C- and GluN2D-containing NMDA receptors. The first subunit-selective potentiators were also disclosed in the last two years. Significant research has been directed toward the indirect modulation of the NMDA receptor by targeting protein-protein interactions which disrupt or enhance NMDA function. One company, Auspex Pharmaceuticals, has focused on enhancing the pharmacokinetic properties of known NMDA receptor antagonists by incorporating deuterium in place of hydrogen. Interestingly, several new uses have emerged in recent years for NMDA receptor modulators including tinnitus and PBA associated with MS, highlighted by the clinical trials of Neramexane and Nuedexta.
13. Expert Opinion
The recent patents filed involving ligands acting at NMDA receptors emphasize at least four important points regarding current thinking of the therapeutic utility of the NMDA receptor as a target. First, it seems widely accepted and intuitively reasonable that subunit-selective ligands may enhance efficacy while diminishing side effects. This is a strategy that will continue to be pursued, supported by basic research delineating the roles of various NMDA receptor subunits in normal brain function and neurological disease. A growing body of clinical and basic research data now supports this trend, and several of the patents described here focus on subunit-selective agents. As the number of compounds that are selective against various receptor subtypes continues to increase, the field should be poised not only for rapid growth toward an enhanced understanding of the physiology of the receptors, but also poised for discoveries that exploit these previously unrecognized sites in pre-clinical and clinical settings [8,10,45]. Second and perhaps related, it seems probable that drug development efforts may shift further toward allosteric modulators. These compounds often bind to regions of the ligand-gated channels with less amino acid conservation than the ligand binding site or channel pore. Ligands acting at these sites can either potentiate or inhibit receptor function, and it is typically thought that there can be a continuum of activity at any site. Although there are few examples of this for the NMDA receptor, there are numerous examples for GABA receptors (benzodiazepine site) and G-protein coupled receptors (mGluRs, biased signaling) [168,169]. Moreover, by preserving some function even at saturating concentrations of ligands, negative allosteric regulators should have a better safety profile than previously studied competitive antagonists and channel blockers that eliminate all activity at all subunits at saturating levels. Third, there is renewed interest in channel blockers and GluN2B selective agents, driven by the clinical data that both can relieve symptoms of treatment-resistant depression [65,66]. This fascinating observation coupled with the generally safe and well-tolerated profile of low affinity and rapidly reversing channel blockers such as memantine (2) has renewed interest in these compounds, not long ago considered clinically a dead-end by many in the field. Fourth, the recent description of a range of drug-like small molecules capable of enhancing NMDA receptor function will likely stimulate further development, given the numerous neurological conditions (schizophrenia, cognitive impairment, learning and memory) that have been proposed to be amenable to treatment with NMDA receptor potentiators [77]. As these compounds enter the mainstream academic literature and data regarding their utility emerges, it seems very likely that work and progress will accelerate.
Article Highlights.
An overview of the structure and function of the NMDA receptor is given with binding sites for known NMDA receptor modulators identified in the text.
A historical perspective on the therapeutic potential of NMDA receptor modulators is presented with particular attention paid to the new applications in the treatment of pain, treatment-resistant depression and schizophrenia.
A review of the patent literature around subunit-selective negative allosteric modulators disclosed since 2010 is described. Significant advances have been made in this timeframe in the discovery of negative allosteric modulators selective for GluN2A-, GluN2C- and/or GluN2D-containing NMDA receptors.
The first classes of subunit-selective positive allosteric modulators have been disclosed and are reviewed in detail.
Due to recent promising clinical data, interest has been directed toward NMDA receptor channel blockers and GluN2B selective antagonists for use in treatment-resistant depression.
Acknowledgements
The authors would like to thank Kevin Ogden and Frank Menniti for their critical feedback and helpful suggestions. This work was supported by the NIH-NINDS(NS071802 TMA, NS065371, NS036654 SFT) and NIH Graduate training in the Pharmacological Sciences [GM008602 TMA]
Footnotes
Declaration of interest
Some of the authors (RMS, TMA, SSZ, SFT, DCL) are inventors on Emory-owned IP involving NMDA receptor ligands. Some of the authors have an equity position (SFT, DCL), are Board members (DCL), or paid consultants (SFT) for NeurOp Inc., which is developing NMDA receptor modulators for the treatment of neurological disease.
Bibliography
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1**.Traynelis SF, Wollmuth LP, McBain CJ, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharm Rev. 2010;62(3):405–96. doi: 10.1124/pr.109.002451. A detailed and historical perspective on ionotropic glutamate receptors covering structure, function and pharmacology.
- 2.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325(6104):529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 3.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309(5965):261–63. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 4.Nowak L, Bregestovski P, Ascher P, et al. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307(5950):462–65. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 5.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7(1):39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Paoletti P. Molecular basis of NMDA receptor functional diversity. Eur J Neurosci. 2011;33(8):1351–65. doi: 10.1111/j.1460-9568.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- 7.Ogden KK, Traynelis SF. New advances in NMDA receptor pharmacology. Trends Pharmacol Sci. 2011;32(12):726–33. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acker TM, Yuan H, Hansen KB, et al. Mechanism for Noncompetitive Inhibition by Novel GluN2C/D N-Methyl-D-Aspartate Receptor Subunit-Selective Modulators. Mol Pharm. 2011;80(5):782–95. doi: 10.1124/mol.111.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa BM, Irvine MW, Fang G, et al. A Novel Family of Negative and Positive Allosteric Modulators of NMDA Receptors. J Pharmacol Exp Ther. 2010;335(3):614–21. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosley CA, Acker TM, Hansen KB, et al. Quinazolin-4-one Derivatives: A Novel Class of Noncompetitive NR2C/D Subunit-Selective N-Methyl-d-aspartate Receptor Antagonists. J Med Chem. 2010;53(15):5476–90. doi: 10.1021/jm100027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gielen M, Retchless BS, Mony L, et al. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009;459(7247):703–07. doi: 10.1038/nature07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan H, Hansen KB, Vance KM, et al. Control of NMDA Receptor Function by the NR2 Subunit Amino-Terminal Domain. J Neurosci. 2009;29(39):12045–58. doi: 10.1523/JNEUROSCI.1365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475(7355):249–53. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi Y-B, Lipton SA. Identification and Mechanism of Action of Two Histidine Residues Underlying High-Affinity Zn2+ Inhibition of the NMDA Receptor. Neuron. 1999;23(1):171–80. doi: 10.1016/s0896-6273(00)80763-1. [DOI] [PubMed] [Google Scholar]
- 15.Fayyazuddin A, Villarroel A, Le Goff A, et al. Four Residues of the Extracellular N-Terminal Domain of the NR2A Subunit Control High-Affinity Zn2+ Binding to NMDA Receptors. Neuron. 2000;25(3):683–94. doi: 10.1016/s0896-6273(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 16.Mony L, Zhu S, Carvalho S, Paoletti P. Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J. 2011;30(15):3134–46. doi: 10.1038/emboj.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao J, Seiler N, Renault J, et al. N1-Dansyl-Spermine and N1-(n-Octanesulfonyl)-Spermine, Novel Glutamate Receptor Antagonists: Block and Permeation of N-Methyl-D-Aspartate Receptors. Mol Pharm. 1997;51(5):861–71. doi: 10.1124/mol.51.5.861. [DOI] [PubMed] [Google Scholar]
- 18.Low C-M, Zheng F, Lyuboslavsky P, Traynelis SF. Molecular Determinants of Coordinated Proton and Zinc Inhibition of N-methyl-D-aspartate NR1/NR2A Receptors. Proc Nat Acad Sci USA. 2000;97(20):11062–67. doi: 10.1073/pnas.180307497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong N, Sun Y, Chen G-Q, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395(6705):913–17. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438(7065):185–92. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 21.Kinarsky L, Feng B, Skifter DA, et al. Identification of Subunit- and Antagonist-Specific Amino Acid Residues in the N-Methyl-D-aspartate Receptor Glutamate-Binding Pocket. J Pharm Exp Ther. 2005;313(3):1066–74. doi: 10.1124/jpet.104.082990. [DOI] [PubMed] [Google Scholar]
- 22.Bonaccorso C, Micale N, Ettari R, et al. Glutamate Binding-Site Ligands of NMDA Receptors. Curr Med Chem. 2011;18(36):5483–506. doi: 10.2174/092986711798347225. [DOI] [PubMed] [Google Scholar]
- 23.Menniti FS, Chenard BL, Collins MB, et al. Characterization of the binding site for a novel class of noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonists. Mol Pharm. 2000;58(6):1310–7. doi: 10.1124/mol.58.6.1310. [DOI] [PubMed] [Google Scholar]
- 24.Hansen KB, Traynelis SF. Structural and Mechanistic Determinants of a Novel Site for Noncompetitive Inhibition of GluN2D-Containing NMDA Receptors. J Neurosci. 2011;31(10):3650–61. doi: 10.1523/JNEUROSCI.5565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horak M, Vlcek K, Chodounska H, Vyklicky L., Jr Subtype-dependence of N-methyl-D-aspartate receptor modulation by pregnenolone sulfate. Neurosci. 2006;137(1):93–102. doi: 10.1016/j.neuroscience.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 26.Jang M-K, Mierke DF, Russek SJ, Farb DH. A steroid modulatory domain on NR2B controls N-methyl-D-aspartate receptor proton sensitivity. Proc Nat Acad Sci USA. 2004;101(21):8198–203. doi: 10.1073/pnas.0401838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettini E, Sava A, Griffante C, et al. Identification and Characterization of Novel NMDA Receptor Antagonists Selective for NR2A-over NR2B-Containing Receptors. J Pharmacol Exp Ther. 2010;335(3):636–44. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- 28.Hansen KB, Ogden KK, Traynelis SF. Subunit-Selective Allosteric Inhibition of Glycine Binding to NMDA Receptors. J Neurosci. 2012;32(18):6197–208. doi: 10.1523/JNEUROSCI.5757-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edman S, McKay S, MacDonald LJ, et al. TCN 201 selectively blocks GluN2A-containing NMDARs in a GluN1 co-agonist dependent but non-competitive manner. Neuropharmacology. 2012;63(3):441–49. doi: 10.1016/j.neuropharm.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462(7274):745–56. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talukder I, Borker P, Wollmuth LP. Specific Sites within the Ligand-Binding Domain and Ion Channel Linkers Modulate NMDA Receptor Gating. J Neurosci. 2010;30(35):11792–804. doi: 10.1523/JNEUROSCI.5382-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gielen M, Le Goff A, Stroebel D, et al. Structural Rearrangements of NR1/NR2A NMDA Receptors during Allosteric Inhibition. Neuron. 2008;57(1):80–93. doi: 10.1016/j.neuron.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Low C-M, Lyuboslavsky P, French A, et al. Molecular Determinants of Proton-Sensitive N-Methyl-D-aspartate Receptor Gating. Mol Pharm. 2003;63(6) doi: 10.1124/mol.63.6.1212. [DOI] [PubMed] [Google Scholar]
- 34.Lodge D, Johnson KM. Noncompetitive excitatory amino acid receptor antagonists. Trends Pharmacol Sci. 1990;11(2):81–86. doi: 10.1016/0165-6147(90)90323-z. [DOI] [PubMed] [Google Scholar]
- 35.Yamakura T, Mori H, Masaki H, et al. Different sensitivities of NMDA receptor channel subtypes to non-competitive antagonists. Neuroreport. 1993;4(6):687–90. doi: 10.1097/00001756-199306000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Kashiwagi K, Masuko T, Nguyen CD, et al. Channel Blockers Acting at N-Methyl-D-aspartate Receptors: Differential Effects of Mutations in the Vestibule and Ion Channel Pore. Mol Pharm. 2002;61(3):533–45. doi: 10.1124/mol.61.3.533. [DOI] [PubMed] [Google Scholar]
- 37.LePage KT, Ishmael JE, Low CM, et al. Differential binding properties of [3H]dextrorphan and [3H]MK-801 in heterologously expressed NMDA receptors. Neuropharmacology. 2005;49(1):1–16. doi: 10.1016/j.neuropharm.2005.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin L, Sugiyama H, Takigawa M, et al. Comparative Studies of Anthraquinone- and Anthracene-Tetraamines as Blockers of N-Methyl-D-aspartate Receptors. J Pharmacol Exp Ther. 2007;320(1):47–55. doi: 10.1124/jpet.106.110528. [DOI] [PubMed] [Google Scholar]
- 39.Burnashev N, Schoepfer R, Monyer H, et al. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257(5075):1415–9. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- 40.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuorRx. 2004;1(1):101–10. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen HS, Lipton SA. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol. 1997;499:27–46. doi: 10.1113/jphysiol.1997.sp021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Chen H-SV, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97(6):1611–26. doi: 10.1111/j.1471-4159.2006.03991.x. Excellent overview of therapeutically relevant NMDA receptor antagonists.
- 43.Sobolevsky AI, Koshelev SG, Khodorov BI. Interaction of memantine and amantadine with agonist-unbound NMDA-receptor channels in acutely isolated rat hippocampal neurons. J Physiol. 1998;512(1):47–60. doi: 10.1111/j.1469-7793.1998.047bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolshakov KV, Gmiro VE, Tikhonov DB, Magazanik LG. Determinants of trapping block of N-methyl-D-aspartate receptor channels. J Neurochem. 2003;87(1):56–65. doi: 10.1046/j.1471-4159.2003.01956.x. [DOI] [PubMed] [Google Scholar]
- 45.Mullasseril P, Hansen K, Vance K, et al. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nature Commun. 2010;1:90. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traynelis SF, Liotta DC, Santangelo RM, Garnier EC. Subunit Selective NMDA Receptor Potentiators For The Treatment Of Neurological Conditions. 2012. US 2012/0028977 A1. [Google Scholar]
- 47.Monaghan DT, Jane DE, Costa BM, et al. Positive and negative modulators of NMDA receptors. 2012. WO 2012/019106 A2. [Google Scholar]
- 48.Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain Interaction between NMDA Receptor Subunits and the Postsynaptic Density Protein PSD-95. Science. 1995;269(5231):1737–40. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 49.Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16(7):2157–63. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483(7388):213–17. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- 51.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7(8):742–55. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchan A. Do NMDA antagonists protect against cerebral ischemia: are clinical trials warranted? Cerebrovasc Brain Metab Rev. 1990;2(1):1–26. [PubMed] [Google Scholar]
- 53.Choi DW. Excitotoxic cell death. J Neurobio. 1992;23(9):1261–76. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 54.Hartley Z, Dubinsky JM. Changes in intracellular pH associated with glutamate excitotoxicity. J Neurosci. 1993;13(11):4690–9. doi: 10.1523/JNEUROSCI.13-11-04690.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitt F, Ryan M, Cooper G. A brief review of the pharmacologic and therapeutic aspects of memantine in Alzheimer’s disease. Exp Opin Drug Metab Toxicol. 2007;3(1):135–41. doi: 10.1517/17425255.3.1.135. [DOI] [PubMed] [Google Scholar]
- 56.Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2004;102(2):155–74. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1(6):383–6. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 58**.Hoyte L, Barber PA, Buchan AM, Hill MD. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004;4(2):131–36. doi: 10.2174/1566524043479248. A detailed discussion of the failures of NMDA receptor antagonist in stroke.
- 59.Dawson DA, Wadsworth G, Palmer AM. A comparative assessment of the efficacy and side-effect liability of neuroprotective compounds in experimental stroke. Brain Res. 2001;892(2):344–50. doi: 10.1016/s0006-8993(00)03269-8. [DOI] [PubMed] [Google Scholar]
- 60.Davis SM, Lees KR, Albers GW, et al. Selfotel in Acute Ischemic Stroke. Stroke. 2000;31(2):347. doi: 10.1161/01.str.31.2.347. [DOI] [PubMed] [Google Scholar]
- 61.Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6(1):53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Muir KW, Lees KR, Ford I, Davis S. IMEiSS, Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. Lancet. 2004;363(9407):439–45. doi: 10.1016/S0140-6736(04)15490-1. [DOI] [PubMed] [Google Scholar]
- 63.Danysz W, Parsons C. Neuroprotective potential of ionotropic glutamate receptor antagonists. Neurotox Res. 2002;4(2):119–26. doi: 10.1080/10298420290015872. [DOI] [PubMed] [Google Scholar]
- 64.Doppenberg EM, Choi SC, Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J Neurosurg Anesthesiol. 2004;16(1):87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 65.Preskorn SH, Baker B, Kolluri S, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631–37. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 66.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Bio Psychiatry. 2000;47(4):351–54. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 67.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38(6):735–67. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 68.Schwab RS, England AC, Jr., Poskanzer DC, Young RR. Amantadine in the treatment of Parkinson’s disease. JAMA. 1969;208(7):1168–70. [PubMed] [Google Scholar]
- 69.Luby E, Cohen BD, Rosenbaum G, et al. Study of a new schizophrenomimetic drug—Sernyl. Arch Neurol Psychiatry. 1959;81(3):363–69. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 70.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatric Res. 1999;33(6):523–33. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 71.Krystal JH, Anand A, Moghaddam B. Effects of NMDA receptor antagonists: Implications for the pathophysiology of schizophrenia. Arch Gen Psychiatry. 2002;59(7):663–64. doi: 10.1001/archpsyc.59.7.663. [DOI] [PubMed] [Google Scholar]
- 72.Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Ann Rev Pharmacol Toxicol. 2002;42:165–79. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 73.Depoortere R, Dargazanli G, Estenne-Bouhtou G, et al. Neurochemical, Electrophysiological and Pharmacological Profiles of the Selective Inhibitor of the Glycine Transporter-1 SSR504734, a Potential New Type of Antipsychotic. Neuropsychopharmacology. 2005;30(11):1963–85. doi: 10.1038/sj.npp.1300772. [DOI] [PubMed] [Google Scholar]
- 74.Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology. 2004;174(1):32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- 75.Shim S, Hammonds M, Kee B. Potentiation of the NMDA receptor in the treatment of schizophrenia: focused on the glycine site. Eur Arch Psychiatry Clin Neurosci. 2007;258(1):16–27. doi: 10.1007/s00406-007-0757-8. [DOI] [PubMed] [Google Scholar]
- 76.Tuominen HJ, Tiihonen J, Wahlbeck K. Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophrenia Res. 2005;72(2-3):225–34. doi: 10.1016/j.schres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 77*.Collingridge GL, Volianskis A, Bannister N, et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.06.051. ASAP, http://dx.doi.org/10.1016/j.neuropharm.2012.06.051. An overview of recent advances in the field of positive modulators of NMDA receptor function
- 78.Tang Y-P, Shimizu E, Dube GR, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401(6748):63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 79.Tang YP, Wang H, Feng R, et al. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41(6):779–90. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 80.Von Engelhardt J, Doganci B, Jensen V, et al. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron. 2008;60(5):846–60. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 81.Kutsuwada T, Sakimura K, Manabe T, et al. Impairment of Suckling Response, Trigeminal Neuronal Pattern Formation, and Hippocampal LTD in NMDA Receptor ε2 Subunit Mutant Mice. Neuron. 1996;16(2):333–44. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 82.Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78(4):535–38. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- 83.Morris R. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9(9):3040–57. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sison M, Gerlai R. Associative learning performance is impaired in zebrafish (Danio rerio) by the NMDA-R antagonist MK-801. Neurobiol Learn Mem. 2011;96(2):230–7. doi: 10.1016/j.nlm.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van der Staay FJ, Rutten K, Erb C, Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav Brain Res. 2011;220(1):215–29. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 86.Willner J, Gallagher M, Graham PW, Crooks GB., Jr. N-methyl-D-aspartate antagonist D-APV selectively disrupts taste-potentiated odor aversion learning. Behav Neurosci. 1992;106(2):315–23. doi: 10.1037//0735-7044.106.2.315. [DOI] [PubMed] [Google Scholar]
- 87.Davis S, Butcher SP, Morris RG. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci. 1992;12(1):21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rockstroh S, Emre M, Tarral A, Pokorny R. Effects of the novel NMDA-receptor antagonist SDZ EAA 494 on memory and attention in humans. Psychopharmacology. 1996;124(3):261–6. doi: 10.1007/BF02246666. [DOI] [PubMed] [Google Scholar]
- 89.Morgan CJA, Riccelli M, Maitland CH, Curran HV. Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug Alcohol Dependence. 2004;75(3):301–08. doi: 10.1016/j.drugalcdep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 90.Rowland LM, Astur RS, Jung RE, et al. Selective Cognitive Impairments Associated with NMDA Receptor Blockade in Humans. Neuropsychopharmacology. 2005;30(3):633–39. doi: 10.1038/sj.npp.1300642. [DOI] [PubMed] [Google Scholar]
- 91.Dravid SM, Burger PB, Prakash A, et al. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 2010;30(7):2741–2754. doi: 10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001;41(2):151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 93.Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive Enhancers as Adjuncts to PsychotherapyUse of D-Cycloserine in Phobic Individuals to Facilitate Extinctionof Fear. Arch Gen Psychiatry. 2004;61(11):1136–44. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 94.Richardson R, Ledgerwood L, Cranney J. Facilitation of Fear Extinction by D-Cycloserine: Theoretical and Clinical Implications. Learning & Memory. 2004;11(5):510–16. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- 95.Vervliet B. Learning and memory in conditioned fear extinction: Effects of D-cycloserine. Acta Psychologica. 2008;127(3):601–13. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 96.Hofmann SG, Pollack MH, Otto MW. Augmentation Treatment of Psychotherapy for Anxiety Disorders with D-Cycloserine. CNS Drug Rev. 2006;12(3-4):208–17. doi: 10.1111/j.1527-3458.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koller M, Urwyler S. Novel N-methyl-D-aspartate receptor antagonists: a review of compounds patented since 2006. Expert Opin Ther Pat. 2010;20(12):1683–702. doi: 10.1517/13543776.2010.533656. [DOI] [PubMed] [Google Scholar]
- 98.Adejare AMNJ. Non-competitive NMDA receptor antagonists. 2012. US 8129414. [Google Scholar]
- 99.Ogunbadeniyi AM, Adejare A. Syntheses of fluorinated phencyclidine analogs. J Fluor Chem. 2002;114(1):39–42. [Google Scholar]
- 100.Adejare A, Nie JY, Hebel D, et al. Effect of fluorine substitution on the adrenergic properties of 3-(tert-butylamino)-1-(3,4-dihydroxyphenoxy)-2-propanol. J Med Chem. 1991;34(3):1063–68. doi: 10.1021/jm00107a027. [DOI] [PubMed] [Google Scholar]
- 101.Adejare A, Sciberras SS. Synthesis and β-Adrenergic Activities of R-Fluoronaphthyloxypropanolamine. Pharm Res. 1997;14(4):533–36. doi: 10.1023/a:1012172121453. [DOI] [PubMed] [Google Scholar]
- 102.Igarashi K, Takayama H. Compounds blocking the NMDA receptor channel and pharmaceutical agent using the same. 2011 EP 2039354. [Google Scholar]
- 103.Igarashi K, Shirahata A, Pahk AJ, et al. Benzyl-polyamines: Novel, Potent N-Methyl-D-Aspartate Receptor Antagonists. J Pharmacol Exp Ther. 1997;283(2):533–40. [PubMed] [Google Scholar]
- 104.Chen J, Graham S, Moroni F, Simon R. A study of the dose dependency of a glycine receptor antagonist in focal ischemia. J Pharmacol Exp Ther. 1993;267(2):937–41. [PubMed] [Google Scholar]
- 105.Koek W, Colpaert FC. Selective blockade of N-methyl-D-aspartate (NMDA)-induced convulsions by NMDA antagonists and putative glycine antagonists: relationship with phencyclidine-like behavioral effects. J Pharmacol Exp Ther. 1990;252(1):349–57. [PubMed] [Google Scholar]
- 106*.Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50(4):597–664. Overview of therapeutic potential of targeting the glycine site
- 107.Bristow LJ, Flatman KL, Hutson PH, et al. The atypical neuroleptic profile of the glycine/N-methyl-D-aspartate receptor antagonist, L-701,324, in rodents. J Pharmacol Exp Ther. 1996;277(2):578–85. [PubMed] [Google Scholar]
- 108.Berger P, Farrel K, Sharp F, Skolnick P. Drugs acting at the strychnine-insensitive glycine receptor do not induce HSP-70 protein in the cingulate cortex. Neurosci Lett. 1994;168(1-2):147–50. doi: 10.1016/0304-3940(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 109.Chiamulera C, Costa S, Reggiani A. Effect of NMDA- and strychnine-insensitive glycine site antagonists on NMDA-mediated convulsions and learning. Psychopharmacology. 1990;102(4):551–52. doi: 10.1007/BF02247140. [DOI] [PubMed] [Google Scholar]
- 110.Murata S, Kawasaki K. Common and uncommon behavioural effects of antagonists for different modulatory sites in the NMDA receptor/channel complex. Eur J Pharmacol. 1993;239(1-3):9–15. doi: 10.1016/0014-2999(93)90969-o. [DOI] [PubMed] [Google Scholar]
- 111.Nicholson K, Balster R. The discriminative stimulus effects of N-methyl-D-aspartate glycine-site ligands in NMDA antagonist-trained rats. Psychopharmacology. 2009;203(2):441–51. doi: 10.1007/s00213-009-1469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Henrich M, Bauer A, Nagel J, et al. Glycine B Antagonists. 2010. WO 2010/139483 A1. [Google Scholar]
- 113.Henrich M, Bauer A, Nagel J, et al. Glycine B Antagonists. 2010. WO 2010/139481 A1. [Google Scholar]
- 114.Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157(8):1301–17. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hansen KB, Furukawa H, Traynelis SF. Control of Assembly and Function of Glutamate Receptors by the Amino-Terminal Domain. Mol Pharmacol. 2010;78(4):535–49. doi: 10.1124/mol.110.067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buettelmann B, Neidhart M-P, Jaeschke G, Pinard E. Substituted imidazol-pyridazine derivatives. 2003. US 2003/0229096 A1. [Google Scholar]
- 117.Kemp JA, Tasker T. Methods for treating disorders using NMDA NR2B-subtype selective antagonist. 2011. US 2011/0053951 A1. [Google Scholar]
- 118.Wünsch B, Tewes B, Schepmann D. NR2B-selective NMDA-receptor antagonists. 2010. WO 2010/122134 A1. [DOI] [PubMed] [Google Scholar]
- 119.Yano T, Kanemasa T, Yamamoto S. Piperidine derivative having NMDA receptor antagonistic activity. 2010. US 7786140. [Google Scholar]
- 120.Albers DS, Weiss SW, Iadarola MJ, Standaert DG. Immunohistochemical localization of N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor subunits in the substantia nigra pars compacta of the rat. Neurosci. 1999;89(1):209–20. doi: 10.1016/s0306-4522(98)00328-5. [DOI] [PubMed] [Google Scholar]
- 121.Standaert DG, Bernhard Landwehrmeyer G, Kerner JA, et al. Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Mol Brain Res. 1996;42(1):89–102. doi: 10.1016/s0169-328x(96)00117-9. [DOI] [PubMed] [Google Scholar]
- 122.Traynelis SF, Liotta DC, Mosley C, et al. Subunit Selective NMDA Receptor Antagonists For The Treatment Of Neurological Conditions. 2011. US 2011/0319416 A1. [Google Scholar]
- 123.Monaghan DT, Jane DE, Tse HW. Phenanthryl piperazinyl dicarboxylic acids as selective NMDA receptor modulating agents. 2005. US 6916816. [Google Scholar]
- 124.Monaghan DT, Jane DE, Tse HW. Phenanthryl piperazinyl dicarboxylic acids as selective NMDA receptor modulating agents. 2001. AU 783141 B2. [Google Scholar]
- 125.Monaghan DT, Irvine MW, Costa BM, et al. Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators. Neurochem Int. 2012 doi: 10.1016/j.neuint.2012.01.004. doi:10.1016/j.neuint.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Costa BM, Irvine MW, Fang G, et al. Structure-activity relationships for allosteric NMDA receptor inhibitors based on 2-naphthoic acid. Neuropharmacology. 2012;62(4):1730–36. doi: 10.1016/j.neuropharm.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li J, Zhao L, Cui B, et al. Multiple signaling pathways involved in stimulation of osteoblast differentiation by N-methyl-D-Aspartate receptors activation in vitro. Acta Pharmacol Sinica. 2011;32:895–903. doi: 10.1038/aps.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu F, Gibbs TT, Farb DH. Pregnenolone Sulfate: A positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40(3):333–36. [PubMed] [Google Scholar]
- 129.Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. Sensitivity of the N-Methyl-D-Aspartate Receptor to Polyamines is Controlled by the NR2 Subunits. Mol Pharmacol. 1994;45:803–09. [PubMed] [Google Scholar]
- 130.Zhang L, Zheng X, Paupard MC, et al. Spermine potentiation of recombinant N-methyl-D-aspartate receptors is affected by subunit composition. Proc Nat Acad Sci. 1994;91(23):10883–87. doi: 10.1073/pnas.91.23.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Traynelis SF, Hartley M, Heinemann SF. Control of Proton Sensitivity of the NMDA Receptor by RNA Splicing and Polyamines. Science. 1995;268(5212):873–76. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- 132.Hollmann M, Boulter J, Maron C, et al. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993 May;10(5):943–54. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- 133.Gavazzo P, Mazzolini M, Tedesco M, Marchetti C. Nickel differentially affects NMDA receptor channels in developing cultured rat neurons. Brain Res. 2006 Mar 17;1078(1):71–9. doi: 10.1016/j.brainres.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 134.Segal JA, Harris BD, Kustova Y, et al. Aminoglycoside neurotoxicity involves NMDA receptor activation. Brain Res. 1999;815(2):270–7. doi: 10.1016/s0006-8993(98)01123-8. [DOI] [PubMed] [Google Scholar]
- 135.Irvine MW, Costa BM, Volianskis A, et al. Coumarin-3-carboxylic acid derivatives as potentiators and inhibitors of recombinant and native N-methyl-D-aspartate receptors. Neurochem International. 2012 doi: 10.1016/j.neuint.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu F. Compositions and methods for modulating nicotinic/NMDA receptor function. 2011. US 2011/0097324 A1. [Google Scholar]
- 137.Tymianski M. Method of reducing injury to mammalian cells. 2011. US 8071548. [Google Scholar]
- 138.Tasker A, Doucette T, Tymianski M, et al. Treatment for anxiety. 2011. US 8008253. [Google Scholar]
- 139.Tymianski M. Agents for reducing injury to mammalian cells. 2010. US 7846897. [Google Scholar]
- 140.Tasker A, Doucette T, Tymianski M, et al. Treatment for anxiety. 2012. US 2012/0083449 A1. [Google Scholar]
- 141.Gurd J, Dykstra C, Tymianski M. Treatment for epilepsy. 2010. US 2010/0160240 A1. [Google Scholar]
- 142.Tymianski M. Method of reducing injury to mammalian cells. 2010. US 2010/0137224 A1. [Google Scholar]
- 143.Nakanishi N, Tong G, Tu S. p16 mediated regulation of NMDA receptors. 2012. US 2012/0046446 A1. [Google Scholar]
- 144.Tymianski M. Method of screening peptides useful in treating traumatic injury to the brain or spinal cord. 2009. US 7510824. [Google Scholar]
- 145.Kosley RW, Macdonald D, Sher R. Dipyrazole compounds and their use as central nervous system agents. 2011 US 8053458. [Google Scholar]
- 146.Snyder GL, Fienberg AA, Huganir RL, Greengard P. A Dopamine/D1 Receptor/Protein Kinase A/Dopamine- and cAMP-Regulated Phosphoprotein (Mr 32 kDa)/Protein Phosphatase-1 Pathway Regulates Dephosphorylation of the NMDA Receptor. J Neurosci. 1998;18(24):10297–303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Feng J, Yan Z, Ferreira A, et al. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci USA. 2000;97(16):9287–92. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148**.Field JR, Walker AG, Conn PJ. Targeting glutamate synapses in schizophrenia. Trends Mol Med. 2011;17(12):689–98. doi: 10.1016/j.molmed.2011.08.004. An excellent review covering strategies targeting the glutamatergic pathways.
- 149.Cordi A, Desos P, Lestage P, Danober L. Benzothiadiazepine compounds, a process for their preparation and pharmaceutical compositions containing them. 2010. US 2010/0240635 A1. [Google Scholar]
- 150.Ip NY-Y, Ip FC-F, Hu Y, Ye WC. Receptor modulators exhibiting neuroprotective and memory enhancing activities. 2010. US 2010/0222286 A1. [Google Scholar]
- 151.Van Langenhove A. Isotope effects: definitions and consequences for pharmacologic studies. J Clin Pharmacol. 1986;26(6):383–89. doi: 10.1002/j.1552-4604.1986.tb03545.x. [DOI] [PubMed] [Google Scholar]
- 152.Foster AB. Deuterium isotope effects in studies of drug metabolism. Trends Pharmacol Sci. 1984;5:524–27. [Google Scholar]
- 153.Gant TG, Sarshar S. Ethanolamine modulators of NMDA receptor and muscarinic acetylcholine receptor. 2010. US 2010/0130617 A1. [Google Scholar]
- 154*.Murrough JW. Ketamine as a novel antidepressant: from synapse to behavior. Clin Pharmacol Ther. 2012;91(2):303–9. doi: 10.1038/clpt.2011.244. Although beyond the scope of this review, an in depth discussion of the use of ketamine in depression.
- 155*.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30(11):563–9. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 156.Ellers-Lenz B, Rosenberg T, Kruger H, Al Thaus M. 1-Aminocyclohexane derivatives for the treatment of cochlear tinnitus. 2011. US 2011/0077304 A1. [Google Scholar]
- 157.Yakatan G, Berg J, Pope LE, Smith RA. Pharmaceutical compositions comprising dextromethorphan and quinidine for the treatment of neurological disorders. 2010. US 7659282. [Google Scholar]
- 158.Makovec F, Caselli G, Rovati LC, Giordani A. Use of neboglamine in the treatment of toxicodependency. 2011. US 2011/0288173 A1. [Google Scholar]
- 159.Makovec F, Rovati LC. Use of neboglamine (CR 2249) as an antipsychotic and neuroprotective. 2010. US 7737180. [Google Scholar]
- 160.Jastreboff PJ, Jastreboff MM. Tinnitus Retraining Therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J Am Acad Audiol. 2000;11(3):162–77. [PubMed] [Google Scholar]
- 161.Guitton MJ, Caston J, Ruel J, et al. Salicylate Induces Tinnitus through Activation of Cochlear NMDA Receptors. J Neurosci. 2003;23(9):3944–52. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. [Accessed 2 August 2012]; Available from: http://clinicaltrials.gov/ct2/results?term=Neramexane.
- 163.FDA Approved Drugs. FDA; Washington, DC: [accessed 29 July 2012]. 2012. Drugs @ FDA. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. [Google Scholar]
- 164.NUEDEXTA® [Accessed 29 July 2012];(dextromethorphan hydrobromide and quinidine sulfate) capsules. Available at: https://www.nuedexta.com/
- 165.Pioro EP, Brooks BR, Cummings J, et al. Dextromethorphan Plus Ultra Low-Dose Quinidine Reduces Pseudobulbar Affect. Annals of Neurology. 2010;68(5):693–702. doi: 10.1002/ana.22093. [DOI] [PubMed] [Google Scholar]
- 166. [Accessed 2 August 2012]; Available from: http://clinicaltrials.gov/ct2/results?term=AVP-923.
- 167.Lanza M, Bonnafous C, Colombo S, et al. Characterization of a novel putative cognition enhancer mediating facilitation of glycine effect on strychnine-resistant sites coupled to NMDA receptor complex. Neuropharmacology. 1997;36(8):1057–64. doi: 10.1016/s0028-3908(97)00092-0. [DOI] [PubMed] [Google Scholar]
- 168.Bowery NG, Bettler B, Froestl W, et al. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. 2002;54(2):247–64. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- 169.Langmead CJ, Christopoulos A. Allosteric agonists of 7TM receptors: expanding the pharmacological toolbox. Trends in Pharmacol Sci. 2006;27(9):475–81. doi: 10.1016/j.tips.2006.07.009. [DOI] [PubMed] [Google Scholar]