Abstract
Background
Massive perioperative blood product transfusion may be required with thoracic aortic operations and is associated with poor outcomes. Our objective was to determine the independent predictors of massive transfusion in thoracic aortic surgery patients undergoing deep hypothermic circulatory arrest (DHCA).
Methods
The study consisted of 168 consecutive patients undergoing open thoracic aortic procedure utilizing DHCA between July 2005 and August 2008. We identified 26 preoperative and procedural variables as being potentially related to blood product usage. We tested the variables for association with total blood products transfused using a multivariate linear regression model and then constructed a logistic regression model for massive transfusion, defined as requiring 5 or more units of transfused packed red blood cells between incision and 48 hours postoperatively.
Results
Multivariate linear regression determined six significant variables as accounting for 42% of the variation in total blood products transfused: age (P=0.008), preoperative hemoglobin (P=0.04), weight (P=0.02), cardiopulmonary bypass time (P<0.0001), emergent status (P<0.0001), and re-do median sternotomy (P<0.0001). A final predictive logistic regression model associated every 1 g/dL increase in preoperative hemoglobin OR=0.54 [0.43, 0.69], P<0.0001; every 10 minute increase in CPB time, OR=1.15 [1.05, 1.26], P=0.0026; and emergent status OR=4.02 [1.53, 10.55], P=0.0047 with massive transfusion.
Conclusions
Our model described CPB time, emergent status, and preoperative hemoglobin as independent predictors of massive transfusion. These variables, along with weight, age, and re-do median sternotomy are associated with total blood product usage in thoracic aortic operations involving DHCA.
INTRODUCTION
Massive perioperative transfusion may occur with thoracic aortic operations. While there is certainly no substitute for meticulous surgical technique, the etiology of unusually high transfusion requirement is often multifactorial. Hemostatic derangements in aortic surgery are caused by a multitude of interrelated factors including interference with the vascular integrity, surgical dissection, deep hypothermia for circulatory arrest, ischemia and reperfusion, dilution of coagulation factors from large-volume fluid resuscitation, transient need for heparinization, and the use of cardiopulmonary bypass (CPB) (1). Patient-level factors include age, sex, diabetes, preoperative hemoglobin, platelets, prothrombin time, and partial thromboplastin time (2–4).
There exists a paucity of clinical data addressing predictors of massive transfusion specific to operations for disease of the thoracic aorta, particularly those involving deep hypothermic circulatory arrest (DHCA) (5, 6). The DHCA employed for neuroprotection in thoracic aortic surgery slows coagulation cascade activity, reduces coagulation factor synthesis, increases fibrinolysis, decreases platelet count, and impairs platelet function (7). The prosthetic grafts used for aortic repair consume platelets and other factors (8). Aortic disease itself may also predispose to coagulopathy via the exposure of tissue factor and other mechanisms (9, 10).
Adverse outcomes attributable to the sequelae of massive perioperative transfusion are well recognized in the cardiac surgical literature, including a strong, independent association with mortality (11, 12). Identification of patients at high risk for requiring massive transfusion will not only allow for improved risk stratification and preoperative counseling but may also create an opportunity for therapeutic modalities targeted to individual patients in the perioperative period. Our objective was to determine the predictors of massive perioperative transfusion with open thoracic aortic procedures requiring DHCA. Therefore, we tested the hypothesis that patient and procedural characteristics could be used to model the need for transfusion with thoracic aortic surgery.
METHODS
Data Source
The Duke Thoracic Aortic Surgery Database is a prospectively maintained, comprehensive clinical registry of all patients who have undergone a thoracic aortic procedure at Duke University Medical Center (Durham, NC) since 2005. The present study included all patients who underwent a thoracic aortic procedure with DHCA between July 2005 and August 2008, excluding only patients undergoing a separate procedure in the perioperative period resulting in massive transfusion. All procedures were performed by a single surgeon (GCH) thus minimizing the potential contribution of variation in surgical technique to the findings. The current study was approved by the Duke University Institutional Review Board (IRB) and the IRB waived the need for individual patient consent. The Duke Thoracic Aortic Surgery Database provided baseline characteristics, clinical variables, and surgical procedure details. Review of individual medical records was undertaken to complete any missing clinical data points and blood bank records provided precise information regarding number of blood products administered.
Conduct of Procedures and Anesthesia
Non-pulsatile CPB was conducted for each case using a membrane oxygenator following a crystalloid and mannitol prime and using an arterial line filter. Porcine heparin was administered as a bolus of 300 U/kg and supplemented to maintain an activated clotting time of greater than 480 seconds. A 5000 unit bolus of heparin was given prior to circulatory arrest. During CPB, temperature adjusted flow rates of 2.5 L/min/m2 were used and mean arterial pressure was generally maintained between 50–70 mmHg. Anesthesia was maintained using isoflurane (0.5–1.0%) via the oxygenator. Alpha stat management for maintenance of normal pH, pO2 and pCO2 values was used. Antegrade and retrograde cold blood cardioplegia solution were used for myocardial protection. Our institutional preference is to perform open thoracoabdominal (Extent I–III) and descending aortic aneurysm repair using DHCA.
Prior to the portion of the aortic reconstruction requiring DHCA, the patient was cooled on CPB until electrocerebral inactivity (ECI) was detected on electroencephalogram (EEG) using previously described techniques (13); ECI was usually reached at a nasopharyngeal temperature between 14 and 18°C. Once ECI was achieved via hypothermia, the circulation was stopped. Antegrade cerebral perfusion (5–15 ml/kg/min) was typically used for adjunctive cerebral perfusion during the period of systemic DHCA. Following aortic reconstruction, CPB was reinstituted, and the patient gradually re-warmed to a normal temperature following a 5-minute period of cold reperfusion for free radical washout. Four units of fresh frozen plasma (FFP) were typically added to the circuit to ensure adequate anticoagulant factors during DHCA. A hematocrit of 0.18 to 0.20 was generally acceptable during CPB, although this was increased to greater than 0.20 for separation from CPB. Protamine sulfate was administered after separation from CPB to reverse heparin anticoagulation until the activated clotting time returned to baseline or the ratio of 1 mg to 100 units of heparin was reached. We maintained a general approach to avoid unnecessary use of blood products, which were not administered unless bleeding or anemia was observed. After separation from CPB, red blood cells were transfused depending on the patient’s preoperative condition, volume status and hemoglobin concentration.
Standard practice included intraoperative administration of an antifibrinolytic therapy; prior to withdrawal from the US market, aprotinin (Bayer Corporation, West Haven, CT) was used (2MU bolus and 0.5Mu/hr infusion until bleeding cessation). Subsequently, epsilon aminocaproic acid was administered as a 10g bolus followed by a 1g/hr infusion with an additional 5g bolus prior to separation from CPB to account for that lost during hemofiltration, which was performed on all patients to remove excess crystalloid prior to separation from CPB. The return of washed, shed red blood cells (BRAT II cell saver, Cobe, Arvada, CO) to the patient was routine. Transfusion decisions in the perioperative period were aided by local guidelines and use of chest tube output, activated clotting time, platelet count, fibrinogen level, thromboelastogram, prothrombin, and partial thromboplastin time tests as recommended by the American Society of Anesthesiologists published guidelines (14). Clopidogrel or other P2Y12 inhibitors, regardless of dose, were held 7 days prior to operation. Aspirin 325mg was held 5 days prior to operation. Aspirin 81mg was not held prior to operation. All antiplatelet agents were restarted at preoperative dose on postoperative day 1 unless active bleeding was present.
Outcome Measures
The 26 candidate variables for the analysis included age, sex, race, American Society of Anesthesiologists (ASA) grade, diabetes, preoperative creatinine level, coumadin use, partial thromboplastin time (PTT), platelet count, international normalized ratio (INR), hemoglobin concentration, weight, height, body surface area, blood pressure, aprotinin use, prior cardiac surgical procedure, emergency status, concomitant cardiac procedure, total arch versus hemi-arch repair, thoracoabdominal aortic aneurysm (TAAA) type, aortic cross-clamp time, cerebral circulatory arrest time (period when brain receiving less than 100% of normal cerebral blood flow; includes period of antegrade or retrograde cerebral perfusion), systemic circulatory arrest time (period during which lower body not perfused; longer than cerebral circulatory arrest time for total arch replacement and open descending/thoracoabdominal aortic repairs; equal to cerebral circulatory arrest time for hemi-arch repairs), and cardiopulmonary bypass time. Allogeneic blood product usage included all products given from the time of incision through postoperative days 0, 1, and 2, including any at the time of take-back for bleeding. Volume of intraoperative cell-saver transfusion was not included in this calculation as it is not allogeneic. Considering need for massive transfusion as a dichotomous outcome, we used a previously described definition of massive transfusion (11). Patients who received >=5 units of PRBCs were defined as having had massive transfusion (referred to as “MT”).
Statistical Analysis
Patient and operative characteristics are summarized based on presence or absence of massive transfusion. Table 1 presents categorical variables as percentages; continuous variables are presented as means and standard deviations unless otherwise stated. For comparisons, the Wilcoxon Rank Sum test was used for continuous variables, and the Chi-squared test for categorical variables, with an alternative hypothesis that the rates across columns were not equal.
Table 1.
Patient/Operative Variables
| Characteristic | Overall (n=168) |
MT (n=49) |
Non-MT (n=119) |
P-value |
|---|---|---|---|---|
| Age | 57.4 +/− 14.2 | 60.6 +/− 15.5 | 56.1 +/− 13.4 | 0.06 |
| Female | 29 (49) | 38 (18) | 25 (30) | 0.09 |
| Non-white | 6 (10) | 9 (4) | 5 (6) | 0.27 |
| ASA grade | 3.5 +/− 0.5 | 3.8 +/− 0.4 | 3.4 +/− 0.5 | <0.0001 |
| Diabetes | 3 (5) | 4 (2) | 2 (3) | 0.66 |
| Preoperative creatinine | 1.3 +/− 1.3 | 1.6 +/− 1.8 | 1.2 +/− 0.9 | 0.11 |
| Preoperative Coumadin | 16 (26) | 20 (10) | 14 (16) | 0.31 |
| Preoperative PTT | 32.6 +/− 9.6 | 31.5 +/− 9.6 | 33.1 +/− 9.7 | 0.32 |
| Preoperative platelets | 214.6 +/− 58.1 | 216.1 +/− 62.2 | 213.9 +/− 56.5 | 0.82 |
| Preoperative INR | 1.05 +/− 0.16 | 1.12 +/− 0.26 | 1.03 +/− 0.09 | 0.001 |
| Preoperative hemoglobin | 13.5 +/− 2.0 | 12.0 +/− 2.1 | 14.1 +/− 1.7 | <0.0001 |
| Weight | 83.3 +/− 18.9 | 78.6 +/− 16.4 | 85.3 +/− 19.6 | 0.03 |
| Height | 174.7 +/− 11.2 | 172.0 +/− 12.7 | 175.8 +/− 10.4 | 0.07 |
| Body Surface Area | 1.98 +/− 0.24 | 1.91 +/− 0.23 | 2.01 +/− 0.25 | 0.01 |
| Total Blood Products [units]: | ||||
| Median (25th–75th percentile) | 13 (8–20) | 22 (18–31) | 9 (6–13) | <0.0001 |
| Recombinant Factor VIIa | 3 (5) | 10 (5) | 0.00 (0) | <0.0001 |
| Aprotinin | 48 (81) | 48 (24) | 48 (57) | 0.97 |
| Maximum aortic diameter | 5.85 +/− 1.01 | 6.04 +/− 1.19 | 5.77 +/− 0.92 | 0.14 |
| Reoperation (re-do median sternotomy) | 27 (45) | 39 (21) | 21 (24) | 0.01 |
| Emergent status | 24 (40) | 39 (20) | 17 (20) | 0.001 |
| Cross-clamp time | 136.9 +/− 51.1 | 142.2 +/− 57.3 | 136.6 +/− 48.2 | 0.38 |
| Cerebral circulatory arrest time | 22.2 +/− 7.4 | 24.6 +/− 7.4 | 21.1 +/− 7.1 | 0.004 |
| Systemic circulatory arrest time | 28.0 +/− 19.6 | 34.8 +/− 19.6 | 25.1 +/− 17.4 | 0.001 |
| Cardiopulmonary bypass time | 225.8 +/− 60.5 | 248.7 +/− 85.8 | 216.1 +/− 42.3 | 0.01 |
| Concomitant procedure | 22 (36) | 34 (17) | 16 (19) | 0.007 |
| Location of Aortic Repair: | 0.004 | |||
| Root/Ascending only | 1.2 (2) | 2 (1) | 0.8 (1) | |
| Arch involvement | 89 (150) | 82 (40) | 92 (110) | |
| Total arch | 9.5 (16) | 16 (8) | 6.7 (8) | |
| Descending/Thoracoabdominal | 10 (16) | 20 (10) | 5 (6) | |
| Cell saver volume | 537 +/− 475 | 715 +/− 663 | 464 +/− 351 | 0.01 |
| 12 Hour Chest Tube Drainage | 664 +/− 568 | 1050 +/− 864 | 517 +/− 341 | 0.0003 |
| Take-back for bleeding | 6 (10) | 18.4 (9) | 0.84 (1) | <0.0001 |
| 30-day Deaths | 3.0 (5) | 4.1 (2) | 2.5 (3) | 0.09 |
NOTE. Categorical data are given as percentages with the number of patients in parentheses. Continuous data are given as mean +/− standard deviation.
Abbreviations: MT=patients receiving massive transfusion, Non-MT=patients not receiving massive transfusion, PTT=partial thromboplastin time, INR=international normalized ratio.
We evaluated the association of patient and procedural characteristics with the need for transfusion in two ways. Primarily, a linear regression model was developed with total blood products transfused as the outcome variable. Total blood products included all allogeneic products from the case start time through 48 hours postoperatively. Because blood product usage is not normally distributed, a log transform was performed prior to analysis. Secondarily, we developed a logistic regression model for massive transfusion (greater than or equal to five units of PRBCs) as a dichotomous outcome.
We identified 26 preoperative and procedural variables as being potentially related to blood product use. Univariate associations were assessed with one-way ANOVA for categorical variables and correlation tests for continuous variables. Race was defined as white versus non-white. Of the 26 variables, 16 had p values < 0.05 when testing for a difference between the groups. These 16 variables were included in a backward stepwise procedure that eliminated non-significant variables individually until only significant variables remained. In this manner, a final predictive model was developed (Table 3). To create the linear regression models for total blood products and CPB time (Tables 2 and 4), linear univariate association tests were used, with non-significant variables dropped from the multivariate model one at a time until only significant variables remained in the model.
Table 3.
Predictors of Massive transfusion (logistic regression model)
| Variable | Odds Ratio |
95% Wald Confidence Interval |
Predictor P value |
|---|---|---|---|
| Preoperative Hemoglobin (per 1g/dl increment) | 0.543 | 0.428, 0.688 | <.0001 |
| CPB Time (per 10 minute increase) | 1.15 | 1.05, 1.26 | 0.0026 |
| Emergent Status | 4.02 | 1.532, 10.553 | 0.0047 |
Abbreviations: CPB=cardiopulmonary bypass
Table 2.
Multivariate Linear Regression Model for log(Total Blood Products)
| Variable | Regression Slope | P value |
|---|---|---|
| Age | 0.009 | 0.008 |
| Preoperative Hemoglobin | −0.05 | 0.04 |
| Weight | −0.006 | 0.02 |
| CPB Time | 0.004 | <0.0001 |
| Emergent Status | 0.52 | <0.0001 |
| Reoperation | 0.47 | <0.0001 |
Abbreviations: CPB=cardiopulmonary bypass
Table 4.
Predictors of CPB Time (linear regression model)
| Variable | Regression Slope |
Standard Error |
t Value | P Value |
|---|---|---|---|---|
| BSA | 49.56 | 16.85 | 2.94 | 0.0037 |
| Concomitant Procedure | 53.70 | 10.24 | 5.24 | <.0001 |
| Reoperation | 35.30 | 9.766 | 3.61 | 0.0004 |
Abbreviations: BSA=body surface area
Lastly, using Pearson correlation coefficients, we conducted a post hoc assessment of the relationship of blood product transfusion with other metrics of perioperative bleeding by examining correlations with volume of processed blood (cell saver) returned to the patient intraoperatively and chest tube drainage from time of ICU arrival to 12 hours postoperatively. We also evaluated the proportion of total blood products given while on CPB in order to distinguish between anemia during CPB and continued bleeding after CPB as reasons for requiring transfusion. All statistical analyses were performed using SAS version 9.1.3.
RESULTS
Patient and Operative Variables
One hundred and seventy thoracic aortic procedures with deep hypothermic circulatory arrest were performed during the period of study. Only two patients were excluded, both of whom experienced major blood loss and massive transfusion from another procedure in the perioperative period. Thus, 168 thoracic aortic procedures were available for data abstraction and fulfilled selection criteria, representing a single-center, single-surgeon experience.
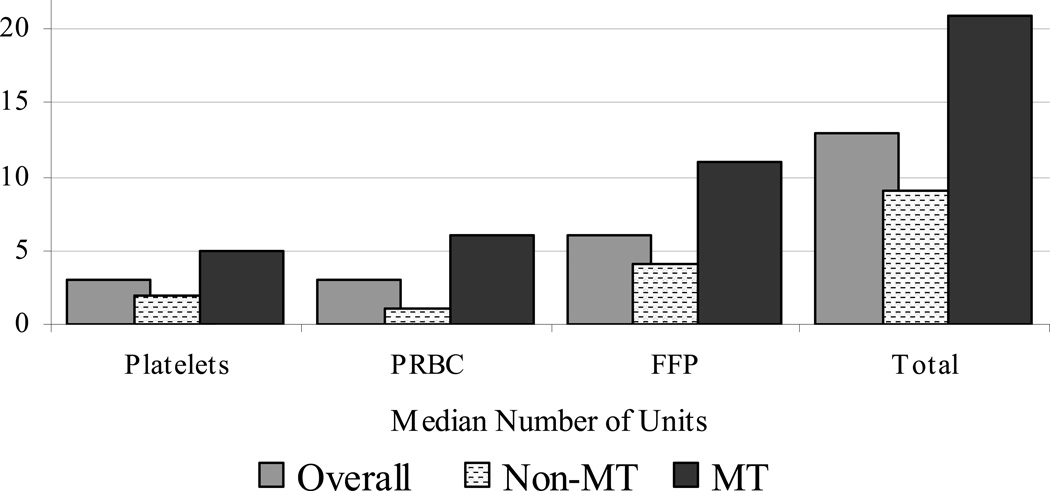
Figure 1 displays the use of the various blood products in the 168 study patients, with medians as well as upper and lower quartiles for PRBC [median=3 units; interquartile range (IQR) 1 to 6], platelets [3; 2 to 4], cryoprecipitate [0; 0 to1], fresh frozen plasma (FFP) [6; 4 to 10], and total units of blood product transfused [13; 8 to 20]. We classified 49 patients (29%) as having massive transfusion (MT) for receiving >= 5 units of transfused PRBCs. The remaining 119 patients were classified as not receiving massive transfusion (non-MT).
Figure 1. Blood Products Transfused.
NOTE. Y axis values represent units of blood product; “Total” represents total units transfused. Cryoprecipitate is not included in the graphical display since the median number of units transfused was zero overall.
Abbreviations: PRBC=packed red blood cells, FFP= fresh frozen plasma, MT=patients receiving massive transfusion, Non-MT=patients not receiving massive transfusion.
Table 1 describes patient and operative variables. Recombinant Activated Factor VII (NovoSeven®, Novo Nordisk Inc., Princeton, New Jersey) was given to only five patients, all of whom had received MT (10% of this cohort). Females comprised 29% of the patients overall (38% MT vs. 25% non-MT), 6% were of races other than white (9% MT vs. 5% non-MT), and only 3% were diabetics (4% MT and 2% non-MT). The mean age of the study group was 57, with a range of 20 to 87 [mean 61 for MT patients and 56 for non-MT (P=0.06)]. Weight and body surface area was lower for those experiencing MT (79 kg versus 85 kg; p = 0.03) and (1.91 m2 versus 2.01 m2, P=0.01).
The MT group had significantly lower preoperative hemoglobin levels (12 g/dL vs. 14 g/dL, P<0.0001) and higher preoperative INR levels (1.12 vs. 1.03, P=0.001). Use of the pharmacologic agents coumadin preoperatively (20% vs. 14%, P=0.31) and aprotinin intraoperatively (48.0% vs. 48.4%, P=0.97) were similar between the groups. Among the 40 emergent cases, none were taking clopidogrel preoperatively and only one was taking aspirin 325mg; aspirin 81mg is not held prior to elective cases.
Overall, the type of aortic repair was root/ascending only in 1.2% (2% MT vs. 0.8% non-MT), hemi- or total arch in 89% (82% MT vs. 92% non-MT) with 9.5% being total arch (16% MT vs. 6.7% non-MT), and descending or thoracoabdominal in 10% (20% MT vs. 5% non-MT). The procedure was classified as re-do median sternotomy (reoperation) for 39% of MT patients compared to 21% for non-MT (P=0.01); there were no redo descending/TAAA procedures. MT patients were also more likely to undergo concomitant procedures (34% vs. 16%, P=0.001) and more likely to have had emergent operations (39% vs. 17%, P=0.001). The thirty six cases with concomitant procedures included CABG (n=24, 67%), MAZE (n=3, 8%), ASD repair (n=3, 8%), and other (n=6, 16%). The indication for emergent operation was acute Type A aortic dissection in all cases. Aortic cross-clamp times (142 vs. 137 minutes, P=0.38) were equivalent between the groups, while systemic circulatory arrest time (35 vs. 25 minutes, P=0.001) and cardiopulmonary bypass time (249 vs. 216 minutes, P=0.007), were significantly longer for the MT group.
Outcome Metrics
Take-back to the operating room for bleeding was required in 6% of cases. The take-back rate for bleeding among the massive transfusion group was 18% versus 0.8% among the non-MT group (P<0.0001). Of note, mean cell saver volume returned to patients intraoperatively was 715ml for MT versus 464ml for non-MT patients (p=0.01) and mean 12 hour chest tube drainage was 1050ml for MT versus 517ml for non-MT patients (p=0.0003). Mortality at 30 days was 3% overall, higher (4%) among MT than among non-MT (2.5%) patients.
The Pearson correlation coefficients between total blood products transfused and volume of cell saver returned intraoperatively was 0.43 (p=0.001) and 0.54 (p<0.0001) between total blood products transfused and 12 hour chest tube drainage, while cell saver volume and chest tube drainage had no association (r=0.049; p=0.55). The mean PRBC units given on-CPB was 1.3 units (SD 2.1, range 0 to 16) compared to 3.9 PRBC units (SD 4.7, range 0 to 26) given from procedure start time to 48 hours postoperatively.
Multivariate Models and Predictors
The correlation coefficient for PRBC and total blood product transfusion was >0.90, with total blood products used as the primary, continuous outcome variable. The linear regression model is described in Table 2 with six significant variables independently associated with total blood product transfusion: age (P=0.008), preoperative hemoglobin (P=0.04), weight (P=0.02), cardiopulmonary bypass time (P<0.0001), emergent status (P<0.0001), and reoperation status (P<0.0001). The adjusted r-square for this model is 0.42, indicating that these variables account for 42% of the variability in blood products transfused.
The logistic regression model for the outcome massive transfusion is indicated by Table 3. The odds ratios for the outcome MT are as follows: for every 1 g/dL increase in preoperative hemoglobin OR=0.54 [95% CI (0.43, 0.69), P<0.0001], for every 10 minute increase in CPB time, OR=1.15 [95% CI (1.05, 1.26), P=0.0026], and for emergent status OR=4.02 [95% CI (1.53, 10.55), P=0.0047]. Many procedural factors correlate with CPB time and therefore are not independent predictors of bleeding. These include cross-clamp time, BSA, concomitant procedure, and reoperation; the univariate associations with massive transfusion may be noted in Table 1. A multivariable regression analysis predicting CPB time (R-squared for the model is 0.26) showed that increased BSA, concomitant procedure, and reoperation are all significant predictors (Table 4).
DISCUSSION
In this observational study of consecutive patients undergoing thoracic aortic procedures utilizing DHCA from 2005 through 2008, we were able to identify the patient and procedural characteristics associated with both overall blood product usage and massive transfusion (MT). First, independent predictors of total blood products transfused include age, preoperative hemoglobin, weight, CPB time, and emergent status. Second, a logistic regression model determined massive perioperative transfusion to be independently predicted by three variables: preoperative hemoglobin level, CPB time, and emergent operation status.
An initial descriptive analysis of patient variables found the MT group tended to be older, smaller (lower preoperative weight and BSA), and sicker (higher ASA grade, lower preoperative hematocrit). Duration of CPB and both systemic and cerebral circulatory arrest were longer for the massive transfusion group. Many of the variables show expected univariate associations with blood product usage, but co-linearity of variables confounds independent association persisting in a multivariate model. For example, while it remained as an independent predictor in the linear regression model, reoperation did not remain in the final logistic regression model as it did not independently contribute more to the model of massive transfusion than CPB time alone.
CPB time and emergent operation are recognized predictors of transfusion (2, 3, 5) and the time required to achieve deep hypothermia further prolonged CPB time likely causing platelet and enzyme dysfunction (1, 3, 7). The value of preoperative anemia as a prognosticator for adverse outcomes has been replicated by several groups (11, 15–17); however, prior studies have not examined anemia in a model for transfusion requirement in thoracic aortic surgery with DHCA. In our patient sample, hemoglobin may be a marker of broader physiologic reserve and consumption of platelets and coagulation factors by various aortic pathologies.
Several limitations should be considered when interpreting the findings of this study. First, this was a retrospective, observational study for which causality cannot be inferred. The effects of unknown or unmeasured confounders on the observed associations cannot be excluded. A major limitation of other studies investigating predictors of perioperative blood loss in cardiac surgery has been that the investigations compared cases across different surgical, anesthesia, and intensive care unit (ICU) teams, resulting in wide, unadjusted variation in surgical practices and perioperative management strategies (2, 3, 18). We reduced but did not eliminate surgical technique and institutional practice as confounders by reporting a large series of patients undergoing aortic surgery with DHCA conducted by a single surgeon and post-surgical care unit with a protocol-driven approach to transfusion.
While the present study represents the largest to date examining factors associated with transfusion in the DHCA population, a relatively large number of variables were determined a priori to be of clinical importance in considering predictors of massive transfusion, which increases the chance of a type II error. We chose not to employ a principal components strategy to reduce the degrees of freedom by combining potential predictors due to the resulting inability to interpret individual coefficients. Also of note, a systematic change in the pharmacologic management of bleeding occurred during the study period. When aprotinin was officially removed from the United States market in late 2007, patients received epsilon aminocaproic acid as an alternative agent, although aprotinin use was similar in those who were and those who were not massively transfused.
The present analysis does not discriminate between transfusion to treat anemia or to treat bleeding which could be regarded as a weakness or strength of the design; we elected to evaluate overall transfusion requirements, because deleterious effects of transfusion are related to the total number of units transfused. For the same reason, we extended our outcome to transfusion in the perioperative period rather than the day of surgery. While there was a highly significant correlation between units of PRBCs transfused and both intraoperative bleeding (cell saver volume transfused) and postoperative bleeding (chest tube drainage), approximately one third of the PRBC units transfused were given prior to separation from CPB to treat preexisting anemia, acute blood loss prior to CPB or hemodilution during CPB. Intraoperative transfusions prior to closure of the chest have been excluded by other investigators based on the premise that excessive bleeding during this part of the operation frequently reflects inadequate surgical hemostasis and may not reflect the hemostatic defect induced by CPB (2, 3). However, while deposition of platelets and circulating blood proteins, as well as non-surgical bleeding related to graft porosity and needle hole injury, occur less with the polyethylene-terephthalate (Dacron) used in our series than with polytetrafluoroethylene and other graft materials, these interactions remain an important consideration (19). The use of large prosthetic vascular conduits and DHCA is unique to aortic surgery and has an immediate impact on the coagulation cascade, which would be lost without inclusion of intraoperative transfusions and the volume of cell saver blood returned intraoperatively correlated closely with total blood product usage.
Our model for predicting massive perioperative transfusion may allow for more focused utilization of both established and novel strategies for reducing perioperative blood loss, including correction of hypothermia and utilization of perioperative pharmacological interventions available for reducing the coagulation dysfunction associated with CPB and aortic surgery (20, 21). Typical pharmacologic means have focused on anti-fibrinolytic agents but could also focus on intraoperative adjuncts for the prevention of platelet dysfunction (7, 22) or the promotion of hemostasis with fibrinogen concentrate (21) or recombinant factor VII (23). The etiology of preoperative anemia in patients undergoing elective cardiac operations has been described and it is suggested that in the majority of patients the anemia is preventable (24). Specifically, diagnosing chronic anemia and correcting it through iron supplementation or erythropoietin (25) for elective cases is worth investigating with further clinical studies.
CONCLUSIONS
Our data support a model of CPB time, emergent status, and preoperative hemoglobin as independent predictors of massive perioperative transfusion in thoracic aortic procedures utilizing DHCA, creating an opportunity for clinical investigations towards targeted therapeutic maneuvers for high risk patients.
REFERENCES
- 1.Shore-Lesserson LRD, Silvay G, Griepp RB. Hemostasis in aortic and cardiothoracic surgery. J Cardiovasc Surg. 1997;12:232–237. [PubMed] [Google Scholar]
- 2.Despotis GJ, Filos KS, Zoys TN, Hogue CW, Jr, Spitznagel E, Lappas DG. Factors associated with excessive postoperative blood loss and hemostatic transfusion requirements: a multivariate analysis in cardiac surgical patients. Anesth Analg. 1996;82:13–21. doi: 10.1097/00000539-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Parr KG, Patel MA, Dekker R, Levin R, Glynn R, Avorn J, et al. Multivariate predictors of blood product use in cardiac surgery. J Cardiothorac Vasc Anesth. 2003;17:176–181. doi: 10.1053/jcan.2003.44. [DOI] [PubMed] [Google Scholar]
- 4.Welsby IJ, Podgoreanu MV, Phillips-Bute B, Mathew JP, Smith PK, Newman MF, et al. Genetic factors contribute to bleeding after cardiac surgery. J Thromb Haemost. 2005;3:1206–1212. doi: 10.1111/j.1538-7836.2005.01337.x. [DOI] [PubMed] [Google Scholar]
- 5.Wahba A, Rothe G, Lodes H, Barlage S, Schmitz G, Birnbaum DE. Predictors of blood loss after coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 1997;11:824–827. doi: 10.1016/s1053-0770(97)90113-0. [DOI] [PubMed] [Google Scholar]
- 6.Harrington DKLJ, Rooney SJ, Bonser RS. Nonneurologic morbidity and profound hypothermia in aortic surgery. Ann Thorac Surg. 2004;78:596–501. doi: 10.1016/j.athoracsur.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Kestin AS, Valeri CR, Khuri SF, Loscalzo J, Ellis PA, MacGregor H, et al. The platelet function defect of cardiopulmonary bypass. Blood. 1993;82:107–117. [PubMed] [Google Scholar]
- 8.Harker LA, Slichter SJ, Sauvage LR. Platelet consumption by arterial prostheses: the effects of endothelialization and pharmacologic inhibition of platelet function. Ann Surg. 1977;186:594–501. doi: 10.1097/00000658-197711000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher D, Yawn D, Crawford E. Preoperative disseminated intravascular coagulation associated with aortic aneurysms. Arch Surg. 1983;118:1252–1255. doi: 10.1001/archsurg.1983.01390110010002. [DOI] [PubMed] [Google Scholar]
- 10.ten Cate JWTH, Becker AE. Coagulopathy in Ruptured or Dissecting Aortic Aneurysms. Am J Med. 1975;59:171–176. doi: 10.1016/0002-9343(75)90351-4. [DOI] [PubMed] [Google Scholar]
- 11.Karkouti K, Wijeysundera DN, Yau TM, Beattie WS, Abdelnaem E, McCluskey SA, et al. The independent association of massive transfusion with mortality in cardiac surgery. Transfusion. 2004;44:1453–1462. doi: 10.1111/j.1537-2995.2004.04144.x. [DOI] [PubMed] [Google Scholar]
- 12.Unsworth-White MJ, Herriot A, Valencia O, Poloniecki J, Smith EE, Murday AJ, et al. Resternotomy for bleeding after cardiac operation: a marker for increased morbidity and mortality. Ann Thorac Surg. 1995;59:664–667. doi: 10.1016/0003-4975(94)00995-3. [DOI] [PubMed] [Google Scholar]
- 13.Husain AM, Ashton KH, GC H. A practical approach to neurophysiogic intraoperative monitoring. New York: Demos Medical Publishing, LLC; 2008. [Google Scholar]
- 14.Therapies. ASoATFoPBTaA. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–108. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Zindrou DTK, Bagger JP. Preoperative haemoglobin concentration and mortality rate after coronary artery bypass surgery. Lancet. 2002;359:1747–1748. doi: 10.1016/S0140-6736(02)08614-2. [DOI] [PubMed] [Google Scholar]
- 16.Cladellas M, Bruguera J, Comin J, Vila J, de Jaime E, Marti J, et al. Is pre-operative anaemia a risk marker for in-hospital mortality and morbidity after valve replacement? Eur Heart J. 2006;27:1093–1099. doi: 10.1093/eurheartj/ehi830. [DOI] [PubMed] [Google Scholar]
- 17.De Santo L, Romano G, Della Corte A, de Simone V, Grimaldi F, Cotrufo M, et al. Preoperative anemia in patients undergoing coronary artery bypass grafting predicts acute kidney injury. J Thorac Cardiovasc Surg. 2009;138:965–970. doi: 10.1016/j.jtcvs.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Ferraris VA, Ferraris SP, Saha SP, Hessel EA, 2nd, Haan CK, Royston BD, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83:S27–S86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 19.Roald HE, Barstad RM, Bakken IJ, Roald B, Lyberg T, KS S. Initial interactions of platelets and plasma proteins in flowing non-anticoagulated human blood with the artificial surfaces Dacron and PTFE. Blood Coagul Fibrinolysis. 1994;5:355–363. [PubMed] [Google Scholar]
- 20.Mannucci PM ML. Prevention and Treatment of Major Blood Loss. N Engl J Med. 2007;356:2301–2311. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 21.Rahe-Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138(3):694–702. doi: 10.1016/j.jtcvs.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 22.Tabata SYS, Nagamine H, Tomita S, Arai S, Takemura H, Watanabe G. Efficacy of FK633, an ultra-short acting glycoprotein IIb/IIIa antagonist on platelet preservation during and after cardiopulmonary bypass. Eur J Cardiothorac Surg. 2004;26:289–293. doi: 10.1016/j.ejcts.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 23.Gill R HM, Vuylsteke A, Olsen PS, von Heymann C, Mythen M, Sellke F, Booth F, Schmidt TA. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120(1):21–27. doi: 10.1161/CIRCULATIONAHA.108.834275. [DOI] [PubMed] [Google Scholar]
- 24.Karski JM, Mathieu M, Cheng D, Carroll J, Scott GJ. Etiology of preoperative anemia in patients undergoing scheduled cardiac surgery. Can J Anaesth. 1999;46:979–982. doi: 10.1007/BF03013135. [DOI] [PubMed] [Google Scholar]
- 25.Weltert L, D'Alessandro S, Nardella S, Girola F, Bellisario A, Maselli D, De Paulis R. Preoperative very short-term, high-dose erythropoietin administration diminishes blood transfusion rate in off-pump coronary artery bypass: a randomized blind controlled study. J Thorac Cardiovasc Surg. 2010;139(3):621–626. doi: 10.1016/j.jtcvs.2009.10.012. [DOI] [PubMed] [Google Scholar]