Abstract
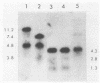
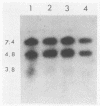
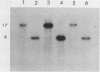
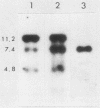
DNA in the polyploid macronucleus of the ciliated protozoan Tetrahymena thermophila contains the modified base N6-methyladenine. We identified two GATC sites which are methylated in most or all of the 45 copies of the macronuclear genome. One site is 2 kilobases 5' to the histone H4-I gene, and the other is 5 kilobases 3' to the 73-kilodalton heat shock protein gene. These sites are de novo methylated between 10 and 16 h after initiation of conjugation, during macronuclear anlage development. The methylation states of these two GATC sites and four other unmethylated GATC sites do not change in the DNA of cells cultured under conditions which change the activity of the genes, including logarithmic growth, starvation, and heat shock.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Burdon R. H. DNA methylation in eukaryotes. CRC Crit Rev Biochem. 1982;13(4):349–384. doi: 10.3109/10409238209108714. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Dennison D. K. Identification and purification of young macronuclear anlagen from conjugating cells of Tetrahymena thermophila. Dev Biol. 1982 Oct;93(2):519–533. doi: 10.1016/0012-1606(82)90139-7. [DOI] [PubMed] [Google Scholar]
- Ammermann D., Steinbrück G., Baur R., Wohlert H. Methylated bases in the DNA of the ciliate Stylonychia mytilus. Eur J Cell Biol. 1981 Apr;24(1):154–156. [PubMed] [Google Scholar]
- Bannon G. A., Bowen J. K., Yao M. C., Gorovsky M. A. Tetrahymena H4 genes: structure, evolution and organization in macro- and micronuclei. Nucleic Acids Res. 1984 Feb 24;12(4):1961–1975. doi: 10.1093/nar/12.4.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon G. A., Calzone F. J., Bowen J. K., Allis C. D., Gorovsky M. A. Multiple, independently regulated, polyadenylated messages for histone H3 and H4 in Tetrahymena. Nucleic Acids Res. 1983 Jun 25;11(12):3903–3917. doi: 10.1093/nar/11.12.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Pan W. C., Johnson C. C. Methylation of ribosomal RNA genes in the macronucleus of Tetrahymena thermophila. Nucleic Acids Res. 1983 Aug 11;11(15):5131–5145. doi: 10.1093/nar/11.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg S., Pratt K., Hattman S. Sequence specificity of DNA adenine methylase in the protozoan Tetrahymena thermophila. J Bacteriol. 1982 May;150(2):993–996. doi: 10.1128/jb.150.2.993-996.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. E., Blumenthal R. M., Gingeras T. R. The isolation and characterization of the Escherichia coli DNA adenine methylase (dam) gene. Nucleic Acids Res. 1983 Feb 11;11(3):837–851. doi: 10.1093/nar/11.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns P. J., Brussard T. B. Pair formation in tetrahymena pyriformis, an inducible developmental system. J Exp Zool. 1974 Jun;188(3):337–344. doi: 10.1002/jez.1401880309. [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Brussard T. B. Positive selection for mating with functional heterokaryons in Tetrahymena pyriformis. Genetics. 1974 Nov;78(3):831–841. doi: 10.1093/genetics/78.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartinhour S. W., Herrick G. A. Three different macronuclear DNAs in Oxytricha fallax share a common sequence block. Mol Cell Biol. 1984 May;4(5):931–938. doi: 10.1128/mcb.4.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Karrer K. M. Chromatin structure of the ribosomal RNA genes of Tetrahymena thermophila as analyzed by trimethylpsoralen crosslinking in vivo. J Mol Biol. 1980 Feb 5;136(4):395–416. doi: 10.1016/0022-2836(80)90397-6. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Tait A., Goddard J. M. Methylated bases in DNA from Paramecium aurelia. Biochim Biophys Acta. 1974 Nov 20;374(1):1–11. doi: 10.1016/0005-2787(74)90194-4. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Findly R. C., Pederson T. Regulated transcription of the genes for actin and heat-shock proteins in cultured Drosophila cells. J Cell Biol. 1981 Feb;88(2):323–328. doi: 10.1083/jcb.88.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frado L. L., Annunziato A. T., Woodcock C. L. Structural repeating units in chromatin. III. A comparison of chromatin subunits from vertebrate, cilliate and angiosperm species. Biochim Biophys Acta. 1977 Apr 4;475(3):514–520. doi: 10.1016/0005-2787(77)90066-1. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Free ribosomal RNA genes in the macronucleus of Tetrahymena. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3078–3081. doi: 10.1073/pnas.71.8.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Glover C., Johmann C. A., Keevert J. B., Mathis D. J., Samuelson M. Histones and chromatin structure in Tetrahymena macro- and micronuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):493–503. doi: 10.1101/sqb.1978.042.01.052. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Hattman S., Pleger G. L. ( 6 N)methyl adenine in the nuclear DNA of a eucaryote, Tetrahymena pyriformis. J Cell Biol. 1973 Mar;56(3):697–701. doi: 10.1083/jcb.56.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Hallberg R. L., Kraus K. W., Findly R. C. Starved Tetrahymena thermophila cells that are unable to mount an effective heat shock response selectively degrade their rRNA. Mol Cell Biol. 1984 Oct;4(10):2170–2179. doi: 10.1128/mcb.4.10.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison G. S., Karrer K. M. DNA synthesis, methylation and degradation during conjugation in Tetrahymena thermophila. Nucleic Acids Res. 1985 Jan 11;13(1):73–87. doi: 10.1093/nar/13.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S., Kenny C., Berger L., Pratt K. Comparative study of DNA methylation in three unicellular eucaryotes. J Bacteriol. 1978 Sep;135(3):1156–1157. doi: 10.1128/jb.135.3.1156-1157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard E. A., Blackburn E. H. Reproducible and variable genomic rearrangements occur in the developing somatic nucleus of the ciliate Tetrahymena thermophila. Mol Cell Biol. 1985 Aug;5(8):2039–2050. doi: 10.1128/mcb.5.8.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer K. M. Germ line-specific DNA sequences are present on all five micronuclear chromosomes in Tetrahymena thermophila. Mol Cell Biol. 1983 Nov;3(11):1909–1919. doi: 10.1128/mcb.3.11.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. A deoxyribonuclease of Diplococcus pneumoniae specific for methylated DNA. J Biol Chem. 1975 Jun 10;250(11):4060–4066. [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J Mol Biol. 1977 Jul;114(1):153–168. doi: 10.1016/0022-2836(77)90289-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D. W., Allis C. D., Bruns P. J. Conjugation in Tetrahymena thermophila. A temporal analysis of cytological stages. Exp Cell Res. 1982 Jul;140(1):227–236. doi: 10.1016/0014-4827(82)90172-0. [DOI] [PubMed] [Google Scholar]
- Martindale D. W., Martindale H. M., Bruns P. J. Tetrahymena conjugation-induced genes: structure and organization in macro- and micronuclei. Nucleic Acids Res. 1986 Feb 11;14(3):1341–1354. doi: 10.1093/nar/14.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M. Purification and characterization of two new modification methylases: MClaI from Caryophanon latum L and MTaqI from Thermus aquaticus YTI. Nucleic Acids Res. 1981 Dec 21;9(24):6795–6804. doi: 10.1093/nar/9.24.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt K., Hattman S. Deoxyribonucleic acid methylation and chromatin organization in Tetrahymena thermophila. Mol Cell Biol. 1981 Jul;1(7):600–608. doi: 10.1128/mcb.1.7.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY D. S., HANAWALT P. C. PROPERTIES OF THE SATELLITE DNA ASSOCIATED WITH THE CHLOROPLASTS OF EUGLENA GRACILIS. J Mol Biol. 1964 Sep;9:812–824. doi: 10.1016/s0022-2836(64)80187-x. [DOI] [PubMed] [Google Scholar]
- Rae P. M., Spear B. B. Macronuclear DNA of the hypotrichous ciliate Oxytricha fallax. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4992–4996. doi: 10.1073/pnas.75.10.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Cedar H. Distribution of 5-methylcytosine in chromatin. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2725–2728. doi: 10.1073/pnas.74.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Friedman J. DNA methylation and its possible biological roles. Prog Nucleic Acid Res Mol Biol. 1981;25:33–52. doi: 10.1016/s0079-6603(08)60482-1. [DOI] [PubMed] [Google Scholar]
- Solage A., Cedar H. Organization of 5-methylcytosine in chromosomal DNA. Biochemistry. 1978 Jul 11;17(14):2934–2938. doi: 10.1021/bi00607a036. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Schuster A. M., Girton L., Burbank D. E., Swinton D., Hattman S. DNA methylation of viruses infecting a eukaryotic Chlorella-like green alga. Nucleic Acids Res. 1985 May 24;13(10):3471–3478. doi: 10.1093/nar/13.10.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenkert D., Allis C. D. Timing of the appearance of macronuclear-specific histone variant hv1 and gene expression in developing new macronuclei of Tetrahymena thermophila. J Cell Biol. 1984 Jun;98(6):2107–2117. doi: 10.1083/jcb.98.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Gorovsky M. A. Comparison of the sequences of macro- and micronuclear DNA of Tetrahymena pyriformis. Chromosoma. 1974;48(1):1–18. doi: 10.1007/BF00284863. [DOI] [PubMed] [Google Scholar]