Abstract
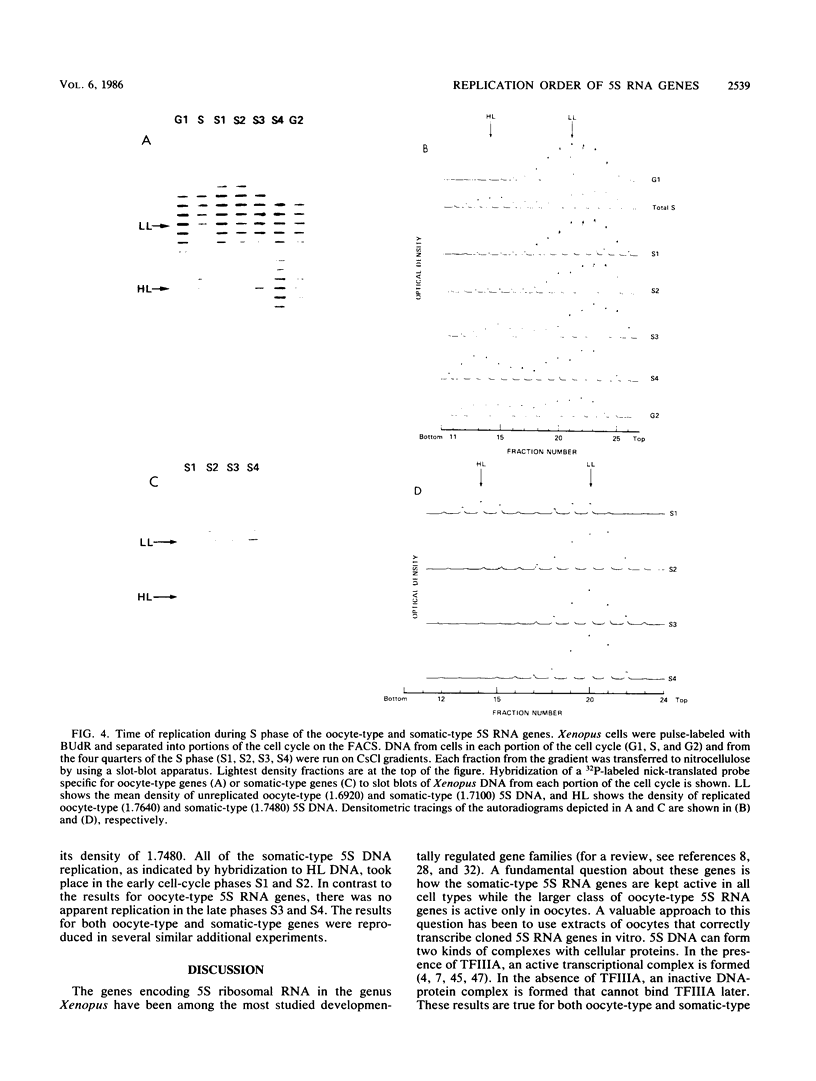
In Xenopus laevis there are two multigene families of 5S RNA genes: the oocyte-type 5S RNA genes which are expressed only in oocytes and the somatic-type 5S RNA genes which are expressed throughout development. The Xenopus 5S RNA replication-expression model of Gottesfeld and Bloomer (Cell 28:781-791, 1982) and Wormington et al. (Cold Spring Harbor Symp. Quant. Biol. 47:879-884, 1983) predicts that the somatic-type 5S RNA genes replicate earlier in the cell cycle than do the oocyte-type genes. Hence, the somatic-type 5S RNA genes have a competitive advantage in binding the transcription factor TFIIIA in somatic cells and are thereby expressed to the exclusion of the oocyte-type genes. To test the replication-expression model, we determined the order of replication of the oocyte- and somatic-type 5S RNA genes. Xenopus cells were labeled with bromodeoxyuridine, stained for DNA content, and then sorted into fractions of S phase by using a fluorescence-activated cell sorter. The newly replicated DNA containing bromodeoxyuridine was separated from the lighter, unreplicated DNA by equilibrium centrifugation and was hybridized with DNA probes specific for the oocyte- and somatic-type 5S RNA genes. In this way we found that the somatic-type 5S RNA genes replicate early in S phase, whereas the oocyte-type 5S RNA genes replicate late in S phase, demonstrating a key aspect of the replication-expression model.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaldi F., Giacomoni D., Zito-Bignami R. On the duplication of ribosomal RNA cistrons in Chinese hamster cells. Eur J Biochem. 1969 Dec;11(3):419–423. doi: 10.1111/j.1432-1033.1969.tb00790.x. [DOI] [PubMed] [Google Scholar]
- BERLOWITZ L. CORRELATION OF GENETIC ACTIVITY, HETEROCHROMATIZATION, AND RNA METABOLISM. Proc Natl Acad Sci U S A. 1965 Jan;53:68–73. doi: 10.1073/pnas.53.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs I., Schildkraut C. L. DNA replication in synchronized cultured mammalian cells. VI. The temporal replication of ribosomal cistrons in synchronized cell lines. Exp Cell Res. 1976 Sep;101(2):307–314. doi: 10.1016/0014-4827(76)90382-7. [DOI] [PubMed] [Google Scholar]
- Bieker J. J., Martin P. L., Roeder R. G. Formation of a rate-limiting intermediate in 5S RNA gene transcription. Cell. 1985 Jan;40(1):119–127. doi: 10.1016/0092-8674(85)90315-0. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Brown D. D., Jordan E. A nuclear extract of Xenopus laevis oocytes that accurately transcribes 5S RNA genes. Cell. 1978 Nov;15(3):1077–1086. doi: 10.1016/0092-8674(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Sakonju S., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3' border of the region. Cell. 1980 Jan;19(1):27–35. doi: 10.1016/0092-8674(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Wormington W. M., Brown D. D. Stable transcription complexes of Xenopus 5S RNA genes: a means to maintain the differentiated state. Cell. 1982 Feb;28(2):413–421. doi: 10.1016/0092-8674(82)90359-2. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Schlissel M. S. A positive transcription factor controls the differential expression of two 5S RNA genes. Cell. 1985 Oct;42(3):759–767. doi: 10.1016/0092-8674(85)90272-7. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Sugimoto K. The structure and evolution of ribosomal and 5S DNAs in Xenopus laevis and Xenopus mulleri. Cold Spring Harb Symp Quant Biol. 1974;38:501–505. doi: 10.1101/sqb.1974.038.01.054. [DOI] [PubMed] [Google Scholar]
- Brown D. D. The role of stable complexes that repress and activate eucaryotic genes. Cell. 1984 Jun;37(2):359–365. doi: 10.1016/0092-8674(84)90366-0. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Tlsty T. D., Schimke R. T. Enhancement of methotrexate resistance and dihydrofolate reductase gene amplification by treatment of mouse 3T6 cells with hydroxyurea. Mol Cell Biol. 1983 Jun;3(6):1097–1107. doi: 10.1128/mcb.3.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. W. Heterochromatin. Science. 1966 Jan 28;151(3709):417–425. doi: 10.1126/science.151.3709.417. [DOI] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Tantravahi U., Lalande M., Perle M. A., Latt S. A. High resolution analysis of the timing of replication of specific DNA sequences during S phase of mammalian cells. Nucleic Acids Res. 1983 Jul 25;11(14):4753–4774. doi: 10.1093/nar/11.14.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke D. R., Ng S. Y., Shastry B. S., Roeder R. G. Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell. 1980 Mar;19(3):717–728. doi: 10.1016/s0092-8674(80)80048-1. [DOI] [PubMed] [Google Scholar]
- Epner E., Rifkind R. A., Marks P. A. Replication of alpha and beta globin DNA sequences occurs during early S phase in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3058–3062. doi: 10.1073/pnas.78.5.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. I. The AT-rich spacer. Cell. 1978 Apr;13(4):701–716. doi: 10.1016/0092-8674(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Fedoroff N. V., Brown D. D. The nucleotide sequence of the repeating unit in the oocyte 5S ribosomal DNA of Xenopus laevis. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1195–1200. doi: 10.1101/sqb.1978.042.01.120. [DOI] [PubMed] [Google Scholar]
- Ford P. J., Brown R. D. Sequences of 5S ribosomal RNA from Xenopus mulleri and the evolution of 5S gene-coding sequences. Cell. 1976 Aug;8(4):485–493. doi: 10.1016/0092-8674(76)90216-6. [DOI] [PubMed] [Google Scholar]
- Ford P. J., Mathieson T. Control of 5S RNA synthesis in Xenopus laevis. Nature. 1976 Jun 3;261(5559):433–435. doi: 10.1038/261433a0. [DOI] [PubMed] [Google Scholar]
- Ford P. J., Southern E. M. Different sequences for 5S RNA in kidney cells and ovaries of Xenopus laevis. Nat New Biol. 1973 Jan 3;241(105):7–12. doi: 10.1038/newbio241007a0. [DOI] [PubMed] [Google Scholar]
- Furst A., Brown E. H., Braunstein J. D., Schildkraut C. L. alpha-Globulin sequences are located in a region of early-replicating DNA in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1023–1027. doi: 10.1073/pnas.78.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
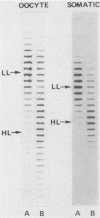
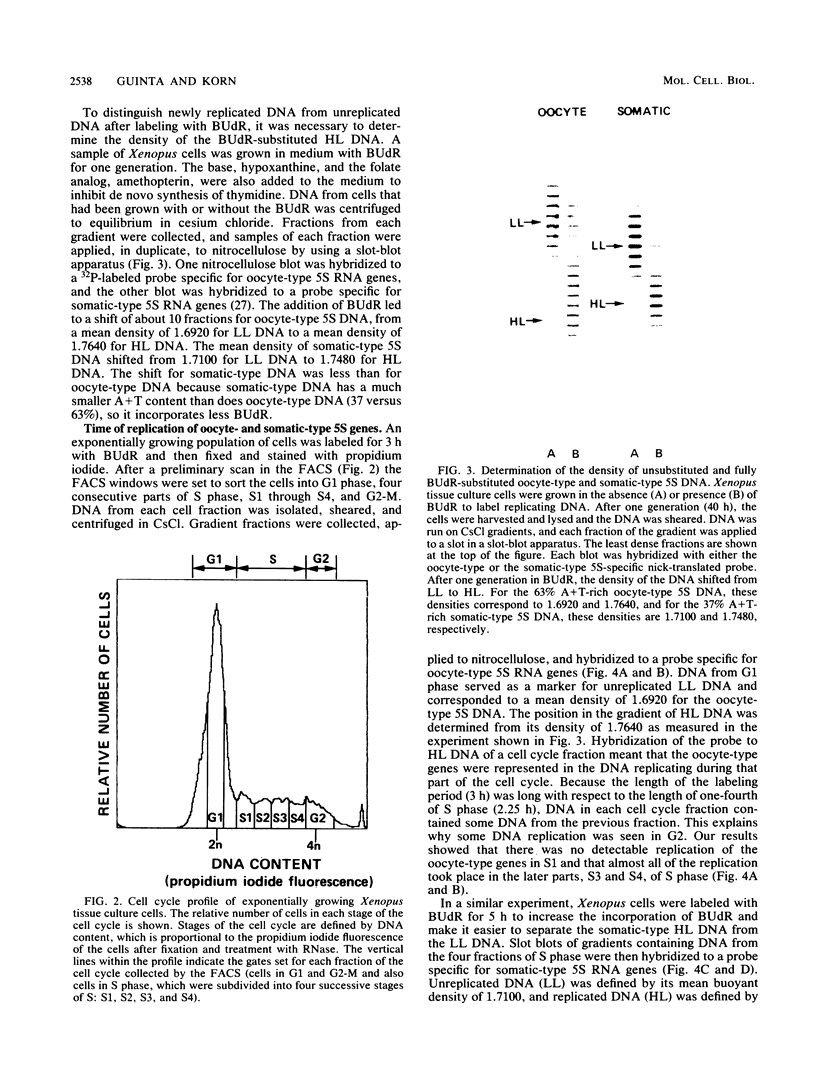
- Gilbert D. M. Temporal order of replication of Xenopus laevis 5S ribosomal RNA genes in somatic cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2924–2928. doi: 10.1073/pnas.83.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J., Bloomer L. S. Assembly of transcriptionally active 5S RNA gene chromatin in vitro. Cell. 1982 Apr;28(4):781–791. doi: 10.1016/0092-8674(82)90057-5. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Price J., Korn L. J. Chromosomal mapping of Xenopus 5S genes: somatic-type versus oocyte-type. Nucleic Acids Res. 1983 Apr 25;11(8):2313–2323. doi: 10.1093/nar/11.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Birkenmeier E. H., Brown D. D. Transcription initiation of Xenopus 5S ribosomal RNA genes in vitro. Nucleic Acids Res. 1979 Oct 25;7(4):947–958. doi: 10.1093/nar/7.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Brown D. D. Nucleotide sequence of Xenopus borealis oocyte 5S DNA: comparison of sequences that flank several related eucaryotic genes. Cell. 1978 Dec;15(4):1145–1156. doi: 10.1016/0092-8674(78)90042-9. [DOI] [PubMed] [Google Scholar]
- Korn L. J. Transcription of Xenopus 5S ribosomal RNA genes. Nature. 1982 Jan 14;295(5845):101–105. doi: 10.1038/295101a0. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Martin P. L., Roeder R. G. Transcription of class III genes: formation of preinitiation complexes. Science. 1983 Nov 18;222(4625):740–748. doi: 10.1126/science.6356356. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Schimke R. T. Gene amplification in a single cell cycle in Chinese hamster ovary cells. J Biol Chem. 1984 Feb 10;259(3):1901–1910. [PubMed] [Google Scholar]
- Miller J. R., Cartwright E. M., Brownlee G. G., Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. II. The GC-rich region. Cell. 1978 Apr;13(4):717–725. doi: 10.1016/0092-8674(78)90221-0. [DOI] [PubMed] [Google Scholar]
- Ng S. Y., Parker C. S., Roeder R. G. Transcription of cloned Xenopus 5S RNA genes by X. laevis RNA polymerase III in reconstituted systems. Proc Natl Acad Sci U S A. 1979 Jan;76(1):136–140. doi: 10.1073/pnas.76.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Brown D. D., Birnstiel M. L. Location of the genes for 5S ribosomal RNA in Xenopus laevis. Chromosoma. 1973;42(2):191–203. doi: 10.1007/BF00320940. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Brown D. D. A specific transcription factor that can bind either the 5S RNA gene or 5S RNA. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4170–4174. doi: 10.1073/pnas.77.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Wormington W. M., Brown D. D. Related 5S RNA transcription factors in Xenopus oocytes and somatic cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1760–1764. doi: 10.1073/pnas.78.3.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. C., Doering J. L., Brown D. D. Characterization of two xenopus somatic 5S DNAs and one minor oocyte-specific 5S DNA. Cell. 1980 May;20(1):131–141. doi: 10.1016/0092-8674(80)90241-x. [DOI] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Sakonju S., Brown D. D. Contact points between a positive transcription factor and the Xenopus 5S RNA gene. Cell. 1982 Dec;31(2 Pt 1):395–405. doi: 10.1016/0092-8674(82)90133-7. [DOI] [PubMed] [Google Scholar]
- Sakonju S., Brown D. D., Engelke D., Ng S. Y., Shastry B. S., Roeder R. G. The binding of a transcription factor to deletion mutants of a 5S ribosomal RNA gene. Cell. 1981 Mar;23(3):665–669. doi: 10.1016/0092-8674(81)90429-3. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Brown D. D. The transcriptional regulation of Xenopus 5s RNA genes in chromatin: the roles of active stable transcription complexes and histone H1. Cell. 1984 Jul;37(3):903–913. doi: 10.1016/0092-8674(84)90425-2. [DOI] [PubMed] [Google Scholar]
- Segall J., Matsui T., Roeder R. G. Multiple factors are required for the accurate transcription of purified genes by RNA polymerase III. J Biol Chem. 1980 Dec 25;255(24):11986–11991. [PubMed] [Google Scholar]
- Setzer D. R., Brown D. D. Formation and stability of the 5 S RNA transcription complex. J Biol Chem. 1985 Feb 25;260(4):2483–2492. [PubMed] [Google Scholar]
- Shastry B. S., Honda B. M., Roeder R. G. Altered levels of a 5 S gene-specific transcription factor (TFIIIA) during oogenesis and embryonic development of Xenopus laevis. J Biol Chem. 1984 Sep 25;259(18):11373–11382. [PubMed] [Google Scholar]
- Shastry B. S., Ng S. Y., Roeder R. G. Multiple factors involved in the transcription of class III genes in Xenopus laevis. J Biol Chem. 1982 Nov 10;257(21):12979–12986. [PubMed] [Google Scholar]
- Stambrook P. J. The temporal replication of ribosomal genes in synchronized Chinese hamster cells. J Mol Biol. 1974 Jan 25;82(3):303–313. doi: 10.1016/0022-2836(74)90592-0. [DOI] [PubMed] [Google Scholar]
- Wegnez M., Monier R., Denis H. Sequence heterogeneity of 5 S RNA in Xenopus laevis. FEBS Lett. 1972 Sep 1;25(1):13–20. doi: 10.1016/0014-5793(72)80443-5. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Latt S. A. Analysis of deoxyribonucleic acid replication in human X chromosomes by fluorescence microscopy. Am J Hum Genet. 1976 May;28(3):213–227. [PMC free article] [PubMed] [Google Scholar]
- Wormington W. M., Bogenhagen D. F., Jordan E., Brown D. D. A quantitative assay for Xenopus 5S RNA gene transcription in vitro. Cell. 1981 Jun;24(3):809–817. doi: 10.1016/0092-8674(81)90106-9. [DOI] [PubMed] [Google Scholar]
- Wormington W. M., Brown D. D. Onset of 5 S RNA gene regulation during Xenopus embryogenesis. Dev Biol. 1983 Sep;99(1):248–257. doi: 10.1016/0012-1606(83)90273-7. [DOI] [PubMed] [Google Scholar]
- Wormington W. M., Schlissel M., Brown D. D. Developmental regulation of Xenopus 5S RNA genes. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):879–884. doi: 10.1101/sqb.1983.047.01.101. [DOI] [PubMed] [Google Scholar]