Abstract
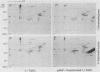
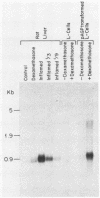
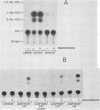
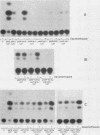
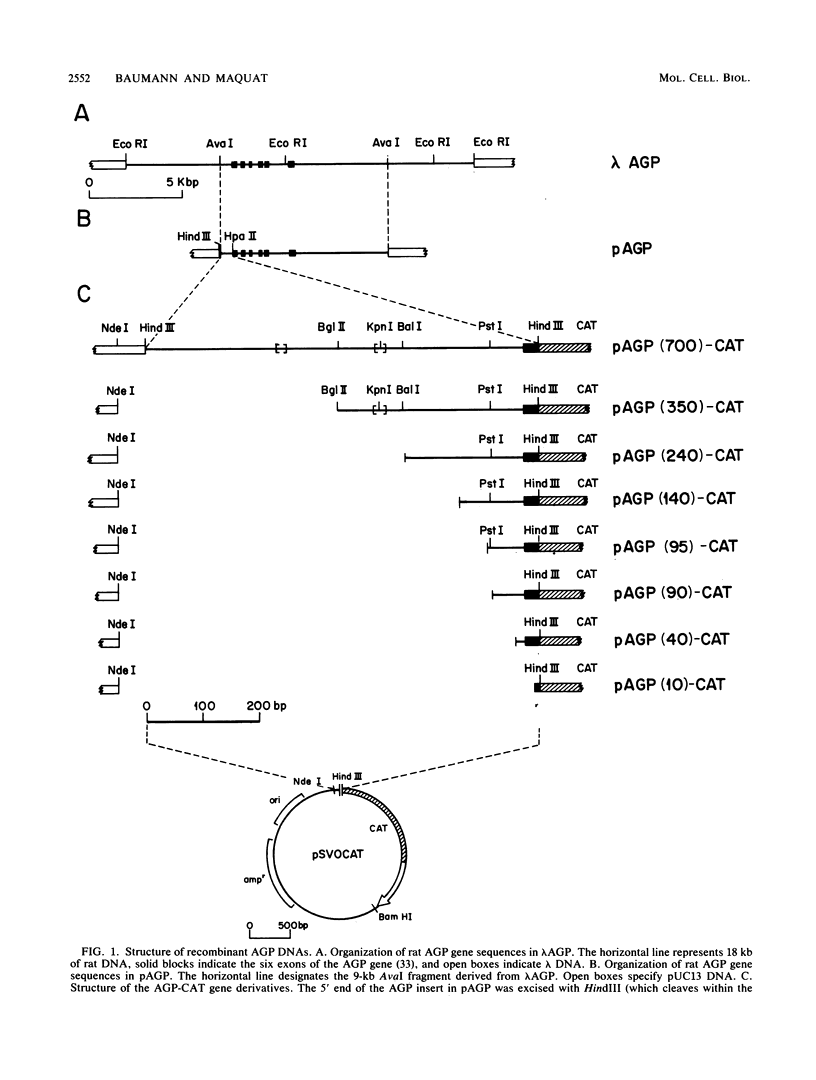
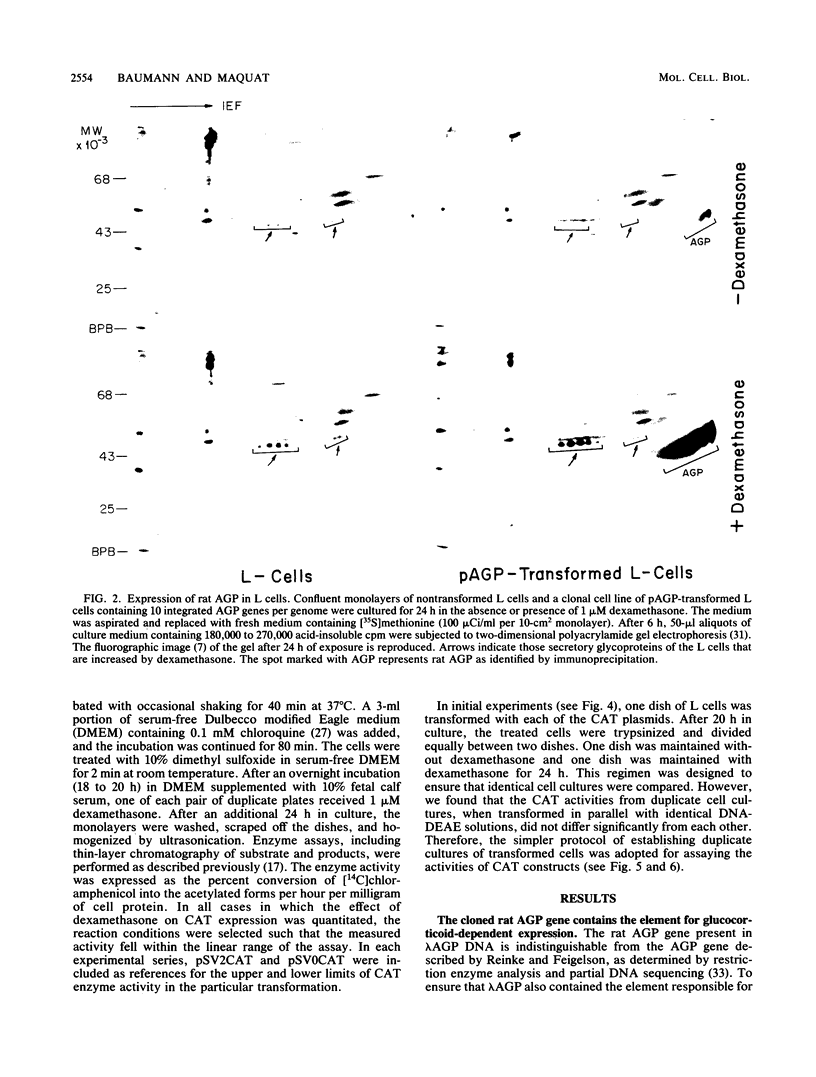
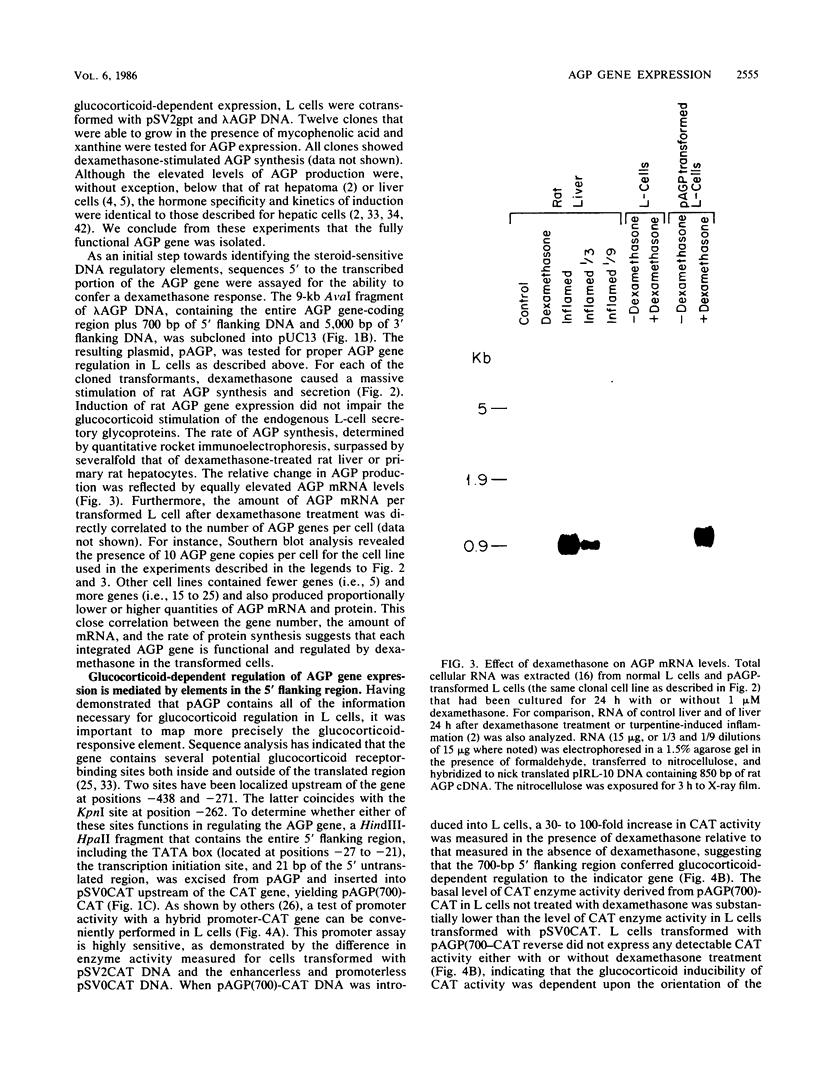
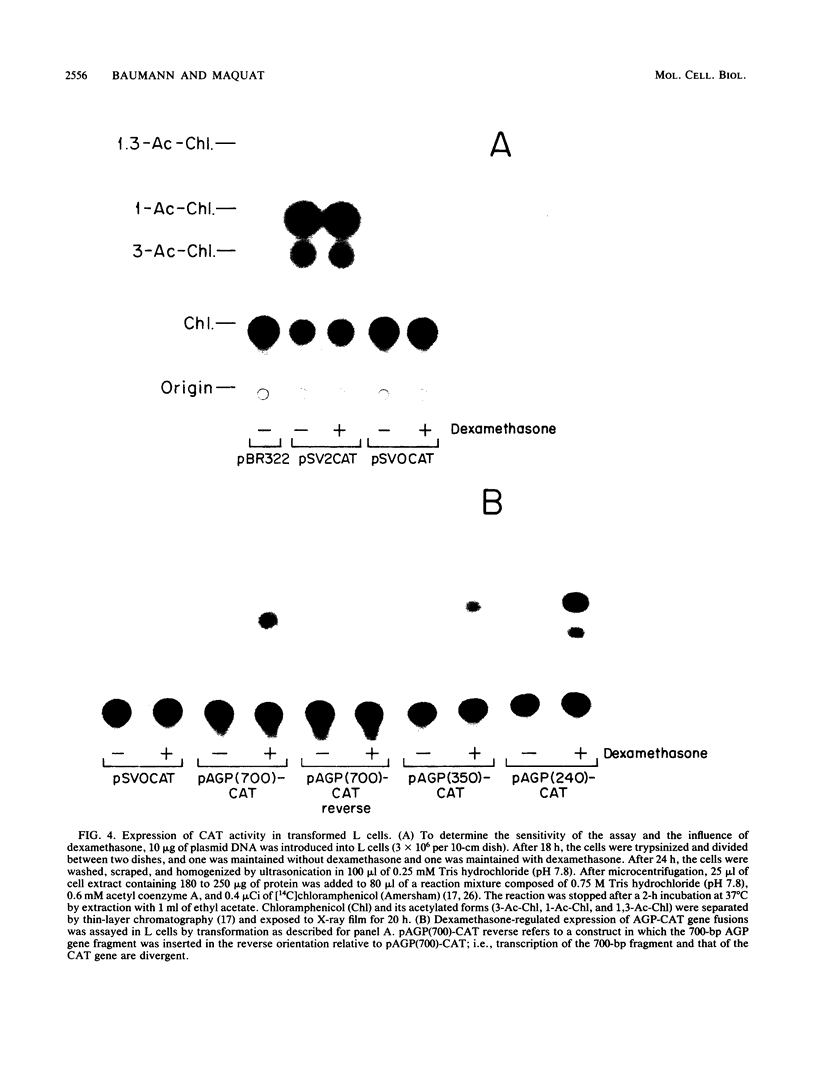
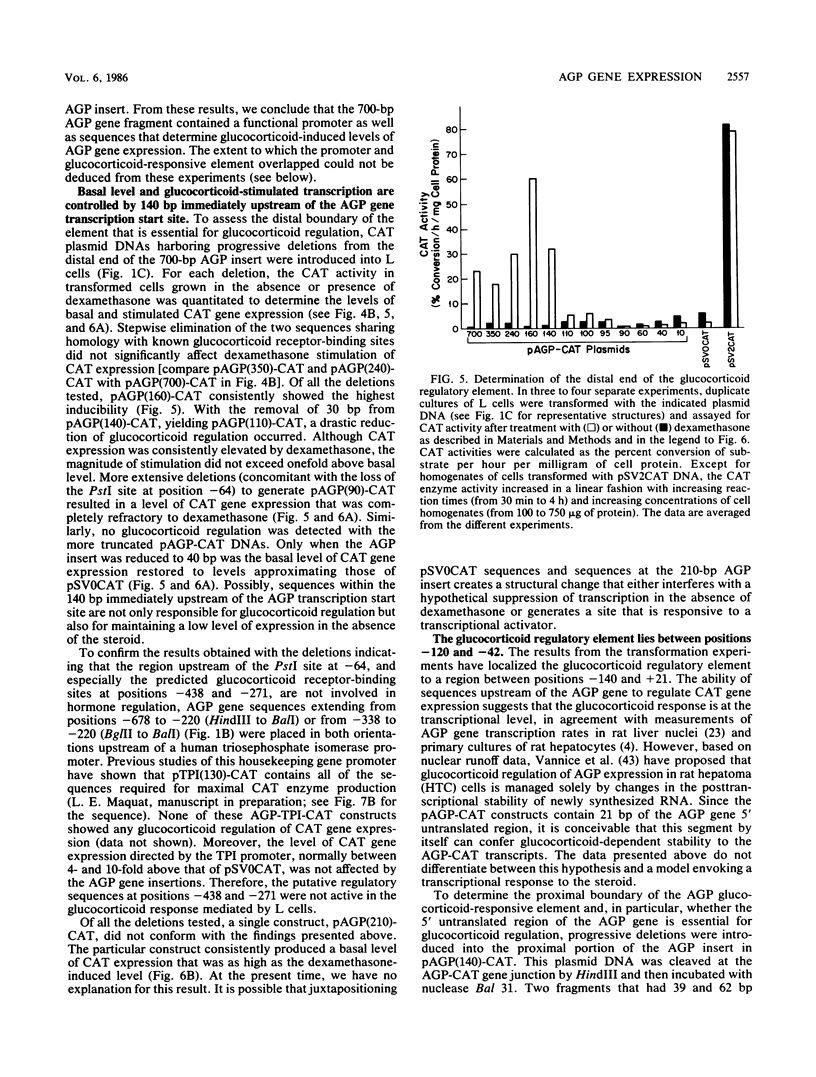
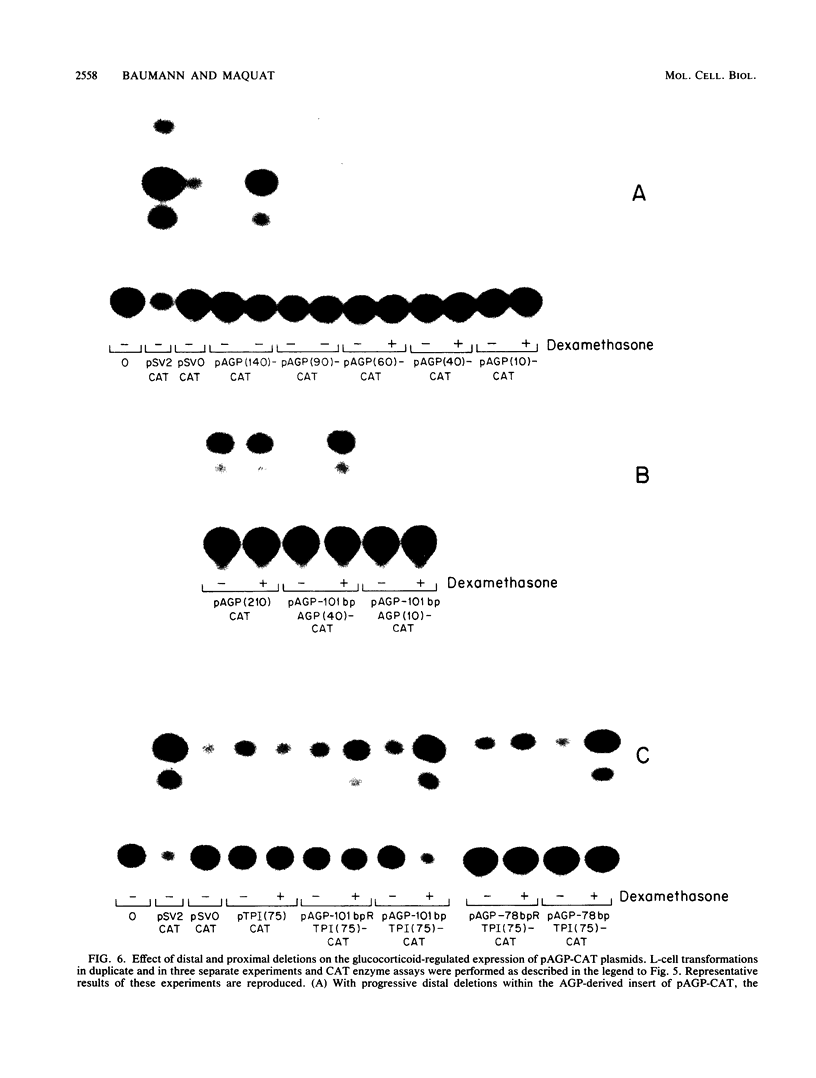
Synthesis of rat alpha 1-acid glycoprotein (AGP), one of the major inflammation-induced plasma proteins, is positively regulated by dexamethasone. To define the dexamethasone-responsive genetic element, we isolated and tested AGP gene sequences for the ability to confer glucocorticoid induction to the bacterial chloramphenicol acetyltransferase (CAT) gene in L cells. A 141-base-pair region of the AGP gene, including 120 base pairs of DNA upstream from the start site of transcription and 21 base pairs of the 5' untranslated region, was sufficient for maximal CAT gene induction by dexamethasone. To localize more precisely the AGP glucocorticoid-responsive element, parts of this 141-base-pair region were inserted 5' to either an AGP promoter-CAT gene or a human triosephosphate isomerase promoter-CAT gene, both of which lacked a response to the steroid. The AGP gene region between 120 and 42 base pairs upstream from the start site of transcription was found to mediate maximal dexamethasone induction of CAT enzyme levels. This result was unexpected because this region does not contain sequence homologies to known glucocorticoid receptor-binding sites and those AGP gene regions that lay further upstream and were homologous to other glucocorticoid receptor-binding sites were inactive in the CAT assay. The fact that the AGP gene region mediating dexamethasone regulation was distinct from the transcribed region indicates that glucocorticoids increase AGP gene expression primarily at the transcriptional rather than the posttranscriptional level.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Berger F. G. Genetics and evolution of the acute phase proteins in mice. Mol Gen Genet. 1985;201(3):505–512. doi: 10.1007/BF00331347. [DOI] [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Baumann H., Held W. A. Biosynthesis and hormone-regulated expression of secretory glycoproteins in rat liver and hepatoma cells. Effect of glucocorticoids and inflammation. J Biol Chem. 1981 Oct 10;256(19):10145–10155. [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Bennett M., Schmid K. Immunosuppression by human plasma alpha 1-acid glycoprotein: importance of the carbohydrate moiety. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6109–6113. doi: 10.1073/pnas.77.10.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown J. R., Daar I. O., Krug J. R., Maquat L. E. Characterization of the functional gene and several processed pseudogenes in the human triosephosphate isomerase gene family. Mol Cell Biol. 1985 Jul;5(7):1694–1706. doi: 10.1128/mcb.5.7.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Haynes D. H., Distasio J. A. Interaction of an acute phase reactant, alpha 1-acid glycoprotein (orosomucoid), with the lymphoid cell surface: a model for non-specific immune suppression. Immunology. 1984 Mar;51(3):541–548. [PMC free article] [PubMed] [Google Scholar]
- Chiu K. M., Mortensen R. F., Osmand A. P., Gewurz H. Interactions of alpha1-acid glycoprotein with the immune system. I. Purification and effects upon lymphocyte responsiveness. Immunology. 1977 Jun;32(6):997–1005. [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R., Kant J. A. Coordinate accumulation of the mRNAs for the alpha, beta, and gamma chains of rat fibrinogen following defibrination. J Biol Chem. 1982 Jul 10;257(13):7277–7279. [PubMed] [Google Scholar]
- Davidson E. H., Jacobs H. T., Britten R. J. Very short repeats and coordinate induction of genes. Nature. 1983 Feb 10;301(5900):468–470. doi: 10.1038/301468a0. [DOI] [PubMed] [Google Scholar]
- Fowlkes D. M., Mullis N. T., Comeau C. M., Crabtree G. R. Potential basis for regulation of the coordinately expressed fibrinogen genes: homology in the 5' flanking regions. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2313–2316. doi: 10.1073/pnas.81.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. J. Control of malaria virulence by alpha 1-acid glycoprotein (orosomucoid), an acute-phase (inflammatory) reactant. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5421–5424. doi: 10.1073/pnas.80.17.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Koj A., Dubin A., Kasperczyk H., Bereta J., Gordon A. H. Changes in the blood level and affinity to concanavalin A of rat plasma glycoproteins during acute inflammation and hepatoma growth. Biochem J. 1982 Sep 15;206(3):545–553. doi: 10.1042/bj2060545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A., Gauldie J., Regoeczi E., Sauder D. N., Sweeney G. D. The acute-phase response of cultured rat hepatocytes. System characterization and the effect of human cytokines. Biochem J. 1984 Dec 1;224(2):505–514. doi: 10.1042/bj2240505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Reinke R., Feigelson P. Acute phase mediators and glucocorticoids elevate alpha 1-acid glycoprotein gene transcription. J Biol Chem. 1985 Dec 15;260(29):15386–15389. [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Liao Y. C., Taylor J. M., Vannice J. L., Clawson G. A., Smuckler E. A. Structure of the rat alpha 1-acid glycoprotein gene. Mol Cell Biol. 1985 Dec;5(12):3634–3639. doi: 10.1128/mcb.5.12.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moore D. D., Marks A. R., Buckley D. I., Kapler G., Payvar F., Goodman H. M. The first intron of the human growth hormone gene contains a binding site for glucocorticoid receptor. Proc Natl Acad Sci U S A. 1985 Feb;82(3):699–702. doi: 10.1073/pnas.82.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ponta H., Kennedy N., Skroch P., Hynes N. E., Groner B. Hormonal response region in the mouse mammary tumor virus long terminal repeat can be dissociated from the proviral promoter and has enhancer properties. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1020–1024. doi: 10.1073/pnas.82.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke R., Feigelson P. Rat alpha 1-acid glycoprotein. Gene sequence and regulation by glucocorticoids in transfected L-cells. J Biol Chem. 1985 Apr 10;260(7):4397–4403. [PubMed] [Google Scholar]
- Ricca G. A., Hamilton R. W., McLean J. W., Conn A., Kalinyak J. E., Taylor J. M. Rat alpha 1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. J Biol Chem. 1981 Oct 25;256(20):10362–10368. [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. Hepatocyte-stimulating factor: a monocyte-derived acute-phase regulatory protein. Ann N Y Acad Sci. 1983 Jun 27;408:490–502. doi: 10.1111/j.1749-6632.1983.tb23268.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Vannice J. L., Ringold G. M., McLean J. W., Taylor J. M. Induction of the acute-phase reactant, alpha 1-acid glycoprotein, by glucocorticoids in rat hepatoma cells. DNA. 1983;2(3):205–212. doi: 10.1089/dna.1983.2.205. [DOI] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]