Abstract
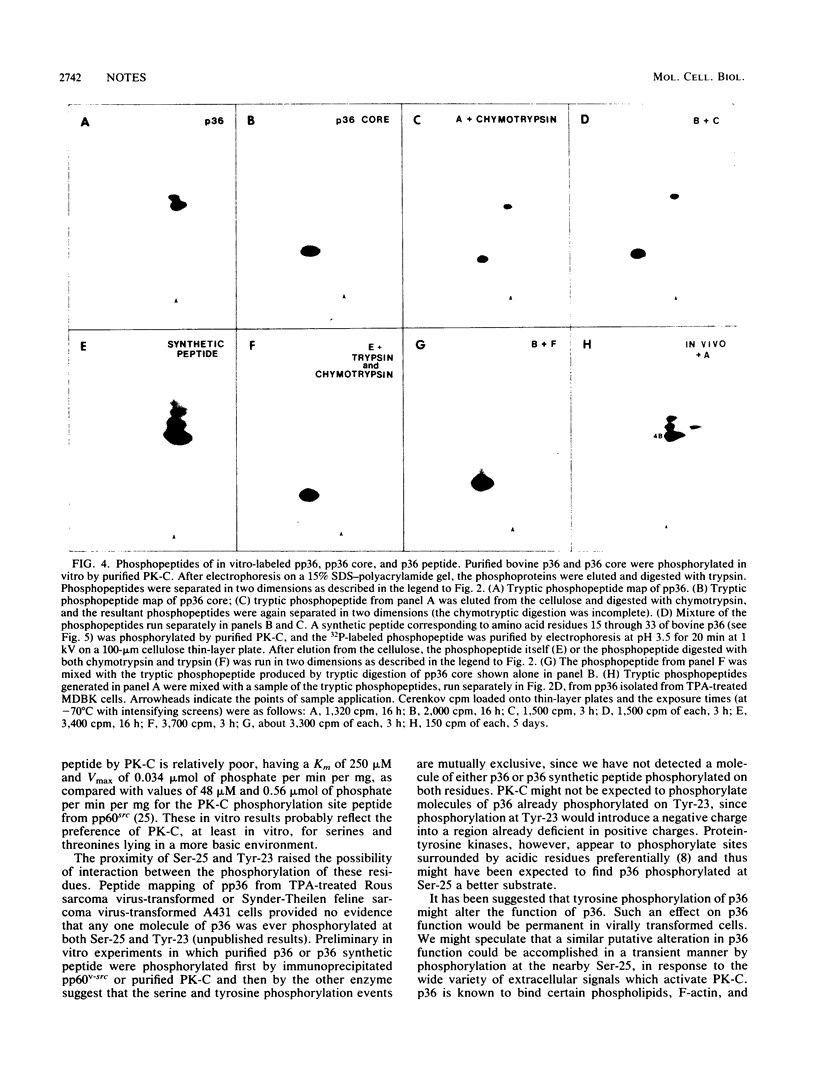
p36, a major in vivo substrate of protein-tyrosine kinases, is shown to be phosphorylated at serine 25, a site very close to the major site of tyrosine phosphorylation by pp60v-src, tyrosine 23 (J. R. Glenney, Jr., and B. F. Tack, Proc. Natl. Acad. Sci. USA 82:7884-7888, 1985). We present evidence suggesting that protein kinase C mediates phosphorylation of serine 25.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashendel C. L. The phorbol ester receptor: a phospholipid-regulated protein kinase. Biochim Biophys Acta. 1985 Sep 9;822(2):219–242. doi: 10.1016/0304-4157(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Identification and characterization of cellular targets for tyrosine protein kinases. J Biol Chem. 1983 Jan 25;258(2):1108–1115. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Regulation of cell growth and transformation by tyrosine-specific protein kinases: the search for important cellular substrate proteins. Curr Top Microbiol Immunol. 1983;107:125–161. doi: 10.1007/978-3-642-69075-4_4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol Cell Biol. 1984 Jan;4(1):30–37. doi: 10.1128/mcb.4.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters cause the phosphorylation of epidermal growth factor receptors in normal human fibroblasts at threonine-654. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1974–1978. doi: 10.1073/pnas.82.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Waterfield M. D., Parker P. J. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J Biol Chem. 1985 Nov 25;260(27):14538–14546. [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson E., Tomasiewicz H. G., Erikson R. L. Biochemical characterization of a 34-kilodalton normal cellular substrate of pp60v-src and an associated 6-kilodalton protein. Mol Cell Biol. 1984 Jan;4(1):77–85. doi: 10.1128/mcb.4.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Marchiori F., Borin G., Pinna L. A. Distinct structural requirements of Ca2+/phospholipid-dependent protein kinase (protein kinase C) and cAMP-dependent protein kinase as evidenced by synthetic peptide substrates. FEBS Lett. 1985 May 6;184(1):72–77. doi: 10.1016/0014-5793(85)80656-6. [DOI] [PubMed] [Google Scholar]
- Friedman B., Frackelton A. R., Jr, Ross A. H., Connors J. M., Fujiki H., Sugimura T., Rosner M. R. Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1984 May;81(10):3034–3038. doi: 10.1073/pnas.81.10.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984 Jan;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Weber K. The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells. EMBO J. 1985 Nov;4(11):2917–2920. doi: 10.1002/j.1460-2075.1985.tb04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Comparison of Ca++-regulated events in the intestinal brush border. J Cell Biol. 1985 Mar;100(3):754–763. doi: 10.1083/jcb.100.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr Phosphorylation of p36 in vitro with pp60src. Regulation by Ca2+ and phospholipid. FEBS Lett. 1985 Nov 11;192(1):79–82. doi: 10.1016/0014-5793(85)80047-8. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B. F. Amino-terminal sequence of p36 and associated p10: identification of the site of tyrosine phosphorylation and homology with S-100. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7884–7888. doi: 10.1073/pnas.82.23.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Cooper J. A., Hunter T. The 46,000-dalton tyrosine protein kinase substrate is widespread, whereas the 36,000-dalton substrate is only expressed at high levels in certain rodent tissues. J Cell Biol. 1984 Feb;98(2):487–497. doi: 10.1083/jcb.98.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Cooper J. A., Buss J. E., Shalloway D., Hunter T. Protein kinase C phosphorylates pp60src at a novel site. Cell. 1985 Oct;42(3):849–857. doi: 10.1016/0092-8674(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Brackenbury R., Edelman G. M. Changes in the distribution of the 34-kdalton tyrosine kinase substrate during differentiation and maturation of chicken tissues. J Cell Biol. 1984 Feb;98(2):473–486. doi: 10.1083/jcb.98.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinase II. Methods Enzymol. 1983;99:317–331. doi: 10.1016/0076-6879(83)99067-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S., Fox C. F. Epidermal growth factor and potent phorbol tumor promoters induce epidermal growth factor receptor phosphorylation in a similar but distinctively different manner in human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Feb 25;259(4):2559–2567. [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D. M., Sege R. D., Kozarsky K. F., Krieger M. DNA-mediated transfer of a human gene required for low-density lipoprotein receptor expression and for multiple Golgi processing pathways. Mol Cell Biol. 1986 Jul;6(7):2734–2737. doi: 10.1128/mcb.6.7.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Nishiyama K., Nakanishi H., Uratsuji Y., Nomura H., Takeyama Y., Nishizuka Y. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1985 Oct 15;260(23):12492–12499. [PubMed] [Google Scholar]
- Kohno M. Diverse mitogenic agents induce rapid phosphorylation of a common set of cellular proteins at tyrosine in quiescent mammalian cells. J Biol Chem. 1985 Feb 10;260(3):1771–1779. [PubMed] [Google Scholar]
- Kuret J., Woodgett J. R., Cohen P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Identification of the sites phosphorylated by casein kinase-I. Eur J Biochem. 1985 Aug 15;151(1):39–48. doi: 10.1111/j.1432-1033.1985.tb09066.x. [DOI] [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Brian C. A., Lawrence D. S., Kaiser E. T., Weinstein I. B. Protein kinase C phosphorylates the synthetic peptide Arg-Arg-Lys-Ala-Ser-Gly-Pro-Pro-Val in the presence of phospholipid plus either Ca2+ or a phorbol ester tumor promoter. Biochem Biophys Res Commun. 1984 Oct 15;124(1):296–302. doi: 10.1016/0006-291x(84)90951-3. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. S., Kemp B. E., Su H. D., Kuo J. F. Substrate specificity of phospholipid/Ca2+-dependent protein kinase as probed with synthetic peptide fragments of the bovine myelin basic protein. J Biol Chem. 1985 Sep 25;260(21):11503–11507. [PubMed] [Google Scholar]
- Werth D. K., Niedel J. E., Pastan I. Vinculin, a cytoskeletal substrate of protein kinase C. J Biol Chem. 1983 Oct 10;258(19):11423–11426. [PubMed] [Google Scholar]
- Werth D. K., Pastan I. Vinculin phosphorylation in response to calcium and phorbol esters in intact cells. J Biol Chem. 1984 Apr 25;259(8):5264–5270. [PubMed] [Google Scholar]