Abstract
The evolution of joints, which afford skeletal mobility, was instrumental in vertebrate success. Here, we explore the molecular genetics and cell biology that govern jaw joint development. Genetic manipulation experiments in zebrafish demonstrate that functional loss, or gain, of the homeobox-containing gene barx1 produces gain, or loss, of joints, respectively. Ectopic joints in barx1 mutant animals are present in every pharyngeal segment, and are associated with disrupted attachment of bone, muscles and teeth. We find that ectopic joints develop at the expense of cartilage. Time-lapse experiments suggest that barx1 controls the skeletal precursor cell choice between differentiating into cartilage versus joint cells. We discovered that barx1 functions in this choice, in part, by regulating the transcription factor hand2. We further show that hand2 feeds back to negatively regulate barx1 expression. We consider the possibility that changes in barx1 function in early vertebrates were among the key innovations fostering the evolution of skeletal joints.
Keywords: barx1, hand2, Cartilage, Joint, Skeleton, Zebrafish
INTRODUCTION
The vertebrate head skeleton, including joints, is derived in large part from pluripotent neural crest cells. Following migration into the pharyngeal arches, this field of cells encounters signaling molecules instructing compartmentalization into dorsal, intermediate and ventral domains, which develop into the upper jaw, the jaw joint and joint-associated tissues, and the lower jaw, respectively (Kimmel et al., 1998; Medeiros and Crump, 2012). The domains are delineated by a transcription factor code, which is likely important for cells to differentiate into either joint or cartilage (Talbot et al., 2010; Walker et al., 2006). A well-characterized signaling system instructing this compartmentalization is the endothelin 1 (Edn1) pathway (Kimmel et al., 2007). Mutations in zebrafish Edn1 pathway genes such as furina, which encodes a protease responsible for Edn1 maturation, and hand2, a transcription factor and Edn1 target gene, disrupt domain partitioning. These, and other Edn1 pathway mutants, do not develop a jaw joint (Miller et al., 2007; Miller et al., 2003; Walker et al., 2006; Walker et al., 2007).
barx1 is another transcription factor expressed in the pharyngeal arches (Tissier-Seta et al., 1995) that is regulated by Edn1 signaling in mice and zebrafish (Clouthier et al., 2000; Walker et al., 2006). In wild-type zebrafish, barx1 expression is restricted to dorsal and ventral domains that develop into cartilage elements, but not in the joint-forming intermediate domain. Conversely, barx1 is ectopically expressed in intermediate domain cells, and joints are lost when Edn1 signaling is weakly lost in furina mutants (Walker et al., 2006). In addition, morpholino knockdown experiments suggest barx1 is required for chondrogenesis in zebrafish (Sperber and Dawid, 2008). These findings all motivate the hypothesis that barx1 represses joints and promotes cartilage. However, reduced function of a joint repressor is predicted to yield gain of joints, but a loss of joint phenotype was reported in morpholino-treated animals (Sperber and Dawid, 2008). Furthermore, it is unknown whether expanded barx1 in Edn1 pathway mutants is functional, and factors functioning downstream of barx1 in the skeleton have not been elucidated. Thus, the role of barx1 in the skeleton remains uncertain.
Here, we provide direct evidence supporting the hypothesis that barx1 functions to repress joints and promote cartilage. We examine newly available mutations, two putative null alleles, in barx1 and show that the pharyngeal cartilages of barx1 mutant zebrafish display focal gaps. The cellular and molecular characteristics of these gaps support our interpretation that they are ectopic joints. We further find that bone, tooth, and muscle attachments are disrupted in barx1 mutants, and altered attachments coincide with sites of ectopic joints. Analysis of compound mutants, as well as direct transgenic misexpression, provides genetic evidence that ectopic barx1 in Edn1 pathways mutants is functionally sufficient for joint repression. Genetic and molecular epistasis experiments together with time-lapse imaging demonstrate that hand2 functions downstream of, and is negatively regulated by, barx1 during differentiation. We also discovered that hand2 feeds back to regulate barx1 expression. Based on our findings, we conclude that barx1 functions to control cartilage versus joint identity in the lower jaw, and likely also in the other pharyngeal segments.
MATERIALS AND METHODS
Zebrafish mutants and transgenic constructs
Fish were raised and staged as described previously (Kimmel et al., 1995). Mutant alleles of barx1 were generated by TILLING (targeting induced local lesions in genomes) (Draper et al., 2004). Both barx1 alleles behaved in a Mendelian recessive fashion with complete penetrance and larval lethality. Genotyping was carried out for barx1fh330 with primers CTTCTGTTTTTCAAATCGTTTTTCT and GGCTAACACACACTCTTATTTCATTC, and digestion with MseI. barx1fh331 was genotyped using ATAGATCTGGCAGAGTCCTTAAGCT and ACCTTCAAAACAATGATAGGTTTACATTT, and digestion with HindIII. The hand2:Crimson reporter line was generated by crossing two independently generated stable transgenic lines Tg(hand2:GAL4VP16)b1228 and Tg(UAS:E2Crimson;cmlc2:EGFP)b1229. We generated the hand2:GAL4VP16 construct with the 2573 nucleotides upstream of the hand2 initiating methionine codon and the Tol2 kit (Kwan et al., 2007). This construct contains a conserved hand2 pharyngeal arch enhancer (Charité et al., 2001), and a control construct lacking this enhancer did not drive pharyngeal arch expression (data not shown). The UAS:E2Crimson;cmlc2:EGFP construct was generated with E2Crimson (Clontech) and the Tol2 kit. The fli1a:NLSmCherry;cmlc2:EGFP construct was generated with the p5E-fli1a-F-hsp70I 5′ entry vector (Das and Crump, 2012) and the Tol2 kit. The fli1a:barx1;cmlc2:EGFP construct was created by first amplifying full-length barx1 cDNA (Open Biosystems) and inserting the product into p221 to generate pME-barx1, which was then recombined with p5E-fli1a-F-hsp70I and the Tol2kit to generate the injection construct. The following lines have been described previously: Df(chr01:hand2)s6 (Yelon et al., 2000), furinatg419 (Walker et al., 2006), sox9azc81Tg (DeLaurier et al., 2012), trps1j127aGt (Talbot et al., 2010), Tg(sox10:mRFP)vu234 (Kirby et al., 2006) and Tg(fli1a:EGFP)y1 (Lawson and Weinstein, 2002).
Tissue labeling
Alcian Blue and Alizarin Red stains on fixed animals and vital staining with Alizarin Red were performed as described previously (Kimmel et al., 2010; Walker and Kimmel, 2007). Staining with fluorescently labeled phalloidin (Invitrogen) was performed overnight. Staining with anti-col2 (II-II6B3-DSHB) and GaM Alexa546 followed treatment with trypsin and hyaluronidase. Staining with SYTO 59 was carried out as described previously (Huycke et al., 2012).
Whole-mount in situ hybridization was carried out as previously described (Talbot et al., 2010) with either fluorescent or immunohistochemical detection. The following in situ probes have been previously described: barx1 (Walker et al., 2006), sox9a (Yan et al., 2002), chd (Schulte-Merker et al., 1997), hand2 (Angelo et al., 2000) and nkx3.2 (Miller et al., 2003).
Microscopy
Skeletal preparations and immunohistochemical in situ stained animals were imaged on a Zeiss Axiophot 2. Static confocal images were captured on either a Zeiss LSM 5 Pascal confocal or a Leica SD6000 spinning disk confocal. Images were assembled in ImageJ and Photoshop, with any adjustments applied to all panels. For time-lapse movies, animals were imaged as described previously (Huycke et al., 2012). Movies were assembled using Metamorph (Molecular Devices) and ImageJ.
RESULTS
barx1 mutant zebrafish exhibit gaps within segmentally homologous pharyngeal cartilages
To identify null alleles of barx1, we searched for mutations by screening genomic DNA from a library of ENU mutagenized zebrafish (Draper et al., 2004). We found 29 mutations in the barx1 gene, including two recovered putative null nonsense mutations conferring early stop codons in the homeobox at L150 (barx1fh330) and L174 (barx1fh331). barx1 homozygous mutant larvae are readily identified by a prominent indentation in their lower jaw, which is visible by light microscopy (Fig. 1B, arrow). Staining the skeleton with Alcian Blue and Alizarin Red to mark cartilage and bone revealed this indentation is due to a prominent cartilage loss, or gap that divides Meckel’s cartilage in mutants (Fig. 1D, arrow). Following dissection and close examination of Alcian Blue Alizarin Red stained skeletons, we discovered a number of remarkable skeletal defects, including gaps dividing Meckel’s cartilage, the ceratohyal cartilage (Fig. 1F, mj, chj) and all five of the ceratobranchial cartilages (supplementary material Fig. S1D, cbj), all of which are considered to be segmentally homologous (Kimmel et al., 2001). Additionally, the dorsal arch 1 pterygoid process is shortened (Fig. 1F, ptp) (supplementary material Table S1) and the dorsal arch 2 hyosymplectic cartilage is variably reduced or notched in barx1 mutants (Fig. 1F, hs) (supplementary material Fig. S1H-J; Table S1). The phenotypes are recessive; heterozygotes appear phenotypically wild type. Importantly, the two putative null alleles demonstrate like phenotypes (supplementary material Table S1) and fail to complement (supplementary material Fig. S1A-D), providing compelling genetic evidence that the phenotypes we observe in these two lines are due to mutations in the barx1 gene. For consistency, we show results with only the barx1fh331 allele in all subsequent figures.
Fig. 1.
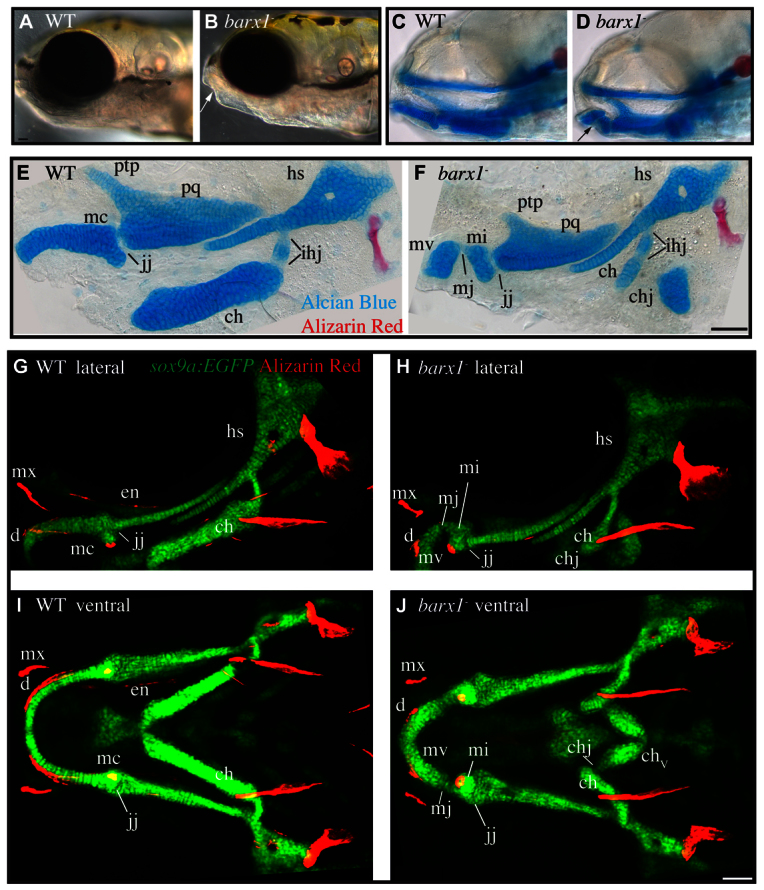
Cartilage and dermal bone phenotypes present in barx1 mutant zebrafish. (A,B) Live 5 days post-fertilization (dpf) zebrafish larvae were imaged with transmitted light. Arrow indicates lower jaw divot. (C,D) 5 dpf zebrafish stained with Alcian Blue and Alizarin Red to label cartilage and bone were imaged with transmitted light. Eyes were removed to allow visualization of skeleton. Arrow indicates lower jaw skeletal gap. (E,F) 4 dpf Alcian Blue and Alizarin Red stained skeletons were dissected and imaged with transmitted light. (G-J) Confocal projections of live 6 dpf larvae with chondrocytes transgenically labeled with EGFP (sox9a:EGFP, green), and bone labeled with Alizarin Red. (A-H) Lateral views, anterior is towards the left, dorsal is upwards. (I,J) Ventral views, anterior is towards the left, whereas left is upwards. Arch 1 elements include the pterygoid process (ptp), palatoquadrate (pq) and Meckel’s (mc) cartilages. The jaw joint (j) and the ectopic Meckel’s joint (mj) are described in cellular detail below. The independent elements resulting from the ectopic arch 1 joint are referred to as Meckel’s cartilage ventral (mv) and Meckel’s cartilage intermediate (mi). Arch 1-derived dermal bones indicated are the dentary (d), maxilla (mx) and entopterygoid (en). Arch 2-derived cartilages include the ceratohyal (ch) and hyosymplectic (hs), which are divided by the interhyal joints (ihj) and the ectopic ceratohyal joint (chj). Scale bars: 50 μm.
To examine chondrocyte arrangement, and the orientation of cartilage elements in live animals, we used the transgenic line sox9a:EGFP, which labels all chondrocytes (Talbot et al., 2012). These studies revealed gaps in sox9a-expressing cells at the jaw joint, as well as the Meckel’s and ceratohyal cartilage gaps in mutants (Fig. 1G-J, jj, mj, chj). In addition, the distalmost segments of the ceratohyals near the midline are reversed along the anterior-posterior axis. Live Alizarin Red staining of larvae revealed a suite of bone defects in barx1 mutants. The arch 1 ventrally and dorsally derived dentary and maxillary dermal bones are dysmorphic, while the arch 1 dorsally derived entopterygoid bone is absent (Fig. 1G-J, d, mx, en). Of note, these dermal bones all associate with the cartilage skeleton near sites of cartilage defects, suggesting the bone defects are secondary consequences of dysmorphic cartilage.
barx1 is required for tooth and musculoskeletal attachment
barx1 is required for proper oral tooth development in mice (Miletich et al., 2011), and barx1 is expressed in the pharyngeal dentition in cichlids and zebrafish (Fraser et al., 2009; Sperber and Dawid, 2008). Zebrafish pharyngeal tooth development progresses through initiation, morphogenesis, matrix deposition and attachment (Van der Heyden and Huysseune, 2000). We find that teeth in barx1 mutants progress thorough matrix deposition normally, yet do not undergo attachment (Fig. 2A,B). Interestingly, at the site where the teeth would normally ankylose to the ceratobranchial 5 bones (Fig. 2B, arrowhead), we observe an ectopic gap (Fig. 2B, arrow). These findings suggest barx1 is not required for tooth initiation and morphogenesis but is required to specify the bone of attachment.
Fig. 2.
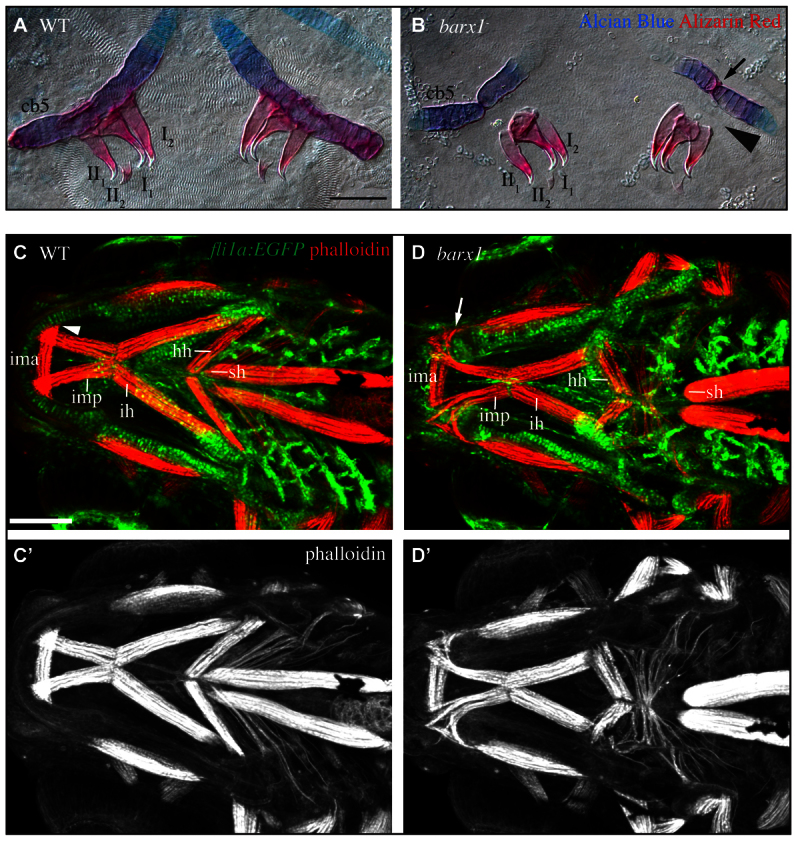
barx1 mutant zebrafish display tooth and muscle phenotypes. (A,B) 5 dpf Alcian Blue- and Alizarin Red-stained ceratobranchial 5 (cb5) and pharyngeal teeth (annotated I1-II2) were dissected and imaged with transmitted light. Arrow indicates ectopic joint in cb5. Arrowhead illustrates missing bone of attachment. Ventral view, anterior is upwards, right is towards the left. Scale bar: 50 μm. (C,D) Confocal projections of 5 dpf larvae with pharyngeal arch derivatives transgenically labeled with EGFP (fli1a:EGFP, green) and muscles stained with phalloidin (red). Ventral view, anterior is towards the left, left is upwards. Labeled muscles include the intermandibularis posterior (imp), intermandibularis anterior (ima), interhyoideus (ih), hyohyoideus (hh) and sternohyoideus (sh). Arrowhead indicates imp attachment site in wild types, arrow indicates disorganized imp invading the ectopic gap in Meckel’s cartilage in mutants. (C′,D′) Single channel phalloidin fluorescence. Scale bar: 100 μm.
Craniofacial muscles also attach to the larval skeleton (Schilling and Kimmel, 1997). We tested the hypothesis that, like teeth, muscles require barx1 for proper association with the skeletal scaffold by labeling craniofacial muscles with phalloidin in fli1a:EGFP larvae. These analyses revealed several muscle groups are out of place in barx1 mutants (Fig. 2C,D). The intramandibular posterior muscles, which in wild types attach to Meckel’s cartilages (Fig. 2C, arrowhead) at or near the site that forms the cartilage gap in mutants, are disorganized and invade the gap (Fig. 2D, arrow). In barx1 mutants, the sternohyal muscles are shorter and fail to attach in the manner we see in wild type (Fig. 2C,D, sh). The hyohyal muscles reverse their orientation along the anterior-posterior axis, similar to the ceratohyal cartilage (Fig. 2C,D, hh). Our data suggest pharyngeal teeth and cranial muscles are properly specified but association with their skeletal scaffold is disrupted in barx1 mutants.
The reported morpholino phenotype (Sperber and Dawid, 2008) very poorly represents loss of function of barx1. None of the mutant phenotypes described above - focal gaps in ventral cartilages, loss of tooth and muscle attachments, and dermal bone phenotypes - were reported in the morpholino knockdown study. Conversely, in our studies with barx1-null mutants we did not see any of the phenotypes of the morpholino-injected animals. For example, we never observed a general loss in Alcian Blue staining, joint fusions or gaps between the hyomandibular and symplectic cartilages in barx1 mutants. Although we do observe a range of phenotypic severities (supplementary material Fig. S1E-P; Table S1), loss of cartilage occurs consistently at discrete locations, at variance with the morpholino knockdown study.
barx1 is expressed in an arch 1 subdomain during chondrocyte differentiation
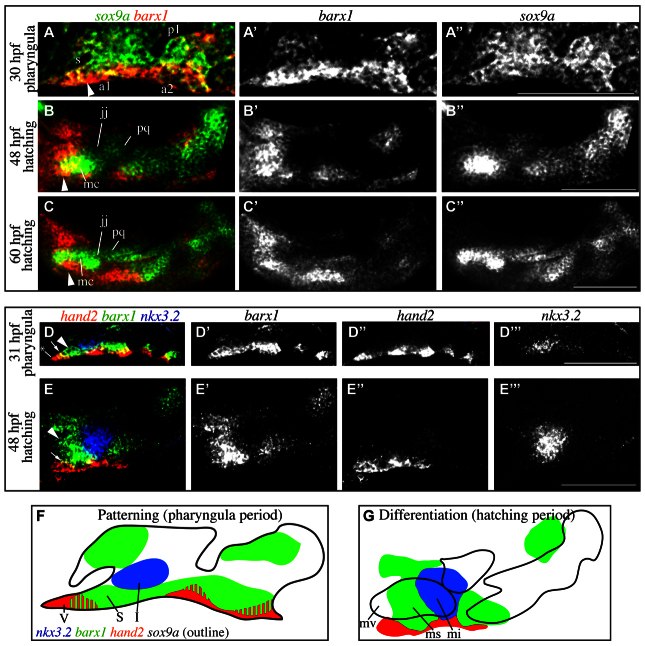
How does expression of barx1, which is known to be restricted to dorsal and ventral cartilage-forming domains, and absent from the joint-forming intermediate domains (Walker et al., 2006), explain the presence of gaps in the ventral cartilages when its function is lost? To help identify its possible function in chondrocyte differentiation, we monitored barx1 expression along with the transcription factor sox9a, which is expressed in both early pharyngeal arches and differentiating chondrocytes (Talbot et al., 2010; Yan et al., 2002). In the pharyngula period (monitored at 30 hpf), broad weak sox9a expression is detected in the ectomesenchyme of the pharyngeal arches, while a strong barx1 expression domain is present ventral to stomodeum (Fig. 3A, arrowhead). During the hatching period (48 hpf) overt cartilage differentiation occurs, evidenced by strongly upregulated sox9a in differentiating cartilage cells of individual skeletal elements (Fig. 3B, mc, pq) relative to weakly labeled joint regions (Fig. 3B, jj). At this stage of differentiation, the strongest barx1 expression domain resolves into a transverse stripe across the developing Meckel’s cartilage distal to the developing retroarticular process near the site where the cartilage gap would develop in barx1 mutants (Fig. 3B, arrowhead). Later in the hatching period (60 hpf), barx1 remains undetectable in the arch 1 joint region. Strong barx1 expression is maintained distal to the joint in cells sheathing a region of Meckel’s cartilage (Fig. 3C, arrowhead).
Fig. 3.
barx1 is excluded from differentiating joint cells but maintained in the subintermediate domain. (A-C′) In situ hybridization was carried out to reveal wild-type barx1 expression (red) in the context of sox9a expression (green). (D-E′′) Fluorescent in situ hybridization revealed wild-type barx1 expression (green) in the context of hand2 (red) and nkx3.2 (blue) expression. Labeled pharyngula period anatomy includes the stomodeum (s), pharyngeal arch 1 (a1), pharyngeal pouch 1 (p1) and pharyngeal arch 2 (a2). Labeled hatching period anatomy includes the Meckel’s (mc) and palatoquadrate cartilages (pq), which are divided by the jaw joint (jj). Arrowheads indicate barx1 expression distal to the jaw joint in the subintermediate domain. Arrows indicate overlap between barx1 and hand2. Asterisk in D marks the ventral-most aspect of hand2 expression. In all images, anterior is towards the left, dorsal is upwards. All images are single confocal sections. Scale bars: 100 μm. (F,G) Outlines of gene expression data in A-E′′. Intermediate (I), subintermediate (S) and ventral (V) domains, and Meckel’s cartilage intermediate (mi), subintermediate (ms) and ventral (mv) are indicated.
Previous works have identified markers, nkx3.2 (formerly bapx1) and hand2, as functional regulators expressed in the intermediate and ventral domains, respectively (Miller et al., 2003; Talbot et al., 2010). Based on these studies, we were surprised to discover that hand2 and nkx3.2 do not directly juxtapose in ventral arch 1 at 31 hpf. Instead, barx1 is expressed in the region between hand2 and nkx3.2 (Fig. 3D, arrowhead), in addition to partially overlapping with hand2 (Fig. 3D, arrow). barx1 expression does not extend all the way to the ventral-most aspect of the first arch, as hand2 does (Fig. 3D, asterisk). By 48 hpf, nkx3.2, barx1 and hand2 expression domains have mostly resolved to be exclusive of one another (Fig. 3E). These expression results reveal the presence of a previously not recognized ‘subintermediate’ domain in the ventral first arch, between the intermediate and ventral domains. We identify the subintermediate domain by expression of barx1 in the absence of both nkx3.2 and hand2.
Meckel’s cartilage gaps exhibit hallmarks of joint identity
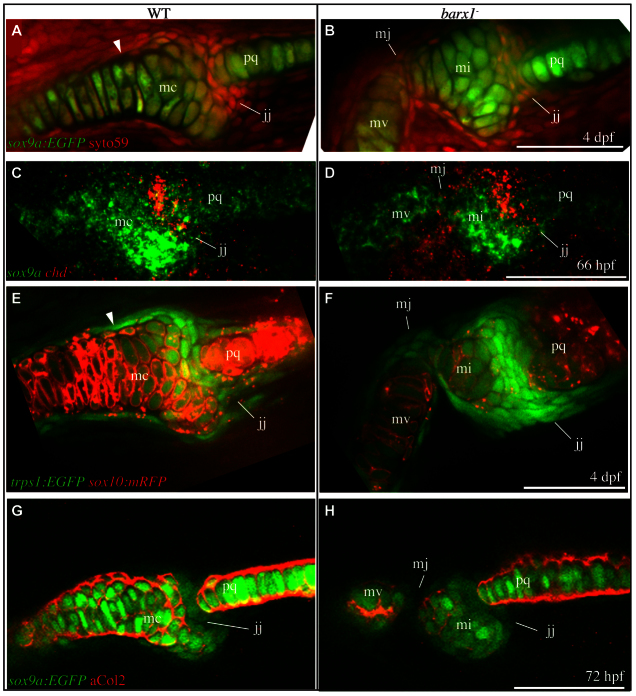
Loss of barx1 expression in the first arch subintermediate domain might explain the presence of the Meckel’s cartilage gap at what would seem to be the corresponding location in barx1 mutants after skeletal differentiation. In this scenario, the loss of subintermediate expression would mimic the absence of barx1 expression in the wild-type intermediate domain and confer joint rather than cartilage identity, yielding a ‘gap’ that may in fact be an ectopic joint. We queried several aspects of cartilage and joint cellular identity during late hatching and early larval periods to examine this hypothesis. Initially, we examined the morphology of cells near the jaw joint by fluorescently labeling all cells with the nucleic acid stain SYTO59, in combination with the transgenic chondrocyte marker sox9a:EGFP at 4 days post-fertilization. Inspection of wild-type animals revealed large chondrocytes labeled with EGFP, bounded by flat perichondrial cells brightly labeled with the nucleic acid stain. In addition, a population of densely packed, flattened cells populates the interface between Meckel’s and palatoquadrate cartilages in wild type (Fig. 4A, jj). Adhering to our previous definition of the mesenchyme connecting early larval skeletal elements (Talbot et al., 2010), we refer to these as joint cells. The morphology of these cells is similar to that described for appendicular (limb) interzone joint precursor cells in other vertebrates (Hartmann and Tabin, 2001; Mitrovic, 1977). We have observed direct kinematic evidence that the larval jaw joint is freely mobile in zebrafish at the time of this analysis (4 dpf, supplementary material Movie 1). In barx1 mutant animals, we find an additional population of flattened joint cells at the Meckel’s gap, supporting that the gap is an ectopic Meckel’s joint (Fig. 4B, mj), and kinematic studies in non-anesthetized fish demonstrate some mobility mediated by the ectopic joint (supplementary material Movie 1).
Fig. 4.
Ectopic joint cells are present in barx1 mutant zebrafish. (A,B) All cells were stained with SYTO59 (red), and chondrocytes transgenically labeled with EGFP (sox9a:EGFP, green) in 4 dpf animals. (C,D) 66 hpf larvae were subjected to in situ hybridization to label cartilage (sox9a, green) and joint cells (chd, red). (E,F) Live larvae with chondrocyte cell membranes transgenically labeled with RFP (sox10:mRFP, red) and joint region cells labeled with EGFP (trps1:EGFP, green) were imaged. (G,H) 72 hpf larvae with chondrocytes transgenically labeled with EGFP (sox9a:EGFP, green) were stained with anti-collagen type II antibody (red). Skeletal elements are oriented and labeled as in Fig. 1F. Arrowhead indicates boundary between distinct chondrocyte populations. All images are single confocal sections. Scale bars: 50 μm.
Close examination of wild types revealed that Meckel’s cartilage chondrocytes can be divided into two distinct populations based on morphology. The jaw joint-adjacent population of chondrocytes consists of rounded interlocking cells, whereas cells more distal to the joint exhibit a columnar and stacked morphology. Of particular interest, the boundary between these two populations (Fig. 4A, arrowhead) corresponds to the location of the ectopic Meckel’s joint in barx1 mutants.
Late in the hatching period in wild-type fish, chondrocytes express high levels of sox9a and joint cells express high levels of chordin (Fig. 4C). Hence, these markers reveal cells executing either the cartilage or joint program. At the site of the ectopic Meckel’s joint in mutants, sox9a expression is minimally detected, consistent with our findings with sox9a:EGFP. Meanwhile, ectopic chordin expression is detected in mutants in and around the ectopic Meckel’s joint (Fig. 4D, mj). Comparison with wild-type controls suggests cells that would express sox9a in wild types express chordin in mutants.
The transgenic line sox10:mRFP marks cartilage cell membranes with red-fluorescent protein. Conversely, cells in the wild-type jaw joint region are labeled in the transgenic line trps1:EGFP. Imaging live double transgenic wild-type animals during the larval period revealed the brightest trps1 expressing cells to be joint cells populating the interface between Meckel’s and palatoquadrate cartilages (Fig. 4E, jj). In transgenic barx1 mutants, sox10:mRFP expression is reduced in conjunction with increased trps1:EGFP expression, when compared with wild type (Fig. 4F, mj). trps1:EGFP also weakly labels wild-type chondrocytes proximal to the joint (for example, chondrocytes of the retroarticular process), indicating these joint-adjacent chondrocytes have an intermediate identity distinct from more ventral chondrocytes, which are minimally labeled. The boundary between these two types of chondrocytes in wild type is located at the site of the ectopic Meckel’s joint in barx1 mutants (Fig. 4E, arrowhead).
The reduced sox9a and sox10 expression at the ectopic Meckel’s joint in barx1 mutants (Fig. 4D,F) strongly suggests that the chondrogenic program has failed to initiate. To test whether chondrocyte cellular function is also absent, we immunostained for collagen type II, a hallmark of cartilage matrix (Kronenberg, 2003), in conjunction with transgenic labeling with the chondrocyte-specific transgene sox9a:EGFP (Fig. 4G, jj). At 72 hpf, transgenically labeled chondrocytes are present in wild-type Meckel’s and palatoquadrate cartilages, which are divided by the weakly sox9a:EGFP-expressing joint region. Chondrocytes actively secrete collagen type II at this time, indicated by immunoreactivity surrounding the cartilage cells (Fig. 4G, mc, pq), whereas collagen type II is not detected in the jaw joint (Fig. 4G, jj). In barx1 mutants, transgenically labeled chondrocytes of the palatoquadrate as well as both the independent elements of the divided Meckel’s cartilage (Fig. 4H, mi, mv) secrete collagen type II, whereas collagen is undetectable in both the endogenous and ectopic joints (Fig. 4H, jj, mj).
These analyses of chondrocyte and joint cell identity suggest that joint cells are gained at the expense of chondrocytes in barx1 mutants. This conclusion motivates the hypothesis that undifferentiated precursor cells in the subintermediate domain have the potential to differentiate into either chondrocytes (as they do in wild type), or joint cells (as in barx1 mutants). To test this hypothesis, we monitored ectomesenchyme cells as they differentiated into joint cells by monitoring trps1:EGFP expression (supplementary material Movie 2, green). Analysis of wild-type animals revealed that the trps1:EGFP reporter is initially broadly expressed at low levels in precursor cells (50-53 hpf). By 60 hpf in wild types, differentiating joint cells begin to adopt their characteristic morphology and upregulate trps1:EGFP. By 70 hpf, a stripe of very bright trps1:EGFP-positive joint cells can be seen adjacent to the more weakly expressing chondrocytes of Meckel’s cartilage intermediate region, as well as the palatoquadrate. In barx1 mutants, the broad weak trps1 reporter expression from 50-53 hpf in undifferentiated cells looks similar to that in wild types. However, by 60 hpf, barx1 mutants begin to display an excess of brightly trps1:EGFP-expressing cells compared with wild types, and by 70 hpf a bulbous accumulation of bright trps1:EGFP cells, which we interpret to be ectopic joint cells, is present in barx1 mutants.
To see whether tissue drift or cell migration contributes to the ectopic joint cells, we enlarged single confocal sections from the recordings and tracked small groups of isolated hand2:Crimson-positive undifferentiated cells and saw no such movement (supplementary material Movie 3, arrow). Specifically, as tracked cells differentiated they remained distal to the developing jaw joint at positions where cartilage or joints develop in wild types or mutants, respectively (Fig. 4A-H). Hence, these findings suggest that cell rearrangement does not contribute to the phenotypic changes between wild types and mutants, and that subintermediate domain barx1 is necessary for ectomesenchyme to differentiate into cartilage versus joints.
Joint loss in furina mutants requires barx1
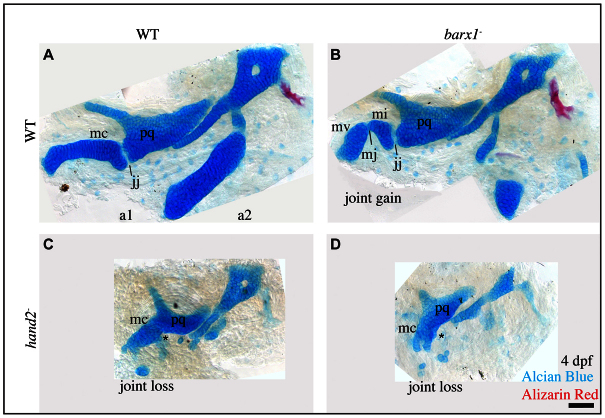
The hypothesis that barx1 represses joint formation, predicts that upregulation of barx1 in the intermediate domain should lead to the loss of the endogenous jaw joint. We can examine this prediction genetically: both expanded barx1 and joint loss are present when Edn1 signaling is partially lost, as in furina mutants (Walker et al., 2006), and our hypothesis predicts that the joint loss in these mutants depends on barx1 being functional in the furina mutant background. It follows that barx1;furina double mutants should not show joint loss. Consistent with our previous studies, we found that furina single mutants have a joint-loss phenotype (Fig. 5C, asterisk), as opposed to the joint-gain phenotype in barx1 single mutants (Fig. 5B, mj). Strikingly, we discovered that mutations in barx1 partially rescue the joint-loss phenotype seen in furina single mutants (Fig. 5D, jj; supplementary material Table S2). These findings suggest that the loss of joints in furina mutants is, at least in part, due to ectopic barx1. Moreover, the discovery that barx1 is epistatic to furina in compound mutants is consistent with our understanding of the order of gene activity: barx1 acts downstream of furina.
Fig. 5.
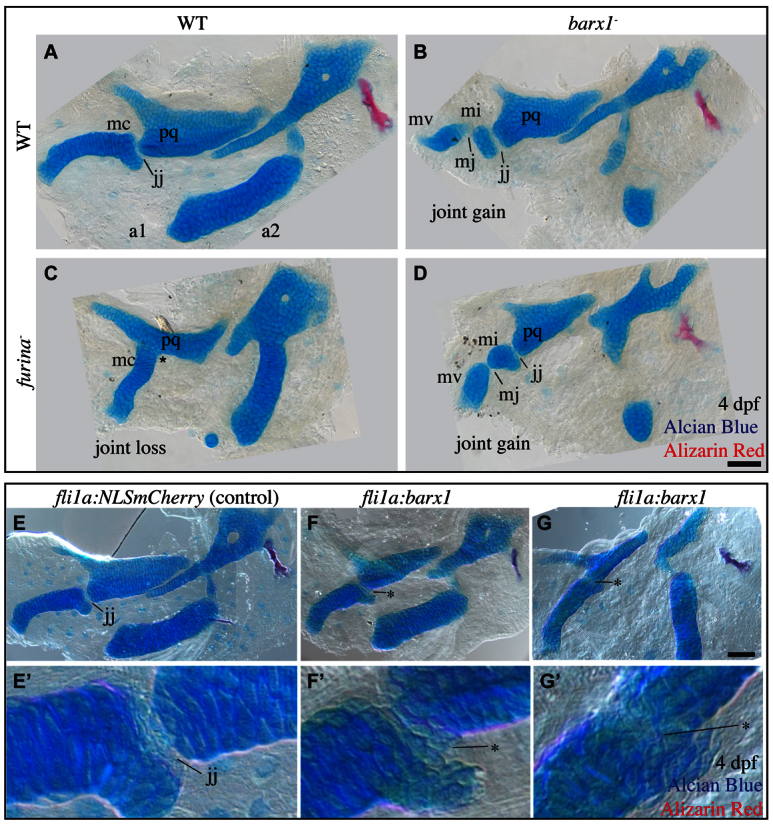
Ectopic barx1 represses the jaw joint. (A-D) Alcian Blue- (cartilage) and Alizarin Red- (bone) stained 4 dpf larval zebrafish skeletons were dissected and imaged with transmitted light. An asterisk indicates jaw joint loss. Skeletal elements are oriented and labeled and as in Fig. 1F. Anterior is towards the left; dorsal is upwards in all panels. Scale bar: 50 μm. (E-G′) 4 dpf larval zebrafish skeletons from animals injected with misexpression constructs and stained with Alcian Blue (cartilage) and Alizarin Red (bone) were imaged with transmitted light. Asterisks indicate joint loss. Scale bar: 50 μm.
To further test the hypothesis that barx1 is sufficient to repress joint formation, we designed an experiment to directly overexpress barx1. Injection of a control construct encoding nuclear mCherry protein under the control of the fli1a promoter (Das and Crump, 2012) results in mosaics in which clones of transgenic cells populate the pharyngeal arches, including occasionally the intermediate domain (data not shown). Animals injected with a construct encoding barx1 driven by the fli1a promoter, display variable joint loss, including clones of ectopic chondrocytes in the jaw joint (Fig. 5F, asterisk), as well as complete jaw joint fusions (Fig. 5G, asterisk). We conclude barx1 is both necessary (Fig. 4) and sufficient (Fig. 5) for joint repression.
hand2 is required for endogenous and ectopic joints, and is negatively regulated by barx1
The above analyses show barx1 functions in the Edn1 signaling pathway to repress joints. By contrast, the Edn1 target gene hand2, in addition to its being required for ventral cartilage development, is required for jaw joint formation (Miller et al., 2003), suggesting the two transcription factors might negatively interact. Epistasis analysis supports such interaction: in hand2;barx1 double mutants, the ectopic Meckel’s joint characteristic of barx1 single mutants is rescued (Fig. 6D). Furthermore, the endogenous jaw joint is lost as in the hand2 single mutants (Fig. 6C, asterisk). Hence, the two genes interact genetically, with hand2 epistatic to barx1 for both phenotypes (supplementary material Table S3). These data place hand2 downstream to barx1 in a negative regulatory pathway (Avery and Wasserman, 1992).
Fig. 6.
hand2 is epistatic to barx1. (A-D) Alcian Blue- (cartilage) and Alizarin Red- (bone) stained 4 dpf larval zebrafish skeletons were dissected and imaged with transmitted light. An asterisk indicates jaw joint loss. Skeletal elements are oriented and labeled and as in Fig. 1F. Anterior is towards the left; dorsal is upwards in all panels. Scale bar: 50 μm.
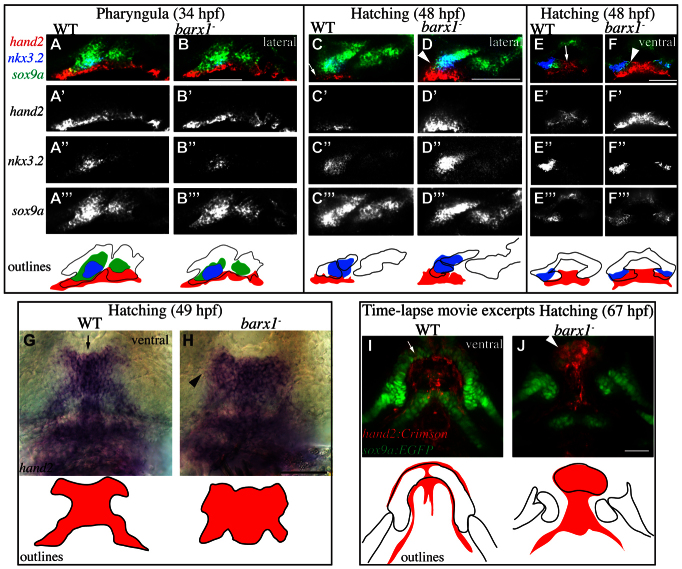
Negative regulation of hand2 by barx1 is also indicated by in situ hybridization. We observed no consistent difference in hand2 expression between wild types and barx1 mutants during the pharyngula period (Fig. 7A,B). By contrast, during the hatching period, hand2 expression is dorsally expanded in barx1 mutants (Fig. 7C-H). Specifically, in wild-type animals, hand2 expression is restricted to ventral undifferentiated midline cells of the developing first arch skeleton (Fig. 7C,E,G, arrows), whereas in barx1 mutants hand2 is upregulated and dorsally expanded towards the joint (Fig. 7D,F,H, arrowheads). Interestingly, hand2 expands to fill the domain normally occupied by barx1 (Fig. 3E, arrowhead), resulting in an ectopic boundary between hand2 and the intermediate domain gene nkx3.2 (Fig. 7D,F, arrowhead). These results strongly suggest that barx1 represses hand2.
Fig. 7.
barx1 negatively regulates hand2. (A-F′′) Wild-type and barx1 mutant animals were subjected to fluorescent in situ hybridization to reveal sox9a, nkx3.2 and hand2 expression during the pharyngula (A-B′′) and hatching (C-F′′) periods. Arrow indicates ventrally restricted hand2; arrowhead indicates expanded hand2 expression. All panels are single confocal sections. (G,H) Animals were subjected to in situ hybridization to reveal midline-restricted hand2 expression in wild type (arrow) and expanded hand2 expression in barx1 mutants (arrowhead). (I,J) hand2-expressing cells were transgenically labeled with E2Crimson (hand2:Crimson, red) and chondrocytes with EGFP (sox9a:EGFP, green), and imaged by time-lapse microscopy. Movie excerpts are z-projections from the ventral perspective. Arrow indicates downregulated hand2 reporter in differentiated cartilages; arrowhead indicates persistent hand2 reporter. Dorsal is upwards and anterior is towards the left in A-D; anterior is towards the top, while right is towards the left in E-J. Scale bars: 100 μm.
That hand2 expression is unchanged in barx1 mutants in the pharyngula period, but is expanded during differentiation in the hatching period, motivates the hypothesis that barx1 downregulates hand2 only as cells differentiate, as we could examine by time-lapse analysis with transgenic reporters. In wild types before chondrocyte differentiation begins, our hand2 reporter hand2:Crimson is strongly expressed in the ventral undifferentiated ectomesenchyme (supplementary material Movie 4, note lack of the green chondrocyte reporter sox9a:EGFP in early frames). As chondrocyte differentiation initiates and the sox9a reporter is upregulated (in addition to the expressing cells organizing and enlarging into their characteristic chondrocyte morphology), the hand2 reporter downregulates in the differentiating chondrocytes of Meckel’s cartilage, compared with the strong expression in the surrounding perichondrium (Fig. 7I, arrow; supplementary material Movie 4). In barx1 mutants, by contrast, hand2 fails to downregulate as efficiently, resulting in an expanded hand2 domain (Fig. 7J, arrowhead), consistent with the results of our static in situ experiments.
hand2 regulates barx1 expression
Expression of hand2 and barx1 partially overlap during the pharyngula period, but expression resolves into exclusive domains during the hatching period, as might be explained by reciprocal negative interaction, and as we examined by in situ hybridization for barx1 in hand2 mutants. During the pharyngula period, we found decreased ventral barx1 expression in hand2 mutants (Fig. 8B, arrow). By contrast, we observed a large expansion in ventral barx1 expression during the hatching period (Fig. 8D, arrowhead). Hence, repression is dynamic and complex: hand2 activates ventral barx1 expression during the pharyngula stage, yet represses barx1 expression during the hatching period.
Fig. 8.
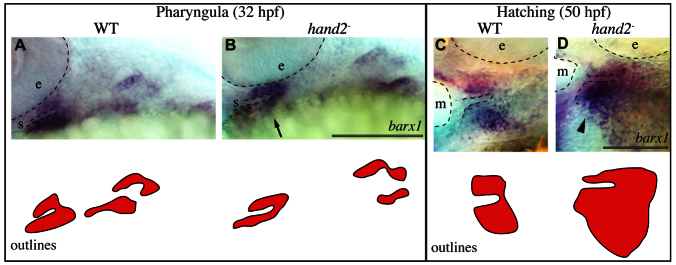
hand2 regulates barx1 positively and negatively early and late, respectively. (A-D) barx1 expression was revealed by in situ hybridization and imaged with transmitted light. Labeled pharyngula period anatomy includes the stomodeum (s) and the eye (e). Labeled hatching period anatomy includes the mouth (m) and the eye (e). Arrow and arrowhead indicate reduced and expanded ventral barx1 expression, respectively. Scale bars: 100 μm.
DISCUSSION
The evidence presented here supports our conclusion that barx1 functions in skeletal precursor cells to promote cartilage development, and repress joints, such that loss of barx1 function yields decreases in cartilage identity and increases in joint identity. Furthermore, gain of barx1 function yields increases in cartilage identity and decreases in joint identity. In at least the first pharyngeal arch, and probably also in the other arches, the ectopic joint-forming site is likely located at the boundary between the intermediate domain and our newly described subintermediate domain, in which barx1 is expressed exclusively of both nkx3.2 (in arch one) and hand2 (Fig. 9). We provide genetic evidence that hand2 functions downstream of barx1 and is not only required for the endogenous jaw joint, as previously shown (Miller et al., 2003), but also for the ectopic joint in barx1 mutants. Our molecular analyses reveal a reciprocal regulatory circuit in which hand2 and barx1 regulate one another. These findings all support a model in which barx1 acts in the endothelin 1 pathway to repress joint development. In this model, barx1 joint repressive function must be removed from the endogenous joint anlage to allow the articulation to develop. Otherwise, the cells of the endogenous joint region develop as chondrocytes, just as they normally do at the intermediate-subintermediate domain boundary in the wild-type embryo.
Fig. 9.
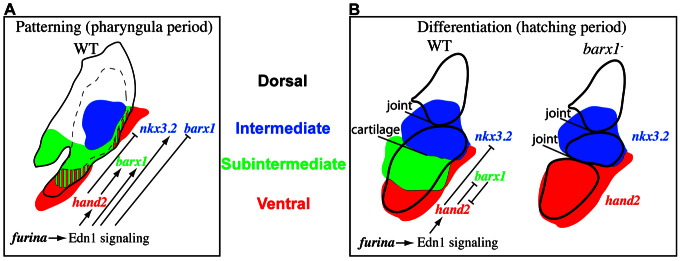
Model for domain patterning and joint versus cartilage cell differentiation. (A) Schematic of patterning domains and gene interactions in wild-type animals during the pharyngula period. (B) Schematic of patterning domains, gene interactions and differentiating cell types in wild-type and barx1 mutant animals during the hatching period.
barx1 repression prevents skeletal boundaries from developing into joints
We discovered that two separate populations of chondrocytes exist in Meckel’s cartilage. A population located adjacent to the jaw joint, and distinct from the joint cells themselves, consists of cells that are trps1 and nkx3.2 positive, and have a rounded morphology. A second population distal to the joint is trps1 and nkx3.2 negative, with stacked, columnar cell morphology. We propose that a cryptic boundary with the potential to develop into a joint separates these two cartilage modules in wild-type animals. Moreover, that organ attachment sites correlate with ectopic joint sites suggest some unknown singular characteristic is present in wild types that marks the location where ectopic joints form in mutants. Through removal of barx1, the singularity of this location is clearly revealed, as joint repression is lifted and the cryptic joint is unveiled. A simple body plan (hidden boundaries/joints) seemingly is layered on top of a more complex one.
The data presented here demonstrate that during patterning, Edn1 signaling precisely positions barx1 such that the joint-repressive properties of barx1 function to prevent the ectopic Meckel’s joint, but not the jaw joint from developing (Fig. 9A). Other Edn1 pathway mutants, such as edn1, plcb3 and mef2ca, also have ectopic barx1 in the intermediate domain and lack joints (Miller et al., 2000; Miller et al., 2007; Walker et al., 2006; Walker et al., 2007). Our model predicts that removing barx1 function from these mutants (by generating compound mutants) would rescue the joint loss phenotype similar to the results obtained in barx1;furina double mutants. The model in Fig. 9 is incomplete however, for in addition to the endothelin 1 pathway, a number of other signaling systems such as BMP and Notch signaling are also crucial for zebrafish joint development. Specifically, misexpression of the BMP ligand bmp4 (Zuniga et al., 2011), a dominant-negative BMP receptor dnBmpr1a (Alexander et al., 2011) or the Notch ligand JAG1 (Zuniga et al., 2010), all result in joint loss. It would be interesting to learn whether these joint loss phenotypes also involve ectopic barx1 in the intermediate domain. In fact, evidence already exists that barx1 expression can be regulated by BMP (Barlow et al., 1999; Sperber and Dawid, 2008; Tucker et al., 1998) or Notch (Mitsiadis et al., 2010) signaling. In this scenario, barx1 would serve as a node for crosstalk between several signaling pathways that sculpt the joint.
We have previously demonstrated that trps1:EGFP expands in hand2 mutants (Talbot et al., 2010), and here we show trps1:EGFP also expands in barx1 mutants. Yet, we conclude these genes, barx1 and hand2, have opposite effects on cell fate: hand2 is required for joints, whereas barx1 represses joints. This apparent paradox can be reconciled by the fact that in addition to joint cells, trps1:EGFP also marks cartilage cells near the joint (e.g. cartilage of the retroarticular process is trps1:EGFP positive). Therefore, it seems likely that the expanded trps1:EGFP in hand2 mutants represents an expansion of intermediate domain cartilage, not of joint cells. Further supporting this model, cells positive for the intermediate marker nkx3.2 and the cartilage marker sox9a are ventrally expanded in hand2 mutants (Talbot et al., 2010). Yet another line of evidence that joint-adjacent cartilage is expanded in hand2 mutants comes from examining the morphology of ventral cells in hand2 mutants. Morphological joints are invariably lost in hand2 mutants, and rounded interlocking chondrocytes constitute what remains of Meckel’s cartilage in hand2 mutant animals, reminiscent of the Meckel’s cartilage intermediate domain morphology (Fig. 6D).
Support for the hinge and caps model
During the hatching period, the mandibular region of the developing jaw can be subdivided into three nonoverlapping domains (Fig. 9B). The distalmost hand2-positive domain and the proximal nkx3.2 domain flank the subintermediate barx1 expression domain. This configuration fits nicely with the recently proposed hinge and caps model of jaw development (Depew and Compagnucci, 2008; Medeiros and Crump, 2012). Briefly, this model posits a hinge region connects the mandibular and maxillary aspects of arch 1. The maxillary and mandibular regions of the arch have an inherent polarity with a cap region at their distalmost end. In such a model, the barx1 domain between the nkx3.2 hinge and hand2 cap is a module that can be altered during the course of evolution. barx1 (this work) as well as several other genes (Miller et al., 2003; Talbot et al., 2010) are dramatically expanded when the hand2 cap is disrupted, with disastrous consequences for jaw development. Our work supports a model in which ventral-most hand2 expression maintains a cap of undifferentiated cells that serves to keep the cartilage modules dorsally contained during chondrocyte differentiation. That barx1 reciprocally represses hand2 suggests that the differentiating cartilage modules also feed back to restrict the cap. In fact, in barx1 mutants the hand2 cap is dorsally expanded such that it directly juxtaposes the nkx3.2 hinge, suggesting the subintermediate domain module is completely absent in these mutant conditions (Fig. 9B).
barx1 function in the evolution of development
The ability to develop a jointed jaw fostered a revolution in feeding strategies among early vertebrates (Janvier, 1996; Mallatt, 1996). In this study, we propose a model in which barx1 functions as a joint repressor such that increased barx1 leads to joint loss, and decreased barx1 results in joint gain. Therefore, it is tempting to hypothesize that changes in Barx1 function may be relevant to the advent of jaw hinges in evolution. The only available developmental model for the assemblage of jawless stem vertebrates from which gnathostomes likely evolved is the lamprey. Modern approaches complement classical studies to reveal that larval lamprey develop cartilaginous mouth support structures, but do not develop dorsal-ventral articulations, i.e. jaw joints (Cattell et al., 2011; Johnels and Sundström-Schött, 1948). Interestingly, Barx is expressed in intermediate domain cells in lamprey, which do not develop into joint cells (Cerny et al., 2010). One might speculate, then, that exclusion of Barx from the intermediate domain of early gnathostomes, perhaps resulting from an increase in Edn1 signaling, was a crucial step in the evolution of the jaw joint. By this scenario, the absence of joints and expanded barx1 in zebrafish furina mutants would be atavistic, resembling the primitive jawless condition seen in lamprey.
The peculiar arrangement of cartilage and joints in barx1 mutant zebrafish is reminiscent of that seen in anuran tadpoles: two joints form in the first arch larval skeleton (the jaw joint and intramandibular joint) carving out three independent elements (the palatoquadrate, Meckel’s and infrarostral cartilages) (de Beer, 1938; Svensson and Haas, 2005). It would be interesting to test the hypothesis that development of the second (intramandibular) joint in anurans involves changes in barx1 function.
One might predict that the Barx1 mutant mouse skeleton will exhibit disruptions similar to those we report here for zebrafish. Indeed, Barx1 mutant mice display a gap in the stapes (Paul Sharpe, personal communication) reminiscent of the gap we report for the zebrafish equivalent, the hyosymplectic (supplementary material Fig. S1J). These phenotypes speak to the conservation of at least some aspects of Barx1 function across vertebrates. However, arch 1 Barx1 expression was reported to be unaffected by losses in Edn1 signaling in mice (Clouthier et al., 2000), highlighting potential differences across vertebrates in the genetic network we propose.
Our findings extend several studies describing remarkable roles for Barx1 in cell fate specification. First, Barx1 was reported to promote the development of molars versus incisors (Miletich et al., 2005; Tucker et al., 1998). More recently, Barx1 was shown to promote stomach versus intestine development (Kim et al., 2005). In keeping with this theme, we now provide evidence that Barx1 functions to promote cartilage versus joint development. A tantalizing idea has been put forth proposing that evolutionary changes in Barx1 could have had profound changes in feeding strategy in mammals by simultaneously altering two organs involved in feeding: the dentition and digestive system (Miletich et al., 2005). Our findings broaden this idea to include vertebrates beyond mammals, and add the origin and subsequent modification of the hinged jaw skeleton to the list of Barx1-controlled feeding anatomy. It is intriguing to consider that Barx was instrumental in the success of early vertebrates.
Supplementary Material
Acknowledgments
We thank John Dowd and the University of Oregon fish facility for animal care.
Footnotes
Funding
The research was supported by the National Institutes of Health (NIH) [RO1 DE13834 and PO1HD22486], and a NIH postdoctoral fellowship [F32-086027 to J.T.N.]. TILLING of barx1 was carried out with the support of the NIH [R01 HG002995 to C.B.M.]. The work was greatly improved by the suggestions of three anonymous reviewers. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
J.T.N. and C.B.K concieved and designed experiments. J.T.N. performed experiments. J.T.N., C.B.K. and C.B.M. analyzed the data. L.P. and C.B.M. performed TILLING. J.T.N. wrote the paper.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.090639/-/DC1
References
- Alexander C., Zuniga E., Blitz I. L., Wada N., Le Pabic P., Javidan Y., Zhang T. L., Cho K. W., Crump J. G., Schilling T. F. (2011). Combinatorial roles for BMPs and Endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton. Development 138, 5135–5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo S., Lohr J., Lee K. H., Ticho B. S., Breitbart R. E., Hill S., Yost H. J., Srivastava D. (2000). Conservation of sequence and expression of Xenopus and zebrafish dHAND during cardiac, branchial arch and lateral mesoderm development. Mech. Dev. 95, 231–237 [DOI] [PubMed] [Google Scholar]
- Avery L., Wasserman S. (1992). Ordering gene function: the interpretation of epistasis in regulatory hierarchies. Trends Genet. 8, 312–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A. J., Bogardi J. P., Ladher R., Francis-West P. H. (1999). Expression of chick Barx-1 and its differential regulation by FGF-8 and BMP signaling in the maxillary primordia. Dev. Dyn. 214, 291–302 [DOI] [PubMed] [Google Scholar]
- Cattell M., Lai S., Cerny R., Medeiros D. M. (2011). A new mechanistic scenario for the origin and evolution of vertebrate cartilage. PLoS ONE 6, e22474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny R., Cattell M., Sauka-Spengler T., Bronner-Fraser M., Yu F. Q., Medeiros D. M. (2010). Evidence for the prepattern/cooption model of vertebrate jaw evolution. Proc. Natl. Acad. Sci. USA 107, 17262–17267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charité J., McFadden D. G., Merlo G., Levi G., Clouthier D. E., Yanagisawa M., Richardson J. A., Olson E. N. (2001). Role of Dlx6 in regulation of an endothelin-1-dependent, dHAND branchial arch enhancer. Genes Dev. 15, 3039–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouthier D. E., Williams S. C., Yanagisawa H., Wieduwilt M., Richardson J. A., Yanagisawa M. (2000). Signaling pathways crucial for craniofacial development revealed by endothelin-A receptor-deficient mice. Dev. Biol. 217, 10–24 [DOI] [PubMed] [Google Scholar]
- Das A., Crump J. G. (2012). Bmps and id2a act upstream of Twist1 to restrict ectomesenchyme potential of the cranial neural crest. PLoS Genet. 8, e1002710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer G. (1938). The development of the vertebrate skull. J Anat. 72, 639–640 [Google Scholar]
- DeLaurier A., Nakamura Y., Braasch I., Khanna V., Kato H., Wakitani S., Postlethwait J. H., Kimmel C. B. (2012). Histone deacetylase-4 is required during early cranial neural crest development for generation of the zebrafish palatal skeleton. BMC Dev. Biol. 12, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew M. J., Compagnucci C. (2008). Tweaking the hinge and caps: testing a model of the organization of jaws. J. Exp. Zool. B 310, 315–335 [DOI] [PubMed] [Google Scholar]
- Draper B. W., McCallum C. M., Stout J. L., Slade A. J., Moens C. B. (2004). A high-throughput method for identifying N-ethyl-N-nitrosourea (ENU)-induced point mutations in zebrafish. Methods Cell Biol. 77, 91–112 [DOI] [PubMed] [Google Scholar]
- Fraser G. J., Hulsey C. D., Bloomquist R. F., Uyesugi K., Manley N. R., Streelman J. T. (2009). An ancient gene network is co-opted for teeth on old and new jaws. PLoS Biol. 7, e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C., Tabin C. J. (2001). Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell 104, 341–351 [DOI] [PubMed] [Google Scholar]
- Huycke T. R., Eames B. F., Kimmel C. B. (2012). Hedgehog-dependent proliferation drives modular growth during morphogenesis of a dermal bone. Development 139, 2371–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier P. (1996). Early Vertebrates. Oxford: Oxford University Press; [Google Scholar]
- Johnels A. G., Sundström-Schött U. (1948). On The Development And Morphology Of The Skeleton Of The Head Of Petromyzon. Stockholm: A. Bonniers; [Google Scholar]
- Kim B. M., Buchner G., Miletich I., Sharpe P. T., Shivdasani R. A. (2005). The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev. Cell 8, 611–622 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Miller C. T., Kruze G., Ullmann B., BreMiller R. A., Larison K. D., Snyder H. C. (1998). The shaping of pharyngeal cartilages during early development of the zebrafish. Dev. Biol. 203, 245–263 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Miller C. T., Keynes R. J. (2001). Neural crest patterning and the evolution of the jaw. J. Anat. 199, 105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Walker M. B., Miller C. T. (2007). Morphing the hyomandibular skeleton in development and evolution. J. Exp. Zool. B 308, 609–624 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., DeLaurier A., Ullmann B., Dowd J., McFadden M. (2010). Modes of developmental outgrowth and shaping of a craniofacial bone in zebrafish. PLoS ONE 5, e9475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby B. B., Takada N., Latimer A. J., Shin J., Carney T. J., Kelsh R. N., Appel B. (2006). In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 9, 1506–1511 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332–336 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Weinstein B. M. (2002). In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 [DOI] [PubMed] [Google Scholar]
- Mallatt J. (1996). Ventilation and the origin of jawed vertebrates: A new mouth. Zool. J. Linn. Soc. 117, 329–404 [Google Scholar]
- Medeiros D. M., Crump J. G. (2012). New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev. Biol. 371, 121–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich I., Buchner G., Sharpe P. T. (2005). Barx1 and evolutionary changes in feeding. J. Anat. 207, 619–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich I., Yu W. Y., Zhang R. F., Yang K., Caixeta de Andrade S., Pereira S. F. D., Ohazama A., Mock O. B., Buchner G., Sealby J., et al. (2011). Developmental stalling and organ-autonomous regulation of morphogenesis. Proc. Natl. Acad. Sci. USA 108, 19270–19275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. T., Schilling T. F., Lee K., Parker J., Kimmel C. B. (2000). sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development. Development 127, 3815–3828 [DOI] [PubMed] [Google Scholar]
- Miller C. T., Yelon D., Stainier D. Y., Kimmel C. B. (2003). Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development 130, 1353–1365 [DOI] [PubMed] [Google Scholar]
- Miller C. T., Swartz M. E., Khuu P. A., Walker M. B., Eberhart J. K., Kimmel C. B. (2007). mef2ca is required in cranial neural crest to effect Endothelin1 signaling in zebrafish. Dev. Biol. 308, 144–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic D. R. (1977). Development of the metatarsophalangeal joint of the chick embryo: morphological, ultrastructural and histochemical studies. Am. J. Anat. 150, 333–347 [DOI] [PubMed] [Google Scholar]
- Mitsiadis T. A., Graf D., Luder H., Gridley T., Bluteau G. (2010). BMPs and FGFs target Notch signalling via jagged 2 to regulate tooth morphogenesis and cytodifferentiation. Development 137, 3025–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T. F., Kimmel C. B. (1997). Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development 124, 2945–2960 [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Lee K. J., McMahon A. P., Hammerschmidt M. (1997). The zebrafish organizer requires chordino. Nature 387, 862–863 [DOI] [PubMed] [Google Scholar]
- Sperber S. M., Dawid I. B. (2008). barx1 is necessary for ectomesenchyme proliferation and osteochondroprogenitor condensation in the zebrafish pharyngeal arches. Dev. Biol. 321, 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M. E., Haas A. (2005). Evolutionary innovation in the vertebrate jaw: A derived morphology in anuran tadpoles and its possible developmental origin. Bioessays 27, 526–532 [DOI] [PubMed] [Google Scholar]
- Talbot J. C., Johnson S. L., Kimmel C. B. (2010). hand2 and Dlx genes specify dorsal, intermediate and ventral domains within zebrafish pharyngeal arches. Development 137, 2507–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot J. C., Walker M. B., Carney T. J., Huycke T. R., Yan Y. L., BreMiller R. A., Gai L., Delaurier A., Postlethwait J. H., Hammerschmidt M., et al. (2012). fras1 shapes endodermal pouch 1 and stabilizes zebrafish pharyngeal skeletal development. Development 139, 2804–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier-Seta J. P., Mucchielli M. L., Mark M., Mattei M. G., Goridis C., Brunet J. F. (1995). Barx1, a new mouse homeodomain transcription factor expressed in cranio-facial ectomesenchyme and the stomach. Mech. Dev. 51, 3–15 [DOI] [PubMed] [Google Scholar]
- Tucker A. S., Matthews K. L., Sharpe P. T. (1998). Transformation of tooth type induced by inhibition of BMP signaling. Science 282, 1136–1138 [DOI] [PubMed] [Google Scholar]
- Van der Heyden C., Huysseune A. (2000). Dynamics of tooth formation and replacement in the zebrafish (Danio rerio) (Teleostei, Cyprinidae). Dev. Dyn. 219, 486–496 [DOI] [PubMed] [Google Scholar]
- Walker M. B., Kimmel C. B. (2007). A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 82, 23–28 [DOI] [PubMed] [Google Scholar]
- Walker M. B., Miller C. T., Coffin Talbot J., Stock D. W., Kimmel C. B. (2006). Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning. Dev. Biol. 295, 194–205 [DOI] [PubMed] [Google Scholar]
- Walker M. B., Miller C. T., Swartz M. E., Eberhart J. K., Kimmel C. B. (2007). phospholipase C, beta 3 is required for Endothelin1 regulation of pharyngeal arch patterning in zebrafish. Dev. Biol. 304, 194–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y. L., Miller C. T., Nissen R. M., Singer A., Liu D., Kirn A., Draper B., Willoughby J., Morcos P. A., Amsterdam A., et al. (2002). A zebrafish sox9 gene required for cartilage morphogenesis. Development 129, 5065–5079 [DOI] [PubMed] [Google Scholar]
- Yelon D., Ticho B., Halpern M. E., Ruvinsky I., Ho R. K., Silver L. M., Stainier D. Y. (2000). The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development 127, 2573–2582 [DOI] [PubMed] [Google Scholar]
- Zuniga E., Stellabotte F., Crump J. G. (2010). Jagged-Notch signaling ensures dorsal skeletal identity in the vertebrate face. Development 137, 1843–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga E., Rippen M., Alexander C., Schilling T. F., Crump J. G. (2011). Gremlin 2 regulates distinct roles of BMP and Endothelin 1 signaling in dorsoventral patterning of the facial skeleton. Development 138, 5147–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.