Abstract
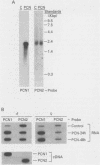
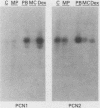
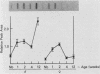
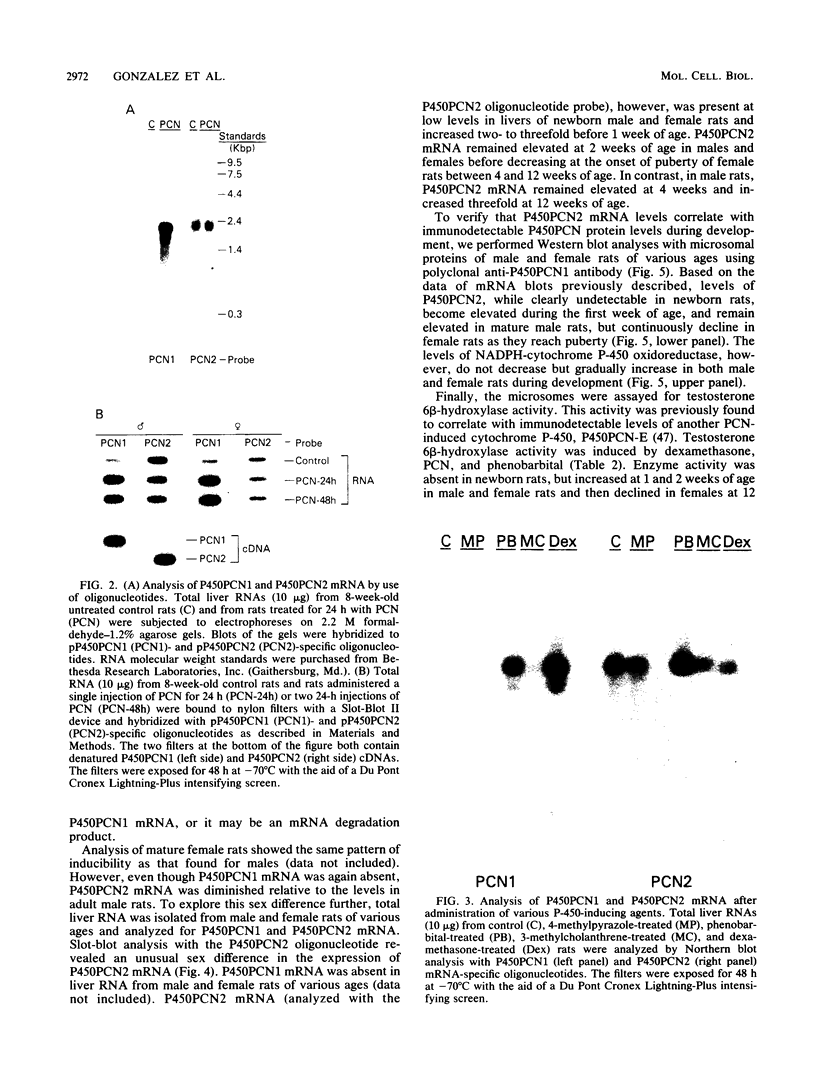
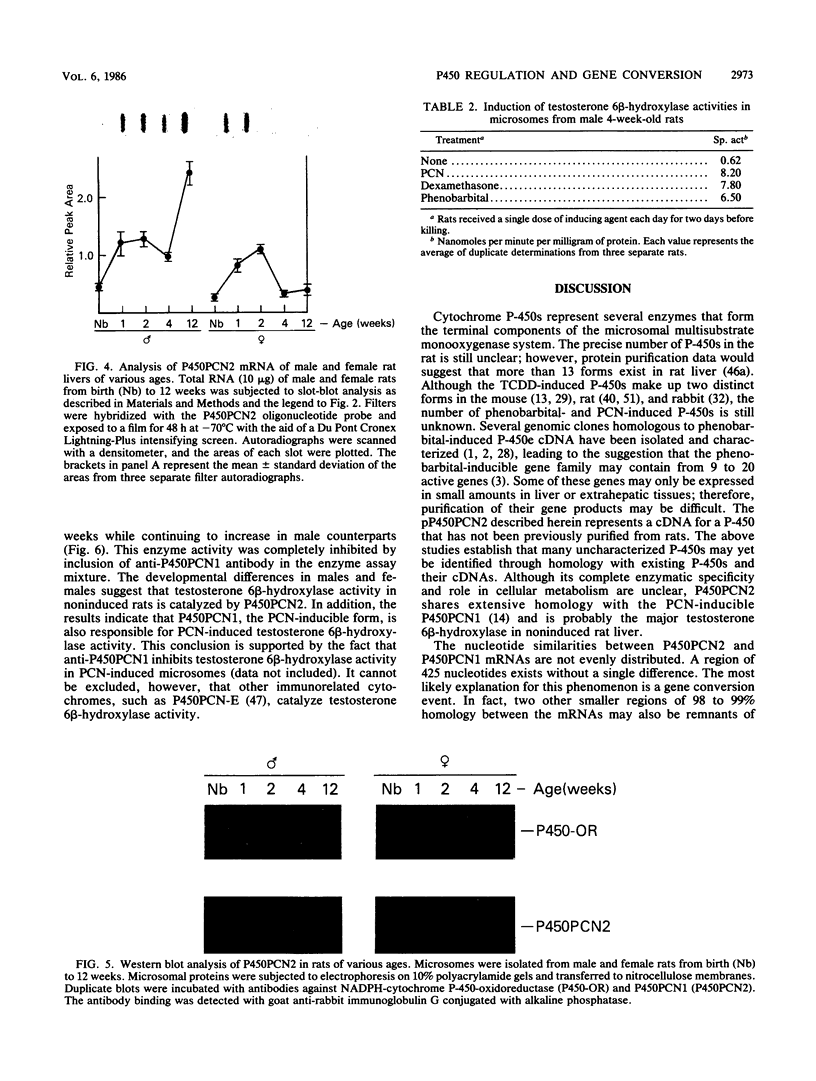
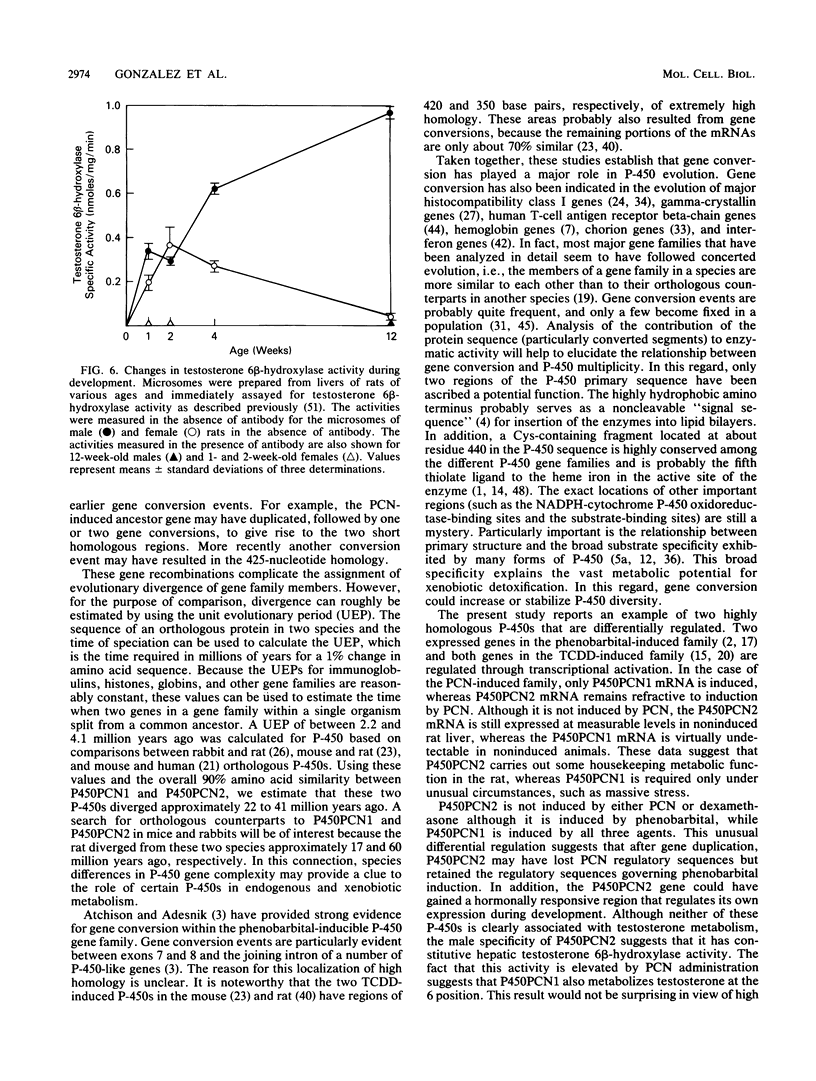
A cytochrome P-450 cDNA clone, designated pP450PCN2, homologous to the previously characterized pregnenolone 16 alpha-carbonitrile (PCN)-induced P-450 cDNA (pP450PCN1; F. J. Gonzalez, D. W. Nebert, J. P. Hardwick, and C. B. Kasper, J. Biol. Chem. 260:7435-7441), was isolated from a rat liver cDNA expression library by use of a polyclonal anti-P450PCN1 antibody. This P-450 cDNA contains 2,014 base pairs and yields an open reading frame of a protein consisting of 504 amino acids (Mr = 57,760). P450PCN2 cDNA and protein shared 90% nucleotide and 89% amino acid similarity with P450PCN1 cDNA and protein, respectively. The 5' untranslated, coding, and 3' untranslated regions between the two cDNAs share 94, 93, and 79% similarities, respectively. Nucleotide differences in the coding regions, however, are not evenly distributed. Complete homology exists between the two mRNAs for 425 nucleotides (positions 346 through 771). Other regions of 93 nucleotides containing only one difference and 147 nucleotides containing two differences exist toward the 3' end of the coding regions. These data suggest the possibility that a gene conversion event(s) have occurred subsequent to duplication of the ancestral P450PCN gene. Oligonucleotide probes unique for P450PCN1 and P450PCN2 cDNAs were used to examine the levels of their respective mRNAs in noninduced and PCN-induced liver cells and in male and female rats of various ages. P450PCN1 mRNA was not detectable in either male or female rats at any ages. In contrast, P450PCN2 mRNA was present at a low level in newborn rats and became elevated in both males and females at 1 week of age. Levels of p450PCN2 mRNA continued to increase in males until 12 weeks, whereas the mRNA in females reached peak levels at 2 weeks of age but declined continuously at the onset of puberty (between 4 and 12 weeks). These levels of P45PCN2 mRNA closely parallel the increases in testosterone 6 beta-hydroxylase activity and P450PCN2 protein level, as analyzed by Western blots. P450PCN1 mRNA was induced by PCN, dexamethasone, and phenobarbital in both male and female rats. P450PCN2 mRNA was not significantly induced by PCN or dexamethasone but was readily induced by phenobarbital. Testosterone 6 beta-hydroxylase activity was also induced severalfold by PCN, dexamethasone, and phenobarbital. These data demonstrate that P450PCN1 and P450PCN2 genes are differentially regulated during development and after administration of inducing compounds and furthermore suggest that both enzymes possess testosterone 6 beta-hydroxylase activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Atchison M. Genes for cytochrome P-450 and their regulation. CRC Crit Rev Biochem. 1986;19(3):247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- Atchison M., Adesnik M. A cytochrome P-450 multigene family. Characterization of a gene activated by phenobarbital administration. J Biol Chem. 1983 Sep 25;258(18):11285–11295. [PubMed] [Google Scholar]
- Atchison M., Adesnik M. Gene conversion in a cytochrome P-450 gene family. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2300–2304. doi: 10.1073/pnas.83.8.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nun S., Kreibich G., Adesnik M., Alterman L., Negishi M., Sabatini D. D. Synthesis and insertion of cytochrome P-450 into endoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):965–969. doi: 10.1073/pnas.77.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. C., Gelboin H. V., Song B. J., Park S. S., Friedman F. K. Detection and purification of cytochromes P-450 in animal tissues with monoclonal antibodies. J Biol Chem. 1984 Oct 10;259(19):12279–12284. [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Elshourbagy N. A., Guzelian P. S. Separation, purification, and characterization of a novel form of hepatic cytochrome P-450 from rats treated with pregnenolone-16 alpha-carbonitrile. J Biol Chem. 1980 Feb 25;255(4):1279–1285. [PubMed] [Google Scholar]
- Fujii-Kuriyama Y., Mizukami Y., Kawajiri K., Sogawa K., Muramatsu M. Primary structure of a cytochrome P-450: coding nucleotide sequence of phenobarbital-inducible cytochrome P-450 cDNA from rat liver. Proc Natl Acad Sci U S A. 1982 May;79(9):2793–2797. doi: 10.1073/pnas.79.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelboin H. V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980 Oct;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Kimura S., Nebert D. W. Comparison of the flanking regions and introns of the mouse 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible cytochrome P1-450 and P3-450 genes. J Biol Chem. 1985 Apr 25;260(8):5040–5049. [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W., Hardwick J. P., Kasper C. B. Complete cDNA and protein sequence of a pregnenolone 16 alpha-carbonitrile-induced cytochrome P-450. A representative of a new gene family. J Biol Chem. 1985 Jun 25;260(12):7435–7441. [PubMed] [Google Scholar]
- Gonzalez F. J., Tukey R. H., Nebert D. W. Structural gene products of the Ah locus. Transcriptional regulation of cytochrome P1-450 and P3-450 mRNA levels by 3-methylcholanthrene. Mol Pharmacol. 1984 Jul;26(1):117–121. [PubMed] [Google Scholar]
- Hardwick J. P., Gonzalez F. J., Kasper C. B. Cloning of DNA complementary to cytochrome P-450 induced by pregnenolone-16 alpha-carbonitrile. Characterization of its mRNA, gene, and induction response. J Biol Chem. 1983 Aug 25;258(16):10182–10186. [PubMed] [Google Scholar]
- Hardwick J. P., Gonzalez F. J., Kasper C. B. Transcriptional regulation of rat liver epoxide hydratase, NADPH-Cytochrome P-450 oxidoreductase, and cytochrome P-450b genes by phenobarbital. J Biol Chem. 1983 Jul 10;258(13):8081–8085. [PubMed] [Google Scholar]
- Hildebrand C. E., Gonzalez F. J., Kozak C. A., Nebert D. W. Regional linkage analysis of the dioxin-inducible P-450 gene family on mouse chromosome 9. Biochem Biophys Res Commun. 1985 Jul 16;130(1):396–406. doi: 10.1016/0006-291x(85)90430-9. [DOI] [PubMed] [Google Scholar]
- Hood L., Campbell J. H., Elgin S. C. The organization, expression, and evolution of antibody genes and other multigene families. Annu Rev Genet. 1975;9:305–353. doi: 10.1146/annurev.ge.09.120175.001513. [DOI] [PubMed] [Google Scholar]
- Israel D. I., Whitlock J. P., Jr Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells. J Biol Chem. 1984 May 10;259(9):5400–5402. [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human dioxin-inducible cytochrome P1-450: complementary DNA and amino acid sequence. Science. 1985 Apr 5;228(4695):80–83. doi: 10.1126/science.3838385. [DOI] [PubMed] [Google Scholar]
- Kato R. Sex-related differences in drug metabolism. Drug Metab Rev. 1974;3(1):1–32. doi: 10.3109/03602537408993737. [DOI] [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. The murine Ah locus. Comparison of the complete cytochrome P1-450 and P3-450 cDNA nucleotide and amino acid sequences. J Biol Chem. 1984 Sep 10;259(17):10705–10713. [PubMed] [Google Scholar]
- Klein J. Gene conversion in MHC genes. Transplantation. 1984 Oct;38(4):327–329. doi: 10.1097/00007890-198410000-00002. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leighton J. K., DeBrunner-Vossbrinck B. A., Kemper B. Isolation and sequence analysis of three cloned cDNAs for rabbit liver proteins that are related to rabbit cytochrome P-450 (form 2), the major phenobarbital-inducible form. Biochemistry. 1984 Jan 17;23(2):204–210. doi: 10.1021/bi00297a005. [DOI] [PubMed] [Google Scholar]
- Meakin S. O., Breitman M. L., Tsui L. C. Structural and evolutionary relationships among five members of the human gamma-crystallin gene family. Mol Cell Biol. 1985 Jun;5(6):1408–1414. doi: 10.1128/mcb.5.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y., Fujii-Kuriyama Y., Muramatsu M. Multiplicity of deoxyribonucleic acid sequences with homology to a cloned complementary deoxyribonucleic acid coding for rat phenobarbital-inducible cytochrome P-450. Biochemistry. 1983 Mar 1;22(5):1223–1229. doi: 10.1021/bi00274a036. [DOI] [PubMed] [Google Scholar]
- Ohta T. Population genetics theory of concerted evolution and its application to the immunoglobulin V gene tree. J Mol Evol. 1984;20(3-4):274–280. doi: 10.1007/BF02104733. [DOI] [PubMed] [Google Scholar]
- Okino S. T., Quattrochi L. C., Barnes H. J., Osanto S., Griffin K. J., Johnson E. F., Tukey R. H. Cloning and characterization of cDNAs encoding 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible rabbit mRNAs for cytochrome P-450 isozymes 4 and 6. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5310–5314. doi: 10.1073/pnas.82.16.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodakis G. C., Lecanidou R., Eickbush T. H. Diversity in a chorion multigene family created by tandem duplications and a putative gene-conversion event. J Mol Evol. 1984;20(3-4):265–273. doi: 10.1007/BF02104732. [DOI] [PubMed] [Google Scholar]
- Ronne H., Widmark E., Rask L., Peterson P. A. Intron sequences reveal evolutionary relationships among major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5860–5864. doi: 10.1073/pnas.82.17.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Misono K. S., Guengerich F. P. Human liver microsomal cytochrome P-450 mephenytoin 4-hydroxylase, a prototype of genetic polymorphism in oxidative drug metabolism. Purification and characterization of two similar forms involved in the reaction. J Biol Chem. 1986 Jan 15;261(2):909–921. [PubMed] [Google Scholar]
- Simmons D. L., Kasper C. B. Genetic polymorphisms for a phenobarbital-inducible cytochrome P-450 map to the Coh locus in mice. J Biol Chem. 1983 Aug 25;258(16):9585–9588. [PubMed] [Google Scholar]
- Simmons D. L., Lalley P. A., Kasper C. B. Chromosomal assignments of genes coding for components of the mixed-function oxidase system in mice. Genetic localization of the cytochrome P-450PCN and P-450PB gene families and the nadph-cytochrome P-450 oxidoreductase and epoxide hydratase genes. J Biol Chem. 1985 Jan 10;260(1):515–521. [PubMed] [Google Scholar]
- Sogawa K., Gotoh O., Kawajiri K., Harada T., Fujii-Kuriyama Y. Complete nucleotide sequence of a methylcholanthrene-inducible cytochrome P-450 (P-450d) gene in the rat. J Biol Chem. 1985 Apr 25;260(8):5026–5032. [PubMed] [Google Scholar]
- Suwa Y., Mizukami Y., Sogawa K., Fujii-Kuriyama Y. Gene structure of a major form of phenobarbital-inducible cytochrome P-450 in rat liver. J Biol Chem. 1985 Jul 5;260(13):7980–7984. [PubMed] [Google Scholar]
- Todokoro K., Kioussis D., Weissmann C. Two non-allelic human interferon alpha genes with identical coding regions. EMBO J. 1984 Aug;3(8):1809–1812. doi: 10.1002/j.1460-2075.1984.tb02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnacliffe A., Kefford R., Milstein C., Forster A., Rabbitts T. H. Sequence and evolution of the human T-cell antigen receptor beta-chain genes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5068–5072. doi: 10.1073/pnas.82.15.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. B. Interaction of selection and biased gene conversion in a multigene family. Proc Natl Acad Sci U S A. 1985 Jan;82(1):153–157. doi: 10.1073/pnas.82.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman M. R., Estabrook R. W. The induction of microsomal electron transport enzymes. Mol Cell Biochem. 1983;53-54(1-2):267–278. doi: 10.1007/BF00225259. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Dannan G. A., Guengerich F. P. Regulation of rat hepatic cytochrome P-450: age-dependent expression, hormonal imprinting, and xenobiotic inducibility of sex-specific isoenzymes. Biochemistry. 1985 Jul 30;24(16):4409–4417. doi: 10.1021/bi00337a023. [DOI] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Maurel P., Schuetz E. G., Watkins P. B., Young B., Guzelian P. S. Identification of the cytochrome P-450 induced by macrolide antibiotics in rat liver as the glucocorticoid responsive cytochrome P-450p. Biochemistry. 1985 Apr 23;24(9):2171–2178. doi: 10.1021/bi00330a010. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Schuetz E. G., Watkins P. B., Maurel P., Barwick J., Bailey B. S., Hartle H. T., Young B., Guzelian P. Demonstration in multiple species of inducible hepatic cytochromes P-450 and their mRNAs related to the glucocorticoid-inducible cytochrome P-450 of the rat. Mol Pharmacol. 1985 Sep;28(3):312–321. [PubMed] [Google Scholar]
- Yabusaki Y., Shimizu M., Murakami H., Nakamura K., Oeda K., Ohkawa H. Nucleotide sequence of a full-length cDNA coding for 3-methylcholanthrene-induced rat liver cytochrome P-450MC. Nucleic Acids Res. 1984 Mar 26;12(6):2929–2938. doi: 10.1093/nar/12.6.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara S., Nagata K., Wada I., Yoshimura H., Kuroki H., Masuda Y. A unique change of steroid metabolism in rat liver microsomes induced with highly toxic polychlorinated biphenyl(PCB) and polychlorinated dibenzofuran(PCDF). J Pharmacobiodyn. 1982 Dec;5(12):994–1004. doi: 10.1248/bpb1978.5.994. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]