Abstract
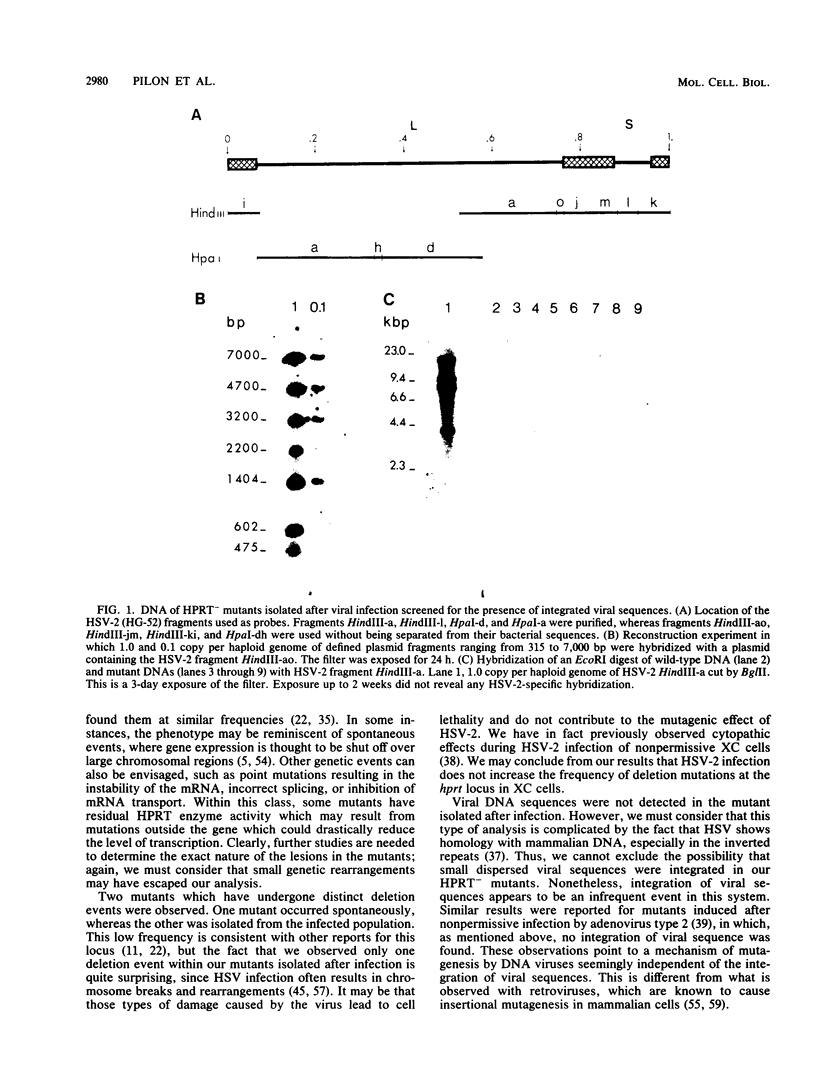
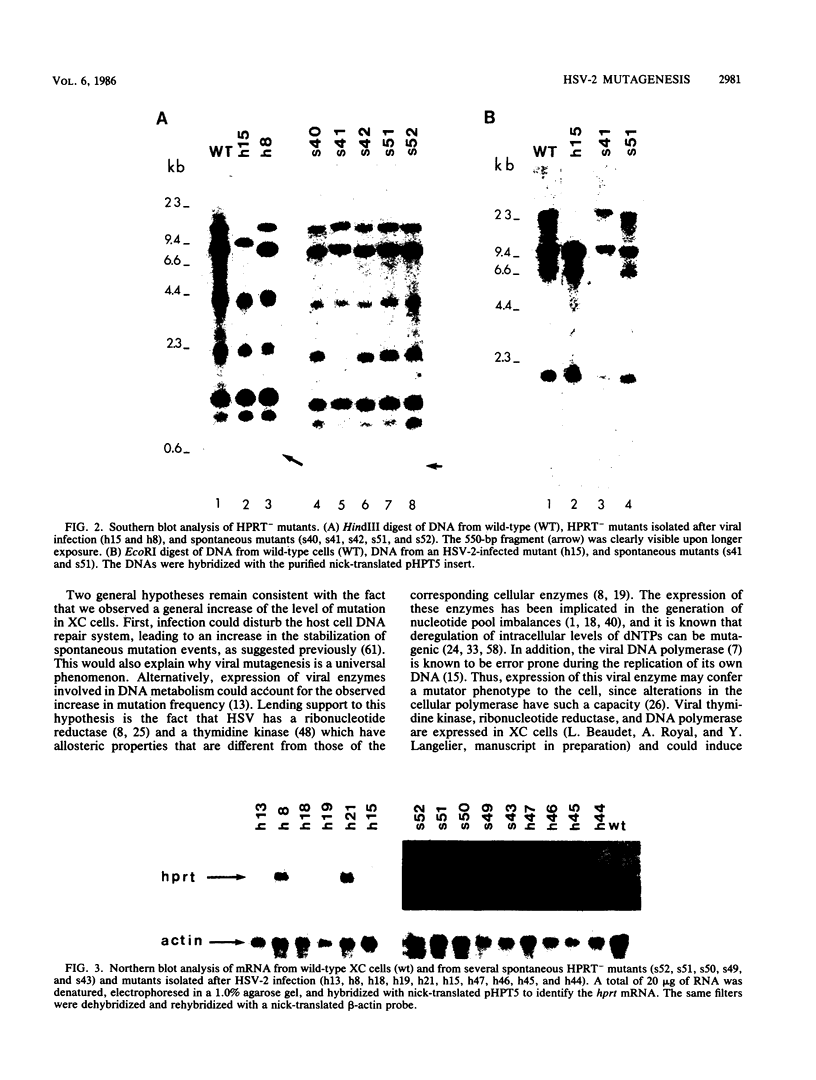
In a previous report, herpes simplex virus type 2 (HSV-2) was shown to increase the frequency of mutation at the hypoxanthine phosphoribosyltransferase (hprt) locus of nonpermissive rat XC cells (L. Pilon, A. Royal, and Y. Langelier, J. Gen. Virol. 66:259-265, 1985). A series of 17 independent mutants were isolated after viral infection together with 12 spontaneous noninfected mutants to characterize the nature of the mutations induced by the virus at the molecular level. The DNA of the mutants isolated after viral infection was probed with cloned HSV-2 fragments representing the entire genome. In these mutants, no authentic HSV-2 hybridization could be detected. This was indicative of a mechanism of mutagenesis which did not require the permanent integration of viral sequences in the host genome. The structure of the hprt gene was determined by the method of Southern (J. Mol. Biol. 98:503-517, 1975), and the level of hprt mRNA was analyzed by Northern blots. Except for the identification of one deletion mutant in each of the two groups, the HPRT- clones showed no evidence of alteration in their hprt gene. A total of 7 of 12 spontaneous mutants and 11 of 15 mutants isolated from the infected population transcribed an hprt mRNA of the same size and abundance as did the wild-type cells. Thus, the majority of the mutants seemed to have a point mutation in their hprt structural gene. Interestingly, the proportion of the different types of mutations was similar in the two groups of mutants. This analysis revealed that HSV-2 infection did not increase the frequency of rearrangements but rather that it probably induced a general increase of the level of mutations in the cells. This type of response is thought to be compatible with the biology of the virus, and the possible mechanisms by which HSV-2 induces somatic mutations in mammalian cells are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baybutt H. N., Murray B. A., Pearson C. K. Aspects of thymidine metabolism and function in cultured mammalian cells infected with herpes simplex virus type 1. J Gen Virol. 1982 Apr;59(Pt 2):223–234. doi: 10.1099/0022-1317-59-2-223. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Boiron M., Tanzer J., Thomas M., Hampe A. Early diffuse chromosome alterations in monkey kidney cells infected in vitro with herpes simplex virus. Nature. 1966 Feb 12;209(5024):737–738. doi: 10.1038/209737a0. [DOI] [PubMed] [Google Scholar]
- Bradley W. E. Mutation at autosomal loci of Chinese hamster ovary cells: involvement of a high-frequency event silencing two linked alleles. Mol Cell Biol. 1983 Jul;3(7):1172–1181. doi: 10.1128/mcb.3.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Kruh G. D. The HPRT locus. Cell. 1979 Jan;16(1):1–9. doi: 10.1016/0092-8674(79)90182-x. [DOI] [PubMed] [Google Scholar]
- Chartrand P., Crumpacker C. S., Schaffer P. A., Wilkie N. M. Physical and genetic analysis of the herpes simplex virus DNA polymerase locus. Virology. 1980 Jun;103(2):311–326. doi: 10.1016/0042-6822(80)90190-7. [DOI] [PubMed] [Google Scholar]
- Cohen G. H. Ribonucleotide reductase activity of synchronized KB cells infected with herpes simplex virus. J Virol. 1972 Mar;9(3):408–418. doi: 10.1128/jvi.9.3.408-418.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuscoe J. C., Fenwick R. G., Jr, Ledbetter D. H., Caskey C. T. Deletion and amplification of the HGPRT locus in Chinese hamster cells. Mol Cell Biol. 1983 Jun;3(6):1086–1096. doi: 10.1128/mcb.3.6.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Copple C. D., McDougall J. K. Analysis of viral DNA sequences in hamster cells transformed by herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1980 Feb;77(2):880–884. doi: 10.1073/pnas.77.2.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Defendi V. Increased mutation rates in doubly viral transformed Chinese hamster cells. Somatic Cell Genet. 1979 Nov;5(6):887–895. doi: 10.1007/BF01542648. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- HAMPAR B., ELLISON S. A. Cellular alterations in the MCH line of Chinese hamster cells following infection with herpes simplex virus. Proc Natl Acad Sci U S A. 1963 Apr;49:474–480. doi: 10.1073/pnas.49.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. D., Coen D. M., Fisher B. L., Weisslitz M., Randall S., Almy R. E., Gelep P. T., Schaffer P. A. Generation of genetic diversity in herpes simplex virus: an antimutator phenotype maps to the DNA polymerase locus. Virology. 1984 Jan 15;132(1):26–37. doi: 10.1016/0042-6822(84)90088-6. [DOI] [PubMed] [Google Scholar]
- Huszar D., Bacchetti S. Is ribonucleotide reductase the transforming function of herpes simplex virus 2? Nature. 1983 Mar 3;302(5903):76–79. doi: 10.1038/302076a0. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Bjursell G. Deoxyribonucleoside triphosphate pools in herpes simplex type 1 infected cells. J Gen Virol. 1976 Apr;31(1):101–113. doi: 10.1099/0022-1317-31-1-101. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Tumorigenic transformation induced by a specific fragment of DNA from herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2279–2283. doi: 10.1073/pnas.77.4.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessous A., Bibor-Hardy V., Suh M., Simard R. Analysis of chromosomes, nucleic acids, and polypeptides in hamster cells transformed by herpes simplex virus type 2. Cancer Res. 1979 Aug;39(8):3225–3234. [PubMed] [Google Scholar]
- King H. W., Brookes P. On the nature of the mutations induced by the diolepoxide of benzo[a]pyrene in mammalian cells. Carcinogenesis. 1984 Jul;5(7):965–970. doi: 10.1093/carcin/5.7.965. [DOI] [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Silber J. R., Loeb L. A. The mutagenic effect of deoxynucleotide substrate imbalances during DNA synthesis with mammalian DNA polymerases. Mutat Res. 1982 Jun;94(2):413–419. doi: 10.1016/0027-5107(82)90304-9. [DOI] [PubMed] [Google Scholar]
- Langelier Y., Buttin G. Characterization of ribonucleotide reductase induction in BHK-21/C13 Syrian hamster cell line upon infection by herpes simplex virus (HSV). J Gen Virol. 1981 Nov;57(Pt 1):21–31. doi: 10.1099/0022-1317-57-1-21. [DOI] [PubMed] [Google Scholar]
- Liu P. K., Chang C. C., Trosko J. E., Dube D. K., Martin G. M., Loeb L. A. Mammalian mutator mutant with an aphidicolin-resistant DNA polymerase alpha. Proc Natl Acad Sci U S A. 1983 Feb;80(3):797–801. doi: 10.1073/pnas.80.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengo C., Mbikay M., Weber J., Thirion J. P. Adenovirus-induced mutations at the hypoxanthine phosphoribosyltransferase locus of Chinese hamster cells. J Virol. 1981 Apr;38(1):184–190. doi: 10.1128/jvi.38.1.184-190.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak M. I., Varshaver N. B., Shapiro N. I. Induction of gene mutations and chromosomal aberrations by simian virus 40 in cultured mammalian cells. Mutat Res. 1975 Dec;30(3):383–396. [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth M., L'Heureux-Huard N., Trudel M. Characterization of a mutator gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6505–6509. doi: 10.1073/pnas.76.12.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Nussbaum R. L., Crowder W. E., Nyhan W. L., Caskey C. T. A three-allele restriction-fragment-length polymorphism at the hypoxanthine phosphoribosyltransferase locus in man. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4035–4039. doi: 10.1073/pnas.80.13.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. I., Nussbaum R. L., gramson P. E., Ledbetter D. H., Caskey C. T., Chinault A. C. Organization of the HPRT gene and related sequences in the human genome. Somat Cell Mol Genet. 1984 Sep;10(5):483–493. doi: 10.1007/BF01534853. [DOI] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Pilon L., Royal A., Langelier Y. Increased mutation frequency after herpes simplex virus type 2 infection in non-permissive XC cells. J Gen Virol. 1985 Feb;66(Pt 2):259–265. doi: 10.1099/0022-1317-66-2-259. [DOI] [PubMed] [Google Scholar]
- Raptis L., de Souza A. C., M'Bikay M., Thirion J. P., Weber J. Stable integration of adenovirus DNA is not required for the induction of mutations in the hypoxanthine phosphoribosyltransferase gene in Chinese hamster cells. Mutat Res. 1982 Nov;105(5):371–375. doi: 10.1016/0165-7992(82)90109-9. [DOI] [PubMed] [Google Scholar]
- Roller B., Cohen G. H. Deoxyribonucleoside triphosphate pools in synchronized human cells infected with herpes simplex virus types 1 and 2. J Virol. 1976 Apr;18(1):58–64. doi: 10.1128/jvi.18.1.58-64.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICH H. F., HSU T. C., RAPP F. VIRUSES AND MAMMALIAN CHROMOSOMES. I. LOCALIZATION OF CHROMOSOME ABERRATIONS AFTER INFECTION WITH HERPES SIMPLEX VIRUS. Virology. 1964 Apr;22:439–445. doi: 10.1016/0042-6822(64)90064-9. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Hausen J. Z. Induction of mutations within the host cell genome by partially inactivated herpes simplex virus type 1. Virology. 1982 Oct 30;122(2):471–475. doi: 10.1016/0042-6822(82)90247-1. [DOI] [PubMed] [Google Scholar]
- Skinner G. R. Transformation of primary hamster embryo fibroblasts by type 2 simplex virus: evidence for a "hit and run" mechanism. Br J Exp Pathol. 1976 Aug;57(4):361–376. [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stout J. T., Caskey C. T. HPRT: gene structure, expression, and mutation. Annu Rev Genet. 1985;19:127–148. doi: 10.1146/annurev.ge.19.120185.001015. [DOI] [PubMed] [Google Scholar]
- Strauss M., Theile M., Eckert R., Geissler E. Detection of antigenically active mutant HGPRT after mutagenesis with Simian virus 40. Mutat Res. 1978 Aug;51(2):297–300. doi: 10.1016/s0027-5107(78)80027-x. [DOI] [PubMed] [Google Scholar]
- Summers W. P., Wagner M., Summers W. C. Possible peptide chain termination mutants in thymide kinase gene of a mammalian virus, herpes simplex virus. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4081–4084. doi: 10.1073/pnas.72.10.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker J. The molecular nature of mutations in cultured mammalian cells: a review. Mutat Res. 1985 Jun-Jul;150(1-2):431–442. doi: 10.1016/0027-5107(85)90140-x. [DOI] [PubMed] [Google Scholar]
- Theile M., Scherneck S., Geissler E. Mutagenesis by simian virus 40. I. detection of mutations in Chinese hamster cell lines using different resistance markers. Mutat Res. 1976 Oct;37(1):111–123. doi: 10.1016/0027-5107(76)90059-2. [DOI] [PubMed] [Google Scholar]
- Theile M., Strauss M., Luebbe L., Scherneck S., Krause H., Geissler E. SV40-induced somatic mutations: possible relevance to viral transformation. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):377–382. doi: 10.1101/sqb.1980.044.01.042. [DOI] [PubMed] [Google Scholar]
- Theile M., Strauss M. Mutagenesis by simian virus 40. II. Changes in substrate affinities in mutant hypoxanthine-guanine phosphoribosyl transferase enzymes at different pH values. Mutat Res. 1977 Oct;45(1):111–123. doi: 10.1016/0027-5107(77)90049-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Turker M. S., Smith A. C., Martin G. M. High frequency "switching" at the adenine phosphoribosyltransferase locus in multipotent mouse teratocarcinoma stem cells. Somat Cell Mol Genet. 1984 Jan;10(1):55–69. doi: 10.1007/BF01534473. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Varshaver N. B., Marshak M. I., Luss E. V., Gorbunova L. V., Shapiro N. I. Spontaneous, chemical and viral mutagenesis in temperature-sensitive glutamine-requiring chinese hamster cells. Mutat Res. 1977 May;43(2):263–278. doi: 10.1016/0027-5107(77)90010-0. [DOI] [PubMed] [Google Scholar]
- Waubke R., Zur Hausen H., Henle W. Chromosomal and autoradiographic studies of cells infected with herpes simplex virus. J Virol. 1968 Oct;2(10):1047–1054. doi: 10.1128/jvi.2.10.1047-1054.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg G., Ullman B., Martin D. W., Jr Mutator phenotypes in mammalian cell mutants with distinct biochemical defects and abnormal deoxyribonucleoside triphosphate pools. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2447–2451. doi: 10.1073/pnas.78.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Rotter V. Inactivation of p53 gene expression by an insertion of Moloney murine leukemia virus-like DNA sequences. Mol Cell Biol. 1984 Jul;4(7):1402–1410. doi: 10.1128/mcb.4.7.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Martin R. G. Relationship of simian virus 40 tumor antigens to virus-induced mutagenesis. Mol Cell Biol. 1983 Mar;3(3):421–428. doi: 10.1128/mcb.3.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. The role of viruses in human tumors. Adv Cancer Res. 1980;33:77–107. doi: 10.1016/s0065-230x(08)60669-7. [DOI] [PubMed] [Google Scholar]