Abstract
Purpose
To determine whether matrix metalloproteinase-7 (MMP-7) that is stably overexpressed by mouse corneal fibroblast cell lines exhibits proteolytic activity against the NC1 fragment of collagen XVIII.
Methods
Corneal fibroblasts isolated from MMP-7 knockout (7ko) mice were subjected to SV40 T-antigen immortalization and stably transfected with a bicistronic retroviral vector encoding green fluorescence protein and active MMP-7. The resulting MMP-7 knock-in fibroblasts (7ko-MMP-7 cells) were isolated and enriched by fluorescence activated cell sorting (FACS). Culture media samples from 7ko and 7ko-MMP-7 cells were then incubated with the recombinant NC1 fragment of collagen XVIII, and NC1 degradation was monitored by immunoblotting.
Results
Immunoblot analysis revealed that MMP-7 was present in lysates and culture media from 7ko-MMP-7 fibroblasts, but not media from immortalized 7ko fibroblasts. Importantly, lower amounts of the NC1 fragment were present in in vitro enzymatic reaction mixtures containing concentrated 7ko-MMP-7 media than in those containing concentrated 7ko media.
Conclusion
Immortalized fibroblasts stably transfected with MMP-7 secrete active MMP-7 with proteolytic activity towards the NC1 fragment of collagen XVIII.
Keywords: Cornea, Gene transfer, Matrilysin, Matrix metalloproteinases, MMP-7
INTRODUCTION
The cornea is transparent and avascular due to its uniquely organized extracellular matrix (ECM).1 Corneal wounding may lead to the loss of ECM organization, the accumulation of altered ECM, or an increase in highly reflective wound healing fibroblasts. These fibroblasts are known to cause corneal opacification and neovascularization (NV).2 Modulation of key regulators involved in corneal transparency, wound healing, corneal scarring, and NV may provide a means of preventing these disorders.3–5
Matrix metalloproteinase-7 (MMP-7), also known as matrilysin, is the smallest known secreted matrix metalloproteinase.6,7 Similar to most matrix metalloproteinases, MMP-7 is secreted as a zymogen (inactive or latent enzyme).7 Cleavage of this proenzyme (proMMP-7) generates an active, 19-kDa fragment (MMP-7).8 Several studies have shown that activated MMP-7 cleaves substrates and regulates their activities, which include ECM remodeling, development of host defense responses,9 tumor survival, and wound healing.10–12 For example, MMP-7 cleaves receptor activator of nuclear factor kappa B ligand (RANKL) to generate soluble RANKL and promote prostate cancer-induced osteolysis.13 MMP-7 cleaves prodefensin to generate active antimicrobial peptides.9 In addition, we have reported that MMP-7 cleaves collagen XVIII to generate a 28-kDa endostatin-containing fragment that has potent antiangiogenic and anti-lymphangiogenic functions in vitro.11,14,15
Up-regulation of MMPs, including MMP-7, has been reported during corneal wound healing. Moreover, Kure et al. have shown that corneal wounding induces substantially greater NV in MMP-7-deficient mice than in their wild type (WT) littermates.16 In the current study, we generated MMP-7 knockout corneal fibroblast cell lines that stably express active MMP-7 and determined whether MMP-7 secreted by these cells exhibits proteolytic activity against the NC1 fragment of collagen XVIII.
MATERIALS AND METHODS
Establishment and Characterization of MMP-7 Knockout Cell Lines
Corneas from WT mice (C57BL/6) and MMP-7-knockout mice (7ko mice, kindly provided by Dr. Lynn M. Matrisian, Vanderbilt-Ingram Cancer Center) were harvested. To loosen epithelial sheets, whole corneas were incubated in Dispase II (5 mg/mL, Roche Diagnostics, Indianapolis, Indiana, USA) in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (penicillin and streptomycin at 100 μg/mL and amphotericin B at 2.5 μg/mL) in 5% CO2 at 37°C. After removing the epithelium, mouse corneas were digested using a sequential collagenase A digestion method as described by Beales et al.17 Briefly, mouse corneas were placed in Dulbecco's modified Eagle's medium (DMEM, Mediatech) containing antibiotics and 3.3 mg/mL collagenase (type L.C8170; Sigma, St. Louis, Missouri, USA) and incubated at 37°C with shaking (126 rpm) for 30 min. The tubes were then vortexed for 30 sec and the tissue pieces separated from the collagenase solution using a cell strainer. Digestion was continued with a second 1 mL aliquot of collagenase for 60 min and with a third 1 mL aliquot of collagenase for 180 min, followed by a repeat of the separation procedure. The cells in the third collagenase digestion were collected by low-speed centrifugation (1400 rpm, 10 min) and the cells resuspended in 5 mL DMEM with 10% FBS.17 The viability and appearance of the cells was determined by trypan blue (Sigma) exclusion, using an inverted microscope and counting the number of the cells by hemocytometer. Cornea cells were immortalized with retrovirus-containing SV40 T-antigen.18 Individual cell lines (including corneal fibroblast) were isolated by a previously described serial dilution method.19
Polymerase chain reaction (PCR) was used to confirm MMP-7 knockout in corneal fibroblast cell lines. Primers for WT and knockout alleles were located in exon 3 (5′-TCAGACTTACCTCGGATCGT-3′) and exon 4 (5′-GTCCTCACCATCAGTCCA G-3′). Primers for mutated alleles were located in the PGK-neo cassette (5′-TTGAGCCTGGCGAACAGT-3′ and 5′-GGCATCGCCATGGGTCACGA-3′). Immortalized WT and 7ko fibroblast cell lines were characterized by immunostaining using polyclonal goat anti-vimentin antibody (Chemicon, Temecula, California, USA), polyclonal goat anti-integrin α5β1 antibody (Chemicon), anti-α-smooth muscle actin (α-SMA) antibody (Sigma, St. Louis, Missouri, USA, Clone 1A4), and mouse anti-epithelial keratin AE1/AE3 antibody (ICN Biomedicals, Irvine, California, USA).20,21
Confocal Analysis of MMP-7 in Corneal Cell Lines
WT fibroblasts, 7ko fibroblasts, and epithelial cells were seeded in chambers and mounted onto glass slides (Lab-Tek II Chamber Slide System, Nunc International, Rochester, New York, USA). After culture media removal, cells were treated with 100% acetone for 10 min at room temperature. Cells were then blocked with 1% BSA and incubated with polyclonal rabbit anti-mouse MMP-7 antibody in 1% BSA for 1 hr at room temperature. Cells were washed with PBS and incubated with FITC-conjugated donkey anti-rabbit IgG. After three 10-min washes with PBS, cells were mounted in Vectashield (Vector Laboratories, Inc., Burlingame, California, USA) plus propidium iodide and digitally imaged using a Leica TCS 4D laser confocal microscope (Wetzlar, Germany).
Generation of Anti-MMP-7 Antibody
New Zealand white rabbits were immunized with a synthetic MMP-7 peptide (CYSEDFSLTKDDIAGIQKLYGKRNTL). Rabbit anti-mouse MMP-7 polyclonal antibodies were affinity purified and used for immunohistochemical and immunoblotting experiments. All animals were used in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
MMP-7 Expression Vectors
A cassette containing a multiple cloning site (MCS), an internal ribosome entry site (IRES), and cDNA encoding enhanced green fluorescent protein (EGFP) was subcloned into the pFB backbone to create pFB-MCS-IRES-EGFP (pFB-EIG). Active MMP-7 cDNA, which contained a leader peptide sequence and the catalytic domain, was generated by PCR to delete the pro sequence from the MMP-7 cDNA. This active MMP-7 cDNA was subcloned into pFB-EIG to create pFB-MMP-7-IRES-EGFP (pFB-EIG-MMP-7).
Production of Infective Replication-Defective Retrovirus
A replication-defective retroviral gene transfer system (from Stratagene, La Jolla, California, USA) was used to create MMP-7 knock-in corneal cell lines. To generate replication defective retrovirus carrying the gene of interest, we co-transfected HEK-293T cells (Effectene Transfection Reagent; Qiagen, Valencia, California, USA) with expression vectors encoding the viral internal structural proteins, reverse transcriptase and integrase (pVPack-Gag-Pol vector), the viral envelope protein (pVPack-Eco vector), and the MMP-7 expression plasmid (pFB-EIG or pFB-EIG-MMP-7). The procedure was performed according to the manufacturer's instructions.
Establishment of MMP-7 Knock-in Cell Lines18
Retroviral supernatants were collected from HEK-293T cells transfected with pFB-EIG or pFB-EIG-MMP-7 and then filtered through a sterile 0.20-μm membrane syringe filter. Next, 7ko fibroblasts were incubated with HEK-293T supernatants in the presence of 4 μg/mL polybrene for 24 hr. Green fluorescent protein (GFP) expression was detected in infected 7ko fibroblasts 3 to 4 days after retroviral infection. Cells were trypsinized with trypsin/EDTA, and suspended in DMEM with 1% fetal calf serum (FCS). GFP-expressing cells were then collected by fluorescence-activated cell sorting (FACS), which was performed using the FITC channel. Uninfected 7ko fibroblasts served as a negative control. Three consecutive sortings were performed to enrich the population of GFP-expressing cells. After sorting, GFP-expressing cells were expanded in DMEM with 20% FCS.
MMP-7 Immunoblotting
Immunoblotting for MMP-7 was performed on culture media and cell lysates collected from 7ko fibroblasts, 7ko fibroblasts expressing pFB-EIG-MMP-7 (MMP-7 knock-in cells, hereafter referred to as 7ko-MMP-7), and 7ko fibroblasts expressing vector only (7ko-vector). Media aliquots (15 mL) were filtered using an Acrodisc syringe filter with a 0.2-μm HT Tuffryn membrane (Gelman Laboratory, Pall corporation, Port Washington, New York, USA). Filtrates were concentrated using a centrifugal filter device with a 10,000-MW cut off membrane (Amicon Ultra-4 centrifugal filter devices with low-binding Ultracel membranes, Millipore, Massachusetts, USA). Cell lysates were prepared using 1× RIPA buffer containing complete protease inhibitors.
Cleavage of the NC1 Fragment of Collagen XVIII
Purified recombinant NC1 fragments (5 ng/μl, provided by Dr. N. Fukai, Harvard Medical School) were incubated with media collected from cultures of 7ko, 7ko-vector, or 7ko-MMP-7 cells. Incubations were performed in the presence of 5 μM ZnSO4 for 4 hr at 37°C. Immunoblot analysis was used to determine the amount of recombinant NC1 fragments that remained unprocessed by MMP-7.
RESULTS
Immortalized 7ko Cell Lines Lack Detectable MMP-7 and Have a Fibroblast-Like Phenotype
Before we could generate 7ko-MMP-7 and 7ko-vector cell lines to investigate MMP-7 activity towards the NC1 fragment of collagen XVIII, we first confirmed that MMP-7 was knocked out in immortalized corneal fibroblasts from 7ko mice. DNA from SV40-immortalized corneal fibroblasts (WT and 7ko mice) was extracted and genotyped using PCR. As shown in Figure 1A, 685- and 535-bp bands were detected in DNA fragments amplified from WT and 7ko cells, respectively, confirming MMP-7 knockout in 7ko cells. Confocal images show that corneal fibroblasts from neither 7ko (Figure 1B) nor WT (Figure 1C) mice exhibited detectable immunostaining for MMP-7. In contrast, corneal epithelial cells did express MMP-7, confirming that the immunostaining technique functioned properly (Figure 1D).
FIGURE 1.
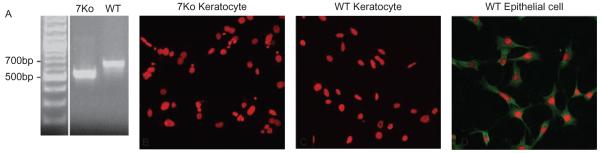
PCR analysis of the MMP-7 allele in 7ko and WT cell lines. Bands of 535 bp and 685 bp were expected for 7ko and WT cell lines, respectively (A). No immunostaining for MMP-7 was observed in WT or 7ko immortalized fibroblasts [(B) and (C); PI nuclei, red], and MMP-7 was localized to the cytoplasm of the immortalized WT corneal epithelial cells (D).
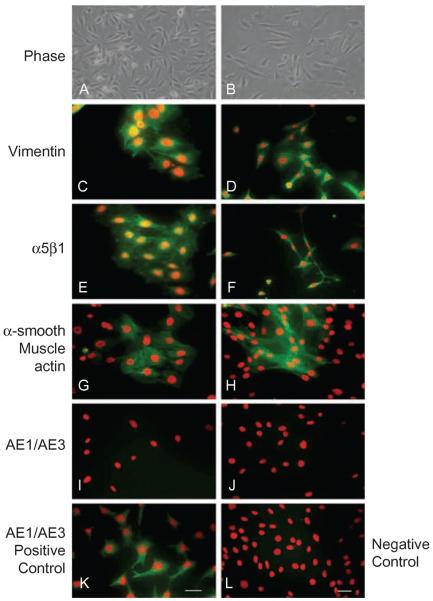
Culture conditions are known to influence keratocyte phenotypes (i.e., their maintenance as keratocytes or transformation into fibroblasts or myofibroblasts).20 For this reason, we analyzed the expression of specific phenotypic markers in WT and 7ko cells (Figure 2). Fibroblasts express both vimentin and prostaglandin D synthase. In the presence of 10% FCS, keratocytes transform into fibroblasts, which express vimentin and integrin α5β1.22 Accordingly, we found that WT and 7ko cell lines were immunopositive for both vimentin (Figure 2C for WT and 2D for 7ko) and integrin α5β1 (Figure 2E for WT and 2F for 7ko). Fibroblasts are known to transform into myofibroblasts after TGF-β stimulation, which promotes α-SMA expression. Stimulation of WT and 7ko cell lines with TGF-β elicited staining for α-SMA (Figure 2G for WT and 2H for 7ko). Our immortalized fibroblast cell lines did not contain any detectable epithelial keratin (a corneal epithelial cell marker), as assessed by staining with AE1/AE3 antibody (Figure 2I for WT and 2J for 7ko). On the other hand, corneal epithelial cells were positive for epithelial keratin (Figure 2K for WT) but did not express detectable α-SMA in response to TGF-β (Figure 2L for WT).
FIGURE 2.
Characterization of immortalized WT and 7ko fibroblasts. WT and 7ko fibroblasts were visualized by phase contrast [WT in (A), 7ko in (B)] and immunostained for vimentin [WT in (C), 7ko in (D)], integrin α5β1 [WT in (E), 7ko in (F)], and epithelial keratin [using AE1/AE3 antibody; WT in (I), 7ko in (J)]. A subset of cells was stimulated with TGF-β and stained for α-SMA (myofibroblast marker) [WT in (G), 7ko in (H)]. Corneal epithelial cells immunostained for epithelial keratin served as a positive control (K), and TGF-β-stimulated corneal epithelial cells immunostained for α-SMA served as a negative control (L). Scale bar, 50 μm.
7ko-MMP-7 Fibroblasts Express and Secrete MMP-7
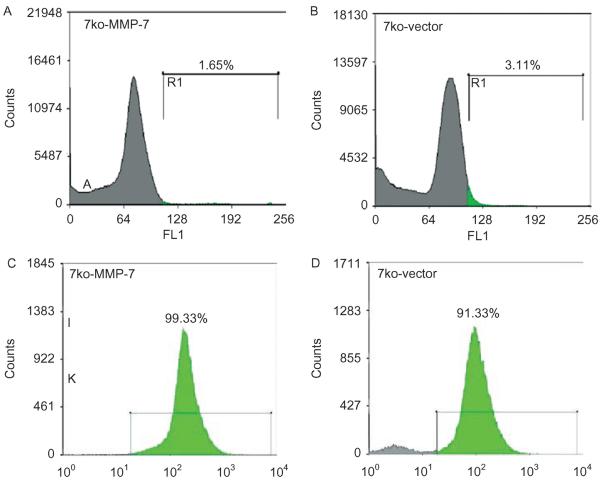
Next, 7ko fibroblast cell lines were infected with supernatants containing pFB-EIG or pFB-EIG-MMP-7 retroviruses. Before FACS, only a small number of 7ko-MMP-7 (Figure 3A) and 7ko-vector (Figure 3B)-infected cells expressed GFP. Efficiency of retroviral infection ranged from 0.95% to 3.11%, as determined by FACS analysis of GFP expression (Figures 3A and 3B). After re-sorting, most fibroblasts expressed GFP (Figures 3C and 3D). GFP expression was clearly visible in 7ko-vector cells (Figures 4B and 4F) and 7ko-MMP-7 cells (Figures 4C and 4G) by fluorescence microscopy. Confocal immunostaining showed MMP-7-ki expression of MMP-7 (Figure 4D) and GFP (Figure 4H). As expected, uninfected 7ko fibroblasts did not express GFP (Figure 4E).
FIGURE 3.
Enrichment of GFP-expressing cells with FACS. Initial sorting yielded only 1.65% GFP-expressing 7ko-MMP-7 fibroblasts (A) and 3.11% 7ko-vector fibroblasts (B). After three sequential sortings, the population of GFP-expressing cells exceeded 90% purity for both the 7ko-MMP-7 (C) and 7ko-vector group (D).
FIGURE 4.
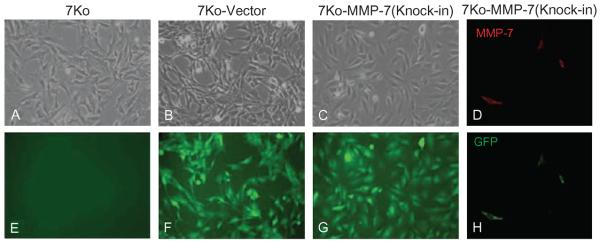
GFP and MMP-7 expression in cultured fibroblasts. Phase contrast images of 7ko (A), 7ko-vector (B), and 7ko-MMP-7 knock-in fibroblasts (C). No GFP expression was observed in 7ko fibroblasts (E). Confocal images of 7ko-vector (F) and 7ko-MMP-7 (knock-in) fibroblasts [(G) and (H)]. There was detectable MMP-7 immunostaining in 7ko-MMP-7 knock-in fibroblasts (D).
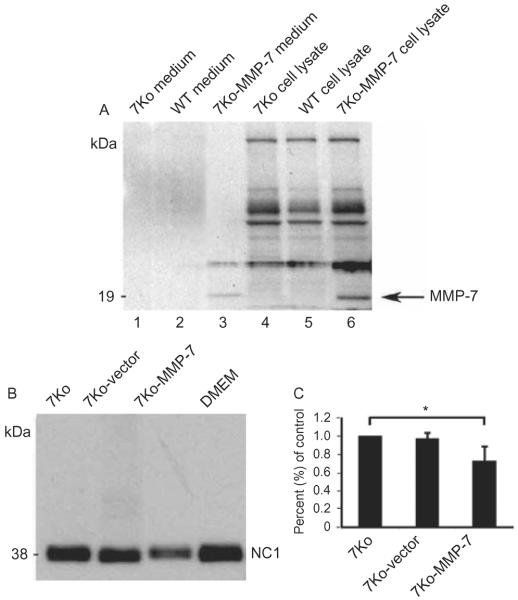
MMP-7 secretion by sorted populations of 7ko-vector and 7ko-MMP-7 cells was then investigated by performing immunoblot analysis of culture media with anti-MMP-7 antibody. A 19-kDa band corresponding to active MMP-7 was detected in media from 7ko-MMP-7 cultures, but not from 7ko or 7ko-vector cultures (Figure 5A). Immunoblot analysis of cell lysates yielded similar results (Figure 5A).
FIGURE 5.
Immunoblot analysis of MMP-7 expression in media (lanes 1–3) and lysates (lanes 4–6) from WT, 7ko, and 7ko-MMP-7 fibroblasts (A). The 7ko and WT groups served as negative controls. Immunoblot analysis of collagen XVIII NC1 degradation induced by media collected from 7ko, 7ko-vector, and 7ko-MMP-7 fibroblast cultures (B). Three independent experiments were performed to quantify the intensity of remaining, unprocessed NC1 fragments. The NC1 fragment density in the 7ko-MMP-7 group is 0.72 ± 0.15% of that of the control 7ko group (C).
MMP-7 Secreted by 7ko-MMP-7 Fibroblasts Cleaves the NC1 Fragment of Collagen XVIII in Vitro
Collagen XVIII is known to be localized to the epithelium, the epithelial basement membrane, and Descemet's membrane in the human cornea. We have previously demonstrated that MMP-7 induces proteolysis of NC1 fragments of collagen XVIII.14 Therefore, we tested whether MMP-7 secreted by 7ko-MMP-7 fibroblasts is capable of cleaving the NC1 fragment in vitro. To do this, we incubated the NC1 fragment of collagen XVIII with conditioned medium collected from 7ko, 7ko-vector, or 7ko-MMP-7 fibroblast cultures. Immunoblot analysis of these reaction mixtures revealed that the amount of NC1 fragment (38 kDa) was comparable in the 7ko and 7ko-vector groups. In contrast, the NC1 fragment band intensity was much lower in the 7ko-MMP-7 group (Figure 5B). Three experiments were performed to quantify NC1 fragment intensity using KODAK Molecular Imaging Software (Kodak, Rochester, New York, USA) and the density of remaining unprocessed NC1 fragments in the 7ko-MMP-7 group was 0.72 ± 0.15% of that of the control 7ko group (Figure 5C). However, the activity of MMP-7 in 7ko-MMP-7 knock-in fibroblasts was not visualized in the casein gel zymograph assay.
DISCUSSION
Our results demonstrate that the MMP-7 gene can be efficiently transferred to MMP-7-deficient corneal fibroblasts using the retroviral system of gene transfer and a bicistronic expression vector. The retroviral system of gene transfer is an excellent system for generating a stable cell line23 and offers many advantages over transient transfection of cDNA probes and constructs. In addition, co-expression of GFP facilitates the monitoring of both infection efficiency and gene expression. It also permits easy enrichment of infected cells with FACS. In our experiments, although the initial infection efficiency was low, populations of GFP-expressing cells that were close to 100% pure were easily obtained by sequential re-sorting with FACS.
In the cornea, MMP-7 has been implicated in epithelial repair, wound healing, and antiangiogenesis. It has been immunolocalized to the basal epithelial layer of the cornea and is upregulated during the migration proliferation phase of corneal wound healing following excimer laser keratectomy.24 Di Girolamo et al. also demonstrated that MMP-7 is immunolocalized to the basal epithelial cells of pterygium specimens, and they suggested that it participates in the pathogenesis of pterygium and angiogenesis associated with this condition.25 We have previously reported that MMP-7 is localized to corneal epithelial cells, but not corneal stromal fibroblasts. However, MMP-7-deficient mice undergoing excimer keratectomy wounding display a higher level of corneal NV and haze than their WT littermates, suggesting that MMP-7 may contribute to corneal avascularity during wound healing.16 Indeed, MMP-7 cleaves collagen XVIII to release a 28-kDa fragment containing the endostatin domain, a known antiangiogenic protein. Here, we focused on the proteolytic activity of MMP-7 produced by fibroblasts, as these cells are known to participate in corneal NV. To do this, we transfected fibroblasts with active MMP-7, an approach that may have drawbacks, such as induction intracellular damage. To avoid such possible damage, we attempted to transfect cells with inactive proMMP and then stimulated proMMP through aminophenylmercuric acetate (APMA) activation. In this approach, overexpressed proMMP is activated outside of the cell, making intracellular damage less likely to occur. However, we were unable to detect MMP-7 activity in the medium after proMMP-7 over-expression despite APMA activation. Support for our strategy of overexpressing active MMP-7 inside the cell comes from Rudolph-Owen et al.26 This group has subcloned three mutated forms of human MMP-7 (WT, active, and inactive) under the control of the murine mammary tumor virus promoter/enhancer (MMTVLTR), which has been used to drive expression in a tissue-specific manner.26 Similar to this construct, the corneal 7ko cell lines are a valuable tool for studying the role of MMP-7. The 7ko fibroblasts expressing active MMP-7 will be of particular value in isolating and characterizing potential MMP-7 substrates in vivo and in vitro.
ACKNOWLEDGMENTS
Supported by National Institutes of Health Grant EY001792, EY10101 (DTA), EY14048 (JHC), MEEI-Schepens Joint Center for Clinical Research Fellowship (MIR).
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- [1].Ellenberg D, Azar DT, Hallak JA, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Azar DT. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- [3].Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Albuquerque RJ, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Daniels JT, Cambrey AD, Occleston NL, et al. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003;44:1104–1110. doi: 10.1167/iovs.02-0412. [DOI] [PubMed] [Google Scholar]
- [6].Seiki M. The cell surface: The stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- [7].Busiek DF, Ross FP, McDonnell S, et al. The matrix metallopro-tease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992;267:9087–9092. [PubMed] [Google Scholar]
- [8].Gaire M, Magbanua Z, McDonnell S, et al. Structure and expression of the human gene for the matrix metalloproteinase matrilysin. J Biol Chem. 1994;269:2032–2040. [PubMed] [Google Scholar]
- [9].Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- [10].Szklarczyk A, Oyler G, McKay R, et al. Cleavage of neuronal synaptosomal-associated protein of 25 kDa by exogenous matrix metalloproteinase-7. J Neurochem. 2007;102:1256–1263. doi: 10.1111/j.1471-4159.2007.04625.x. [DOI] [PubMed] [Google Scholar]
- [11].Chang JH, Javier JA, Chang GY, et al. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579:3601–3606. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- [12].Strand S, Vollmer P, van den Abeelen L, et al. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene. 2004;23:3732–3736. doi: 10.1038/sj.onc.1207387. [DOI] [PubMed] [Google Scholar]
- [13].Lynch CC, Hikosaka A, Acuff HB, et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- [14].Lin HC, Chang JH, Jain S, et al. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- [15].Kojima T, Azar DT, Chang JH. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 2008;582:2515–2520. doi: 10.1016/j.febslet.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kure T, Chang JH, Kato T, et al. Corneal neovascularization after excimer keratectomy wounds in matrilysin-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:137–144. doi: 10.1167/iovs.01-1058. [DOI] [PubMed] [Google Scholar]
- [17].Beales MP, Funderburgh JL, Jester JV, et al. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: Maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40:1658–1663. [PubMed] [Google Scholar]
- [18].Azar DT, Casanova FH, Mimura T, et al. Effect of MT1-MMP deficiency and overexpression in corneal keratocytes on vascular endothelial cell migration and proliferation. Curr Eye Res. 2008;33:954–962. doi: 10.1080/02713680802461106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Onguchi T, Han KY, Chang JH, et al. Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am J Pathol. 2009;174:1564–1571. doi: 10.2353/ajpath.2009.080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jester JV, Huang J, Fisher S, et al. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:1850–1858. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- [21].Chen CC, Chang JH, Lee JB, et al. Human corneal epithelial cell viability and morphology after dilute alcohol exposure. Invest Ophthalmol Vis Sci. 2002;43:2593–2602. [PubMed] [Google Scholar]
- [22].Berryhill BL, Kader R, Kane B, et al. Partial restoration of the keratocyte phenotype to bovine keratocytes made fibroblastic by serum. Invest Ophthalmol Vis Sci. 2002;43:3416–3421. [PubMed] [Google Scholar]
- [23].Clements MO, Godfrey A, Crossley J, et al. Lentiviral manipulation of gene expression in human adult and embryonic stem cells. Tissue Eng. 2006;12:1741–1751. doi: 10.1089/ten.2006.12.1741. [DOI] [PubMed] [Google Scholar]
- [24].Lu PC, Ye H, Maeda M, et al. Immunolocalization and gene expression of matrilysin during corneal wound healing. Invest Ophthalmol Vis Sci. 1999;40:20–27. [PubMed] [Google Scholar]
- [25].Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: Potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001;42:1963–1968. [PubMed] [Google Scholar]
- [26].Rudolph-Owen LA, Cannon P, Matrisian LM. Overexpression of the matrix metalloproteinase matrilysin results in premature mammary gland differentiation and male infertility. Mol Biol Cell. 1998;9:421–435. doi: 10.1091/mbc.9.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]