Abstract
Neuropeptide genes are expressed in specific subsets of large polyploid neurons in Aplysia californica. We have defined the transcription initiation sites of three of these neuropeptide genes (the R14, L11, and ELH genes) and determined the nucleotide sequence of the promoter regions. The genes contain the usual eucaryotic promoter signals as well as other structures of potential regulatory importance, including inverted and direct repeats. The L11 and ELH genes, which are otherwise unrelated, have homology in the promoter regions, while the R14 promoter was distinct. When cloned plasmids were microinjected into Aplysia neurons in organ culture, transitions between supercoiled, relaxed circular, and linear DNAs occurred along with ligation into high-molecular-weight species. About 20% of the microinjected neurons expressed the genes. The promoter region of the R14 gene functioned in expression of the microinjected DNA in all cells studied. When both additional 5' and 3' sequences were included, the gene was specifically expressed only in R14, suggesting that the specificity of expression is generated by a multicomponent repression system. Finally, the R14 peptide could be expressed in L11, demonstrating that it is possible to alter the transmitter phenotype of these neurons by introduction of cloned genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltz G. A., Jacobs K. A., Eickbush T. H., Cherbas P. T., Kafatos F. C. Isolation of multigene families and determination of homologies by filter hybridization methods. Methods Enzymol. 1983;100:266–285. doi: 10.1016/0076-6879(83)00061-0. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Bienz-Tadmor B., Zakut-Houri R., Libresco S., Givol D., Oren M. The 5' region of the p53 gene: evolutionary conservation and evidence for a negative regulatory element. EMBO J. 1985 Dec 1;4(12):3209–3213. doi: 10.1002/j.1460-2075.1985.tb04067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brent R. Repression of transcription in yeast. Cell. 1985 Aug;42(1):3–4. doi: 10.1016/s0092-8674(85)80091-x. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Portmann R., Irminger J. C., Birnstiel M. L. Ubiquitous and gene-specific regulatory 5' sequences in a sea urchin histone DNA clone coding for histone protein variants. Nucleic Acids Res. 1980 Mar 11;8(5):957–977. doi: 10.1093/nar/8.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E., Yaksta B. A., Swartz F. J. A cytophotometric analysis of the DNA in the nucleus of the giant cell, R-2, in Aplysia. Chromosoma. 1970;32(2):205–212. doi: 10.1007/BF00286009. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Etkin L. d., Pearman B., Roberts M., Bektesh S. L. Replication, integration and expression of exogenous DNA injected into fertilized eggs of Xenopus laevis. Differentiation. 1984;26(3):194–202. doi: 10.1111/j.1432-0436.1984.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Everett R. D., Baty D., Chambon P. The repeated GC-rich motifs upstream from the TATA box are important elements of the SV40 early promoter. Nucleic Acids Res. 1983 Apr 25;11(8):2447–2464. doi: 10.1093/nar/11.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Ohno S., Yasumitsu H., Taniguchi T. Delimitation and properties of DNA sequences required for the regulated expression of human interferon-beta gene. Cell. 1985 Jun;41(2):489–496. doi: 10.1016/s0092-8674(85)80022-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W. Autoregulation plus upstream positive and negative control regions associated with transcriptional activation of the mouse P1(450) gene. Nucleic Acids Res. 1985 Oct 25;13(20):7269–7288. doi: 10.1093/nar/13.20.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Jones P. B., Galeazzi D. R., Fisher J. M., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985 Mar 22;227(4693):1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
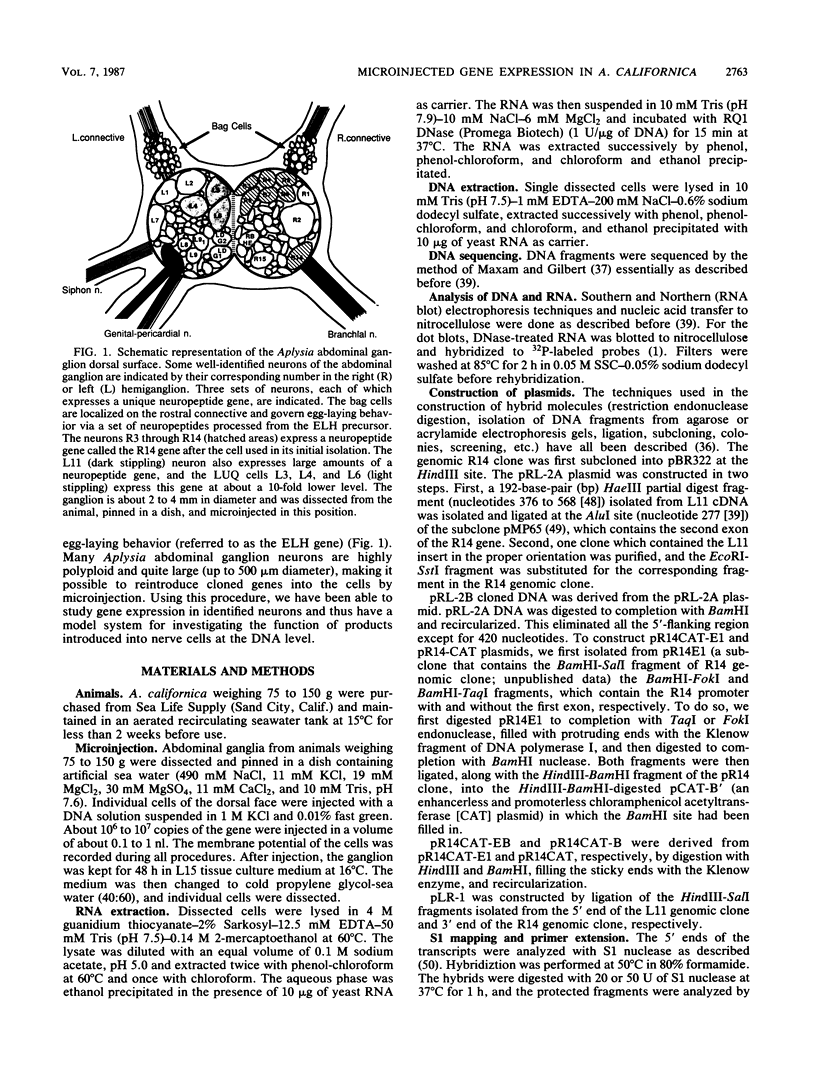
- Kaldany R. R., Nambu J. R., Scheller R. H. Neuropeptides in identified Aplysia neurons. Annu Rev Neurosci. 1985;8:431–455. doi: 10.1146/annurev.ne.08.030185.002243. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner T., Rothbard J. B., Schoolnik G. K., Scheller R. H. Antibodies to synthetic peptides defined by cDNA cloning reveal a network of peptidergic neurons in Aplysia. J Neurosci. 1984 Oct;4(10):2581–2589. doi: 10.1523/JNEUROSCI.04-10-02581.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner T., Sossin W., Scheller R. H. Localization of Aplysia neurosecretory peptides to multiple populations of dense core vesicles. J Cell Biol. 1986 Mar;102(3):769–782. doi: 10.1083/jcb.102.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek R. J., Dower W. J. Aplysia californica: analysis of nuclear DNA in individual nuclei of giant neurons. Science. 1971 Apr 16;172(3980):278–280. doi: 10.1126/science.172.3980.278. [DOI] [PubMed] [Google Scholar]
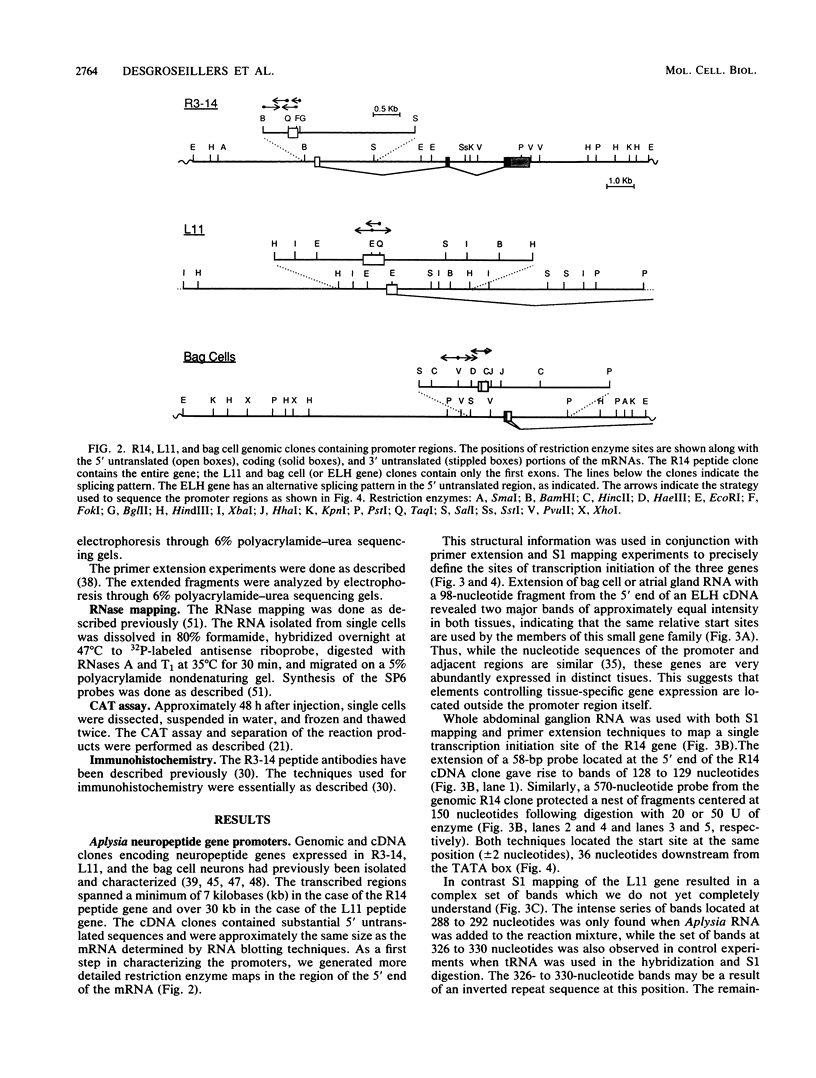
- Mahon A. C., Nambu J. R., Taussig R., Shyamala M., Roach A., Scheller R. H. Structure and expression of the egg-laying hormone gene family in Aplysia. J Neurosci. 1985 Jul;5(7):1872–1880. doi: 10.1523/JNEUROSCI.05-07-01872.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Nambu J. R., Taussig R., Mahon A. C., Scheller R. H. Gene isolation with cDNA probes from identified Aplysia neurons: neuropeptide modulators of cardiovascular physiology. Cell. 1983 Nov;35(1):47–56. doi: 10.1016/0092-8674(83)90206-4. [DOI] [PubMed] [Google Scholar]
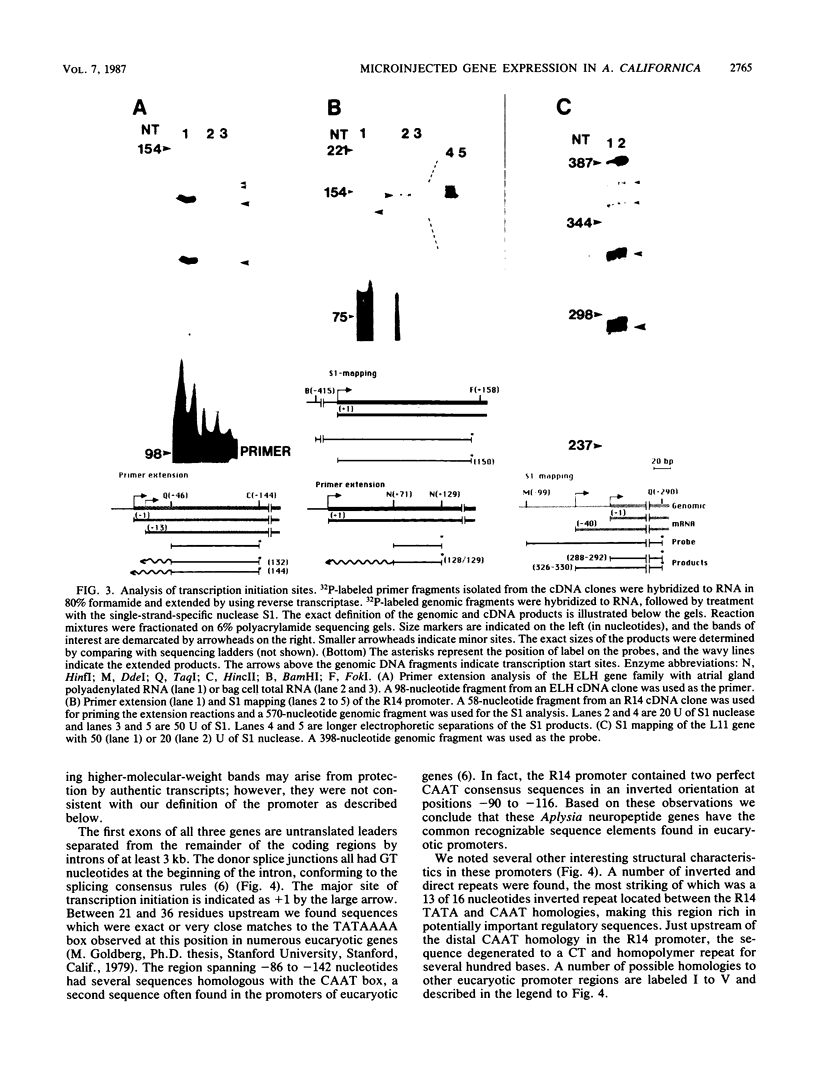
- Newcomb R., Scheller R. H. Proteolytic processing of the Aplysia egg-laying hormone and R3-14 neuropeptide precursors. J Neurosci. 1987 Mar;7(3):854–863. doi: 10.1523/JNEUROSCI.07-03-00854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Ragg H., Weissmann C. Not more than 117 base pairs of 5'-flanking sequence are required for inducible expression of a human IFN-alpha gene. Nature. 1983 Jun 2;303(5916):439–442. doi: 10.1038/303439a0. [DOI] [PubMed] [Google Scholar]
- Remmers E. F., Yang J. Q., Marcu K. B. A negative transcriptional control element located upstream of the murine c-myc gene. EMBO J. 1986 May;5(5):899–904. doi: 10.1002/j.1460-2075.1986.tb04301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Jackson J. F., McAllister L. B., Rothman B. S., Mayeri E., Axel R. A single gene encodes multiple neuropeptides mediating a stereotyped behavior. Cell. 1983 Jan;32(1):7–22. doi: 10.1016/0092-8674(83)90492-0. [DOI] [PubMed] [Google Scholar]
- Taussig R., Kaldany R. R., Rothbard J. B., Schoolnik G., Scheller R. H. Expression of the L11 neuropeptide gene in the Aplysia central nervous system. J Comp Neurol. 1985 Aug 1;238(1):53–64. doi: 10.1002/cne.902380105. [DOI] [PubMed] [Google Scholar]
- Taussig R., Kaldany R. R., Scheller R. H. A cDNA clone encoding neuropeptides isolated from Aplysia neuron L11. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4988–4992. doi: 10.1073/pnas.81.15.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
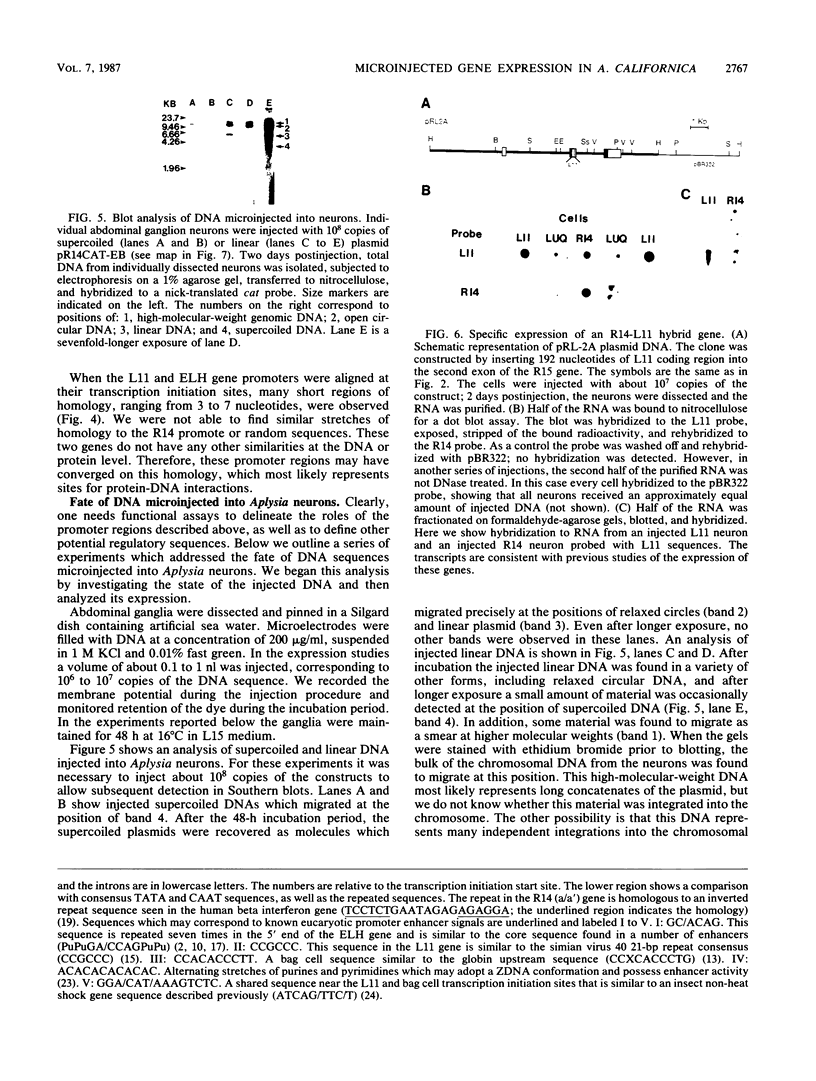
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]