Abstract
Objective
The goal of this study was to identify mutations in 25 known causative genes in 47 unrelated Chinese families with cone-rod dystrophy (CORD).
Methods
Forty-seven probands from unrelated families with CORD were recruited. Genomic DNA prepared from leukocytes was analyzed by whole exome sequencing. Variants in the 25 genes were selected and then validated by Sanger sequencing.
Results
Fourteen potential pathogenic mutations, including nine novel and five known, were identified in 10 of the 47 families (21.28%). Homozygous, compound heterozygous, and hemizygous mutations were detected in three, four, or three families, respectively. The 14 mutations in the 10 families were distributed among CNGB3 (three families), PDE6C (two families), ABCA4 (one family), RPGRIP1 (one family), RPGR (two families), and CACNA1F (one family).
Conclusions
This study provides a brief view on mutation spectrum of the 25 genes in a Chinese cohort with CORD. Identification of novel mutations enriched our understanding of variations in these genes and their associated phenotypes. To our knowledge, this is the first systemic exome-sequencing analysis of all of the 25 CORD-associated genes.
Introduction
Cone-rod dystrophy (CORD) refers to a series of hereditary retinal disorders with a predominantly cone involvement [1]. Rod impairment may occur at the same time as the cone impairment or appear later. Patients with CORD usually have reduced visual acuity, photophobia, and color vision defects.
CORD may be transmitted as an autosomal dominant (adCORD), autosomal recessive (arCORD), or X-linked trait (xlCORD). To date, mutations in at least 25 genes have been reported to be associated with different forms of CORD, including the following: aryl hydrocarbon receptor interacting protein-like 1 (AIPL1) [1]; the cone-rod homeobox containing gene (CRX) [2] ; guanylate cyclase activator 1A (GUCA1A) [3] ; guanylate cyclase 2D (GUCY2D) [4] ; PITPNM family member 3 (PITPNM3) [5] ; prominin 1 (PROM1) [6] ; peripherin 2 (PRPH2) [7] ; regulating synaptic membrane exocytosis 1 (RIMS1) [8] ; sema domain, immunoglobulin domain (Ig), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 4A (SEMA4A) [9] ; unc-119 homolog (UNC119) [10]; ATP-binding cassette, sub-family A (ABC1) member 4 (ABCA4) [11] ; ADAM metallopeptidase domain 9 (ADAM9) [12] ; chromosome 8 open-reading frame 37 (C8ORF37) [13] ; calcium channel voltage-dependent alpha 2/delta subunit 4 (CACNA2D4) [14] ; cadherin-related family member 1 (CDHR1) [15] ; ceramide kinase-like (CERKL) [16] ; cyclic nucleotide gated channel beta 3 (CNGB3) [17] ; cyclin M4 (CNNM4) [18]; potassium channel subfamily V member 2 (KCNV2) [19] ; phosphodiesterase 6C, cGMP-specific, cone, alpha prime (PDE6C) [20] ; retina and anterior neural fold homeobox 2 (RAX2) [21] ; retinol dehydrogenase 5 (RDH5) [22] ; retinitis pigmentosa GTPase regulator interacting protein 1 (RPGRIP1) [23]; calcium channel voltage-dependent L type alpha 1F subunit (CACNA1F) [24] ; and retinitis pigmentosa GTPase regulator (RPGR) [25] (RetNet: https://sph.uth.edu/Retnet/). Of the 25 genes, mutations in the first 10 genes are responsible for adCORD, the next 13 for arCORD, and the last two for xlCORD. The associated genomic information of the 25 genes is listed in Table S1.
In our previous study on CORD, mutations were only detected in 7 of 130 (5.38%) Chinese families with CORD by using cycle sequencing of all coding exons of five genes (CRX, GUCY2D, GUCA1A, PRPH2, and KCNV2) as well as of all exons harboring reported mutations in other 17 CORD-associated genes [26], [27], [28], [29]. Of the seven families, all mutations were identified in genes responsible for adCORD but none in genes for arCORD and xlCORD. The genetic cause for most families (the remaining 123 of 130 (94.62%) Chinese families) was still unknown. In order to identify the additional cause of most CORD and to disclose further the mutation spectrum and frequency of the 25 genes, whole exome sequencing was used to screen for mutations in 47 unrelated Chinese families with CORD.
Materials and Methods
Patients
Forty seven probands from unrelated families with CORD were recruited from the Eye Hospital of Zhongshan Ophthalmic Center, Sun Yat-sen University. Patients with identified mutation who were included in our previous study were excluded from this one. Written informed consents were obtained from the participants or their guardians before the study, which was conforms to the tenets of the Declaration of Helsinki and follows the Guidance of Sample Collection of Human Genetic Diseases (863-plan) by the Ministry of Public Health of China. This study was approved by the Institute Review Board of the Zhongshan Ophthalmic Center. Genomic DNA was prepared from the blood leukocytes as previous described [30].
Exome Sequencing
Exome sequencing was completed by using a commercial service from BGI Shenzhen (http://www.genomics.cn/index.php). The exome sequencing, genotype calling, and SNP calling were in the same way as the methods reported before [31]. In brief, exome capture was carried out by using a NimbleGen SeqCap EZ Exome (44 M) array. Exon-enriched DNA fragments were sequenced by the Illumina Genome Analyzer II. The average sequencing depth was set to 60-fold. SOAP aligner was used to set the sequencing reads to UCSC hg19 [32], [33]. The likelihood of possible genotypes in the target regions was calculated using SOAPsnp [34]. Variants in all the 25 genes detected by exome sequencing were selected for validation. Exome sequencing dataset of the patients with identified mutations in this study have been deposited to NIH (http://www.ncbi.nlm.nih.gov/biosample: accession number SAMN01997562 to SAMN01997571).
Sanger Sequencing
Sanger sequencing was used to validate variants in the 25 genes that resulted from exome sequencing, including heterozygous variants in the adCORD genes, homozygous or compound heterozygous variants in the arCORD genes, or hemizygous variants in the xlCORD genes. Primers (Table S2) used to amplify the regions containing the variants were designed by primer design tool Primer3 (http://frodo.wi.mit.edu/primer3/) [35]. A touch-down polymerase chain reaction (PCR) was used to amplify the fragments with variants, as previously reported [36], and the amplicons were analyzed with an ABI BigDye Terminator cycle sequencing kit v3.1 (Applied Biosystems, Foster City, CA) on an ABI3100 Genetic Analyzer (Applied Biosystems). Sequencing results from patients and controls were compared using the SeqManII program of the Lasergene package (DNAStar Inc, Madison, WI). Detected variants were further sequenced in the available family members. Novel variants were further evaluated in 192 control individuals. The description of mutations was in accordance with the nomenclature for the description of sequence variants [37](HGVS: http://www.hgvs.org/mutnomen/). The conservation of a variation was evaluated by Phastcons_score (http://varianttools.sourceforge.net/Annotation/PhastCons) [38], the effect of a missense variation was analyzed by using SIFT [39] (http://sift.jcvi.org/) and Polyphen-2 [40] (http://genetics.bwh.harvard.edu/pph2/) online tools, and the effect of splicing site changes was predicted by Berkeley Drosophila Genome Project (BDGP) [41] (http://www.fruitfly.org/).
Considering that CORD-causing mutations are rare and the presence of the normal carriers of arCORD gene mutations, we assumed that the affected individuals were likely homozygous or compound heterozygous, so variants absent in the dbSNP134, 1000Genome or with allelic frequencies ≤0.006 were considered to be potentially pathogenic (frequency of heterozygote carriers calculated based on a disease incidence of 1∶40,000, under the hypothesis that a unique arCORD gene would explain the remaining 40% of cases [42]).
Results
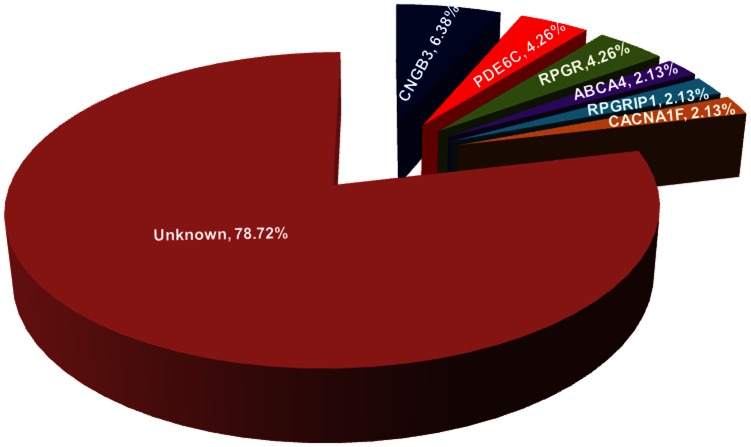
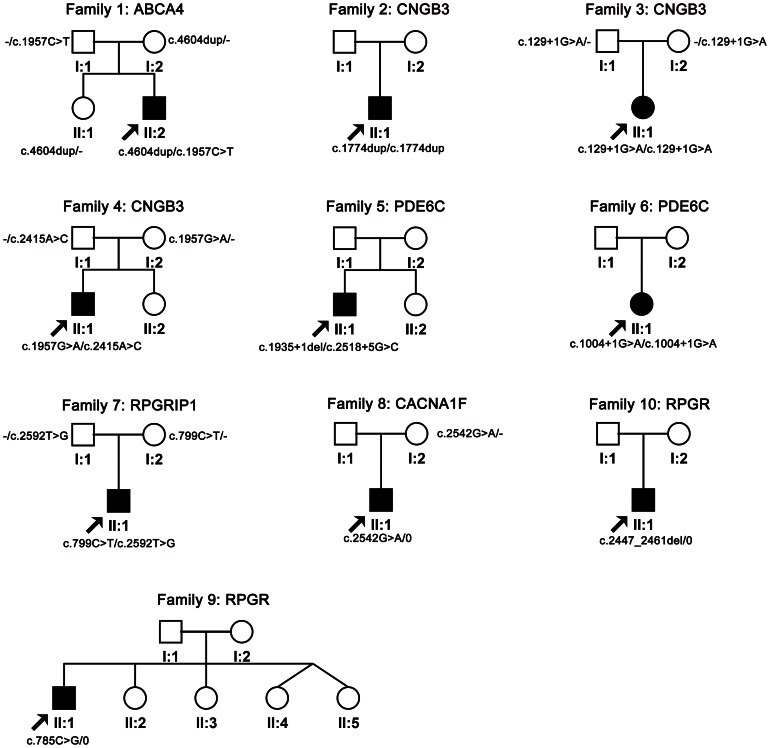
Whole exome sequencing identified 14 potential pathogenic mutations in 10 of the 47 (21.28%) families with CORD (Table 1), including seven homozygous or compound heterozygous mutations in four (ABCA4, CNGB3, PDE6C, and RPGRIP1) of the 13 genes associated with arCORD, and three hemizygous mutations in the two genes (RPGR and CACNA1F) associated with xlCORD. Of the 14 mutations, nine were novel. The 14 mutations in the 10 families involved six of the 25 CORD-associated genes, including CNGB3 (three families), PDE4C (two families), RPGR (two families), ABCA4 (one family), RPGRIP1 (one family), and CACNA1F (one family) (Figure 1), respectively. Sanger sequencing confirmed the 14 mutations in the 10 families (Figure S1). Segregation analysis was available for five of the 10 families in where the mutations co-segregated with the disease in the family (Figure 2). No potential pathogenic mutation was identified in the other 19 genes of the 47 families.
Table 1. Potential pathogenic mutations detected in 10 of the 47 families.
| Family ID | Gene | Variations | Status | Bioinformation analysis | Allele frequency in | Reference | |||||
| DNA | Protein | SIFT | Polyphen-2 | Splice | Phastcons _score | patients | controls | ||||
| Family 1 | ABCA4 | c.4604dup | p.T1537Nfs*18 | hetero | – | – | – | 0.997 | 1/94 | 0/384 | novel |
| Family 1 | ABCA4 | c.1957C>T | p.R653C | hetero | D | PD | – | 1.000 | 1/94 | NA | [38] |
| Family 2 | CNGB3 | c.1774dup | p.E592Gfs*44 | homo | – | – | – | 1.000 | 2/94 | 0/384 | novel |
| Family 3 | CNGB3 | c.129+1G>A | – | homo | – | – | DSA | 1.000 | 2/94 | 0/384 | novel |
| Family 4 | CNGB3 | c.2415A>C | p.E805D | hetero | D | PD | – | 1.000 | 1/94 | NA | rs186448979# |
| Family 4 | CNGB3 | c.1957G>A | p.A653T | hetero | tolerated | benign | – | 0.000 | 1/94 | 0/384 | novel |
| Family 5 | PDE6C | c.1935+1del | – | hetero | – | – | DSA | 1.000 | 1/94 | 0/384 | novel |
| Family 5 | PDE6C | c.2518+5G>C | NA | hetero | – | – | DSA | 0.112 | 1/94 | 0/384 | novel |
| Family 6 | PDE6C | c.1004+1G>A | – | homo | – | – | DSA | 1.000 | 2/94 | 0/384 | novel |
| Family 7 | RPGRIP1 | c.2592T>G | p.Y864* | hetero | – | – | – | 0.994 | 1/94 | 0/384 | novel |
| Family 7 | RPGRIP1 | c.799C>T | p.R267* | hetero | – | – | – | 1.000 | 1/94 | NA | [36] |
| Family 8 | CACNA1F | c.2542G>A | p.G848S | hemi | tolerated | benign | – | 1.000 | 1/94 | 0/384 | novel |
| Family 9 | RPGR | c.785C>G | p.A262G | hemi | tolerated | benign | – | 0.002 | 1/94 | NA | [39] |
| Family10 | RPGR | c.2447_2461del | p.G816_E820del | hemi | – | – | – | NA | 1/94 | NA | [40] |
Note: D = damaging; PD = probably damaging; DSA = donor site abolished.
The variation was found in 1000 Genomes database with the Global minor allele frequency (MAF) of G = 0.001/3 so that the pathogeneity of the variants in this family need to be clarified further.
Figure 1. Prevalence of mutations in the investigated genes in our cohort of 47 CORD patients.
Figure 2. Pedigrees of the 10 families with mutations.
The family numbers and their corresponding mutations were shown just above the pedigrees.
Clinical data of the 10 probands with potential pathogenic mutations are listed in Table 2. All probands with identified mutations had an early onset severe form of retinal dystrophy with predominantly cone involvement. Fundus changes were mainly in the macular regions, showing mild pigmentary changes and a loss of foveal reflex, as well as attenuated retinal arteries in rare cases.
Table 2. Clinical data of the probands with CORD and identified potential pathogenic mutations.
| Family ID | Gene | Nucleotide changes | Sex | Age (year) at | First | Best visual acuity | Fundus changes | ERG responses from | ||||
| exam | onset | symptom | right | left | right | left | rod | cone | ||||
| Family 1 | ABCA4 | c.[1957C>T];[4604dup] | M | 7.0 | 6.0 | PV, PP | 0.20 | 0.15 | MA, TPOD, ARA | MA, TPOD, ARA | Normal | Reduced |
| Family 2 | CNGB3 | c.[1774dup];[1774dup] | M | 6.5 | EC | PV,NYS | 0.20 | 0.20 | MA, TPOD | MA, TPOD | Mildly reduced | Extinguished |
| Family 3 | CNGB3 | c.[129+1G>A];[129+1G>A] | F | 5.0 | 0.3 | PV,NYS, PP | 0.20 | 0.20 | MA, TPOD, ARA | MA, TPOD, ARA | Normal | Extinguished |
| Family 4 | CNGB3 | c.[1957G>A];[2415A>C] | F | 4.5 | 0.5 | PP,NYS | 0.10 | 0.20 | ARA | ARA | Mildly reduced | Severely reduced |
| Family 5 | PDE6C | c.[1935+1del];[2518+5G>C] | M | 7.0 | EC | NYS | 0.05 | 0.05 | High myopic | High myopic | Normal | Severely reduced |
| Family 6 | PDE6C | c.[1004+1G>A];[1004+1G>A] | F | 2.0 | EC | PV,PP,NYS | PO | PO | NA | NA | Mildly reduced | Extinguished |
| Family 7 | RPGRIP1 | c.[799C>T];[2592T>G] | M | 3.6 | FMB | PV, PP, NYS | NA | NA | ARA,CRD | ARA,CRD | Extinguished | Extinguished |
| Family 8 | CACNA1F | c.[2542G>A]; [0] | M | 2.3 | NA | PP, NYS | NA | NA | MA | MA | Severely reduced* | Extinguished |
| Family 9 | RPGR | c.[785C>G]; [0] | M | 28.0 | EC | PV, PP | 0.10 | 0.10 | MA, TPOD, ARA | MA, TPOD, ARA | Moderately reduced | Extinguished |
| Family 10 | RPGR | c.[2447_2461del]; [0] | M | 9.0 | EC | PV, PP | 0.20 | 0.20 | MA | MA | Normal | Extinguished |
Note: F = female; M = male; EC = early childhood; FMB = first few months after birth; PV = poor vision; PP = photophobia; NYS = nystagmus; PO = pursuing object; NA = not available; MA = macular atrophy; TPOD = temporal pallor of optic disc; ARA = attenuated retinal arteries; CRD = carpet-like retinal degeneration.
This patient did not have the “electronegative” ERG in the standard combined response.
Discussion
Based on an initial screening of exome sequencing and the subsequent confirmation of Sanger sequencing, potential pathogenic mutations were identified in 10 of the 47 (21.28%) families with CORD, involving 14 mutations in six of the 25 CORD-associated genes. For the 47 families in this study, the contributions of causative mutations in individual genes are as follows: CNGB3 (6.38%), PDE6C (4.26%), RPGR (4.26%), ABCA4 (2.13%), RPGRIP1 (2.13%), and CACNA1F (2.13%).
The frequency of mutations detected in this study is significantly higher than in our previous study, in which mutations were only detected in seven of 130 (5.38%) Chinese families with CORD through the process of sequencing the coding exons of five genes and all exons with reported mutations in 17 other genes [26], [27], [28], [29]. In addition, in this study, mutations in seven of the 10 families were found in arCORD-associated genes but no mutations were detected in the adCORD-associated genes; in contrast, mutations in all seven families from our previous study were found in adCORD-associated genes. This difference may partly have resulted from 1) several genes associated with adCORD having been systemically analyzed before and all patients with mutations in these genes being excluded from this study; and 2) selective analysis of exons with reported mutations perhaps failing to identify both mutations in arCORD associated genes if one mutation in the exons is not analyzed. The real proportion of mutations in the 25 genes should be higher than 21.28% in Chinese patients with CORD if mutations identified in previous study are taken into account [26], [27], [28], [29]. Further analysis of the results from exome sequencing for those patients without identified mutations in the 25 genes may provide useful clues in the identification of new genes responsible for CORD.
Previously studies on an individual gene or a set of genes have revealed a different frequency of mutation in CORD-associated genes in different populations. Sanger sequencing of 10 adCORD-associated genes identified mutations in 25 of 52 (48%) German families with adCORD, and mutations were found in GUCY2D (24%), PRPH2 (12%), GUCA1A (8%), CRX (4%), and PROM1 (2%) [43]. In other studies for individual genes, mutations in CRX are responsible for a 4.76% proportion of CORD [26], GUCY2D for 11.11% of CORD in a Japanese population [44] and 9.09% in a Spanish one [45], GUCA1A for 16.67% of CORD in a German one [46], PRPH2 for 11% of CORD in another German population [43], AIPL1 for 1.82% of CORD in an American sample [1], PROM1 for 0.93% of CORD in a Dutch population [16], SEMA4A for 8.00% of CORD in a Pakistani group [9], and UNC119 for 5.00% of CORD in another American population [10].
For genes known to be associated with arCORD, their mutations might be responsible for nearly 40% of arCORD [42], whereas mutations in ABCA4 are most frequent in European and American populations, ranging from 16.13% to 65.00% [47], [48], [49], [50]. However, ABCA4 mutations are very rare in Chinese families with CORD. The mutation frequency of CNGB3 [49], PDE6C [20], [49], and PRGRIP1 [23] in other populations are relatively rare, as seen in Chinese sample. For other CORD-associated genes without identified mutations in this study, mutations in other populations are also rare, as with RAX2 in 0.62% of CORD [21], CERKL in 1.85% of Canadian CORD [16], or ADAM9 [12], RDH5 [22], CDHR1 [15], and C8ORF37 [13] in a few reports based on an analysis of a limited number of families.
Supporting Information
Sequence chromatography. Forteen sequence changes detected in the probands with CORD are shown (left column) compared with corresponding normal sequences (right column). Some known mutations were not verified in the normal controls, so the normal sequences are absent.
(TIF)
Genomic information of the 25 genes referred in this study. This table listed the accession numbers of the genomic DNA, mRNA, and protein for each of the 25 genes. The information is based on human genome reference GRCh37.p10.
(XLS)
Sequences of primers used in this study. This table listed 52 primers used to amplify the genomic fragments with variants detected by exome sequencing.
(XLS)
Acknowledgments
The authors are grateful to the patients for their participation.
Funding Statement
This study was supported by the National Natural Science Foundation of China (81170881, U1201221, http://www.nsfc.gov.cn/Portal0/default152.htm), Guangdong Translational Medicine Public Platform, the "985 project" of Sun Yat-sen University, and the Fundamental Research Funds of the State Key Laboratory of Ophthalmology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sohocki MM, Perrault I, Leroy BP, Payne AM, Dharmaraj S, et al. (2000) Prevalence of AIPL1 mutations in inherited retinal degenerative disease. Mol Genet Metab 70: 142–150. [DOI] [PubMed] [Google Scholar]
- 2. Freund CL, Gregory-Evans CY, Furukawa T, Papaioannou M, Looser J, et al. (1997) Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell 91: 543–553. [DOI] [PubMed] [Google Scholar]
- 3. Payne AM, Downes SM, Bessant DA, Taylor R, Holder GE, et al. (1998) A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum Mol Genet 7: 273–277. [DOI] [PubMed] [Google Scholar]
- 4. Kelsell RE, Gregory-Evans K, Payne AM, Perrault I, Kaplan J, et al. (1998) Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant cone-rod dystrophy. Hum Mol Genet 7: 1179–1184. [DOI] [PubMed] [Google Scholar]
- 5. Kohn L, Kadzhaev K, Burstedt MS, Haraldsson S, Hallberg B, et al. (2007) Mutation in the PYK2-binding domain of PITPNM3 causes autosomal dominant cone dystrophy (CORD5) in two Swedish families. Eur J Hum Genet 15: 664–671. [DOI] [PubMed] [Google Scholar]
- 6. Yang Z, Chen Y, Lillo C, Chien J, Yu Z, et al. (2008) Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest 118: 2908–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fishman GA, Stone EM, Alexander KR, Gilbert LD, Derlacki DJ, et al. (1997) Serine-27-phenylalanine mutation within the peripherin/RDS gene in a family with cone dystrophy. Ophthalmology 104: 299–306. [DOI] [PubMed] [Google Scholar]
- 8. Kelsell RE, Gregory-Evans K, Gregory-Evans CY, Holder GE, Jay MR, et al. (1998) Localization of a gene (CORD7) for a dominant cone-rod dystrophy to chromosome 6q. Am J Hum Genet 63: 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abid A, Ismail M, Mehdi SQ, Khaliq S (2006) Identification of novel mutations in the SEMA4A gene associated with retinal degenerative diseases. J Med Genet 43: 378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobayashi A, Higashide T, Hamasaki D, Kubota S, Sakuma H, et al. (2000) HRG4 (UNC119) mutation found in cone-rod dystrophy causes retinal degeneration in a transgenic model. Invest Ophthalmol Vis Sci 41: 3268–3277. [PubMed] [Google Scholar]
- 11. Cremers FP, van de Pol DJ, van Driel M, den Hollander AI, van Haren FJ, et al. (1998) Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet 7: 355–362. [DOI] [PubMed] [Google Scholar]
- 12. Parry DA, Toomes C, Bida L, Danciger M, Towns KV, et al. (2009) Loss of the metalloprotease ADAM9 leads to cone-rod dystrophy in humans and retinal degeneration in mice. Am J Hum Genet 84: 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Estrada-Cuzcano A, Neveling K, Kohl S, Banin E, Rotenstreich Y, et al. (2012) Mutations in C8orf37, encoding a ciliary protein, are associated with autosomal-recessive retinal dystrophies with early macular involvement. Am J Hum Genet 90: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, et al. (2006) Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci 47: 3523–3530. [DOI] [PubMed] [Google Scholar]
- 15. Ostergaard E, Batbayli M, Duno M, Vilhelmsen K, Rosenberg T (2010) Mutations in PCDH21 cause autosomal recessive cone-rod dystrophy. J Med Genet 47: 665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Littink KW, Koenekoop RK, van den Born LI, Collin RW, Moruz L, et al. (2010) Homozygosity mapping in patients with cone-rod dystrophy: novel mutations and clinical characterizations. Invest Ophthalmol Vis Sci 51: 5943–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Michaelides M, Aligianis IA, Ainsworth JR, Good P, Mollon JD, et al. (2004) Progressive cone dystrophy associated with mutation in CNGB3. Invest Ophthalmol Vis Sci 45: 1975–1982. [DOI] [PubMed] [Google Scholar]
- 18. Polok B, Escher P, Ambresin A, Chouery E, Bolay S, et al. (2009) Mutations in CNNM4 cause recessive cone-rod dystrophy with amelogenesis imperfecta. Am J Hum Genet 84: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu H, Cowing JA, Michaelides M, Wilkie SE, Jeffery G, et al. (2006) Mutations in the gene KCNV2 encoding a voltage-gated potassium channel subunit cause "cone dystrophy with supernormal rod electroretinogram" in humans. Am J Hum Genet 79: 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thiadens AA, den Hollander AI, Roosing S, Nabuurs SB, Zekveld-Vroon RC, et al. (2009) Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am J Hum Genet 85: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang QL, Chen S, Esumi N, Swain PK, Haines HS, et al. (2004) QRX, a novel homeobox gene, modulates photoreceptor gene expression. Hum Mol Genet 13: 1025–1040. [DOI] [PubMed] [Google Scholar]
- 22. Wada Y, Abe T, Sato H, Tamai M (2001) A novel Gly35Ser mutation in the RDH5 gene in a Japanese family with fundus albipunctatus associated with cone dystrophy. Arch Ophthalmol 119: 1059–1063. [DOI] [PubMed] [Google Scholar]
- 23. Hameed A, Abid A, Aziz A, Ismail M, Mehdi SQ, et al. (2003) Evidence of RPGRIP1 gene mutations associated with recessive cone-rod dystrophy. J Med Genet 40: 616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jalkanen R, Mantyjarvi M, Tobias R, Isosomppi J, Sankila EM, et al. (2006) X linked cone-rod dystrophy, CORDX3, is caused by a mutation in the CACNA1F gene. J Med Genet 43: 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Demirci FY, Rigatti BW, Wen G, Radak AL, Mah TS, et al. (2002) X-linked cone-rod dystrophy (locus COD1): identification of mutations in RPGR exon ORF15. Am J Hum Genet 70: 1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang L, Xiao X, Li S, Jia X, Wang P, et al. (2012) CRX variants in cone-rod dystrophy and mutation overview. Biochem Biophys Res Commun 426: 498–503. [DOI] [PubMed] [Google Scholar]
- 27. Huang L, Li S, Xiao X, Jia X, Sun W, et al. (2013) Novel GUCA1A mutation identified in a Chinese family with cone-rod dystrophy. Neurosci Lett 541: 179–183. [DOI] [PubMed] [Google Scholar]
- 28. Xiao X, Guo X, Jia X, Li S, Wang P, et al. (2011) A recurrent mutation in GUCY2D associated with autosomal dominant cone dystrophy in a Chinese family. Mol Vis 17: 3271–3278. [PMC free article] [PubMed] [Google Scholar]
- 29. Huang L, Li S, Xiao X, Jia X, Wang P, et al. (2013) Screening for variants in 20 genes in 130 unrelated patients with cone-rod dystrophy. Mol Med Rep 7: 1779–1785. [DOI] [PubMed] [Google Scholar]
- 30. Wang Q, Wang P, Li S, Xiao X, Jia X, et al. (2010) Mitochondrial DNA haplogroup distribution in Chaoshanese with and without myopia. Mol Vis 16: 303–309. [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Vinckenbosch N, Tian G, Huerta-Sanchez E, Jiang T, et al. (2010) Resequencing of 200 human exomes identifies an excess of low-frequency non-synonymous coding variants. Nat Genet 42: 969–972. [DOI] [PubMed] [Google Scholar]
- 32. Li R, Li Y, Kristiansen K, Wang J (2008) SOAP: short oligonucleotide alignment program. Bioinformatics 24: 713–714. [DOI] [PubMed] [Google Scholar]
- 33. Li R, Yu C, Li Y, Lam TW, Yiu SM, et al. (2009) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25: 1966–1967. [DOI] [PubMed] [Google Scholar]
- 34. Li R, Li Y, Fang X, Yang H, Wang J, et al. (2009) SNP detection for massively parallel whole-genome resequencing. Genome Res 19: 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386. [DOI] [PubMed] [Google Scholar]
- 36. Li L, Xiao X, Li S, Jia X, Wang P, et al. (2011) Detection of variants in 15 genes in 87 unrelated Chinese patients with Leber congenital amaurosis. PLoS One 6: e19458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. den Dunnen JT, Antonarakis SE (2000) Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 15: 7–12. [DOI] [PubMed] [Google Scholar]
- 38. Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, et al. (2005) Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res 15: 1034–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4: 1073–1081. [DOI] [PubMed] [Google Scholar]
- 40. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reese MG, Eeckman FH, Kulp D, Haussler D (1997) Improved splice site detection in Genie. J Comput Biol 4: 311–323. [DOI] [PubMed] [Google Scholar]
- 42. den Hollander AI, Black A, Bennett J, Cremers FP (2010) Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies. J Clin Invest 120: 3042–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kohl S, Kitiratschky V, Papke M, Schaich S, Sauer A, et al. (2012) Genes and mutations in autosomal dominant cone and cone-rod dystrophy. Adv Exp Med Biol 723: 337–343. [DOI] [PubMed] [Google Scholar]
- 44. Ito S, Nakamura M, Nuno Y, Ohnishi Y, Nishida T, et al. (2004) Novel complex GUCY2D mutation in Japanese family with cone-rod dystrophy. Invest Ophthalmol Vis Sci 45: 1480–1485. [DOI] [PubMed] [Google Scholar]
- 45. Garcia-Hoyos M, Auz-Alexandre CL, Almoguera B, Cantalapiedra D, Riveiro-Alvarez R, et al. (2011) Mutation analysis at codon 838 of the Guanylate Cyclase 2D gene in Spanish families with autosomal dominant cone, cone-rod, and macular dystrophies. Mol Vis 17: 1103–1109. [PMC free article] [PubMed] [Google Scholar]
- 46. Kitiratschky VB, Behnen P, Kellner U, Heckenlively JR, Zrenner E, et al. (2009) Mutations in the GUCA1A gene involved in hereditary cone dystrophies impair calcium-mediated regulation of guanylate cyclase. Hum Mutat 30: E782–796. [DOI] [PubMed] [Google Scholar]
- 47. Ducroq D, Rozet JM, Gerber S, Perrault I, Barbet D, et al. (2002) The ABCA4 gene in autosomal recessive cone-rod dystrophies. Am J Hum Genet 71: 1480–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fishman GA, Stone EM, Eliason DA, Taylor CM, Lindeman M, et al. (2003) ABCA4 gene sequence variations in patients with autosomal recessive cone-rod dystrophy. Arch Ophthalmol 121: 851–855. [DOI] [PubMed] [Google Scholar]
- 49. Thiadens AA, Phan TM, Zekveld-Vroon RC, Leroy BP, van den Born LI, et al. (2012) Clinical course, genetic etiology, and visual outcome in cone and cone-rod dystrophy. Ophthalmology 119: 819–826. [DOI] [PubMed] [Google Scholar]
- 50. Maugeri A, Klevering BJ, Rohrschneider K, Blankenagel A, Brunner HG, et al. (2000) Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet 67: 960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence chromatography. Forteen sequence changes detected in the probands with CORD are shown (left column) compared with corresponding normal sequences (right column). Some known mutations were not verified in the normal controls, so the normal sequences are absent.
(TIF)
Genomic information of the 25 genes referred in this study. This table listed the accession numbers of the genomic DNA, mRNA, and protein for each of the 25 genes. The information is based on human genome reference GRCh37.p10.
(XLS)
Sequences of primers used in this study. This table listed 52 primers used to amplify the genomic fragments with variants detected by exome sequencing.
(XLS)