Abstract
Purpose
Ocular local anesthetics (OLA’s) currently used in routine clinical practice for corneal anesthesia are short acting and their ability to delay corneal healing makes them unsuitable for long-term use. In this study, we examined the effect on the duration of corneal anesthesia of the site-1 sodium channel blocker tetrodotoxin (TTX), applied with either proparacaine or the chemical permeation enhancer OTAB. The effect of test solutions on corneal healing was also studied.
Methods
Solutions of TTX, proparacaine, and OTAB, singly or in combination were applied topically to the rat cornea. The blink response, an indirect measure of corneal sensitivity, was recorded using a Cochet-Bonnet esthesiometer, and the duration of corneal anesthesia calculated. The effect of test compounds on the rate of corneal epithelialization was studied in vivo following corneal debridement.
Results
Combination of TTX and proparacaine resulted in corneal anesthesia that was 8–10 times longer in duration than that from either drug administered alone, while OTAB did not prolong anesthesia. The rate of corneal healing was moderately delayed following co-administration of TTX and proparacaine.
Conclusion
Co-administration of TTX and proparacaine significantly prolonged corneal anesthesia but in view of delayed corneal re-epithelialization, caution is suggested in use of the combination.
Keywords: Corneal anesthesia, Tetrodotoxin, Proparacaine, corneal toxicity
Introduction
Ocular local anesthetics (OLA’s) are routinely used in ophthalmology for short procedures. Currently used OLAs act by binding to an intracellular domain of the sodium channel.1 The duration of action of typical OLA’s like proparacaine and tetracaine is generally between 10 to 20 minutes.2 Their short durations and the potential for corneal toxicity 3 effectively excludes them from use in chronic pain conditions and for lengthier ophthalmic procedures. Fear of pain and inadequate pain relief are factors that may limit patient enthusiasm for surgical procedures, such as photorefractive keratectomy.4 An effective topical pain-relieving formulation with extended effect is needed in ophthalmology. Ideally, such a formulation would last hours not minutes, and could be used to prevent pain more effectively during surgical procedures.
Tetrodotoxin (TTX) and saxitoxin (STX) are site 1 sodium channel blockers that induce local anesthesia by binding to site 1 on the extracellular part of the sodium channel.5–7 When combined with amino-ester or amino-amide local anesthetics, 7–9 or chemical permeation enhancers (CPEs),10 site 1 sodium channel blockers demonstrate a synergistic effect on local anesthesia. Corneal analgesia from topical application of TTX has been previously reported, 11 with minimal toxicity to the corneal epithelium.12 We hypothesized that combining site 1 sodium channel blocker and a typical OLA, proparacaine, with or without a CPE would result in prolonged corneal anesthesia with toxicity to the corneal epithelium similar to or less than from proparacaine alone.
Materials and Methods
Animal care
Adult male Sprague-Dawley rats (Charles River laboratories International, Inc, Wilmington, MA) weighing 300–350 grams were housed in pairs, allowed standard rat diet and water ad libitum, and maintained on a 7 a.m. – 7 p.m. light/dark cycle. Animal studies were conducted using protocols approved by the Massachusetts Institute of Technology Committee on Animal Care and in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement on the use of animals in ophthalmic research. Rats were chosen at random for inclusion into specific treatment groups.
Drugs and Reagents
Octyltrimethylammoniumbromide (OTAB) and proparacaine (PPC) were obtained from Sigma-Aldrich Corp. (St. Louis, MO) and solutions prepared in 0.9% saline, while tetrodotoxin citrate (Tocris Bioscience (Ellisville, MO) solutions were prepared in 20 mM citrate solution (pH 4.5). All drug solutions were prepared immediately prior to administration. The volume of drug or drug combination that was topically applied on the cornea was 30 µL. For in vitro cell viability assay, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) and phenazine methosulfate (MTS) was purchased from Promega Corp, (Madison, WI). All materials were used as received unless stated otherwise.
Cell viability assay
Immortalized human corneal limbal epithelial (HCLE) cells and immortalized human corneal keratocytes (corneal fibroblasts) were generous gifts from Dr. Ilene Gipson (Schepens Eye Research Center, Harvard Medical School, MA). HCLE were cultured in keratinocyte serum-free medium (KSFM; Invitrogen, Carlsbad, CA) supplemented with epidermal growth factor (EGF) and bovine pituitary extract, until cells reached 50% confluence, then, culturing medium was switched to a 1:1 mixture of KSFM and a combination of 1:1 unsupplemented low-calcium DMEM and F12 Ham's nutrient mixture (Invitrogen, Carlsbad, CA). For differentiation and stratification of HCLE's, cells were exposed to 1:1 DMEM/F12 medium (Mediatech, Manassas, VA) supplemented with newborn calf serum and EGF.
Corneal keratocytes were cultured in Dulbecco’s minimum essential medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All cells were incubated at 37 °C in a 5% CO2 environment. Corneal keratocytes and differentiated HCLE were exposed to media containing (in mM), 15 PPC and 1, 3 and 6 TTX, and cellular viability assessed after 4, 8, 16 and 24 hours, using the MTS colorimetric assay (CellTiter96 Proliferation Assay, Promega, WI). Results are presented as percent viability from four separate experiments, normalized to cultured cells that did not receive drug exposure.
Assessment of corneal touch sensitivity in rats
Towel-restrained rats received drugs in the form of topical drops to the left eye, while the untreated right eye served as untreated control. All animals received a single administration of test solutions (Table 1) in a volume of 30 µL, unless mentioned otherwise. Corneal tactile sensitivity was tested using a Cochet-Bonnet esthesiometer13 (Luneau Ophthalmologie, Chartres, France), which consists of a retractable nylon monofilament that exerts pressure, inversely proportional to its length. At full extension, the monofilament is 6 cm long with a diameter of 0.12 mm. Testing began by gently placing the tip of the fully extended filament perpendicularly to the cornea, followed by application of sufficient force to slightly buckle the filament. A reflexive blink was considered a positive response. In the absence of blink response, the filament length was reduced by 0.5 cm and the animal retested. The process of filament length reduction and retesting was repeated until a positive response was elicited. Both eyes were tested in this manner at least 3 times. Animals were tested 10, 20, and 30 minutes after administration of drops, then every hour for the next 12 hours. Duration of non-responsiveness to filament length of 0.5 cm (block0.5 ) was defined as the duration of complete corneal block. Similarly, non-responsiveness to 2 cm (block2 ) and 2–5 cm (block<6 ) was considered as the duration of dense and partial corneal block respectively.
Table 1.
Effect of proparacaine (PPC), tetrodotoxin (TTX), and octyltrimethylammonium bromide (OTAB) on the duration of corneal anesthesia.
| TTX (mM) |
OTAB (mM) |
PPC (mM) |
Block0.5 (min) |
Block2 (min) |
Block<6 (min) |
|---|---|---|---|---|---|
| - | - | 15 | 17 ± 5 | 22 ± 5.3 | 90 ± 24.4 |
| - | 0.5 | 15 | 10 ± 0.1 | 22 ± 2.6 | 52 ± 15.0 |
| 0.1 | - | - | 0 | 0 | 45 ± 29.5 |
| 0.1 | 0.1 | - | 0 | 4 ± 7.8 | 105 ± 30.0 |
| 0.1 | - | 15 | 30 ± 21.6 | 55 ± 30 | 420 ± 69.2*† |
| 0.1 | 0.1 | 15 | 30 ± 20 | 64 ± 28.8 | 417 ± 5.0*† |
| 1 | - | - | 0 | 13 ± 15.2 | 105 ± 90.0 |
| 1 | 1 | - | 10 ± 14.1 | 20 ± 14.8 | 195 ± 57.4 |
| 1 | - | 15 | 75 ± 30* | 179 ± 21.2 *† | 345 ± 57.4*† |
| 1 | 1 | 15 | 90 ± 34.6* | 180 ± 20.7 *† | 345 ± 57.4*† |
| 3 | - | - | 10 ± 11.7 | 66 ± 55.7 | 210 ± 34.6 |
| 3 | 3 | - | 25 ± 10 | 85 ± 93.4 | 297 ± 5.0* |
| 3 | - | 15 | 90 ± 34.6* | 180 ± 17.2 *† | 375 ± 30.0*† |
| 3 | 3 | 15 | 105 ± 57.4* | 246 ± 134.4 *† | 615 ± 172.3*†‡ |
| 6 | - | - | 7 ± 9.5 | 57 ± 35.45 | 225 ± 90.0 |
| 6 | 6 | - | 75 ± 30 | 64 ± 15.3 | 270 ± 60.0* |
| 6 | - | 15 | 75 ± 30* | 141 ± 18.6 * | 390 ± 34.6*† |
| 6 | 6 | 15 | 82 ± 45* | 163 ± 61.3 *† | 585 ± 157*†‡ |
Block0.5, 2, <6: duration of absent blink response when the cornea was probed with filament length of 0.5 cm (complete blocks), 2 cm (dense blocks) and 6 cm (any degree of block; time to block resolution) respectively. Data are means ± SD, n=4. Groups were compared using one-way ANOVA with Bonferroni post hoc test.
p< 0.05 compared to 15 mM proparacaine,
p < 0.05, compared with rats administered TTX only within each TTX concentration group,
p < 0.05, compared with rats co-administered TTX and proparacaine within each TTX concentration group.
Cornea debridement studies
To assess the effect of TTX and/or proparacaine on the rate of cornea epithelialization, rats were anesthetized with 2% isoflurane and 98% oxygen inhalation. A corneal trephine (3 mm diameter) was placed on the left cornea and filled with 50% ethanol for 90 seconds. This resulted in loosening and detachment of the ethanol-soaked cornea within the trephine. Care was taken to ensure no ethanol leaked outside the trephine. The eye was flushed with warm saline and the detached cornea was gently scraped away using sterile surgical blade.14 Immediately following the lesion, a single dose of 30 µL drug solution was placed in the wounded eye. Photographs were taken after fluorescein (FULGLO strips, Akron Inc, Lake forest, IL) instillation, using a Nikon D60 camera with a 60mm f/2.8 micro-nikkor lens and an attached #8 yellow lens filter (Tiffen company, Hauppauge, NY), every 4 hours till complete re-epithelialization. An external light source with a cobalt-blue filter was used to better visualize the fluorescein filled corneal defect. All images were analyzed using the ImageJ software (NIH, Bethesda) for corneal wound area estimation. The rate of corneal epithelialization was calculated using the following formula:
Statistical Analysis
Data are presented as means ± standard deviations of N observations unless noted otherwise. All in vitro assays were done in duplicates and each experiment was repeated four times (n=4) and the average considered for statistical analysis. Statistical differences in mean corneal block duration and average rate of corneal wound healing between experimental groups were compared using one-way ANOVA with Bonferroni post test. Data from in vitro analysis at various time points were compared using two-way ANOVA with Bonferroni post test; p < 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism version 5.0c for Mac OSX (GraphPad Software, San Diego, CA).
Results
Topical co-application of TTX and proparacaine prolongs corneal analgesia
To determine whether drug combinations could yield prolonged corneal anesthesia with less corneal toxicity, we compared duration of corneal block obtained by topical application of 15 mM proparacaine on the rat cornea with blocks obtained by application of varying concentrations of tetrodotoxin (TTX) with or without OTAB (a CPE that has been previously demonstrated to prolong TTX-induced nerve conduction blocks 10,15) (Table 1). A concentration of 15 mM proparacaine (0.5% w/v) was used throughout since it is commonly used in clinical applications.3 All drug and drug combinations were administered in a volume of 30 µL into the inferior conjunctival cul-de-sac, after retraction of the lower eyelid. This volume completely saturated the cornea, with minimal overflow.
Corneal sensitivity to tactile stimulation was tested by recording the blink reflex in response to gentle probing of the central corneal surface using the Cochet-Bonnet esthesiometer,13 which consists of a length-adjustable nylon monofilament. A filament length of 6 cm (maximum length) is least painful on contact, while a shorter, stiffer filament length of 0.5 cm (the shortest) is most painful. Naïve (no local anesthetic treatment) rats exhibited the normal blink response at 5–6 cm. Dense corneal block was defined here as the absence of blink reflex in response to a filament length of 2 cm (Block2), while non-responsiveness to filament lengths of 2–5 cm was considered as partial block (Block<6). Lack of response to the minimum filament length of 0.5 cm was termed complete block. The duration of block0.5, block2, or block<6 (equivalent to time to return of normal function) was calculated starting from the time of drug administration.
Topical application of 15 mM proparacaine produced block2 that lasted approximately 20 minutes (Table 1). 0.1 mM TTX failed to produce dense blocks (block2 = 0 min), while 1, 3 and 6 mM TTX produced block2 that were similar in duration (p > 0.05, n = 4) to 15 mM proparacaine. However co-administration of 1, 3 or 6 mM TTX with 15 mM proparacaine produced block2 that were considerably longer (7–8 fold, p < 0.05, n = 4) than either drug concentration administered individually. Nonetheless, the prolongation of block2 from the combination of proparacaine and TTX, did not increase with increasing concentration of TTX beyond 1 mM (p > 0.05) (Table 1). Co-administration of OTAB did not increase block2 from proparacaine, TTX, or their combinations (p > 0.05).
Similar to what was seen with block2, the combination of proparacaine and varying concentrations of TTX produced block<6 that were significantly longer than those from individual drugs. However, co-administration of lower concentration of OTAB (0.1 and 1 mM) did not prolong block<6 of proparacaine and TTX (p < 0.05), while higher concentration of OTAB (3 and 6 mM) produced significant prolongation. In all groups, the untreated eye exhibited no changes in corneal sensitivity.
Topical application of 15 mM proparacaine produced corneal blocks that reached complete insensitivity (block0.5) for approximately 15 min. For each TTX concentration, the duration of analgesia from the combination with proparacaine was longer than from proparacaine alone (p < 0.05, n = 4), even though only 1 out of 4 rats tested achieved complete insensitivity. Addition of OTAB to combinations of TTX and proparacaine did not further enhance block0.5 at any of the concentrations tested (Table 1) (p > 0.05, compared to respective concentration of co-administered TTX and proparacaine).
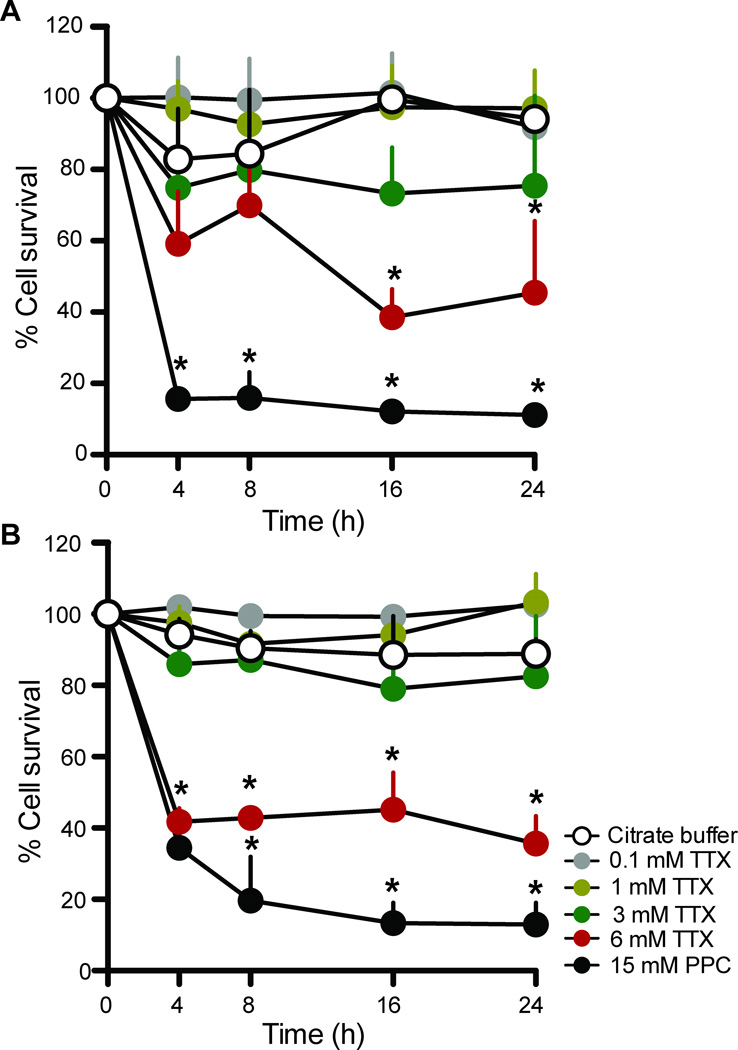
Higher concentrations of TTX decrease cellular viability
To determine the cytotoxicity of TTX, human corneal limbal epithelial (HCLE) cells (Fig. 1A) and human corneal keratocytes (corneal fibroblasts) (Fig. 1B) were incubated in media containing 0.1, 1, 3, 6 mM of TTX or 15 mM proparacaine. Exposure of corneal fibroblasts and epithelial cells to 15 mM proparacaine for 4 hours resulted in 60–70% loss of cellular viability and by 24 hours, only 10% of the cells survived (Fig. 1A,B; p<0.05 compared to the viability of cells exposed to citrate buffer alone (the carrier for all test solutions), at all time points tested; n = 3). Similarly, cells exposed to 6 mM TTX demonstrated decreased cell viability compared to citrate buffer-exposed cells (p < 0.05, n = 3) at all time points tested. In contrast, cells exposed to lower concentrations of TTX (0.1, 1 and 3 mM) exhibited viability similar to that of vehicle-treated cells at all time points (p > 0.05, n = 3).
Figure 1.
Effect of tetrodotoxin (TTX) and proparacaine on cell viability in-vitro. Viability of (A) human corneal limbal epithelial cells and (B) corneal keratocytes was determined using the MTS assay. Data are means ± SD, n = 3. Comparisons at each time point were performed with the two-way ANOVA with Bonferroni post hoc test (* = p < 0.05). Citrate buffer 0.1 M, pH 4.5 was also studied, as it was the buffer in which tetrodotoxin was dissolved.
Co-application of TTX and proparacaine delays the rate of corneal healing
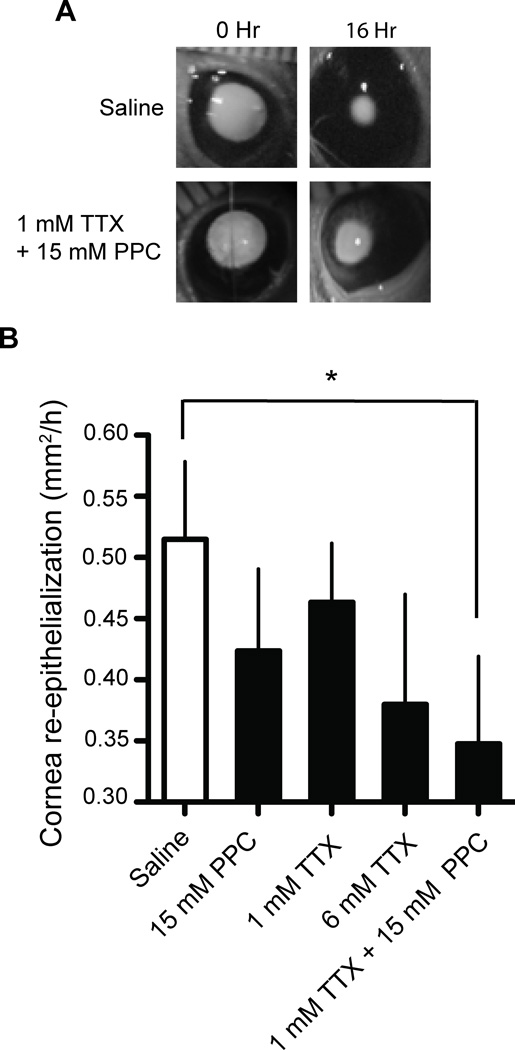
Proparacaine is known to cause corneal epithelial toxicity and delayed healing.16 To assess whether the combination of TTX with proparacaine alters corneal healing, we monitored the rate of corneal epithelialization following debridement of 12–14 mm2 of the corneal epithelial layer (Fig. 2A). Drug solutions were applied topically immediately after the debridement procedure.
Figure 2.
Effect of drugs on rate of corneal re-epithelialization. (A) Representative black-and-white images of the rate of epithelial re-growth in surgically debrided cornea after topical application of saline and 1 mM TTX with 15 mM proparacaine. Images were taken under illumination with a UV lamp to show fluorescein applied to the cornea to enhance visualization of the wound. (B) Effect of test compounds on the rate of corneal re-epithelialization averaged over a period of 36 hours. Data are means ± SD, n = 5. Groups were compared to saline treated controls using one-way ANOVA with Bonferroni post hoc test (* = p < 0.05).
The rate of re-epithelialization was decreased by approximately 18% (over a period of 36 hours) by a single dose of 15 mM proparacaine, which was not significantly different from the re-epithelialization rate in saline-treated eyes (p > 0.05, n = 4; Fig. 2B). TTX at 1 and 6 mM also exhibited corneal re-epithelialization rates that were similar (p > 0.05, n = 4) to saline treated corneas. However, the rate of corneal re-epithelialization following the co-administration of 15 mM proparacaine with 1 mM TTX was decreased (p < 0.05, n = 5) compared to saline administered rats (Fig. 2B & 3).
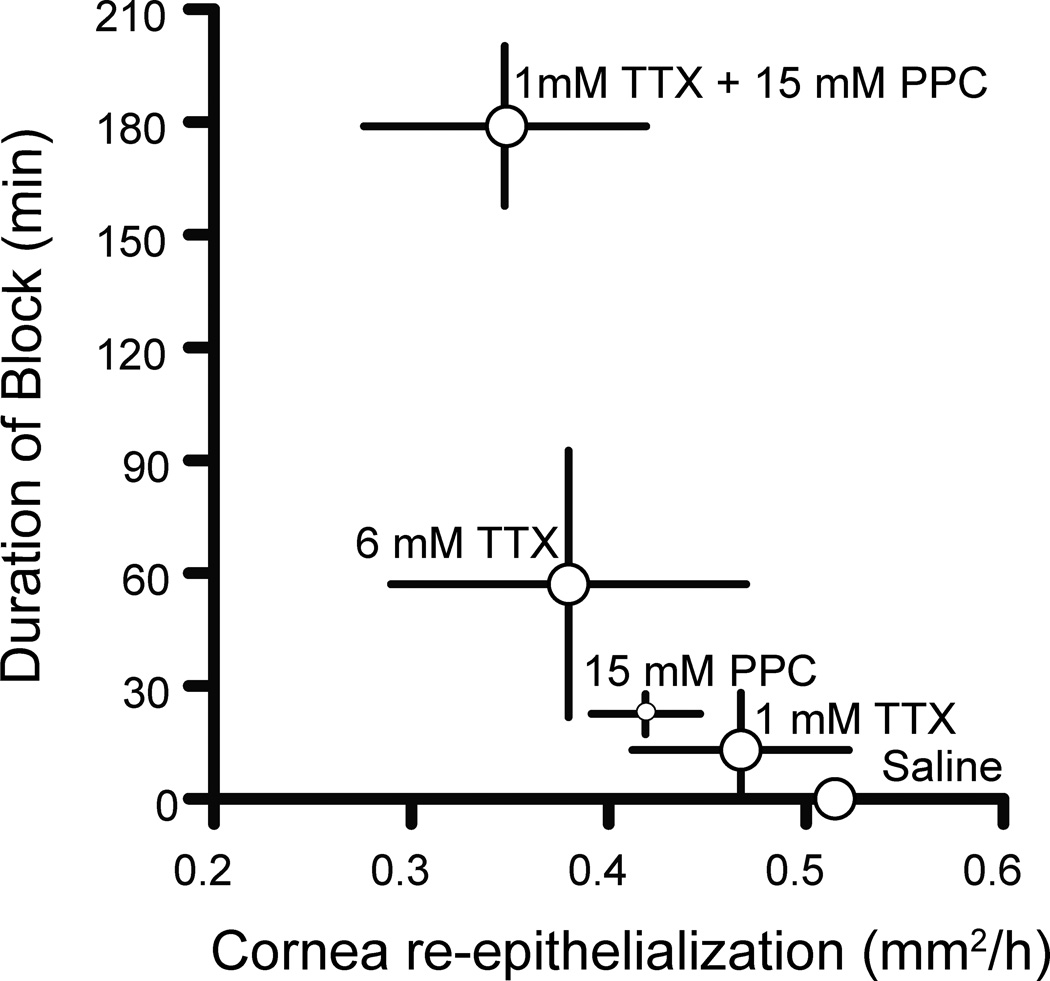
Figure 3.
Relationship between duration of corneal anesthesia and rate of corneal re-epithelialization after topical application of anesthetics. Data are means ± SD.
Discussion
The synergistic action of site 1 sodium channel blockers with amino-amide and amino-ester local anesthetics in the peripheral nerve has been described by others 17,18 and by us.7,9,19 Co-administration of bupivacaine with tetrodotoxin, as a sciatic nerve block injection, produces conduction blocks that are 3–4 times more prolonged than that from either drug alone.9 In the rat cornea, we observed a similar enhancement of nerve blockade between tetrodotoxin and the ocular local anesthetic proparacaine, which together produce corneal blocks that were approximately 7–8 times longer than those from the individual drugs. This interaction may be attributed to the fact that conventional local anesthetics block sodium channels from within the cell, whereas tetrodotoxin acts on the extracellular part of the channel.20 These combinations could provide approximately 3 hours of dense (block2) anesthesia that might be suitable for sharp perioperative pain. Complete recovery of sensation took more than 6 hours, suggesting a useful dosing timeframe for less intense non-surgical pain.
Co-administering tetrodotoxin and proparacaine also enhanced the intensity of block. This is important since proparacaine, a commonly used corneal numbing drug, produced complete block (block 0.5) for only 15 minutes, while the combination of tetrodotoxin and proparacaine produced about 60 minutes of complete block. This finding suggests the possible use of the TTX-proparacaine combination for ophthalmic procedures that require complete corneal numbness for up to an hour.
Corneal anesthesia requires that the ocular anesthetic must penetrate the superficial epithelial layers and bind to the corneal nerve fibers. Chemical permeation enhancers (CPEs) facilitate drug penetration across epithelial layers and can prolong TTX-induced peripheral nerve blockade.10 Tetracaine, an ocular amino-ester anesthetic is a known CPE.22 Proparacaine, a drug related to tetracaine, could act as a CPE and promote TTX binding to corneal sensory axons, and thereby prolong corneal block. However, addition of OTAB, a CPE,10,15 did not prolong block from TTX, suggesting that proparacaine’s prolongation of TTX block was unlikely to be a CPE effect. Since corneal nerve fibers extend to within a few cell layers of the corneal surface,21 TTX by itself maybe be reaching nerve fibers easily, so that CPE's have no effect.
The use of high concentrations of TTX (compared to those used in peripheral nerve, 30–50 µm7,9) raises the issue of the potential systemic toxicity of that compound 23 if absorbed into a wound or inflammation, or after passage into the nasolacrimal duct. However, there were no clinical signs of toxicity in any of the rats in this study. Distribution of TTX within the anterior chamber is another potential concern. TTX does not affect intraocular pressure or change aqueous humor composition,24 but its effects on other structures within the eye are yet to be determined.
The use of tetrodotoxin for corneal analgesia has been reported.11,12,25 In rabbits, topical application of 10 mM TTX produces corneal anesthesia for up to 8 hours.11 Here, rats exposed to 1 mM TTX (or higher concentrations) produced block <6 corneal anesthesia of approximately 3 hours. The dissimilarity in duration of block between the current study and previous reports could be due to differences in rat and rabbit corneal innervation.26 In addition, we used the Cochet-Bonnet esthesiometer, while previous studies use a blink scoring system.11,25
Tetrodotoxin caused concentration-dependent corneal cytotoxicity in vitro, while the same concentrations caused only a mild decrease in corneal epithelial proliferation in vivo. Co-application of a single dose of 1 mM TTX and 15 mM proparacaine significantly decreased corneal re-epithelialization, whereas similar concentrations of TTX and proparacaine administered separately did not significantly alter corneal re-epithelialization. The mechanism for this difference is not clear, but it has been observed that denervation delays corneal healing.27 Co-application of TTX and proparacaine could hinder corneal healing by inhibiting neural feedback; our data suggest a relationship between duration of block and rate of re-epithelialization (Fig. 3). If that is correct, it is interesting that a relatively brief period of blockade would have a lasting effect on wound healing. These findings underscore the need for further investigation of the impact of local anesthetics, including those described here, in models of corneal analgesia.
In conclusion, the combination of TTX and proparacaine can be utilized to produce corneal anesthesia of much longer duration than proparacaine or TTX alone. However, in view of potential corneal toxicity, caution should be exercised in their use in the context of existing injury.
Acknowledgments
Funding source: NIH GM 073626
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ragsdale DS, McPhee JC, Scheuer T, et al. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 2.Bartfield JM, Holmes TJ, Raccio-Robak N. A comparison of proparacaine and tetracaine eye anesthetics. Acad Emerg Med. 1994;1:364–367. doi: 10.1111/j.1553-2712.1994.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu JC, Steinemann TL, McDonald MB, et al. Topical bupivacaine and proparacaine: a comparison of toxicity, onset of action, and duration of action. Cornea. 1993;12:228–232. doi: 10.1097/00003226-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Naroo SA. Factors influencing patient choice of refractive surgery or contact lenses and choice of centre. Cont Lens Anterior Eye. 2006;29:17–23. doi: 10.1016/j.clae.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M-M, Gruszczynski P, Walewska A, et al. Cooccupancy of the outer vestibule of voltage-gated sodium channels by micro-conotoxin KIIIA and saxitoxin or tetrodotoxin. J Neurophysiol. 2010;104:88–97. doi: 10.1152/jn.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohane DS, Lu NT, Gökgöl-Kline AC, et al. The local anesthetic properties and toxicity of saxitonin homologues for rat sciatic nerve block in vivo. Reg Anesth Pain Med. 2000;25:52–59. doi: 10.1016/s1098-7339(00)80011-5. [DOI] [PubMed] [Google Scholar]
- 7.Padera RF, Tse JY, Bellas E, et al. Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity. Muscle Nerve. 2006;34:747–753. doi: 10.1002/mus.20618. [DOI] [PubMed] [Google Scholar]
- 8.Barnet CS, Tse JY, Kohane DS. Site 1 sodium channel blockers prolong the duration of sciatic nerve blockade from tricyclic antidepressants. Pain. 2004;110:432–438. doi: 10.1016/j.pain.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Kohane DS, Yieh J, Lu NT, et al. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology. 1998;89:119–131. doi: 10.1097/00000542-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Simons EJ, Bellas E, Lawlor MW, et al. Effect of chemical permeation enhancers on nerve blockade. Mol Pharm. 2009;6:265–273. doi: 10.1021/mp800167a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz DM, Fields HL, Duncan KG, et al. Experimental study of tetrodotoxin, a long-acting topical anesthetic. Am J Ophthalmol. 1998;125:481–487. doi: 10.1016/s0002-9394(99)80188-3. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz DM, Duncan KG, Duncan JL. Experimental use of tetrodotoxin for corneal pain after excimer laser keratectomy. Cornea. 1998;17:196–199. doi: 10.1097/00003226-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Lawrenson JG, Ruskell GL. Investigation of limbal touch sensitivity using a Cochet- Bonnet aesthesiometer. Br J Ophthalmol. 1993;77:339–343. doi: 10.1136/bjo.77.6.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TI, Chung JL, Hong JP, et al. Bevacizumab Application Delays Epithelial Healing in Rabbit Cornea. Invest Ophthalmol Vis Sci. 2009;50:4653–4659. doi: 10.1167/iovs.08-2805. [DOI] [PubMed] [Google Scholar]
- 15.Sagie I, Kohane DS. Prolonged sensory-selective nerve blockade. Proc Natl Acad Sci U S A. 2010;107:3740–3745. doi: 10.1073/pnas.0911542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med. (Maywood) 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 17.Adams HJ, Blair MR, Takman BH. The local anesthetic activity of tetrodotoxin alone and in combination with vasoconstrictors and local anesthetics. Anesth Analg. 1976;55:568–573. [PubMed] [Google Scholar]
- 18.Staiman AL, Seeman P. Different sites of membrane action for tetrodotoxin and lipid-soluble anesthetics. Can J Physiol Pharmacol. 1975;53:513–524. doi: 10.1139/y75-073. [DOI] [PubMed] [Google Scholar]
- 19.Kohane DS, Smith SE, Louis DN, et al. Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres. Pain. 2003;104:415–421. doi: 10.1016/s0304-3959(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 20.Cahalan MD, Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979;27:39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marfurt C, Cox J, Deek S. Anatomy of the human corneal innervation. Exp. Eye Res. 2010;90:478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Karande P, Jain A, Ergun K, et al. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci U S A. 2005;102:4688–4693. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao CY. Pharmacology of tetrodotoxin and saxitoxin. Fed Proc. 1972;31:1117–1123. [PubMed] [Google Scholar]
- 24.Oksala O, Stjernschantz J, Dickhoff von K. Characterization of the mechanism of acute ocular irritation to YAG laser capsulotomy in rabbits: effects of substance P antagonists, Met-enkephalin, tetracaine and tetrodotoxin. Ophthalmic Res. 1989;21:360–368. doi: 10.1159/000266900. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz DM, Duncan KG, Fields HL, et al. Tetrodotoxin: anesthetic activity in the de-epithelialized cornea. Graefes Arch Clin Exp Ophthalmol. 1998;236:790–794. doi: 10.1007/s004170050160. [DOI] [PubMed] [Google Scholar]
- 26.Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Invest Ophthalmol Vis Sci. 1989;30:461–472. [PubMed] [Google Scholar]
- 27.Araki K, Ohashi Y, Kinoshita S, et al. Epithelial wound healing in the denervated cornea. Curr. Eye Res. 1994;13:203–211. doi: 10.3109/02713689408995778. [DOI] [PubMed] [Google Scholar]