Abstract
Vitamin A was recognized as an essential nutrient 100 years ago. In the 1930s, it became clear that dietary β-carotene was cleaved at its central double to yield vitamin A (retinal or β-apo-15′-carotenal). Thus a great deal of research has focused on the central cleavage of provitamin A carotenoids to form vitamin A (retinoids). The mechanisms of formation and the physiological role(s) of noncentral (eccentric) cleavage of both provitamin A carotenoids and nonprovitamin A carotenoids has been less clear. It is becoming apparent that the apocarotenoids exert unique biological activities themselves. These compounds are found in the diet and thus may be absorbed in the intestine, or they may form from enzymatic or nonenzymatic cleavage of the parent carotenoids. The mechanism of action of apocarotenoids in mammals is not fully worked out. However, as detailed in this review, they have profound effects on gene expression and work, at least in part, through the modulation of ligand-activated nuclear receptors. Understanding the interactions of apocarotenoids with other lipid-binding proteins, chaperones, and metabolizing enzymes will undoubtedly increase our understanding of the biological roles of these carotenoid metabolites.
Keywords: retinoids, vitamin A, β-carotene, lycopene
Carotenoids are isoprenoids, and over 700 are found in nature (1–5). Carotenoids are synthesized biochemically from eight isoprene (C5H8) units. Oxygenated carotenoids are called xanthophylls, and nonoxygenated, hydrocarbon carotenoids are called carotenes. The characteristic feature of the carotenoids is the polyene chain (long conjugated double-bond system) that allows them to absorb light with UV absorption maxima between 450 and 570 nM. Chlorophyll also absorbs light in this range, and carotenoids can serve as accessory pigments to enhance light harvesting in photosynthesis. In 1930, Moore demonstrated that orally fed carotene was converted into the colorless form of vitamin A found in the liver of rats (6). At the same time, the Swiss organic chemist Karrer elucidated the structures of β-carotene and vitamin A (7).
Carotenoids occur naturally in fruits and vegetables, and they are synthesized in plants and microorganisms. The first C40 carotenoid in the biosynthetic pathway is phytoene. Phytoene is dehydrogenated to other acyclic carotenoids, including lycopene, and ultimately cyclized to carotenes (2). β-Carotene, α-carotene, β-cryptoxanthin, lycopene, and lutein are the primary carotenoids found in human plasma (8). Among them, β-carotene, α-carotene, and β-cryptoxanthin are provitamin A carotenoids, the others are nonprovitamin A carotenoids; lycopene is the acyclic carotenoid and lutein has two hydroxlated rings. To exhibit provitamin A activity, the carotenoid molecule must have at least one unsubstituted β-ionone ring and the correct number and position of methyl groups in the polyene chain (9). Hence, α-carotene and β-cryptoxanthin show 30–50% of provitamin A activity (10, 11), and 9-cis and 13-cis isomers of β-carotene less than 10% (12) of provitamin A activity of all-trans-β-carotene.
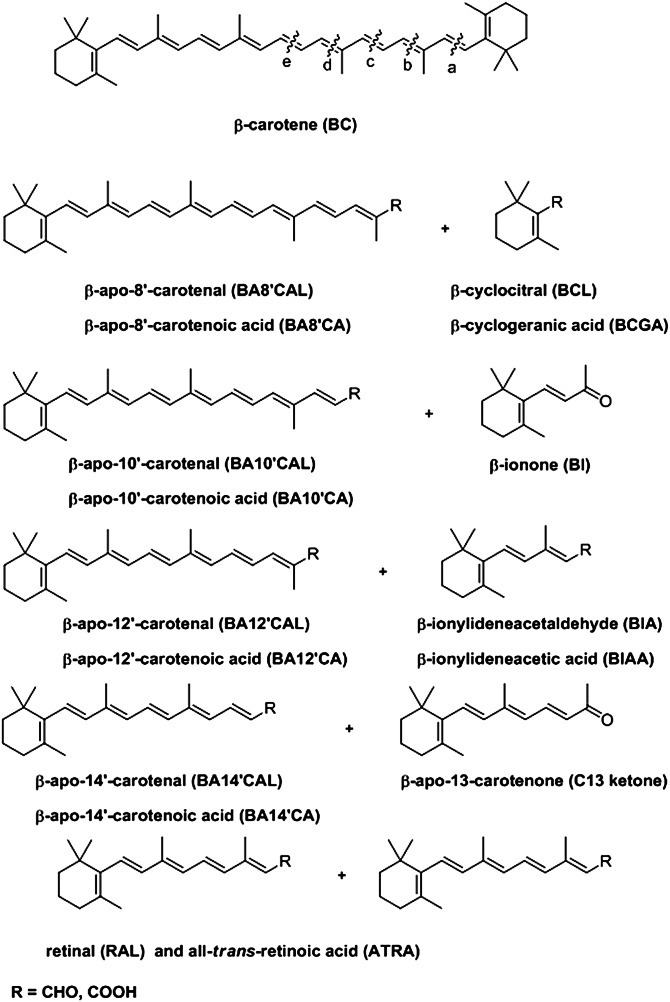
This review focusses on the metabolism of carotenoids, especially β-carotene, in higher vertebrates, including man, with a particular focus on the formation, occurrence, and function of apocarotenoids. The term “apocarotenoid” is a trivial, nonsystematic nomenclature that refers to any cleavage product of a parent 40-carbon carotenoid. Fig. 1 shows all of the possible cleavage products of β-carotene that can arise from oxidative cleavage of the double bonds in the polyene chain. The initial products are aldehydes or ketones. Thus, “β-apo-8′-carotenal” (8′-apo-β-caroten-8′-al) is the longer fragment that arises from cleavage of the 7′, 8′ double bond of β-carotene; the shorter fragment is β-cyclocitral. The naming would be the same for other carotenoids; for example, zeaxanthin, the symmetric xanthophyll that differs from β-carotene by having hydroxyl groups on both β-ionone rings, when cleaved at the 7′, 8′ double bond yields 3-hydroxy-β-apo-8′-carotenal and 3-hydroxycylocitral.
Fig. 1.
Structures and nomenclature of the β-apocarotenoids. All possible β-apocarotenoids arising from oxidative cleavage of β-carotene. Initial products are β-apocarotenals (aldehydes, R = CHO) and, in some cases, β-apocarotenones (ketones). (Top) Cleavages are shown between carbon atoms at the following positions in the β-carotene molecule: (a) 7′, 8′ (b) 9′, 10′ (c) 11′, 12′ (d) 13′, 14′ and (e) 15, 15′. It is possible that in cells the aldehydes can be reduced to the corresponding alcohols by aldehyde reductases and/or alcohol dehydrogenases and can be oxidized to the corresponding carboxylic acids (R = COOH) by aldehyde dehydrogenases.
ABSORPTION, METABOLISM, TRANSPORT, AND TISSUE DELIVERY OF β-CAROTENE AND VITAMIN A
Provitamin A carotenoids are partly converted to vitamin A (as retinyl esters) in the intestinal mucosa. In the enterocytes, both carotenoids and retinyl esters are incorporated into chylomicrons and secreted into lymph for delivery to the blood (13, 14). Following consumption of carotenoid-containing foods, carotenoids are released from their food matrix and incorporated into mixed micelles consisting of lipids and bile components (14). The intestinal absorption of carotenoids occurs via passive diffusion or through facilitated transport via scavenger receptor class B, type 1 (SR-B1) (15–18). In humans, some of the absorbed β-carotene is cleaved oxidatively by β,β-carotene-15,15′-monooxygenase 1 (BCMO1) into two molecules of all-trans-retinal (atRAL), which can be either further oxidized irreversibly to all-trans-retinoic acid (atRA) or reduced reversibly to all-trans-retinol (18). The intact, absorbed β-carotene molecule can accumulate in humans in the blood and tissues, which also occurs in other mammals, such as horses and ferrets (19). In rodents, it has been reported that almost all of the β-carotene present in foods is converted to vitamin A (20).
After being absorbed, vitamin A (retinol), either preformed or resulting from cleavage of β-carotene, undergoes esterification with long-chain fatty acids, particularly with palmitic acid, by the enzyme lecithin:retinol acyltransferase (LRAT), which utilizes the acyl group at the sn-1 position of phosphatidylcholine as a source of fatty acids (18, 21–23). A second enzyme activity has been named and described, acyl-CoA:retinol acyltransferase (ARAT), which utilizes fatty acyl-CoAs, and is likely due to the enzyme diacylglycerol acyltransferase 1 (DGAT1) (24, 25). LRAT clearly functions when physiological amounts of vitamin A are consumed, and DGAT1 may play are role in the reesterification of very large (pharmacological) doses of vitamin A (25). Retinol binds with high affinity to specific cytoplasmic proteins, the cellular retinol-binding proteins, CRBPI and CRBPII (26). CRBPII plays primary roles in the regulation of retinol absorption into enterocytes and its intracellular metabolism, also acting on the reaction of retinaldehyde reduction to retinol (27–29). CRBPII directs the RAL more specifically toward microsomal reductase, thus preventing its free access to cytosol reductases (28).
Retinyl esters formed in the intestine are incorporated into chylomicrons for absorption into the lymph (18, 30). The carotenoids present in the chylomicron remnants may be converted to retinoids in the liver or be incorporated into very low density lipoproteins (VLDL) and transported to peripheral cells (31). Low-density lipoprotein (LDL) is the major carrier of β-carotene in fasting plasma (8).
It has been observed that the liver is the major site of β-carotene accumulation in the organism when diets supplemented with this carotenoid are administered to various species, such as ferrets, rats, and chickens (20, 32). Liver is also the major site of vitamin A storage in the animal. Stellate cells are the major cellular site of vitamin A storage in the liver, containing 80–90% of total hepatic retinol, which is present in the form of retinyl esters (33). For retinol to be released from stellate cells into the circulation, the stored retinyl esters must first be hydrolyzed. Retinol then associates with the plasma retinol-binding protein (RBP) in hepatocytes and is secreted from the liver. The retinol-RBP complex (holo RBP) in the blood associates with the thyroxine-binding protein transthyretin (TTR) (34, 35). It is in this form that retinol is delivered to peripheral sites of action. The metabolism and transport of vitamin A are thoroughly discussed in the accompanying review by O'Byrne and Blaner (35).
Recently, the stimulated by retinoic acid gene 6 protein (STRA6) was identified as the RBP receptor that is a widely expressed, multitransmembrane domain protein (36). It mediates cellular uptake of vitamin A by removing retinol from the RBP complex and transporting it across the plasma membrane, where it can be metabolized. Additionally, STRA6 was found to localize to the cellular locations expected of the RBP receptor in tissues (36). It is likely that nonreceptor-mediated pathways are also involved in retinol delivery to cells.
After delivery to peripheral cells, retinol can be converted to the biologically active hormone atRA. After 1987, with the important discovery of the existence of nuclear retinoic acid receptors (37, 38), researchers started to devote more time to studies related to the mechanisms involved in the regulation of the intracellular concentrations of atRA. Conversion of retinol to atRA initially involves the oxidation to atRAL and subsequent oxidation of the retinal to atRA. In this respect, the enzymes alcohol dehydrogenases (ADH) and retinol dehydrogenases (RDH) have been reported to act in the formation of retinal (39). Retinal dehydrogenases (RALDH) catalyze the oxidation of retinal to retinoic acid. Collectively, all these enzymes, including ADHs, RDHs, and RALDHs, are involved in the interconversion of various isomers of ROL, RAL, and atRA. The pathways and enzymes involved in the conversion of retinol to atRA have been recently reviewed (40) and are presented in more detail in the accompanying review by Kedishvili (39).
CLEAVAGE AND OXIDATION OF CAROTENOIDS TO APOCAROTENOIDS
Apocarotenoids are molecules resulting from the oxidative cleavage of double bonds in the carotenoid molecule. These apocarotenoids are formed by chemical reactions in foods that contain carotenoids or by enzymatic cleavage of intact carotenoids. To date, a plethora of apocarotenoids has been identified in both plants and animals. In plants, abscisic acid (ABA) is the physiologically important phytohormone derived from 9-cis-violoxanthin and 9-cis-neoxanthin (41). Another example of plant apocarotenoids are strigolactones that can serve as signaling molecules and as shoot branching regulators (42–44). In animals, the best-known apocarotenoids are vitamin A and its derivatives (retinoids). The first study that a carotenoid is the precursor of vitamin A came from Moore (6) where he described conversion of β-carotene to vitamin A in the rat. Then, the structure of β-carotene was elucidated and the central cleavage mechanism at the central carbon double bond (15′, 15′) of β-carotene for its conversion to vitamin A was proposed (7). Goodman and Huang (45) and Olson and Hayaishi (46) characterized the respective enzymatic activity in cell-free homogenates from rat small intestine and showed that the activity required iron.
Enzymatic cleavage of carotenoids
The molecular characterization of a carotenoid-cleaving enzyme was achieved first in plants (41) by analyzing the ABA-deficient maize mutant, vp14 (viviparous 14). ABA is a plant growth regulator involved in the induction of seed dormancy and in adaptation to various stresses, such as drought (47). Vp14 protein cleaves 9-cis-neoxanthin and 9-cis-violaxanthin to form xanthoxin and a C25 by-product in the ABA synthesis pathway (41). The product of this cleavage reaction is cis-xanthoxin, which is readily converted to ABA. The molecular identification of vp14 led to the findings of related carotenoid cleavage enzymes in other organisms, including bacteria (48), fungi (49), and animals (50, 51). These are nonheme iron oxygenases containing a ferrous iron as an essential cofactor for the reaction as well as four conserved histidines and a glutamate residue. There are three different family members of these enzymes encoded in mammals. First, the retinal pigment epithelium protein of 65 kDa (RPE65), which catalyzes the concerted hydrolysis and isomerization of all-trans-retinyl palmitate to 11-cis-retinol, was identified (52). The other two members incorporate molecular oxygen into their substrates and catalyze the oxidative cleavage of double bonds of the polyene chain of carotenoids. Mammalian BCMO1 uses mostly provitamin A carotenoids as substrates, and it cleaves them at the central 15,15′ double bond to yield RAL (Fig. 2). It has been reported that BCMO1 only catalyzes the cleavage of β-carotene, α-carotene, and β-cryptoxanthin and requires substrates having at least one nonsubstituted β-ionone ring (53).
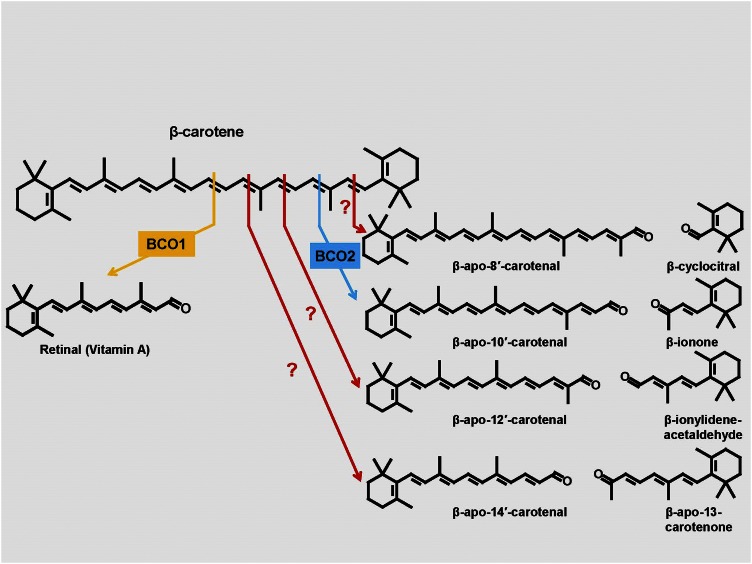
Fig. 2.
Central and eccentric cleavages of β-carotene. Oxidative cleavage of β-carotene at the 15, 15′ double bond is catalyzed by the enzyme β-carotene 15, 15′-oxygenase 1 (BCO1) and leads to the generation of two molecules of retinal (β-apo-15-carotenal). Cleavage at other double bonds leads to the formation of β-apocarotenals and β-apocarotenones. For example, the cleavage at the 9′, 10′ double bond is catalyzed by β-carotene 9′10′-oxygenase 2 (BCO2) and leads to the formation of β-apo-10′-carotenal and β-ionone. Eccentric cleavage at other double bonds may occur nonenzymatically or may be enzyme catalyzed.
The other carotenoid-cleaving oxygenase catalyzes the eccentric (asymmetric) cleavage of β-carotene and is known as β-carotene-9′-10′-dioxygenase (BCDO2). This reaction results in the formation of β-apo-10′-carotenal and β-ionone (54) (Fig. 2). Later on, it was found that this enzyme could also catalyze the eccentric cleavage of xanthophylls, such as zeaxanthin and lutein (55, 56), and the acyclic carotenoid lycopene (57). The cellular localization of these two enzymes is different; whereas the central cleavage enzyme, BCMO1, is a cytosolic protein (53), the eccentric cleavage enzyme, BCDO2, is localized to mitochondria (55).
The mechanism of the central cleavage is still unclear. Originally, the enzyme was thought to be a dioxygenase (46, 47). However, one study presented evidence that it acts as a monooxygenase (58). In this latter proposed reaction, a number of points still need to be clarified, such as the nature of the metal complex involved in the epoxidation as well as the extents of oxygen exchanges between species during the 7.5 h enzyme reaction at 37°C. Indeed, it has been reported that most of the oxygen of retinal exchanged with water during 14 days in H218O (59). It is clear that more detailed information is needed on the catalytic mechanism of both the central cleavage enzyme and the eccentric cleavage enzyme. It is unfortunate that the genes for these enzymes were named with “monooxygenase” and “dioxygenase,” respectively, as this has given rise to a somewhat confusing nomenclature in which authors use different names for these enzymes. Given the lack of definitive information on the reaction mechanisms, we use β-carotene-oxygenase 1 (BCO1) for BCMO1 and β-carotene-oxygenase 2 (BCO2) for BCDO2. BCO1 has been cloned from the Drosophila melanogaster (51), chicken (50), mouse (60–62), and human (63). BCO2 has been identified and cloned from mice, humans, and zebrafish (54).
First, it was thought that the eccentric cleavage of β-carotene was an alternative pathway to produce retinoids and vitamin A (64). However, BCO1-knockout mice that are fed β-carotene become vitamin A deficient despite the expression of BCO2, suggesting that BCO1 is the primary enzyme for retinoid production and BCO2 is responsible for different physiological functions (65). Also, it was seen that while both BCO1 and BCO2 are expressed in human tissues in kidney tubules, exocrine pancreas, Leydig and Sertoli cells in the testis, small intestinal and stomach mucosa, adrenal gland, and epithelium in the eye, only BCO2 is expressed in cardiac and skeletal muscle, prostate and endometrial connective tissue, and the endocrine pancreas, suggesting that BCO2 has a function independent of vitamin A production (66). Two excellent recent reviews discuss in detail the regulation and physiological functions of the mammalian carotenoid oxygenases (67, 68).
It has been recently shown that there is negative feedback regulation of β-carotene conversion and intestinal absorption. This may occur via regulation of intestine-specific homeobox (ISX), an intestine-specific transcription factor (69). It was found that BCO1 and SR-BI expression in the intestine was increased in ISX-deficient mice (70). Thus, intestinal β-carotene absorption and β-carotene conversion to retinoids were inhibited in the presence of increasing cellular levels of atRA (71).
RETINOIC ACID RECEPTORS AND RETINOID X RECEPTORS
atRA acts through ligand-regulated trans-acting transcription factors that bind to cis-acting DNA regulatory elements in the promoter regions of target genes. Retinoic acid receptors (RAR) are members of the steroid/thyroid hormone family of nuclear receptor that consist of RARα (NR1B1), RARβ (NR1B2), and RARγ (NR1B3) (36, 37, 72–74), which form heterodimers with retinoid X receptors (RXR). atRA is the physiological ligand of RARs, and upon binding to receptor, atRA signaling occurs, which leads to the transcription of target genes involved in various cellular events, such as cellular differentiation, proliferation, and apoptosis (74). atRA is the major biologically active retinoid by virtue of activating the RARs globally and thereby exerting pleiotropic events in cell growth and differentiation in embryonic development and adult physiology (75, 76). Mechanisms of RAR signaling are reviewed in detail in the accompanying review by Al Tanoury et al. (74).
Nuclear receptors, including RARs and RXRs, are composed of modular structural/functional domains denoted A to F (74, 77), with different regions corresponding to autonomous functional domains that can be interchanged between related receptors without loss of function. The N-terminal A/B region contains a ligand-independent activation function (AF-1). The DNA-binding domain (DBD) is the most conserved domain of nuclear receptors, and it is responsible of recognition of specific DNA sequences called hormone response elements: retinoic acid response elements (RARE) and retinoid X response elements (RXRE). The E region contains the moderately conserved and largest domain, the ligand-binding domain (LBD). It consists of a ligand-binding pocket, a dimerization surface, and a ligand-dependent transcriptional activation function (AF-2). The short D region (linker domain) is not well conserved among the different receptors and serves as a hinge between the DBD and the LBD, allowing rotation of the DBD. The C-terminal F domain is present in RARs but not in RXRs and has no clear function.
Like other nuclear receptors, retinoid receptors regulate transcription by binding to specific DNA sequences in target genes known as hormone response elements (HRE). RARs and RXRs recognize response elements composed of two direct repeats of the consensus sequence 5′-AGGTCA-3′ separated by one to five base pairs, known as the 1–5 rule (77, 78). In the case of RAR-RXR heterodimers, the response element can be distinguished as either DR1 or DR5; the preferred HRE for the RARs is DR5. RAREs are found in the promoters of a large number of atRA target genes implicated in a wide variety of functions.
The LBDs are formed by 12 conserved α-helical regions (H1 to H12) and a β-turn (situated between H5 and H6). The LBD contains the ligand-binding pocket (LBP), the main dimerization domain, and the ligand-dependent activation function-2 (AF-2). LBP consists of hydrophobic residues. It is involved in selectivity of ligand binding, maximizing hydrophobic contacts, and its shape matches the volume of the ligand (79–81). The C-terminal helix 12 or H12 (AF-2) is the key mediator between active (recruitment of coactivators) and inactive configuration (presence of corepressors) of these nuclear receptors. Upon agonist binding to receptor, there is a conformational change in H12 that induces an α-strand to β-helix transition leading to H11 formation, which in turn stimulates release of corepressors, and H12 repositions itself so it can contact coactivators through their LXXLL motifs (82, 83), where L represents leucine and X represents any amino acid.
Because β-apocarotenoids are retinoid analogs, it is possible that they can serve as ligands for the retinoid receptors. Evidence for this possible role is discussed below, although the exact modes of interaction with the receptor LBDs are not known.
OCCURRENCE AND FUNCTIONS OF APOLYCOPENOIDS AND β-APOCAROTENOIDS IN FOODS AND IN MAMMALIAN TISSUES AND PLASMA
Occurrence of apolycopenoids
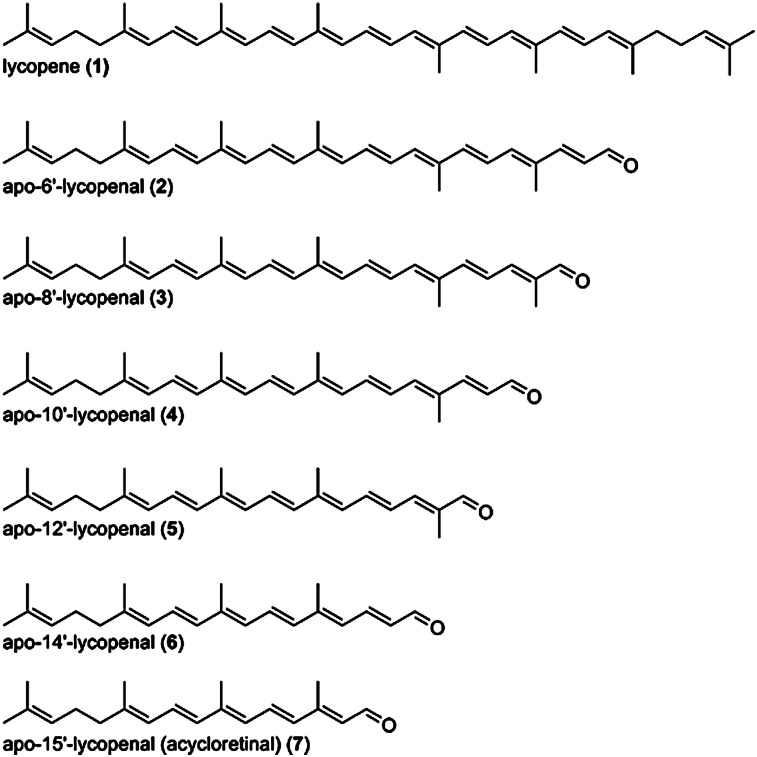
The occurrence of apolycopenoids such as apo-6′-lycopenal and apo-8′-lycopenal in tomato extracts and tomato paste (84) and in raw tomatoes (85) was reported some time ago. Recently, in addition to apo-6′- and 8′-lycopenal, apo-10′-, 12′-, and 14′-lycopenals were found in both raw and processed food products (86) (Fig. 3). The sum of the lycopenals was 6.5 µg/100 g Roma tomato and 73.4 µg/100 g tomato paste. These amounts are about 0.2% of the lycopene content of these foods. Also, in the same study, all of these apolycopenals (apo-6′-, 8′-, 10′-, 12′-, and 14′-lycopenal) were detected in human plasma at a total concentration of about 2 nM, also about 0.2% of the plasma lycopene concentration. Whether the plasma apolycopenals originated from enzymatic cleavage of lycopene or from consumption of apolycopenal-containing fruits and vegetables is not clear.
Fig. 3.
Series of apolycopenals. The apo-6′-, apo-8′-, apo-10′-, apo-12′-, and apo-14′-lycopenals were found in tomato products and in human plasma (154).
Another study revealed the presence of apo-8′-lycopenal and, putatively, apo-12′-lycopenal in the liver of 14C-lycopene-fed rats (87). The authors estimated the concentration of apo-8′-lycopenal at about 250 ng/g liver, or about 0.4% of the hepatic lycopene concentration. Apo-10′-lycopenal was not detected in this study. Apo-10′-lycopenal was likewise not detected in the lung tissues of lycopene-supplemented ferrets, but the reduction product of this apolycopenoid, apo-10′-lycopenol, was observed at a concentration of about 8 pmol/g tissue (57).
Biological activities of apolycopenoids
Lycopene oxidation products, including apolycopenoids, may be responsible for some of the biological activities attributed to lycopene (88, 89). There are number of reports indicating that apolycopenoids are biologically active compounds both in vitro and in vivo.
Gap junction communication (GJC) plays a role in the control of cell growth via differentiation, proliferation, and apoptosis (90). The central cleavage of lycopene yields acycloretinoic acid (ACR), the linear analog of atRA, which was shown to enhance GJC at supraphysiological concentrations (50 μM) in human fetal skin fibroblasts (91). ACR was also found to reduce cell viability by inducing apoptosis in human prostate cancer cells but not at physiological concentrations (92). In another cell-based study, this metabolite was shown to inhibit cell growth in human mammary MCF-7 cells (at 1 µM and above); in the same study, it was observed that ACR activates RARE but at a 100-fold lower potency than atRA (93).
The eccentric cleavage of lycopene catalyzed by BCO2 yields apo-10′-lycopenal that can be either further oxidized into apo-10′-lycopenoic acid or reduced to apo-10′-lycopenol, depending on whether NAD+ or NADH is present as a cofactor (57). Apo-10′-lycopenoic acid is implicated as the biologically active apolycopenoid, particularly in lung carcinogenesis, both in vitro and in vivo by inducing phase II enzymes (94); a process is mediated through antioxidant response elements (ARE) and activation of ARE transcription factor nuclear factor E2-related factor 2 (Nrf2). These effects were generally found with concentrations of the metabolite in the micromolar range. The same group studied the effects of apo-10′-lycopenoic acid on adipose tissue. In this study, they found that apo-10′-lycopenoic acid induced RAR activation both in vitro and in vivo and that this apolycopenoid displayed a similar effect as atRA in adipocyte tissue biology. However, although this apolycopenoid showed an anti-inflammatory response in adipose tissue, it did not have any effect on adipogenesis (95). All of these experiments utilized a 2 µM concentration of apo-10′-lycopenoic acid or atRA.
Sirtuin 1 (SIRT1), a key regulator of lipid metabolism (96), is involved in attenuating adipogenesis and inducing fat mobilization (97, 98). In a recent study, it was reported that apo-10′-lycopenoic acid led to the increase in SIRT1 enzyme activity by treatment with this metabolite in ob/ob mice, resulting in prevention of fatty liver (99). In this study the compound was fed in the diet at 40 µg/g diet (or 240 µg/kg body weight/day).
Interestingly, there is accumulating evidence that modulation of BCO2 activity in the intact animal alters lycopene metabolism and has profound effects on whole-body physiology (67, 68, 88, 89).
Occurrence of β-apocarotenoids
In 1970s, Ganguly and coworkers (100, 101) reported studies regarding the existence and possible biological activities of β-apocarotenoids. In one of them, β-apo-8′-, β-apo-10′-, and β-apo-12′-carotenal were tested in the curative-growth assay in rats. The assay was performed in which the total gain in weight of vitamin A-deficient rats receiving β-carotene and β-apo-8′-, β-apo-10′-, and β-apo-12′-carotenal was assessed and the biopotencies of these β-apocarotenoids were calculated against β-carotene taken as 100%, on a molar basis. The biopotencies found were 72 ± 4, 78 ± 3, and 72 ± 5 for β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-12′-carotenal, respectively (100). In the same study, they demonstrated that β-apocarotenals can be oxidized to the corresponding β-apocarotenoic acids in mitochondrial and microsomal fractions of the homogenates of rat and chicken liver and that addition of either NAD+ or NADP+ produced an increase in the aldehyde oxidase activity in both species. Another study by the same group identified retinal and β-apo-8′-, β-apo-10′-, and β-apo-12′-carotenal from the intestine of chickens fed β-carotene using thin-layer chromatography and spectroscopic analysis (101).
The formation of β-apocarotenoids can occur enzymatically, as shown in a study that utilized human, monkey, ferret, and rat tissue homogenates. These homogenates were incubated with β-carotene in the presence of NAD and dithiothreitol. Significant amounts of β-apo-8′-, β-apo-10′-, and β-apo-12′-carotenal as well as retinal and retinoic acid were found (102). There were no β-apocarotenals or retinoids detected in the control incubations without tissue homogenates. Moreover, the amounts of β-apocarotenals and retinoids formed were markedly reduced when NADH or when dithiothreitol and cofactors were deleted from the incubation and replaced by NAD. Both β-apocarotenals and retinoid production were inhibited completely by adding disulfiram, an inhibitor of sulfydryl-containing enzymes (102).
Other investigators detected β-apocarotenoids (β-apo-8′, β-apo-10′, β-apo-12′, and β-apo-14′ carotenal) in intestinal preparations from vitamin A-deficient rats fed a single dose of β-carotene, but they were present in amounts less than 5% of the retinoids formed in the same 3 h period (103). Collectively, all the studies mentioned above suggest that β-apocarotenals are formed as products of β-carotene oxidation in vivo.
β-Apocarotenoids can also be formed via autoxidation, thermal degradation of β-carotene, or during food processing. The spontaneous autoxidation of toluene solutions of β-carotene with 100% oxygen at 60°C for 120 min yielded a homologous series of carbonyl cleavage products of β-carotene, including RAL, β-apo-13-carotenone, β-apo-14′-carotenal, β-apo-12′-carotenal, and β-apo-10′-carotenal that were separated by reverse-phase HPLC, and individual peaks were characterized with an online diode array detector (104).
In a simulated deodorization using purified palm oil and β-carotene in which the individual compounds in fractions were isolated using a silicic acid column, β-apo-13-carotenone, β-apo-14′-carotenal, and RAL were identified by infrared spectroscopy and mass spectrometry (105). In another food-related study, thermal degradation (various heat treatments) of β-carotene yielded β-apo-8′-carotenal, β-apo-10′-carotenal, β-apo-12′-carotenal, and β-apo-14′-carotenal; these oxidation products were found to be 5% of initial β-carotene (106).
β-Apocarotenoids are present in the diet (107, 108). Processed mango juice, acerola juice, and dried apricots were analyzed by TLC, HPLC, and visible absorption spectra obtained by a photodiode array detector and mass spectra. These processed foods were tested for the presence of β-apocarotenals, including β-apo-8′-, β-apo-10′-, β-apo-12′-, and β-apo-14′-carotenal derived from β-carotene. None of these oxidation products was detected in acerola juice or dried apricots, and only β-apo-12′-carotenal was found in mango juice (107).
We recently reported the existence of β-apocarotenoids in orange-fleshed melons. The levels of β-apo-13-carotenone and β-apocarotenals, including β-apo-8′-, β-apo-10′-, β-apo-12′-, and β-apo-14′-carotenal, in melons (a cantaloupe and a greenhouse-grown Orange Dew) were measured by LC/MS (108). In both melons, the five β-apocarotenoids were each present at approximately 30 pmole/g wet weight and in total represented about 1.5% the level of β-carotene in the melons.
More recently, our collaborators developed sensitive LC/MS procedures for detection of β-apocarotenoids in human plasma. The specificity and sensitivity was assured by multiple-reaction monitoring. Human plasma samples from six free-living individuals were analyzed and the plasma concentration of β-apo-13-caroteneone was found to be about 4 nM (109).
Additionally, β-apocarotenoids can arise in humans and rats from β-carotene metabolism. In one study, β-apo-8′-carotenal was detected in the plasma of a healthy man three days following ingestion of a small oral tracer dose of [14C]β-carotene (110). Given that this analysis used accelerator mass spectrometry (and thus detected only 14CO2 in combusted samples), it was not possible to quantify the level of the β-apocarotenoid in plasma. Our group undertook a study in which the concentrations of β-carotene and β-apo-8′-, β-apo-10′-, β-apo-12′-, and β-apo-14′-carotenal were measured both in serum and liver of wild-type and BCO1 knockout mice that were on a β-carotene-containing diet (111). All four β-apocarotenals were found in the diet (at 0.1, 0.2, 0.2, and 0.3 nmol/g, respectively). However, only β-apo-10′- and β-apo-12′-carotenal were detected in serum (0.2–0.7 nM) and livers from wild-type and BCO1 knockout mice, and only β-apo-12′-carotenal levels were found to be significantly higher in the liver of knockout mice compared with wild-type (111).
Biological activities of β-apocarotenoids in mammals
β-Carotene is one of the substances used in chemoprevention clinical trials against various types of cancers (112). Chemoprevention can be defined as the application of natural or synthetic molecules to prevent, inhibit, or reverse the carcinogenic machinery (113). Epidemiologic studies demonstrated that individuals eating more fruits and vegetables that are rich in carotenoids and people having higher serum β-carotene levels have a lower risk of cancer, particularly lung cancer (114). Two large randomized intervention trials of β-carotene supplementation were designed to test whether β-carotene could reduce the risk of lung cancer: the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study and the Beta Carotene and Retinol Efficacy Trial (CARET).
In 1994, ATBC reported its findings on more than 29,000 participants of a double-blinded, placebo-controlled trial on the prevention of lung cancer and other cancers by supplementation with micronutrients (115). The results for β-carotene given at a dose of 20 mg in one capsule taken daily for five to eight years were not expected because they provided no evidence for benefit in the prevention of lung cancer in older male cigarette smokers; instead, there were more incidents of lung cancer diagnosed in those receiving β-carotene supplements.
In the CARET study, β-carotene (30 mg) and retinyl palmitate 25,000 IU (13,664 retinol equivalents) were given to more than 18,000 men and women with a history of smoking or asbestos exposure (116). Among them, 388 developed lung cancer, with a 28% increase in lung cancer incidence in those who received the β-carotene and retinyl palmitate combination daily for an average of four years compared with participants who received placebo. The study was halted ahead of schedule for this reason.
The reason for the adverse effects of β-carotene observed in the CARET and ATBC trials may be that the subject group in these trials was restricted to smokers. It is possible that β-carotene could be easily oxidized under the conditions of smoking and asbestos exposure. Also, the doses given in these trials were supraphysiological levels (20 mg in ATBC, and 30 mg in CARET).
The biological activities of β-apocarotenoids were examined in a number of studies based on considering the biological properties of the parental compound, i.e., β-carotene. In one study, the interaction between benzo[a]pyrene (an important carcinogen found in cigarette smoke) and β-carotene or β-apo-14′-carotenoic acid in normal human bronchial epithelial (NHBE) cells was examined (117). Also, tested was whether benzo[a]pyrene, β-carotene, or β-apo-14′-carotenoic acid affected the expression levels of RARβ in NHBE cells. It was found that, although a carcinogen benzo[a]pyrene treatment led to the downregulation of RARβ, β-carotene or β-apo-14′-carotenoic acid treatment, at 30 µM and 10 µM, respectively, doubled the levels of RARβ. Additionally, it was observed that RARβ downregulated by carcinogen was completely reversed by treatment of β-carotene or β-apo-14′-carotenoic acid. In the same study, it was demonstrated that β-apo-14′-carotenoic acid can transactivate RARβ2, but that it was 10- to 100-fold less than active than the physiological agonist atRA. However, this transactivation seen by β-apo-14′-carotenoic acid was suggested to be due to its metabolism to atRA. The authors tested another β-apocarotenoid, β-apo-13-carotenone, in the same study, and this compound did not display a significant transcriptional activation of RARβ.
It is known that aRA exhibits anticancer activities by repressing activator protein-1 (AP-1) (118). The ability of β-apocarotenoic acids, including β-apo-8′-, β-apo-10′-, β-apo-12′-, and β-apo-14′-carotenoic acid, to inhibit breast tumor cell growth in both estrogen receptor-positive (MCF-7) and estrogen receptor-negative (Hs578T and MDA-MB-231) human breast cancer cells, which express RARs (119), was tested. It was observed that after two to six days of treatment, none of the β-apocarotenoic acids and atRA at various concentrations (10 nM, 100 nM, and 1 µM) induced cell growth inhibition; however, nine days of treatment with 1 µM β-apo-12′- and β-apo-14′-carotenoic acid displayed half of the inhibition elicited by 1 µM atRA in MCF-7 cells on the basis of live cell calculations. Additionally, 1 µM β-apo-14′-carotenoic acid exerted the growth-inhibitory response in Hs578T cells. The induced growth-inhibitory effect of β-apocarotenoids was associated with repression of AP-1 activity, similar to the action of atRA, but not dependent on RAR proteins. The authors concluded that these β-apocarotenoic acids display antiproliferative effects through anti-AP-1 activity and that these compounds are biologically active in breast tumor cells.
Acute myeloid leukemia occurs as a result of the propagation of cell clones that have a block in their differentiation pathway to functional mature granulocytes or monocytes (120). The human cell line HL-60 was developed (121) and has been widely used to examine the compounds that induce maturation of these cells. atRA inhibits proliferation of HL-60 cells and induces them to differentiate into morphologically functional mature granulocytes (122, 123). β-carotene and some of its metabolites were tested in promyelocytic leukemia cell lines, such as HL-60 cells and U937 cells, for their ability to function as biologically active compounds on myeloid differentiation. β-Carotene inhibited proliferation and induced differentiation of HL60 cells (125) and U937 cells (125). β-Carotene cleavage products, such as β-apo-14′-carotenoic acid, were found to stimulate the differentiation of U937 leukemia cells (126), and β-apo-12′-carotenoic acid was capable of inhibiting the proliferation of HL-60 cells (127).
In addition to these ascribed anticarcinogenic properties of the parent compound, i.e., β-carotene, it was shown that β-carotene can be an activator of the human pregnane receptor (PXR) (128). PXR, a member of the nuclear receptor family of ligand-activated transcription factors, is involved in xenobiotic detoxification to prevent accumulation of toxic substances in liver (129). It was tested whether β-carotene cleavage products are biologically active in terms of eliciting PXR activity. β-Apocarotenals, including β-apo-8′- and 12′-carotenal and β-carotene (all at 10 µM), and a known PXR agonist (rifampicin) were tested in reporter assays using Hep G2 liver cells. Although β-carotene itself elicited a 5- to 6-fold reporter gene activity increase, β-apocarotenals exerted less or no activation compared with a 7-fold induction by rifampicin (130).
Whether β-apocarotenals are biologically active was also studied by Ziouzenkova et al. (131). They tested whether eccentric cleavage products of β-carotene, such as β-apo-8′-, β-apo-12′-, and β-apo-14′-carotenal, display any biological activity and whether they have any effect on gene expression. Based on atRA being a suppressor in adipocyte differentiation via peroxisome proliferator-activated receptor (PPAR)γ (132), they tested whether β-apocarotenals displayed any activity on adipocyte differentiation assays using 3TC3-L1 cells. They found that only β-apo-14′-carotenal decreased adipogenesis (lipid accumulation) in a concentration-dependent manner (1–20 µM). Next, they tested whether this suppression of adipogenesis is through association with RARs, and they found that β-apo-14′-carotenal was a weak partial agonist (in the µM concentration range), suggesting a RARα-independent mechanism leading to β-apo-14′-carotenal's repression of adipogenesis. Since adipogenesis is also regulated by activation of RXR and RXR-PPAR or RXR partnering with other nuclear receptors (133), they tested RXRα activation in the presence of a synthetic agonist of RXR and β-apo-8′-, β-apo-12′-, and β-apo-14′-carotenal in human bovine cells. Only β-apo-14′-carotenal effectively inhibited agonist-induced RXRα activation with inhibition constant (Ki) value of 500 nM. In addition, RXR partner nuclear receptors were tested, including PPARα, PPARδ, and PPARγ. It was found that β-apo-14′-carotenal decreased agonist-induced PPARα and PPARγ activation very effectively, and PPARδ modestly. Also, they found that β-apo-14′-carotenal displays an inflammatory response via PPARα inhibition in vivo in wild-type mice and that this response was not observed in PPARα-deficient mice. Most of the experiments in this study were conducted with 1–10 µM β-apo-14′-carotenal.
The possibility that the β-apocarotenoids might function as antagonists rather than agonists of nuclear receptors has been supported by the results of recent studies in the authors’ laboratory. In one study, we investigated the effects of β-apocarotenoids on RXRα signaling (134). Transactivation assays were performed to test whether β-apocarotenoids activate or antagonize RXRα. Reporter gene constructs (RXRE-Luciferase) and RXRα were transfected into Cos-1 cells and used to perform these assays. None of the β-apocarotenoids tested activated RXRα. Among the compounds tested, β-apo-13-carotenone was found to antagonize the activation of RXRα by 9-cis-RA and was effective at concentrations as low as 1 nM. β-Apo-14′-carotenal and β-apo-8′-carotenal were also found to be antagonists of RXRα but with less potency than β-apo-13-carotenone. Molecular modeling studies revealed that β-apo-13-carotenone makes molecular interactions like an antagonist of RXRα.
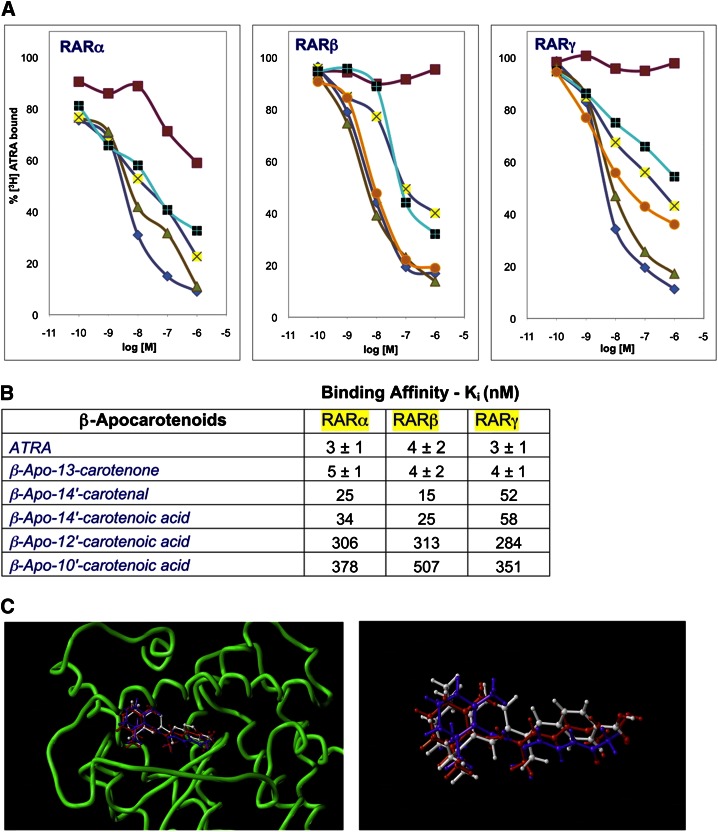
In another study (109), we found that β-apo-14′-carotenal, β-apo-14′-carotenoic acid, and β-apo-13-carotenone antagonized atRA-induced transactivation of all three of the RARs and were effective at nanomolar concentrations. Competitive radioligand binding assays demonstrated that these putative RAR antagonists compete directly with retinoic acid for high-affinity binding to purified receptors. The binding affinity for β-apo-13-carotenone was 4–5 nM, close to that of atRA itself, while that of β-apo-14′-carotenal and β-apo-14′carotenoic acid was 5–10 times lower (Fig. 4). Molecular modeling studies confirmed that β-apo-13-carotenone can directly interact with the ligand-binding site of the retinoid receptors. β-Apo-13-carotenone and the β-apo-14′-carotenoids inhibited ATRA-induced expression of retinoid-responsive genes in Hep G2 cells. Finally, as previously mentioned, we found that 3–5 nM β-apo-13-carotenone was present in human plasma. These findings suggest that β-apocarotenoids function as naturally occurring retinoid antagonists. The antagonism of retinoid signaling by these metabolites may have implications for the activities of dietary β-carotene as a provitamin A and as a modulator of risk for cardiovascular disease and cancer.
Fig. 4.
β-Apo-13-carotenone is a high-affinity ligand for purified retinoic acid receptors and fits into the ligand binding site. (A) Competitive displacement of 5 nM tritiated atRA from purified RAR proteins by unlabeled atRA (filled diamond) as a positive control, β-apo-13-carotenone (filled triangle), 14′-CA (plus), 14′-AL (cross), and 13-cis-RA (filled square) as a negative control for RARα (left) experiment, and CD 2665 (filled circle) and retinyl acetate (filled square) as a negative control for RARβ (middle) and RARγ (right) experiments. Points shown are means of n = 3 with a variance of less than 10%. (B) Binding affinities (in nM) of β-apocarotenoids to RARs calculated from the data shown in (A) and additional experiments with β-apo-12′-and β-apo-10′-carotenoic acids. For atRA and β-apo-13-carotenone, variance shown is for three independent experiments. (C) Molecular modeling of the docking of atRA (red) and β-apo-13-carotenone (purple) into the ligand-binding site (protein backbone in green) of RARβ (PDB entry:1xap) (left). Shown on the right is the energy minimized then docked conformations of atRA (red) and β-apo-13-carotenone (purple) overlaid onto the conformation of the agonist TTNPB (white) as observed in the X-ray structure (177).
CONCLUSION AND PROSPECTIVE
It is clear that much remains to be discovered about the formation, occurrence, and function of apocarotenoids in humans and other mammals. More information is needed on the mechanisms of enzymatic and nonenzymatic formation of the apocarotenoids. It is also important to have a better quantitative assessment of the amounts of these compounds present in the diet and the mechanisms of their intestinal absorption. More needs to be known about the metabolism of the apocarotenoids themselves. Very little information is available on the concentrations of apocarotenoids in plasma and tissues, but the available evidence suggests that these will be in the nanomolar or submicromolar range. Thus, proposed mechanisms of action, such as nuclear receptor binding, must include those that are sensitive to these low concentrations. Finally, it will be important to understand the interactions of apocarotenoids with other lipid-activated receptors, lipid-binding proteins, and lipid-metabolizing enzymes.
Acknowledgments
The authors thank the students, fellows, and collaborators who contributed to this work.
Footnotes
Abbreviations:
- atRA
- all-trans-retinoic acid
- atRAL
- all-trans-retinal
- RAR
- retinoic acid receptor
- RBP
- retinol-binding protein
- RXR
- retinoid X receptor
- ABA
- abscisic acid
- BCDO2
- β-carotene-9′-10′-dioxygenase
- BCMO1
- β,β-carotene-15,15′-monooxygenase 1
- BCO1
- β-carotene-oxygenase 1
- BCO2
- β-carotene-oxygenase 2
- LBD
- ligand-binding domain
- LRAT
- lecithin:retinol acyltransferase
- PPAR
- peroxisome proliferator-activated receptor
- RARE
- retinoic acid response element
- RXRE
- retinoid X response element
Work done in the authors’ laboratory was supported by National Institutes of Health Grants R01 DK-44498 and R01 HL-49879 and by funds from the Ohio Agricultural Research and Development Center.
REFERENCES
- 1.Britton G., Liaaen-Jensen S., Pfander H., editors. 2008. Carotenoids: Natural Functions. Vol. 4. Birkhäuser, Basel. [Google Scholar]
- 2.Lu S., Li L. 2008. Carotenoid metabolism: biosynthesis, regulation, and beyond. J. Integr. Plant Biol. 50: 778–785 [DOI] [PubMed] [Google Scholar]
- 3.Maresca J. A., Graham J. E., Bryant A. D. 2008. The biochemical basis for structural diversity in the carotenoids of chlorophototrophic bacteria. Photosynth. Res. 97: 121–140 [DOI] [PubMed] [Google Scholar]
- 4.Takaichi S., Mochimaru M. 2007. Carotenoids and carotenogenesis in cyanobacteria: unique ketocarotenoids and carotenoid glycosides. Cell. Mol. Life Sci. 64: 2607–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Z. W., Jiang J. G., Wu G. H. 2008. Biosynthesis and regulation of carotenoids in Dunaliella: progresses and prospects. Biotechnol. Adv. 26: 352–360 [DOI] [PubMed] [Google Scholar]
- 6.Moore T. 1930. Vitamin A and carotene: the conversion of carotene to vitamin A in vivo. Biochem. J. 24: 692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karrer P., Helfenstein A., Wehrli H., Wettstein A. 1930. Über die konstitution des lycopins und carotins. Helv. Chim. Acta. 13: 1084–1099 [Google Scholar]
- 8.Romanchik J. E., Morel D. W., Harrison E. H. 1995. Distributions of carotenoids and α-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J. Nutr. 125: 2610–2617 [DOI] [PubMed] [Google Scholar]
- 9.Wirtz G. M., et al. 2001. The substrate specificity of β,β-carotene 15,15’- monooxygenase. Helv. Chim. Acta. 84: 2301–2315 [Google Scholar]
- 10.Bauernfeind J. C. 1972. Carotenoid vitamin A precursors and analogs in food and feeds. J. Agric. Food Chem. 20: 456–473 [DOI] [PubMed] [Google Scholar]
- 11.van Vliet T., van Schaik F., Schreurs W. H. P., van den Berg H. 1996. In vitro measurement of β-carotene cleavage activity: methodological considerations and the effect of other carotenoids on β-carotene cleavage. Int. J. Vitam. Nutr. Res. 66: 77–85 [PubMed] [Google Scholar]
- 12.Nagao A., Olson J. A. 1994. Enzymatic formation of 9-cis, 13-cis, and all-trans retinals from isomers of β-carotene. FASEB J. 8: 968–973 [DOI] [PubMed] [Google Scholar]
- 13.Olson J. A. 1989. Provitamin A function of carotenoids: the conversion of β-carotene into vitamin A. J. Nutr. 119: 105–108 [DOI] [PubMed] [Google Scholar]
- 14.Parker R. S. 1996. Absorption, metabolism, and transport of carotenoids. FASEB J. 10: 542–551 [PubMed] [Google Scholar]
- 15.Moussa M., Landrier J. F., Reboul E., Ghiringhelli O., Coméra C., Collet X., Fröhlich K., Böhm V., Borel P. 2008. Lycopene absorption in human intestinal cells and in mice involves scavenger receptor class B type I but not Niemann-Pick C1-like. J. Nutr. 138: 1432–1436 [DOI] [PubMed] [Google Scholar]
- 16.van Bennekum A., Werder M., Thuahnai S. T., Han C. H., Duong P., Williams D. L., Wettstein P., Schulthess G., Phillips M. C., Hauser H. 2005. Class B scavenger receptor-mediated intestinal absorption of dietary β-carotene and cholesterol. Biochemistry. 44: 4517–4525 [DOI] [PubMed] [Google Scholar]
- 17.During A., Harrison E. H. 2007. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J. Lipid Res. 48: 2283–2294 [DOI] [PubMed] [Google Scholar]
- 18.Harrison E. H. 2012. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta. 1821: 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bondi A., Sklan D. 1984. Vitamin A and carotene in animal nutrition. Prog. Food Nutr. Sci. 8: 165–191 [PubMed] [Google Scholar]
- 20.Ribaya-Mercado J. D., Holmgren S. C., Fox J. G., Russell R. M. 1989. Dietary β-carotene absorption and metabolism in ferrets and rats. J. Nutr. 119: 665–668 [DOI] [PubMed] [Google Scholar]
- 21.O'Byrne S. M., Wongsiriroj N., Libien J., Vogel S., Goldberg I. J., Baehr W., Palczewski K., Blaner W. S. 2005. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J. Biol. Chem. 280: 35647–35657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H. S., Goodman D. S. 1965. Vitamin A and carotenoids. 1. Intestinal absorption and metabolism of 14C-labelled vitamin A alcohol and β-carotene in the rat. J. Biol. Chem. 240: 2839–2844 [PubMed] [Google Scholar]
- 23.MacDonald P. N., Ong D. E. 1988. Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine. J. Biol. Chem. 263: 12478–12482 [PubMed] [Google Scholar]
- 24.Helgerud P., Petersen L. B., Norum K. R. 1983. Retinol esterification by microssomes from the mucosa of human small intestine: evidence for acyl- coenzym A retinol acyltransferase activity. J. Clin. Invest. 71: 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wongsiriroj N., Piantedosi R., Palczewski K., Goldberg I. J., Johnston T. P., Li E., Blaner W. S. 2008. The molecular basis of retinoid absorption: a genetic dissection. J. Biol. Chem. 283: 13510–13519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ong D. E. 1994. Cellular transport and metabolism of vitamin A: roles of the cellular retinoid-binding proteins. Nutr. Rev. 52: S24–S31 [DOI] [PubMed] [Google Scholar]
- 27.Wolf G. 1991. The intracellular vitamin A-binding proteins: an overview of their functions. Nutr. Rev. 49: 1–12 [DOI] [PubMed] [Google Scholar]
- 28.Kakkad B.P., Ong D.E. 1988. Reduction of retinaldehyde bound to cellular retinol- binding protein (type II) by microsomes from rat small intestine. J. Biol. Chem. 263: 12916–12919 [PubMed] [Google Scholar]
- 29.Napoli J. L. 1996. Retinoic acid biosynthesis and metabolism. FASEB J. 10: 993–1001 [DOI] [PubMed] [Google Scholar]
- 30.Blomhoff R., Green M. H., Green J. B., Berg T., Norum K. R. 1991. Vitamin A metabolism: new perspectives on absorption, transport and storage. Physiol. Rev. 71: 951–990 [DOI] [PubMed] [Google Scholar]
- 31.Blomhoff R. 1994. Transport and metabolism of vitamin A. Nutr. Rev. 52: S13–S23 [DOI] [PubMed] [Google Scholar]
- 32.Levin G., Amotz A. B., Mokady S. 1994. Liver accumulation of soluble all-trans or 9-cis β-carotene in rats and chicks. Comp. Biochem. Physiol. 107: 203–207 [Google Scholar]
- 33.Matsuura T., Gad M. Z., Harrison E. H., Ross A. C. 1997. Lecithin:retinol acyltransferase and retinyl ester hydrolase activities are differentially regulated by retinoids and have distinct distributions between hepatocytes and non-parenchymal cell fractions of rat liver. J. Nutr. 127: 218–224 [DOI] [PubMed] [Google Scholar]
- 34.Navab M., Smith J. E., Goodman D. S. 1977. Rat plasma prealbumin: metabolic studies on effects of vitamin A status and on tissue distribution. J. Biol. Chem. 252: 5107–5114 [PubMed] [Google Scholar]
- 35.O'Byrne S. M., Blaner W. S. 2013. Retinol and retinyl esters: biochemistry and physiology. J. Lipid Res. 54: 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawaguchi R., Yu J., Honda J., Hu J., Whitelegge J., Ping P., Wiita P., Bok D., Sun H. 2007. A membrane receptor for retinol-binding protein mediates cellular uptake of vitamin A. Science. 315: 820–825 [DOI] [PubMed] [Google Scholar]
- 37.Giguère V., Ong E. S., Segui P., Evans R. M. 1987. Identification of a receptor for the morphogen retinoic acid. Nature. 330: 624–629 [DOI] [PubMed] [Google Scholar]
- 38.Petkovich M., Brand N. J., Krust A., Chambon P. 1987. A human retinoic acid receptor which belongs to the family of nuclear receptor. Nature. 330: 444–450 [DOI] [PubMed] [Google Scholar]
- 39.Kedishvili N. Y. 2013. Enzymology of retinoic acid biosynthesis and degradation. J. Lipid Res. 54: 1744–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Napoli J. L. 2012. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta. 1821: 152–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz S. H., Tan B. C., Gage D. A., Zeevaart J. A. D., McCarty D. R. 1997. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- 42.Bouwmeester H. J., Roux C., Lopez-Raez J. A., Becard G. 2007. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 12: 224–230 [DOI] [PubMed] [Google Scholar]
- 43.Gomez-Roldan V., Fermas S., Brewer P. B., Puech-Pagès V., Dun E. A., Pillot J. P., Letisse F., Matusova R., Danoun S., Portais J. C., et al. 2008. Strigolactone inhibition of shoot branching. Nature. 455: 189–194 [DOI] [PubMed] [Google Scholar]
- 44.Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., Magome H., Kamiya Y., Shirasu K., Yoneyama K., et al. 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 455: 195–200 [DOI] [PubMed] [Google Scholar]
- 45.Goodman D. S., Huang H. S. 1965. Biosynthesis of vitamin A with rat intestinal enzyme. Science. 149: 879–880 [DOI] [PubMed] [Google Scholar]
- 46.Olson J. A., Hayaishi O. 1965. The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc. Natl. Acad. Sci. USA. 54: 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeevart J. A. D., Creelman R. A. 1988. Metabolism and physiology of abscisic acid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39: 439–473 [Google Scholar]
- 48.Ruch S., Beyer P., Ernst H., Al-Babili S. 2005. Retinal biosynthesis in Eubacteria: in vitro characterization of a novel carotenoid oxygenase from Synechocystis sp. PCC 6803. Mol. Microbiol. 55: 1015–1024 [DOI] [PubMed] [Google Scholar]
- 49.Prado-Cabrero A., Scherzinger D., Avalos J., Al-Babili S. 2007. Retinal biosynthesis in fungi: characterization of the carotenoid oxygenase CarX from Fusarium fujikuroi. Eukaryot. Cell. 6: 650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyss A., Wirtz G., Woggon W., Brugger R., Wyss M., Friedlein A., Bachmann H., Hunziker W. 2000. Cloning and expression of beta,beta-carotene 15,15’-dioxygenase. Biochem. Biophys. Res. Commun. 271: 334–336 [DOI] [PubMed] [Google Scholar]
- 51.von Lintig J., Vogt K. 2000. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J. Biol. Chem. 275: 11915–11920 [DOI] [PubMed] [Google Scholar]
- 52.Kiser P. D., Golczak M., Maeda A., Palczewski K. 2012. Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim. Biophys. Acta. 1821: 137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindqvist A., Andersson S. 2002. Biochemical properties of purified recombinant human β-carotene 15,15’-monooxygenase. J. Biol. Chem. 277: 23942–23948 [DOI] [PubMed] [Google Scholar]
- 54.Kiefer C., Hessel S., Lampert J. M., Vogt K., Lederer M. O., Breithaupt D. E., von Lintig J. 2001. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 276: 14110–14116 [DOI] [PubMed] [Google Scholar]
- 55.Amengual J., Lobo G. P., Golczak M., Li H. N., Klimova T., Hoppel C. L., Wyss A., Palczewski K., von Lintig J. 2011. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 25: 948–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mein J. R., Dolnikowski G. G., Ernst H., Russell R. M., Wang X. D. 2011. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and β-cryptoxanthin by ferret carotene-9’,10’-monooxygenase. Arch. Biochem. Biophys. 506: 109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu K. Q., Liu C., Ernst H., Krinsky N. I., Russell R. M., Wang X. D. 2006. The biochemical characterization of ferret carotene-9’,10’-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J. Biol. Chem. 281: 19327–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leuenberger M. G., Engeloch-Jarret C., Woggon W. D. 2001. The reaction mechanism of the enzyme-catalyzed central cleavage of β-carotene to retinal. Angew. Chem. Int. Ed. Engl. 40: 2613–2617 [DOI] [PubMed] [Google Scholar]
- 59.Devery J., Milborrow B. V. 1994. beta-Carotene-15,15’-dioxygenase (EC 1.13.11.21) isolation, reaction mechanism, and an improved assay procedure. Br. J. Nutr. 72: 397–414 [DOI] [PubMed] [Google Scholar]
- 60.Redmond T. M., Gentleman S., Duncan T., Yu S., Wiggert B., Gantt E., Cunningham F. X., Jr 2001. Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15’-dioxygenase. J. Biol. Chem. 276: 6560–6565 [DOI] [PubMed] [Google Scholar]
- 61.Wyss A., Wirtz G. M., Woggon W. D., Brugger R., Wyss M., Friedlein A., Riss G., Bachmann H., Hunziker W. 2001. Expression pattern and localization of beta-beta carotene 15,15’- dioygenase in different tissues. Biochem. J. 354: 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paik J., During A., Harrison E. H., Mendelsohn C. L., Lai K., Blaner W. S. 2001. Expression and characterization of a murine enzyme able to cleave β-carotene: the formation of retinoids. J. Biol. Chem. 276: 32160–32168 [DOI] [PubMed] [Google Scholar]
- 63.Yan W., Jang G. F., Haeseleer F., Esumi N., Chang J., Kerrigan M., Campochiaro M., Campochiaro P., Palczewski K., Zack D. J. 2001. Cloning and characterization of a human β,β-carotene-15,15’- dioxygenase that is highly expressed in the retinal pigment epithelium. Genomics. 72: 193–202 [DOI] [PubMed] [Google Scholar]
- 64.Wang X. D., Russell R. M., Liu C., Stickel F., Smith D. E., Krinsky N. I. 1996. Beta-oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from beta-apocarotenoic acids. J. Biol. Chem. 271: 26490–26498 [PubMed] [Google Scholar]
- 65.Hessel S., Eichinger A., Isken A., Amengual J., Hunzelmann S., Hoeller U., Elste V., Hunziker W., Goralczyk R., Oberhauser V., et al. 2007. CMO1 deficiency abolishes vitamin A production from beta- carotene and alters lipid metabolism in mice. J. Biol. Chem. 282: 33553–33561 [DOI] [PubMed] [Google Scholar]
- 66.Lindqvist A., He Y. G., Andersson S. 2005. Cell type-specific expression of beta- carotene 9’,10’-monooxygenase in human tissues. J. Histochem. Cytochem. 53: 1403–1412 [DOI] [PubMed] [Google Scholar]
- 67.von Lintig J. 2010. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 30: 35–56 [DOI] [PubMed] [Google Scholar]
- 68.Lobo G. P., Amengual J., Palczewski G., Babino D., von Lintig J. 2012. Mammalian carotenoid oxygeneases: Key players for carotenoid function and homeostasis. Biochim. Biophys. Acta. 1821: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi M. Y., Romer A. I., Hu M., Lepourcelet M., Mechoor A., Yesilaltay A., Krieger M., Gray P. A., Shivdasani R. A. 2006. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development. 133: 4119–4129 [DOI] [PubMed] [Google Scholar]
- 70.Seino Y., Miki T., Kiyonari H., Abe T., Fujimoto W., Kimura K., Takeuchi A., Takahashi Y., Oiso Y., Iwanaga T., Seino S. 2008. Isx participates in the maintenance of vitamin A metabolism by regulation of β-carotene 15,15’-monooxygenase (Bcmo1) expression. J. Biol. Chem. 283: 4905–4911 [DOI] [PubMed] [Google Scholar]
- 71.Lobo G. P., Hessel S., Eichinger A., Noy N., Moise A. R., Wyss A., Palczewski K., von Lintig J. 2010. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β,β-carotene absorption and vitamin A production. FASEB J. 24: 1656–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Germain P., Chambon P., Eichele G., Evans R. M., Lazar M. A., Leid M., De Lera A. R., Lotan R., Mangelsdorf D. J., Gronemeyer H. 2006. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol. Rev. 58: 712–725 [DOI] [PubMed] [Google Scholar]
- 73.Germain P., Staels B., Dacquet C., Spedding M., Laudet V. 2006. Overview of nomenclature of nuclear receptors. Pharmacol. Rev. 58: 685–704 [DOI] [PubMed] [Google Scholar]
- 74.Al Tanoury A., Piskunov A., Rochette-Egly C. 2013. Vitamin A and retinoid signaling: genomic and non-genomic effects. J. Lipid Res. 54: 1761–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chambon P. 1996. A decade of molecular biology of retinoic acid receptors. FASEB J. 10: 940–954 [PubMed] [Google Scholar]
- 76.Mark M., Ghyselinck N. B., Chambon P. 2009. Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 7: e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dawson M. I., Xia Z. 2012. The retinoid X receptors and their ligands. Biochim. Biophys. Acta. 1821: 21–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rastinejad F. 2001. Retinoid X receptor and its partners in the nuclear receptor family. Curr. Opin. Struct. Biol. 11: 33–38 [DOI] [PubMed] [Google Scholar]
- 79.Géhin M., Vivat V., Wurtz J. M., Losson R., Chambon P., Moras D., Gronemeyer H. 1999. Structural basis for engineering of retinoic acid receptor isotype- selective agonists and antagonists. Chem. Biol. 6: 519–529 [DOI] [PubMed] [Google Scholar]
- 80.Klaholz B. P., Mitschler A., Moras D. 2000. Structural basis for isotype selectivity of the human retinoic acid nuclear receptor. J. Mol. Biol. 302: 155–170 [DOI] [PubMed] [Google Scholar]
- 81.Klein E. S., Pino M. E., Johnson A. T., Davies P. J., Nagpal S., Thacher S. M., Krasinski G., Chandraratna R. A. 1996. Identification and functional separation of retinoic acid receptor neutral antagonists and inverse agonists. J. Biol. Chem. 271: 22692–22696 [DOI] [PubMed] [Google Scholar]
- 82.le Maire A., Teyssier C., Erb C., Grimaldi M., Alvarez S., de Lera A. R., Balaguer P., Gronemeyer H., Royer C. A., Germain P., Bourguet W. 2010. A unique secondary-structure switch controls constitutive gene repression by retinoic acid receptor. Nat. Struct. Mol. Biol. 17: 801–807 [DOI] [PubMed] [Google Scholar]
- 83.Perez E., Bourguet W., Gronemeyer H., de Lera A. R. 2012. Modulation of RXR function through ligand design. Biochim. Biophys. Acta. 1821: 57–69 [DOI] [PubMed] [Google Scholar]
- 84.Winterstein A. 1960. Neuere ergebnisse der carotenoid-forschung. Angew. Chem. 72: 902–910 [Google Scholar]
- 85.Ben-Aziz A., Britton G., Goodwin T. W. 1973. Carotene epoxides of Lycopersicon esculentum. Phytochemistry. 12: 2759–2764 [Google Scholar]
- 86.Kopec R. E., et al. 2010. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J. Agric. Food Chem. 58: 3290–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gajic M., Zaripheh S., Sun F., Erdman J. W. 2006. Apo-8’-lycopenal and apo- 12’-lycopenal are metabolic products of lycopene in rat liver. J. Nutr. 136: 1552–1557 [DOI] [PubMed] [Google Scholar]
- 88.Lindshield B. L., Canene-Adams K., Erdman J. W. 2007. Lycopenoids: are lycopene metabolites bioactive? Arch. Biochem. Biophys. 458: 136–140 [DOI] [PubMed] [Google Scholar]
- 89.Erdman J. W., Ford N. A., Lindshield B. L. 2009. Are the health attributes of lycopene related to its antioxidant function? Arch. Biochem. Biophys. 483: 229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trosko J. E., Chang C. C., Upham B., Wilson M. 1998. Epigenetic toxicology as toxicant-induced changes in intracellular signalling leading to altered gap junctional intercellular communication. Toxicol. Lett. 102–103: 71–78 [DOI] [PubMed] [Google Scholar]
- 91.Stahl W., Von Laar J., Martin H. D., Emmerich T., Sies H. 2000. Stimulation of gap junctional communication: comparison of acyclo-retinoic acid and lycopene. Arch. Biochem. Biophys. 373: 271–274 [DOI] [PubMed] [Google Scholar]
- 92.Kotake-Nara E., Kim S. J., Kobori M., Miyashita K., Nagao A. 2002. Acyclo-retinoic acid induces apoptosis in human prostate cancer cells. Anticancer Res. 22: 689–695 [PubMed] [Google Scholar]
- 93.Ben-Dor A., et al. 2001. Effects of acyclo-retinoic acid and lycopene on activation of the retinoic acid receptor and proliferation of mammary cancer cells. Arch. Biochem. Biophys. 391: 295–302 [DOI] [PubMed] [Google Scholar]
- 94.Lian F., Smith D. E., Ernst H., Russell R. M., Wang X. D. 2007. Apo-10’-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 28: 1567–1574 [DOI] [PubMed] [Google Scholar]
- 95.Gouranton E., et al. 2011. Apo-10’-lycopenoic acid impacts adipose tissue biology via the retinoic acid receptors. Biochim. Biophys. Acta. 1811: 1105–1114 [DOI] [PubMed] [Google Scholar]
- 96.Lomb D. J., Laurent G., Haigis M. C. 2010. Sirtuins regulate key aspects of lipid metabolism. Biochim. Biophys. Acta. 1804: 1652–1657 [DOI] [PubMed] [Google Scholar]
- 97.Jin Q., Zhang F., Yan T., Liu Z., Wang C., Ge X., Zhai Q. 2010. C/EBPalpha regulates SIRT1 expression during adipogenesis. Cell Res. 20: 470–479 [DOI] [PubMed] [Google Scholar]
- 98.Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Machado De Oliveira R., Leid M., McBurney M. W., Guarente L. 2004. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 429: 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chung J., Koo K., Lian F., Hu K. Q., Ernst H., Wang X. D. 2012. Apo-10’-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J. Nutr. 142: 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma R. V., Mathur S. N., Ganguly J. 1976. Studies on the relative biopotencies and intestinal absorption of different apo-beta-carotenoids in rats and chickens. Biochem. J. 158: 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma R. V., Mathur S. N., Dmitrovskii A. A., Das R. C., Ganguly J. 1976. Studies on the metabolism of beta-carotene and apo-beta-carotenoids in rats and chickens. Biochim. Biophys. Acta. 486: 183–194 [DOI] [PubMed] [Google Scholar]
- 102.Wang X. D., Tang G. W., Fox J. G., Krinsky N. I., Russell R. M. 1991. Enzymatic conversion of beta-carotene into beta-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues. Arch. Biochem. Biophys. 285: 8–16 [DOI] [PubMed] [Google Scholar]
- 103.Barua A. B., Olson J. A. 2000. β-Carotene is converted primarily to retinoids in rats in vivo. J. Nutr. 130: 1996–2001 [DOI] [PubMed] [Google Scholar]
- 104.Handelman G. J., Van Kuijk F. J., Chatterjee A., Krinsky N. I. 1991. Characterization of products formed during the autoxidation of beta-carotene. Free Radic. Biol. Med. 10: 427–437 [DOI] [PubMed] [Google Scholar]
- 105.Ouyang J. M., Daun H., Chang S. S., Ho C.-T. 1980. Formation of carbonyl compounds from P-carotene during palm oil deodorization. J. Food Sci. 45: 1214–1217 [Google Scholar]
- 106.Marty C., Berset C. 1990. Factors affecting the thermal degradation of all-trans-β-carotene. J. Agric. Food Chem. 38: 1063–1067 [Google Scholar]
- 107.Rodriguez E. B., Rodriguez-Amaya D. B. 2007. Formation of apocarotenals and epoxycarotenoids from β-carotene by chemical reactions and by autoxidation in model systems and processed foods. Food Chem. 101: 563–572 [Google Scholar]
- 108.Fleshman M. K., Lester G. E., Riedl K. M., Kopec R. E., Narayanasamy S., Curley R. W., Jr, Schwartz S. J., Harrison E. H. 2011. Carotene and novel apocarotenoid concentrations in orange-fleshed cucumis melo melons: determinations of β-carotene bioaccessibility and bioavailability. J. Agric. Food Chem. 59: 4448–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eroglu A., Hruszkewycz D. P., dela Sena C., Narayanasamy S., Riedl K. M., Kopec R. E., Schwartz S. J., Curley R. W., Jr, Harrison E. H. 2012. Naturally occurring eccentric cleavage products of provitamin A carotene β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287: 15886–15895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ho C. C., de Moura F. F., Kim S. H., Clifford A. J. 2007. Excentral cleavage of beta-carotene in vivo in a healthy man. Am. J. Clin. Nutr. 85: 770–777 [DOI] [PubMed] [Google Scholar]
- 111.Shmarakov I., Fleshman M. K., D'Ambrosio D. N., Piantedosi R., Riedl K. M., Schwartz S. J., Curley R. W., Jr, von Lintig J., Rubin L. P., Harrison E. H., Blaner W. S. 2010. Hepatic stellate cells are an important cellular site for β-carotene conversion to retinoid. Arch. Biochem. Biophys. 504: 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malone W. F. 1991. Studies evaluating antioxidants and β-carotene as chemopreventives. Am. J. Clin. Nutr. 53(1 Suppl.): 305S–313S [DOI] [PubMed] [Google Scholar]
- 113.Krishnan K., Campbell S., Abdel-Rahman F., Whaley S., Stone W. L. 2003. Cancer chemoprevention drug targets. Curr. Drug Targets. 4: 45–54 [DOI] [PubMed] [Google Scholar]
- 114.Mayne S. T. 1996. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J. 10: 690–701 [PubMed] [Google Scholar]
- 115.Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group 1994. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 330: 1029–1035 [DOI] [PubMed] [Google Scholar]
- 116.Omenn G. S., Goodman G. E., Thornquist M. D., Balmes J., Cullen M. R., Glass A., Keogh J. P., Meyskens F. L., Valanis B., Williams J. H., et al. 1996. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 334: 1150–1155 [DOI] [PubMed] [Google Scholar]
- 117.Prakash P., Liu C., Hu K. Q., Krinsky N. I., Russell R. M., Wang X. D. 2004. Beta-carotene and beta-apo-14’-carotenoic acid prevent the reduction of retinoic acid receptor beta in benzo[a]pyrene-treated normal human bronchial epithelial cells. J. Nutr. 134: 667–673 [DOI] [PubMed] [Google Scholar]
- 118.Benkoussa M., Brand C., Delmotte M. H., Formstecher P., Lefebvre P. 2002. Retinoic acid receptors inhibit AP1 activation by regulating extracellular signal- regulated kinase and CBP recruitment to an AP1-responsive promoter. Mol. Cell. Biol. 22: 4522–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tibaduiza E. C., Fleet J. C., Russell R. M., Krinsky N. I. 2002. Excentric cleavage products of β-carotene inhibit estrogen receptor positive and negative breast tumor cell growth in vitro and inhibit activator protein-1-mediated transcriptional activation. J. Nutr. 132: 1368–1375 [DOI] [PubMed] [Google Scholar]
- 120.Zile M. H., Cullum M. E., Simpson R. U., Barua A. B., Swartz D. A. 1987. Induction of differentiation of human promyelocytic leukemia cell line HL-60 by retinoyl glucuronide, a biologically active metabolite of vitamin A. Proc. Natl. Acad. Sci. USA. 84: 2208–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collins S. J., Gallo R. C., Gallagher R. E. 1977. Continuous growth and differentiation of human myeloid leukemic cells in suspension culture. Nature. 270: 347–349 [DOI] [PubMed] [Google Scholar]
- 122.Breitman T. R., Selonick S. E., Collins S. J. 1980. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc. Natl. Acad. Sci. USA. 77: 2936–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Breitman T. R., Collins S. J., Keene B. R. 1981. Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood. 57: 1000–1004 [PubMed] [Google Scholar]
- 124.Gross M. D., Bishop T. D., Belcher J. D., Jacobs, Jr D. R. 1997. Induction of HL-60 differentiation by carotenoids. Nutr. Cancer. 27: 169–173 [DOI] [PubMed] [Google Scholar]
- 125.McDevitt T. M., Tchao R., Harrison E. H., Morel D. W. 2005. Carotenoids normally present in serum inhibit proliferation and induce differentiation of a human monocyte/macrophage cell line (U937). J. Nutr. 135: 160–164 [DOI] [PubMed] [Google Scholar]
- 126.Winum J. Y., Kamal M., Defacque H., Commes T., Chavis C., Lucas M., Marti J., Montero J. L. 1997. Synthesis and biological activities of higher homologues of retinoic acid. Farmaco. 52: 39–42 [PubMed] [Google Scholar]
- 127.Suzuki T., Matsui M., Murayama A. 1995. Biological activity of (all-E)-beta-apo-12’-carotenoic acid and the geometrical isomers on human acute promyelocytic leukemia cell line HL-60. J. Nutr. Sci. Vitaminol. (Tokyo). 41: 575–585 [DOI] [PubMed] [Google Scholar]
- 128.Rühl R. 2005. Induction of PXR-mediated metabolism by β-carotene. Biochim. Biophys. Acta. 1740: 162–169 [DOI] [PubMed] [Google Scholar]
- 129.Kliewer S. A. 2003. The nuclear pregnane X receptor regulates xenobiotic detoxification. J. Nutr. 133: 2444S–2447S [DOI] [PubMed] [Google Scholar]
- 130.Rühl R., Sczech R., Landes N., Pfluger P., Kluth D., Schweigert F. J. 2004. Carotenoids and their metabolites are naturally occurring activators of gene expression via the pregnane X receptor. Eur. J. Nutr. 43: 336–343 [DOI] [PubMed] [Google Scholar]
- 131.Ziouzenkova O., Orasanu G., Sukhova G., Lau E., Berger J. P., Tang G., Krinsky N. I., Dolnikowski G. G., Plutzky J. 2007. Asymmetric cleavage of β-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator- activated receptor responses. Mol. Endocrinol. 21: 77–88 [DOI] [PubMed] [Google Scholar]
- 132.Kawada T., Kamei Y., Fujita A., Hida Y., Takahashi N., Sugimoto E., Fushiki T. 2000. Carotenoids and retinoids as suppressors on adipocyte differentiation via nuclear receptors. Biofactors. 13: 103–109 [DOI] [PubMed] [Google Scholar]
- 133.Shulman A. I., Mangelsdorf D. J. 2005. Retinoid X receptor heterodimers in the metabolic syndrome. N. Engl. J. Med. 353: 604–615 [DOI] [PubMed] [Google Scholar]
- 134.Eroglu A., Hruszkewycz D. P., Curley R. W., Harrison E. H. 2010. The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch. Biochem. Biophys. 504: 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]