Abstract
Purpose of review
To provide the reader with a review of contemporary literature describing the evolving understanding of the molecular pathobiology of pseudohypoparathyroidism (PHP).
Recent findings
The features of PHP type 1 reflect imprinting of the GNAS gene, which encodes the α subunit of the heterotrimeric G protein (Gαs) that couples heptahelical receptors to activation of adenylyl cyclase. Transcription of Gαs is biallelic in most cells, but is primarily from the maternal allele in some tissues (e.g. proximal renal tubules, thyroid, pituitary somatotropes, gonads). Patients with PHP 1a have heterozygous mutations within the exons of the maternal GNAS allele that encode Gαs, whereas patients with PHP 1b have methylation defects in the GNAS locus that reduce transcription of Gαs from the maternal allele. In both PHP 1a and PHP 1b, paternal imprinting of Gαs leads to resistance to parathyroid hormone and TSH. Although brachydactyly is characteristic of PHP 1a, it is sometimes present in patients with PHP 1b.
Summary
Molecular studies enable a distinction between PHP 1a and PHP 1b, with different mechanisms accounting for Gαs deficiency. Clinical overlap between these two forms of PHP type 1 is likely due to the variable levels of Gαs activity expressed in specific cell types.
Keywords: GNAS, hormone resistance, imprinting
INTRODUCTION
The term functional hypoparathyroidism refers to a group of metabolic disorders in which hypocalcemia and hyperphosphatemia occur either from a failure of the parathyroid glands to secrete adequate amounts of biologically active parathyroid hormone (PTH) or, less commonly, from an inability of PTH to elicit appropriate biological responses in its target tissues. Plasma concentrations of PTH are low or absent in patients with true hypoparathyroidism. By contrast, plasma concentrations of PTH are elevated in patients with pseudohypoparathyroidism (PHP), and reflect the failure of target tissues to respond appropriately to the biological actions of PTH. In both conditions, a correct diagnosis relies upon the demonstration that serum levels of magnesium are normal, as modest deficiency of magnesium inhibits PTH action whereas more profound deficiency of magnesium impairs PTH secretion.
NOSOLOGY
The term PHP encompasses a heterogeneous group of rare metabolic disorders that share in common end-organ resistance to the action of PTH. Because the PTH receptor requires the heterotrimeric G protein (Gαs) as an intermediary coupling protein to stimulate adenylyl cyclase, measurement of serum and urinary cyclic AMP (cAMP) levels after the injection of PTH permits the differentiation among several variants of PHP. In PHP type 1a blunted cAMP response is observed, whereas in PHP type 2 the cAMP response to PTH is conserved, but no phosphaturic response occurs, indicating that the defect is distal to cAMP generation in the PTH-mediated signal transduction pathway. The blunted nephrogenous cAMP response to PTH in individuals with PHP type 1 is caused by a deficiency of the α subunit of Gs (Gαs), whereas the absent phosphaturic response in patients with PHP type 2 may be a consequence of severe hypocalcemia due to profound vitamin D deficiency, particularly in patients who are taking anticonvulsant medications that induce P450-dependent degradation of vitamin D metabolites [1].
Molecular and biochemical studies have provided a basis for distinguishing between different forms of PHP type 1 (Table 1), although considerable overlap occurs: patients with heterozygous mutations within exons 1–13 of the GNAS gene have a more generalized deficiency of Gαs protein and are classified as PHP type 1a (OMIM 103580), whereas patients with more restricted deficiency of Gαs due to methylation defects in the imprinted GNAS cluster are classified as PHP type 1b (OMIM 603233). PHP 1a and PHP 1b also differ in the pattern of hormone resistance and in the expression of additional somatic features.
Table 1.
Classification of pseudohypoparathyroidism and related disorders
| Molecular defect | Parental origin of GNAS allele | Endocrine defects | Clinical features | Other features | |
|---|---|---|---|---|---|
| PHP 1a | Heterozygous mutations in GNAS gene that reduce expression or function of Gαs | Maternal | Multihormone resistancea | 1. AHOb | Cognitive disability |
| 2. Early onset obesity | |||||
| PPHP | Heterozygous mutations in GNAS gene | Paternal | None | 1. AHO | |
| PHP 1c | Heterozygous mutations in GNAS that impair coupling of heptahelical receptors to adenylyl cyclase | Maternal | Multihormone resistance | 1. AHO | Cognitive disability |
| 2. Early onset obesity | |||||
| Familial PHP type 1b | Heterozygous deletions in STX16, NESP55 and/or AS exons | Maternal | PTH resistance, partial resistance to TSH in some | Mild brachydactyly in some | Loss of methylation in exon A/B |
| Sporadic PHP type 1b | Paternal UPD in some | Maternal | PTH resistance, partial resistance to TSH in some | Mild brachydactyly in some | Global defect in methylation at all three DMRs |
| Progressive osseous heteroplasia | Heterozygous mutations in GNAS gene that reduce expression or function of Gαs | Paternal | None | Progressive heterotopic ossification extending to deep connective tissues | |
| Osteoma cutis | Heterozygous mutations in GNAS gene that reduce expression or function of Gαs | Paternal | None | Heterotopic ossification that is limited to dermis and subcutaneous tissues | |
| PHP type 2 | None | N/A | PTH resistance | Severe hypocalcemia | Vitamin D deficiency |
AHO, Albright hereditary osteodystrophy; DMR, differentially methylated region; PHP, pseudohypoparathyroidism; PTH, parathyroid hormone; TSH, thyrotropin; UPD, uniparental disomy.
Multiple hormone resistance, resistance to PTH, TSH and GHRH, often to gonadotropins as well.
AHO, Albright hereditary osteodystrophy; comprising round face, short stature, brachydactyly/brachymetacarpia, heterotopic ossification.
PHP 1c [2■] appears to be a variant of PHP 1a in which the specific GNAS mutation disrupts receptor-mediated activation of adenylyl cyclase but does not affect receptor-independent activation of the enzyme. Hence, these unusual mutations account for the inability of most conventional in-vitro assays to demonstrate reduced activity of solubilized Gαs (see below).
MOLECULAR STRUCTURE AND EXPRESSION OF GNAS
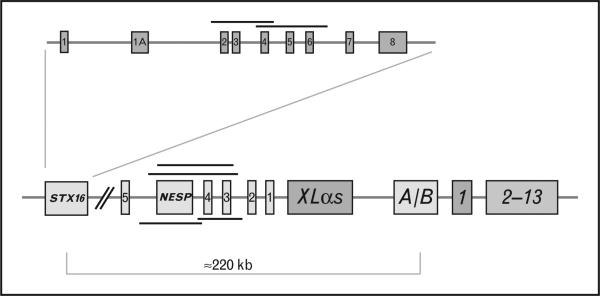
GNAS is a complex transcriptional unit that derives considerable plasticity through use of alternative first exons, alternative splicing of downstream exons, antisense transcripts, and reciprocal imprinting (Fig. 1). Gαs is encoded by exons 1–13, and is synthesized as a 52 or 45 kDa protein based on the inclusion or exclusion of exon 3, respectively. Upstream of exon 1 are three alternative first exons that each splice onto exons 2–13 to create novel transcripts. These include extra large (XL), which is expressed only from the paternal allele and which generates a transcript with overlapping open reading frames that encodes XLαs and ALEX. The two proteins are interacting cofactors, and are specifically expressed in neuroendocrine cells. XLαs is a much larger signaling protein than Gαs (≈78 kDa versus 45–52 kDa) and can interact with receptors for PTH and a variety of other hormones in vitro, but the native receptors that interact with XLαs in vivo are presently unknown. XLαs can mimic or enhance Gαs action in vivo when expressed ectopically in the proximal tubules of transgenic mice [3]. A second alternative promoter encodes the secretory protein Nesp55, which is expressed only from the maternal allele and shares no protein homology with Gαs. An antisense transcript (nespas in mice) comprised of five exons flanks NESP55, and is expressed only from the paternal GNAS allele. The antisense transcript is not translated and has no known function. A second nontranslated transcript utilizes the alternative first exon A/B (associated first exon, also termed exon 1A in mice), which is expressed only from the paternal allele. These alternative first exons and antisense are associated with promoters that contain differentially methylated regions (DMRs), each of which is methylated on the nonexpressed allele. By contrast, Gαs is expressed from both alleles in most cells, but in some cells (e.g. pituitary somatotropes, proximal renal tubular cells, thyroid epithelial cells, gonadal cells) Gαs is primarily expressed from the maternal allele. Recent work by Klenke et al. [4■] now suggests that there may be modest preferential expression of the maternal allele in many other tissues, including the heart. There is no DMR that regulates expression of the Gαs transcript, but cis-acting elements that control tissue-specific paternal imprinting of Gαs appear to be located within the primary imprint region in exon A/B.
FIGURE 1.
GNAS gene. General organization of the GNAS gene complex and the STX16 gene. The GNAS gene complex consists of 13 exons that encode the signaling protein Gαs. Upstream of exon 1 are three alternative first exons that are labeled exon A/B, XLαs, and Nesp55; exons 1–5 for the NESP antisense transcript are also depicted. The three alternative exons are spliced to exons 2–13 to produce unique transcripts (see text). Nesp55 is transcribed exclusively from the maternal allele; XLαs, AS, and exon A/B are transcribed exclusively from the paternal allele. Nesp AS (antisense) and exon A/B transcripts produce noncoding RNAs. Gαs transcripts are biallelically expressed except in a small number of tissues, such as the renal proximal tubules, thyroid, gonads and pituitary somatotrophs, in which expression is preferentially from the maternal allele. The nine coding exons of the STX16 gene are shown above the GNAS locus, and the locations of deletions in STX16, NESP55 and antisense are shown as black lines (see text).
PSEUDOHYPOPARATHYROIDISM TYPE 1A AND PSEUDOHYPOPARATHYROIDISM TYPE 1C
PHP 1a (MIM 103580) is the most readily recognized form of PHP, and is distinguished from PHP 1c (MIM 612462) only by ex-vivo assays of Gαs function (see below). These two forms of PHP type 1 result from heterozygous inactivating mutations on the maternal allele of the GNAS gene (MIM# 610540, 20q13.2-q13.3) that involve exons encoding Gαs. As Gαs is required for normal transmembrane signal transduction by many hormones and neurotransmitters, individuals with PHP 1a and 1c also have resistance to other hormones (e.g. thyroid stimulating hormone, gonadotropins, calcitonin, and growth hormone releasing hormone) whose target tissues show predominant or exclusive expression of Gαs from the maternal GNAS allele. Primary hypothyroidism, without goiter, and growth hormone deficiency are commonly associated endocrinopathies. By contrast, responsiveness to other hormones (e.g. ACTH, vasopressin) is normal in tissues in which GNAS is not imprinted and both parental alleles are expressed.
Patients with PHP 1a (and likely PHP 1c) but not PPHP manifest mild to moderate intellectual disability [5] and early-onset morbid obesity [6,7], which likely reflects the effects of Gαs deficiency in imprinted regions of the brain. Al Salameh et al. [6] showed successful treatment of obesity in a single patient with PHP type 1c using a cannabinoid receptor type 1 (CB1) antagonist, lowering the patient's BMI from 40.5 to 33.5. These results are consistent with the hypothesis that Gαs deficiency in imprinted regions of the hypothalamus impairs melanocortin-4 receptor signaling and leads to upregulation of the CB1 receptor.
Growth hormone replacement may also be beneficial in these children. Mantovani et al. [8] studied the effect of recombinant human growth hormone (rhGH) therapy on height velocity in eight prepubertal children with PHP 1a with proven growth hormone (GH) deficiency. These patients (seven girls and one boy, aged 5.8–12 years) underwent 3–8 years of treatment with rhGH, and showed robust increases in height SD scores from –2.4 ± 0.58 to –1.8 ± 0.47 (P = 0.04) after 12 months. After 3 years of treatment both the height velocity and height velocity SD scores showed sustained significant increases from 3.5 ± 0.6 to 7.0 ± 0.9 cm per year (P < 0.0001) and from –2.8 ± 0.8 to 2.2 ± 1.0 (P < 0.0001), respectively. However, six patients treated for 4–8 years showed a blunted pubertal spurt and did not improve their near-adult height. Although these data suggest that GH replacement can increase growth velocity, earlier treatment may be necessary to overcome the effect of Gαs deficiency to advance maturation of the growth plate and induce premature closure of the epiphyses.
Due to haploinsufficiency of Gαs in non-imprinted tissues, patients with PHP 1a/1c develop additional somatic features; including short stature, round facies, brachydactyly/brachymetacarpia of hands (particularly shortening of III, IV, and V metacarpals and I distal phalanx) and/or feet, and heterotopic ossifications (a clinical constellation termed Albright hereditary osteodystrophy (AHO). A previously unrecognized complication of brachydactyly in AHO appears to be carpal tunnel syndrome [9], which can cause symptomatic paresthesias that may be confused with similar symptoms caused by hypocalcemia. Individuals with paternally inherited GNAS mutations have variable features of AHO without hormonal resistance, and have been described to have pseudopseudohypoparathyroidism (PPHP), progressive osseous heteroplasia (POH) [10], or osteoma cutis [11,12■■,13] based on the clinical phenotype (Table 1).
PSEUDOHYPOPARATHYROIDISM TYPE 1b
Individuals with PHP type 1b (MIM 603233) lack typical features of AHO but may have mild brachydactyly. PTH resistance is the principal manifestation of hormone resistance, but some patients have slightly elevated serum levels of TSH and normal serum concentrations of thyroid hormones as evidence of partial TSH resistance. A growing number of studies now confirm that loss of methylation in the exon A/B DMR of the maternal allele is a consistent finding in patients with PHP 1b, and that this epigenetic defect accounts for decreased Gαs expression from the affected allele [14■■,15■,16■] (Fig. 1). Most cases of autosomal dominant PHP 1b are caused by microdeletions on the maternal allele that include exons 3–5 or 4–6 of the gene encoding syntaxin-16 (STX16) (reviewed in [14■■]). The frequent recurrence of the STX16 deletion of exons 3–5 is likely due to homologous recombination between repeated sequences of 391 bp in introns 2 and 6 that flank the deleted region. Three other maternally inherited microdeletions involving NESP55 and antisense (delNESP55/ASdel3–4) or antisense (delAS3–4) alone [17] have been identified, and these deletions disrupt methylation of the three GNAS DMRs (A/B, XL/antisense and NESP55) (Fig. 1). More recently, Richard et al. [18] identified a new deletion of 18 988 bp covering NESP55 in members of a PHP 1b family. This deletion overlaps with the previously reported delNESP55/delAS3–4 and delAS3–4 by only 342 bp (Fig. 1), and does not disrupt methylation of XL/AS. The gain of methylation in the NESP55 DMR is due to the physical deletion of the unmethylatated maternal DMR in this region, whereas the loss of methylation at exon A/B is a long distance consequence of the deletion. Hence, this deletion may disrupt a cis-acting element on the maternal allele that is required for establishing or maintaining maternal A/B methylation without affecting the XL/AS DMR. Alternatively, loss of this region may reveal an unsuspected function of either the maternally expressed NESP55 transcript or of AS noncoding macro-RNA (ncRNA). This pattern of methylation is similar to that observed in mice with targeted loss of NESP55 [19], but contrasts with the effects of targeted deletion of the NESP55-Nespas 2–4 region in mice [20], which is associated with loss of methylation of both the Nespas/Gnaxl and exon 1A DMRs. These results therefore suggest that the methylation anomalies of XL/AS DMR may be due to loss of antisense rather than NESP55.
The genetic basis for most cases of sporadic PHP 1b remains unknown, and does not appear to be associated with the GNAS locus [21]. These patients have global epigenetic defects in methylation that affect all three DMRs. In some cases, partial [22] or complete [23] paternal uniparental disomy (UPD) for chromosome 20 has been identified, in which two normal copies of GNAS are both derived from the father. Paternal UPD would predict a near complete deficiency of Gαs in imprinted cells and tissues in which Gαs is not transcribed from the paternal allele.
EFFECTS OF Gαs DEFICIENCY ON BONEHOMEOSTASIS
Haploinsufficiency of GNAS is associated with brachydactyly and heterotopic ossification, conditions that reflect the effects of impaired Gαs signaling on osteoblasts or osteoblast precursors. The ectopic calcified tissue is true intramembraneous bone rather than metastatic calcification, and its development and growth are unrelated to prevailing concentrations of extracellular calcium and/or phosphorus. In most patients with GNAS mutations, the formation of heterotopic bone is limited to the dermis and subcutaneous tissues, and is clinically mild; these patients have oteoma cutis, AHO/PPHP, and PHP 1a/c. In one infant with a GNAS mutation inherited from a father with PHP type 1a, linear atrophic skin lesions were noted to precede the development of cutaneous ossifications [11]. In patients with POH, ectopic bone formation progresses to invade deeper connective tissues, often with debilitating effects. Although both patients with osteoma cutis and POH have paternally inherited GNAS defects, the basis for the striking difference in heterotopic ossification remains uncertain.
Heterotopic bone likely reflects misdifferentiation of mesenchymal stem cells. Past studies had suggested that Gαs deficiency can induce ectopic expression of Cbfa1/Runx2, early markers of osteoblast formation, in mesenchymal stem cells [24]. To address the role of Gαs deficiency, two research groups, Pignolo et al. [25] and Huso et al. [26■■] recently used the same heterozygous GNAS exon 1-knockout mouse line, in which only Gαs is deleted, to investigate the development of ectopic ossification. This knockout mouse line forms heterotopic bone that appears to be initiated in subcutaneous adipose tissue. The ossified lesions increase in number and size after birth and are uniformly detected in adult mice by 1 year of age. They are located in both the dermis, often in perifollicular areas, and the subcutis. These lesions are particularly prominent in skin prone to injury or pressure. The ossified lesions comprise mature bone with evidence of mineral deposition and bone marrow elements, and lesions are positive for both osteonectin and osteopontin [26■■]. Pignolo et al. [25] have shown that haploinsufficiency for Gαs abrogates upregulation of multiple GNAS transcripts that normally occurs with osteoblast differentiation in wild-type adipose stromal cells. These transcriptional changes in GNAS+/– mice are accompanied by accelerated osteoblast differentiation of adipose stromal cells in vitro. These workers have suggested that the clinical effects of GNAS-inactivating mutations in patients is not solely dependent on the level of Gαs expression but may be influenced by the expression levels of other GNAS transcripts. As XLαs, like Gαs, activates cAMP signaling, it is possible that paternally inherited mutations result in a larger deficit of cAMP signaling (owing to a reduction of Gαs and the absence of XLαs) compared with maternally inherited mutations (which do not affect expression of XLαs). Hence, the more progressive ossifications that occur in POH might reflect the combined loss of XLαs and Gαs signaling.
Haploinsufficiency of Gαs is also the likely basis for brachydactyly, which appears to be due in part to premature fusion of epiphyses in tubular and long bones, thus implying a requirement of two functional copies of GNAS for normal differentiation and maturation of the growth plate. Brachydactyly also occurs in patients with acrodysostosis (OMIM 101800), a rare skeletal dysplasia in which severe brachydactyly is associated with facial dysostosis, nasal hypoplasia, short stature and often endocrine dysfunction. The recent finding that patients with acrodysostosis harbor germline mutations in the genes encoding PRKAR1A, the cAMP-dependent regulatory subunit of protein kinase A [27,28], and PDE4D [28], which encodes a class IV cAMP-specific phosphodiesterase that regulates cAMP concentration, provides further evidence that loss of cAMP signaling is the basis for the skeletal features of AHO. Patients with acrodysostosis and AHO both have markedly advanced skeletal maturation, which leads to premature fusion of the growth plates and accounts for the development of brachydactyly. Wu et al. [29] investigated the role of Gαs signaling in cells of the early osteoblast lineage in vivo by developing transgenic mice in which Gαs was conditionally deleted from cells expressing osterix, a marker of early osteoblast commitment. Although osteoblast number was decreased, osteogenic differentiation was markedly accelerated in the absence of Gαs, leading to a loss of the normal restraint of osteoblastic differentiation that is necessary to enable osteoblasts to produce bone of optimal mass, quality and strength.
Early studies had documented typical hyperparathyroid bone disease, including osteitisfibrosacystica, in some patients with PHP type 1, particularly when patients had not been treated with sufficient amounts of active vitamin D analogs to prevent chronically elevated secretion of PTH. Neary et al. [30] investigated five patients with PHP 1b who presented with hypercalcemia and elevated PTH, consistent with the diagnosis of tertiary hyperparathyroidism. Bone mineral density (BMD) was normal in most patients, and calvarial hyperostosis was common. Parathyroid surgery was performed in four of the five patients, each of whom had one or two parathyroid adenomas, with resolution of hypercalcemia. This study confirms previous in vitro and clinical reports indicating that bone tissue, in contrast to the proximal renal tubule, is responsive to PTH in patients with PHP type 1. Moreover, other recent clinical studies suggest that PTH may exert an anabolic effect on the skeleton. Long et al. [31■] investigated BMD in 22 children and adults with PHP 1a using dual-energy X-ray absorptiometry (DXA). BMD in total body and LS spine corrected for height-for-age Z-score was significantly greater in affected patients than controls, but there was no correlation between PTH level and BMD Z-score or between BMI and BMD Z-score. Similarly, Sbrocchi et al. [32] evaluated bone density by DXA and bone histomorphometry in two brothers with PHP 1b due to the common 3-kb deletion within STX16 with osteosclerosis of the lumbar spine. BMD was elevated in the lumbar spine (Z-scores + 5.4 and +4.9), and trans-illiac crest bone biopsy showed marked elevations in cortical width, with elevations in bone formation rate on the endocortical and trabecular surfaces, in both brothers. Together, these studies indicate that elevated levels of PTH can have a predominant resorptive or anabolic effect in PHP 1a and 1b, and suggest the possible involvement of non-Gαs signaling pathways.
DIAGNOSTIC ADVANCES
The diagnosis of PHP type 1 is based on the presence of PTH resistance. Although this diagnostic criterion will be readily fulfilled in most patients with hypocalcemia and hyperphosphatemia who have elevated serum levels of PTH in the setting of normal vitamin D status, demonstration of an absent nephrogenous cAMP response after administration of exogenous PTH may be desirable or necessary in some cases. Now Todorova-Koteva et al. [33] provide a simple diagnostic test based on the subcutaneous injection of commercially available recombinant teriparatide (PTH 1–34) that can be used to demonstrate PTH resistance. Although the authors have evaluated this protocol in only a few individuals with PHP type 1, their results extend earlier studies [34] that showed that subcutaneous administration of teriparatide to estrogenized women with post-menopausal osteoporosis generated urinary cAMP and phosphaturic responses that were similar to those achieved after intravenous administration of PTH.
Several biochemical tests have been developed to assess Gαs in vitro, but none are offered by commercial laboratories. These include semi-quantitative immunoblot analysis as well as functional assays of Gαs. The conventional determination of Gαs function is based on an ex-vivo complementation assay in which adenylyl cyclase activity of S49 cyc– murine lymphoma cells, which genetically lack Gαs, is reconstituted with Gαs protein present in detergent-solubilized extracts of human erythrocyte membranes. This method relies on the addition of agents (e.g. GTPγS, aluminum fluoride) that can directly activate Gαs; Gαs-dependent stimulation of adenylyl cyclase via heptahelical receptor activation is not measured by this method. Hence, patients with PHP 1c, who have Gαs defects that impair interaction with receptors, may not be identified using this assay. The initial application of this assay to patients with PHP type 1 demonstrated that patients with AHO and multihormone resistance had an approximately 50% reduction in Gαs activity (i.e. PHP 1a) whereas patients without clinical features of AHO and hormone resistance that was limited to PTH (i.e. PHP 1b) had normal levels of Gαs activity. Subsequently, patients with PPHP and POH, who carry GNAS mutations on their paternal alleles, were also found to have a similar 50% reduction in Gαs activity. These results reflect the effect of GNAS haploinsufficiency in erythrocytes, which normally express both copies of GNAS, leading to a 50% reduction in Gαs activity regardless of the parental origin of the GNAS defect. Normal Gαs activity in PHP 1b represented the absence of paternal imprinting of Gαs in erythrocytes, so both alleles are expressed regardless of an epigenetic methylation defect. The recent description of PHP 1b patients with GNAS methylation defects who have mild features of AHO, most notably brachydactyly [35] or a Madelung-like defect [36], and partial resistance to TSH, has introduced the notion that PHP 1a and 1b may share overlapping phenotypes. Insight into this unexpected finding came from Zazo et al. [37], who used immunoblot analysis and the Gαs complementation assay to examine expression and function of Gαs in erythrocyte membranes from patients with PHP 1b. Zazo et al. [37] found that Gαs was partially reduced in patients who had mild AHO but normal in those without these features, but that there was no correlation between Gαs expression and the extent of the methylation defect.
Freson et al. [38,39] have investigated Gαs function in intact platelets from PHP type 1 patients using an assay that is based on inhibition of platelet aggregation in response to agonist-induced generation of cAMP. Patients with PHP 1a and PPHP with GNAS mutations have reduced inhibition of platelet aggregation [39]. These investigators found that platelet function was normal in most individuals with PHP 1b, but was mildly depressed in those patients with features of AHO [40]. Taken together, these studies of Gαs function in patients with PHP 1b suggest that features of AHO are manifestations of Gαs deficiency in relevant cells and tissues, and argue against the association of AHO with a specific GNAS genotype. Hence, a threshold level of Gαs activity is necessary to assure a normal phenotype. This interpretation implies that Gαs expression is subject to allelic bias in a larger number of tissues than is currently recognized and that the degree of this allelic bias varies among individuals.
Molecular genetic analysis of the GNAS gene provides an important advance to the diagnosis of PHP and can distinguish between various subtypes. Conventional sequencing will identify genetic mutations within exons 1–13 in most patients with PHP 1a and 1c, as well as patients with PPHP, osteoma cutis [12■■,13] and POH [10,41,42], but cannot distinguish between these related disorders or the parental origin of the defective GNAS allele (Table 1).
GNAS epigenetic defects can be identified in patients with PHP 1b by methylation analysis of DMRs in the GNAS locus after bisulfate treatment of genomic DNA. Familial and sporadic forms of PHP 1b have different patterns of methylation of the three DMRs within the GNAS locus, but loss of methylation at the maternal exon A/B DMR is a consistent finding in all patients. GNAS imprinting studies have utilized a variety of non-quantitative or semiquantitive techniques to detect the methylation of single or multiple cytosine phospho guanine (CpG) sites, including Southern blot analysis using methylation-sensitive restriction digestion and bisulfite treatment of DNA followed either by sequencing or by methylation-sensitive restriction analysis. Other more recent methods have utilized PCR-based technologies to provide highly quantitative analyses of methylation at the GNAS DMRs that enable subclassification of forms of inherited and sporadic PHP 1b [43■■]. The most accurate and rapid protocols utilize bisulfite pyrosequencing [44] and SequenomEpiTYPER [45], both of which enable highly accurate and precise quantitative analysis of the methylation status of different CpG sites within all DMRs of GNAS. Multiplex Ligation-dependent Probe Amplification (MS-MLPA) analysis of exon A/B methylation has been developed as a diagnostic test for PHP 1b and is commercially available from the DNA Diagnostics reference laboratory at Johns Hopkins Medical Institutions (http://www.hopkinsmedicine.org/dnadiagnostic/PHP1A_AHO.htm).
It is important to note that methylation defects might be detected in some PHP 1a patients who have large GNAS deletions that extend into the DMRs. In one case, a 850-kb submicroscopic deletion that encompassed the entire GNAS locus was identified in members of a family with PHP 1a [46], whereas in a second case a 30-kb intragenic GNAS deletion was identified that extended from the intronic region between exons XL and A/B to intron 5 [47]. Mantovani et al. [44] performed methylation analyses on 40 patients with sporadic AHO and multihormone resistance who did not have a GNAS mutation. Remarkably, global GNAS imprinting defects were identified in 24 of the 40 patients, none of whom had STX16 deletions. These results further emphasize the growing appreciation of the clinical overlap between PHP 1a and PHP 1b.
The goal of therapy in PHP type 1 is to restore serum calcium as close to normal as possible. The main pharmacologic agents available are activated forms of vitamin D and supplemental calcium. Approximately 1–2 g (25–50 mg/kg) of elemental calcium per day is recommended to supply adequate calcium and to manage dietary phosphorous intake, and optimal results are achieved when calcium supplements are taken with meals. Patients are unable to convert parent compounds of vitamin D to fully active forms as lack of PTH action in the proximal tubule and hyperphosphatemia both inhibit the renal 1-α hydroxylase enzyme that converts 25-hydroxyvitamin D to 1,25-dihydoxyvitamin D. The 1-α hydroxylated vitamin D metabolites (e.g. calcitriol) are the preferred forms of vitamin D as these drugs circumvent the enzymatic block in vitamin D activation. Because calcitriol has a plasma half-life of only hours and body stores do not accumulate, calcitriol must be given several times each day. By contrast, 1-α calcidiol [1α(OH)D3] has a longer half-life and may be given once daily. A variety of parent vitamin D preparations have been used in the past, including vitamin D3 or D2, however, typically very large concentrations are required to raise the serum calcium. In this case, body stores of vitamin D can accumulate in massive amounts, increasing the risk of severe and prolonged vitamin D toxicity. Nevertheless, it seems prudent to ensure that patients have sufficient intake of vitamin D to maintain normal circulating levels of 25(OH)D. Any deficiency of magnesium should be replaced.
Additional endocrine disorders occur principally in patients with PHP 1a/1c, and are addressed in accordance to the usual principles of treatment. Patients should be screened for primary hypothyroidism and growth hormone deficiency, and replaced with appropriate doses of the relevant replacement hormones. Menstrual irregularity is sometimes improved with weight loss, and at present the management of obesity in PHP 1a/1c remains the greatest treatment challenge. The current treatment of obesity in PHP type 1a/1c is limited to the usual recommendations of increasing physical activity and reducing calorie intake. An analysis of resting energy expenditure may provide useful guidance for a recommended daily calorie intake. The clinician should maintain a high index of suspicion for obstructive sleep apnea in very overweight patients.
CONCLUSION
Recent advances in the molecular genetic and biochemical characterization of patients with PHP type 1, as well as characterization of new mouse models, have disclosed novel pathogenic mechanisms that explain the phenotypes of PHP 1a/1c and PHP 1b, and have also led to the unsuspected recognition that these various forms of PHP share considerable overlap with one another. Considerable work remains ahead, however, and at a minimum must address the questions of allelic imbalance in GNAS expression, the genetic modifiers that account for the development of progressive heterotopic bone formation in only some patients, and the genetic basis for the global imprinting defects in patients with sporadic PHP 1b.
KEY POINTS.
PHP type 1 is due to defects in the GNAS gene that impair function or expression of Gαs (PHP 1a) or transcription of Gαs from the maternal allele (PHP 1b) in imprinted tissues.
Clinical and endocrinological overlap occurs between PHP 1a and PHP 1b, and reflects the effect of Gαs deficiency in different tissues.
Heterotopic ossification arises as a result of Gαs haploinsufficiency, with heterozygous mutations on either the maternal or paternal GNAS allele.
Obesity is likely to be a result of Gαs deficiency in imprinted regions of the hypothalamus, and is limited to PHP 1a patients with maternal GNAS mutations.
Acknowledgements
None.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Akin L, Kurtoglu S, Yildiz A, et al. Vitamin D deficiency rickets mimicking pseudohypoparathyroidism. J Clin Res Pediatr Endocrinol. 2010;2:173–175. doi: 10.4274/jcrpe.v2i4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2■.Thiele S, de Sanctis L, Werner R, et al. Functional characterization of GNAS mutations found in patients with pseudohypoparathyroidism type Ic defines a new subgroup of pseudohypoparathyroidism affecting selectively Gsalpha-receptor interaction. Hum Mutat. 2011;32:653–660. doi: 10.1002/humu.21489. [Demonstrates the basis for PHP type 1c, and provides an explanation for previous misclassification.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Segawa H, Aydin C, et al. Transgenic overexpression of the extra-large Gsalpha variant XLalphas enhances Gsalpha-mediated responses in the mouse renal proximal tubule in vivo. Endocrinology. 2011;152:1222–1233. doi: 10.1210/en.2010-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4■.Klenke S, Siffert W, Frey UH. A novel aspect of GNAS imprinting: higher maternal expression of Galphas in human lymphoblasts, peripheral blood mononuclear cells, mammary adipose tissue, and heart. Mol Cell Endocrinol. 2011;341:63–70. doi: 10.1016/j.mce.2011.05.032. [Provides new insights into allelic imbalance of GNAS alleles, which may explain some of the unusual phenotypic features of PHP type 1a.] [DOI] [PubMed] [Google Scholar]
- 5.Mouallem M, Shaharabany M, Weintrob N, et al. Cognitive impairment is prevalent in pseudohypoparathyroidism type Ia, but not in pseudopseudohy-poparathyroidism: possible cerebral imprinting of Gsalpha. Clin Endocrinol (Oxf) 2008;68:233–239. doi: 10.1111/j.1365-2265.2007.03025.x. [DOI] [PubMed] [Google Scholar]
- 6.Al Salameh A, Despert F, Kottler ML, et al. Resistance to epinephrine and hypersensitivity (hyperresponsiveness) to CB1 antagonists in a patient with pseudohypoparathyroidism type Ic. Eur J Endocrinol. 2010;162:819–824. doi: 10.1530/EJE-09-0951. [DOI] [PubMed] [Google Scholar]
- 7.Dekelbab BH, Aughton DJ, Levine MA. Pseudohypoparathyroidism type 1A and morbid obesity in infancy. Endocr Pract. 2009;15:249–253. doi: 10.4158/EP.15.3.249. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani G, Ferrante E, Giavoli C, et al. Recombinant human GH replacement therapy in children with pseudohypoparathyroidism type Ia: first study on the effect on growth. J Clin Endocrinol Metab. 2010;95:5011–5017. doi: 10.1210/jc.2010-1649. [DOI] [PubMed] [Google Scholar]
- 9.Joseph AW, Shoemaker AH, Germain-Lee EL. Increased prevalence of carpal tunnel syndrome in albright hereditary osteodystrophy. J Clin Endocrinol Metab. 2011;96:2065–2073. doi: 10.1210/jc.2011-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebrun M, Richard N, Abeguile G, et al. Progressive osseous heteroplasia: a model for the imprinting effects of GNAS inactivating mutations in humans. J Clin Endocrinol Metab. 2010;95:3028–3038. doi: 10.1210/jc.2009-1451. [DOI] [PubMed] [Google Scholar]
- 11.Lau K, Willig RP, Hiort O, Hoeger PH. Linear skin atrophy preceding calcinosis cutis in pseudo-pseudohypoparathyroidism. Clin Exp Dermatol. 2012;37:646–648. doi: 10.1111/j.1365-2230.2011.04292.x. [DOI] [PubMed] [Google Scholar]
- 12■■.Martin J, Tucker M, Browning JC. Infantile osteoma cutis as a presentation of a GNAS mutation. Pediatr Dermatol. 2012;29:483–484. doi: 10.1111/j.1525-1470.2011.01469.x. [Provides important genetic confirmation of the relationship of osteoma cutis to PHP type 1a.] [DOI] [PubMed] [Google Scholar]
- 13.Ward S, Sugo E, Verge CF, Wargon O. Three cases of osteoma cutis occurring in infancy. A brief overview of osteoma cutis and its association with pseudo-pseudohypoparathyroidism. Australas J Dermatol. 2011;52:127–131. doi: 10.1111/j.1440-0960.2010.00722.x. [DOI] [PubMed] [Google Scholar]
- 14■■.Izzi B, Van Geet C, Freson K. Recent advances in GNAS epigenetic research of pseudohypoparathyroidism. Curr Mol Med. 2012;12:566–573. doi: 10.2174/156652412800619969. [Great review of the nuances in GNAS imprinting that form the basis for understanding the clinical features of PHP as well as the basis of classification.] [DOI] [PubMed] [Google Scholar]
- 15■.Mantovani G. Clinical review: pseudohypoparathyroidism – diagnosis and treatment. J Clin Endocrinol Metab. 2011;96:3020–3030. doi: 10.1210/jc.2011-1048. [This is a superb review of current approach to the diagnosis and management of the endocrine disorders that are common in patients with PHP.] [DOI] [PubMed] [Google Scholar]
- 16■.Mantovani G, Elli FM, Spada A. GNAS epigenetic defects and pseudohy-poparathyroidism: time for a new classification? Horm Metab Res. 2012;00:000–000. doi: 10.1055/s-0032-1314842. [Points out the confusion arising from the clinical overlap between PHP 1a and PHP 1b.] [DOI] [PubMed] [Google Scholar]
- 17.Chillambhi S, Turan S, Hwang DY, et al. Deletion of the noncoding GNAS antisense transcript causes pseudohypoparathyroidism type Ib and biparental defects of GNAS methylation in cis. J Clin Endocrinol Metab. 2010;95:3993–4002. doi: 10.1210/jc.2009-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richard N, Abeguile G, Coudray N, et al. A new deletion ablating NESP55 causes loss of maternal imprint of A/B GNAS and autosomal dominant pseudohypoparathyroidism type Ib. J Clin Endocrinol Metab. 2012;97:E863–E867. doi: 10.1210/jc.2011-2804. [DOI] [PubMed] [Google Scholar]
- 19.Chotalia M, Smallwood SA, Ruf N, et al. Transcription is required for establishment of germline methylation marks at imprinted genes. Genes Dev. 2009;23:105–117. doi: 10.1101/gad.495809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frohlich LF, Mrakovcic M, Steinborn R, et al. Targeted deletion of the Nesp55 DMR defines another Gnas imprinting control region and provides a mouse model of autosomal dominant PHP-Ib. Proc Natl Acad Sci U S A. 2010;107:9275–9280. doi: 10.1073/pnas.0910224107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Rebollo E, Perez dN, Lecumberri B, et al. Exclusion of the GNAS locus in PHP-Ib patients with broad GNAS methylation changes: evidence for an autosomal recessive form of PHP-Ib? J Bone Miner Res. 2011;26:1854–1863. doi: 10.1002/jbmr.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Rebollo E, Lecumberri B, Garin I, et al. New mechanisms involved in paternal 20q disomy associated with pseudohypoparathyroidism. Eur J Endocrinol. 2010;163:953–962. doi: 10.1530/EJE-10-0435. [DOI] [PubMed] [Google Scholar]
- 23.Bastepe M, Altug-Teber O, Agarwal C, et al. Paternal uniparental isodisomy of the entire chromosome 20 as a molecular cause of pseudohypoparathyroidism type Ib (PHP-Ib). Bone. 2011;48:659–662. doi: 10.1016/j.bone.2010.10.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lietman SA, Ding C, Cooke DW, Levine MA. Reduction in Gsalpha induces osteogenic differentiation in human mesenchymal stem cells. Clin Orthop Relat Res. 2005;434:231–238. doi: 10.1097/01.blo.0000153279.90512.38. [DOI] [PubMed] [Google Scholar]
- 25.Pignolo RJ, Xu M, Russell E, et al. Heterozygous inactivation of Gnas in adipose-derived mesenchymal progenitor cells enhances osteoblast differentiation and promotes heterotopic ossification. J Bone Miner Res. 2011;26:2647–2655. doi: 10.1002/jbmr.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26■■.Huso DL, Edie S, Levine MA, et al. Heterotopic ossifications in a mouse model of albright hereditary osteodystrophy. PLoS One. 2011;6:e21755. doi: 10.1371/journal.pone.0021755. [Indicates that deficiency of Gαs is sufficient to induce heterotopic bone formation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linglart A, Menguy C, Couvineau A, et al. Recurrent PRKAR1A mutation in acrodysostosis with hormone resistance. N Engl J Med. 2011;364:2218–2226. doi: 10.1056/NEJMoa1012717. [DOI] [PubMed] [Google Scholar]
- 28.Michot C, Le Goff C, Goldenberg A, et al. Exome sequencing identifies PDE4D mutations as another cause of acrodysostosis. Am J Hum Genet. 2012;90:740–745. doi: 10.1016/j.ajhg.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu JY, Aarnisalo P, Bastepe M, et al. Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121:3492–3504. doi: 10.1172/JCI46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neary NM, El Maouche D, Hopkins R, et al. Development and treatment of tertiary hyperparathyroidism in patients with pseudohypoparathyroidism type 1b. J Clin Endocrinol Metab. 2012;00:000–000. doi: 10.1210/jc.2012-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31■.Long DN, Levine MA, Germain-Lee EL. Bone mineral density in pseudohy-poparathyroidism type 1a. J Clin Endocrinol Metab. 2010;95:4465–4475. doi: 10.1210/jc.2010-0498. [This is a surprising observation that bone density is increased in PHP 1a patients with chronically elevated PTH levels.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sbrocchi AM, Rauch F, Lawson ML, et al. Osteosclerosis in two brothers with autosomal dominant pseudohypoparathyroidism type 1b: bone histomorphometric analysis. Eur J Endocrinol. 2011;164:295–301. doi: 10.1530/EJE-10-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todorova-Koteva K, Wood K, Imam S, Jaume JC. Screening for parathyroid hormone resistance in patients with nonphenotypically evident pseudohypoparathyroidism. Endocr Pract. 2012:1–21. doi: 10.4158/EP12007.OR. [DOI] [PubMed] [Google Scholar]
- 34.Lindsay R, Nieves J, Henneman E, et al. Subcutaneous administration of the amino-terminal fragment of human parathyroid hormone-(1–34): kinetics and biochemical response in estrogenized osteoporotic patients. J Clin Endocrinol Metab. 1993;77:1535–1539. doi: 10.1210/jcem.77.6.8263137. [DOI] [PubMed] [Google Scholar]
- 35.de Nanclares GP, Fernandez-Rebollo E, Santin I, et al. Epigenetic defects of GNAS in patients with pseudohypoparathyroidism and mild features of Albright's hereditary osteodystrophy. J Clin Endocrinol Metab. 2007;92:2370–2373. doi: 10.1210/jc.2006-2287. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez J, Perera E, Jan dB, et al. Madelung-like deformity in pseudohypoparathyroidism type 1b. J Clin Endocrinol Metab. 2011;96:E1507–E1511. doi: 10.1210/jc.2011-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zazo C, Thiele S, Martin C, et al. Gsalpha activity is reduced in erythrocyte membranes of patients with psedohypoparathyroidism due to epigenetic alterations at the GNAS locus. J Bone Miner Res. 2011;26:1864–1870. doi: 10.1002/jbmr.369. [DOI] [PubMed] [Google Scholar]
- 38.Freson K, Thys C, Wittevrongel C, et al. Pseudohypoparathyroidism type Ib with disturbed imprinting in the GNAS1 cluster and Gsalpha deficiency in platelets. Hum Mol Genet. 2002;11:2741–2750. doi: 10.1093/hmg/11.22.2741. [DOI] [PubMed] [Google Scholar]
- 39.Freson K, Izzi B, Labarque V, et al. GNAS defects identified by stimulatory G protein alpha-subunit signalling studies in platelets. J Clin Endocrinol Metab. 2008;93:4851–4859. doi: 10.1210/jc.2008-0883. [DOI] [PubMed] [Google Scholar]
- 40.Izzi B, Francois I, Labarque V, et al. Methylation defect in imprinted genes detected in patients with an Albright's hereditary osteodystrophy like phenotype and platelet Gs hypofunction. PLoS One. 2012;7:e38579. doi: 10.1371/journal.pone.0038579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh GK, Verma V. Progressive osseous heteroplasia in a 10-year-old male child. Indian J Orthop. 2011;45:280–282. doi: 10.4103/0019-5413.80050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adegbite NS, Xu M, Kaplan FS, et al. Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am J Med Genet A. 2008;146A:1788–1796. doi: 10.1002/ajmg.a.32346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43■■.Maupetit-Mehouas S, Mariot V, Reynes C, et al. Quantification of the methylation at the GNAS locus identifies subtypes of sporadic pseudohypoparathyroidism type Ib. J Med Genet. 2011;48:55–63. doi: 10.1136/jmg.2010.081356. [Different patterns of methylation defects relate to different mechanisms for PHP 1b.] [DOI] [PubMed] [Google Scholar]
- 44.Mantovani G, de Sanctis L, Barbieri AM, et al. Pseudohypoparathyroidism and GNAS epigenetic defects: clinical evaluation of albright hereditary osteodystrophy and molecular analysis in 40 patients. J Clin Endocrinol Metab. 2010;95:651–658. doi: 10.1210/jc.2009-0176. [DOI] [PubMed] [Google Scholar]
- 45.Izzi B, Decallonne B, Devriendt K, et al. A new approach to imprinting mutation detection in GNAS by Sequenom EpiTYPER system. Clin Chim Acta. 2010;411:2033–2039. doi: 10.1016/j.cca.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 46.Mitsui T, Nagasaki K, Takagi M, et al. A family of pseudohypoparathyroidism type Ia with an 850-kb submicroscopic deletion encompassing the whole GNAS locus. Am J Med Genet A. 2011 doi: 10.1002/ajmg.a.34393. doi: 10.1002/ajmg.a.34393. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Rebollo E, Garcia-Cuartero B, Garin I, et al. Intragenic GNAS deletion involving exon A/B in pseudohypoparathyroidism type 1A resulting in an apparent loss of exon A/B methylation: potential for misdiagnosis of pseudohypoparathyroidism type 1B. J Clin Endocrinol Metab. 2010;95:765–771. doi: 10.1210/jc.2009-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]