Abstract
Eosinophils are innate immune leukocytes found in relatively low numbers within the blood. Terminal effector functions of eosinophils, deriving from their capacity to release their content of tissue-destructive cationic proteins, have historically been considered primary effector mechanisms against specific parasites, and are likewise implicated in tissue damage accompanying allergic responses such as asthma. However, the past decade has seen dramatic advancements in the field of eosinophil immunobiology, revealing eosinophils to also be key participants in many other facets of innate immunity, from bridging innate and adaptive immune responses to orchestrating tissue remodeling events. Here, we review the multifaceted functions of eosinophils in innate immunity that are currently known, and discuss new avenues in this evolving story.
Keywords: Eosinophil, Innate immunity, Immunomodulation, Tissue remodeling, Piecemeal secretion
Introduction
Eosinophils are innate immune granulocytes best recognized for their cytotoxic effector functions, causing damage to parasitic pathogens in helminth infections, and to host tissues in allergic diseases. However, more recent data have uncovered a much more complex picture, revealing additional roles for eosinophils in regulating inflammation, maintaining epithelial barrier function, affecting tissue remodeling, and bridging innate and adaptive immunity. Many of these functions arise from the capacity of eosinophils to store a preformed armamentarium of cytokines, chemokines and growth factors, available for immediate release.
Eosinophils are generally found in low numbers within circulation (1–4% of total peripheral blood leukocytes), while the majority of eosinophils at baseline reside within mucosal tissues interfacing with the environment and within primary and secondary lymphoid tissues. Localization to mucosal surfaces places eosinophils within ideal sites to identify and respond rapidly to pathogens, and within secondary lymphoid tissues, eosinophils are well-poised for their functions in bridging innate and adaptive immunity. Moreover, in response to allergens or Th2-inducing helminth infections, eosinophils are rapidly recruited to tissue sites. Here, we will review old and new data highlighting the contributions of eosinophils to innate immune processes.
In the first section, the structure, content and membrane receptor repertoire of eosinophils with direct relevance to innate immunity will be introduced. In the following section, how the biology of eosinophils contributes to specific innate immune functions will be described, and in the final two sections, how these specific functions participate in innate immunity during health and within specific infectious settings will be discussed.
Content and receptor expression relevant to innate immunity
Structure and content of eosinophils relating to innate immunity
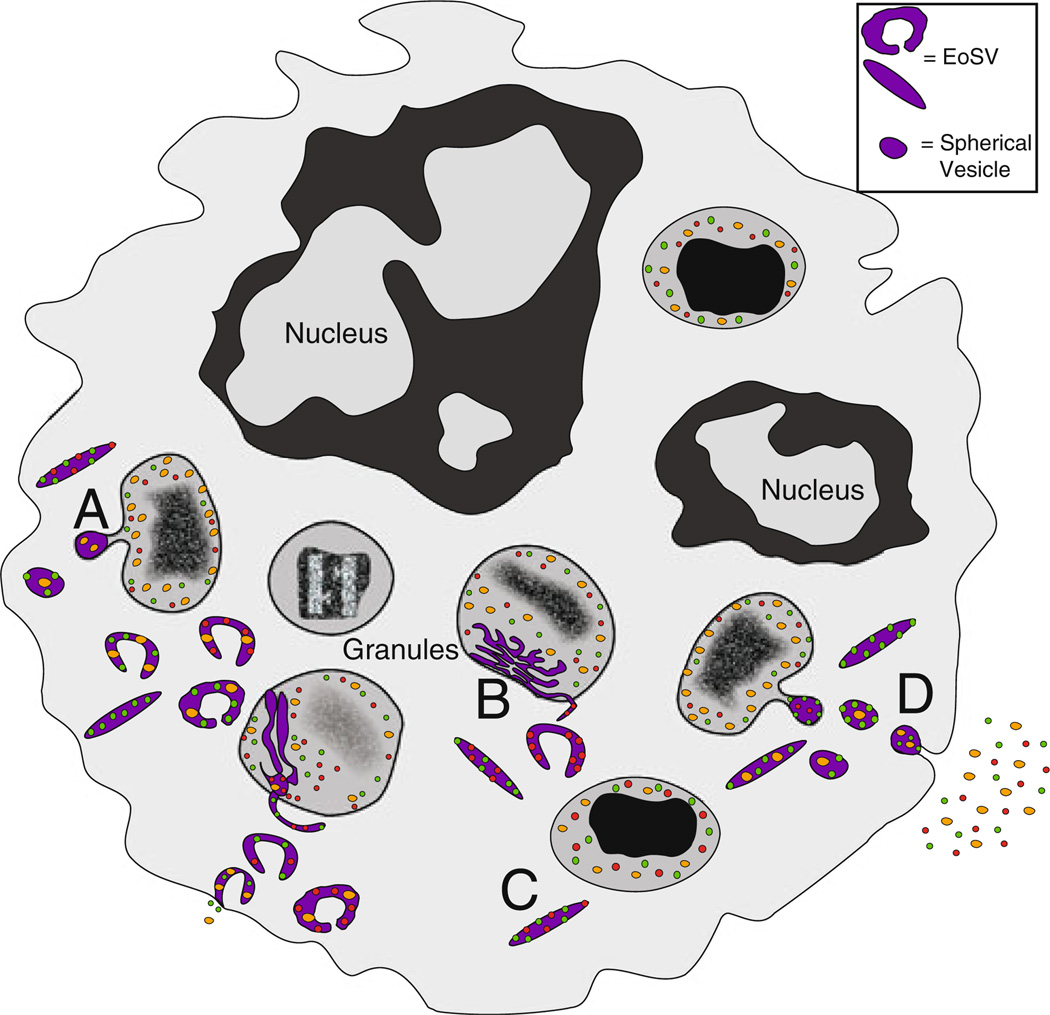
The most characteristic feature of eosinophils is their cytoplasmic content of specific granules. The specific granules, also called secondary or crystalloid granules, have a distinctive dense crystalline core, surrounded by a less dense matrix, and enclosed by a trilaminar membrane. Eosinophil granules are composed mainly of cytotoxic cationic proteins, and also store a plethora of cytokines, chemokines and growth factors, available for very rapid, stimulus-dependent release. The most common physiological means by which eosinophil granule-stored proteins are released involves a regulated process, termed piecemeal degranulation (PMD), by which cargo-laden small spherical vesicles and larger tubular vesicles emerge from mobilized intracellular granules and traffic through the cytoplasm to the cell membrane for release of their transported contents. Docking of vesicles to granules and the plasma membrane is controlled through the selective expression of vesicle- and membrane-specific SNARE (soluble N- ethylmaleimide-sensitive factor attachment protein receptor) molecules (Logan et al. 2006; Moqbel and Coughlin 2006). PMD is evidenced in electron micrographs of activated eosinophils by the presence of numerous intracytoplasmic tubular structures, termed eosinophil sombrero vesicles (EoSVs) in recognition of the donut-shaped configuration these vesicles often display in cross-section (Melo et al. 2008) (Fig. 1). Two more processes of granule protein secretion recognized for eosinophils are classical exocytosis, in which granules fuse with the plasma membrane to release their contents (Moqbel and Lacy 1999), and cytolysis, in which the plasma membrane ruptures and granules are liberated extracellulary (Erjefalt et al. 1998; Ahlstrom-Emanuelsson et al. 2004). Cell free granules deposited within tissues likely remain secretion competent to release their contents upon appropriate stimulation (Neves et al. 2008; Neves and Weller 2009). The capacity of eosinophils to rapidly secrete a wide variety of mediators without the need for de novo protein synthesis distinguishes these cells from many other innate and adaptive immune cells and plays a key role in the innate immune functions of eosinophils. Eosinophil-expressed molecules and surface receptors with relevance to innate immune processes are included in Table 1, Table 2 and Table 3.
Fig. 1.
Preformed cytokines, chemokines and growth factors are secreted from intracellular granules through piecemeal degranulation. Transport of granule-derived proteins occurs by the mechanism of piecemeal degran-ulation, involving budding of small spherical and elongated tubular (EoSV) vesicles from intracellular granules, which then traffic to the plasma membrane for mediator release. a Activation of eosinophils causes mobilization of intracellular granule cores and loss of granule contents, along with an increase in vesicular traffic (vesicles pseudocol-ored in purple). b Intragranular membranous subcompartments (pseu-docolored in purple) are often visualized in conjunction with a mobilized core prior to the emergence of spherical and tubular sombrero vesicles. c Some EoSVs exhibit membrane-bound contents, representing receptor-dependent chaperoning of granule-derived cytokines. d Vesicular contents are released extracellularly following fusion with the plasma membrane. EoSV Eosinophil sombrero vesicle, Gr granule, Nu nucleus
Table 1.
Cytokines, cytokine receptors and growth factors
| Cytokine receptors | Cytokines | Growth factors |
|---|---|---|
|
Cherry et al. 2008; Cheung et al. 2008, 2010; Hogan et al. 2008; Hu et al. 2010; Matsuba-Kitamura et al. 2010; Wang et al. 2007b |
Hogan et al. 2008; Melo et al. 2008; Minshall et al. 2000; Spencer et al. 2009; Wong et al. 2007 | Hogan et al. 2008 |
| IL-2R (CD25/CD122) | IL-1β | Groα (CXCL1) |
| IL-3R (CD123/CD131) | IL-2a | NGF |
| IL-4R (CD124/CD132) | IL-3a | PDGF |
| IL-5R (CD125/CD131 ) | IL-4a | SCF |
| IL-9R (CD129/CD132) | IL-5a | VEGF |
| IL-10Rb | IL-6a | HB-EGF-LBP |
| IL-12Rb | IL-8 | |
| IL-13Rα1 (CD213a1) | IL-10a | |
| IL-17A/F R (IL-17RA/IL-17RC) | IL-11 | |
| IL-23R (IL-23R/IL-12Rβ1) | IL-12a | |
| IL-27R (gp130/WSX-1) | IL-13a | |
| IL-31R (IL-31RA/OSMRβ) | IL-16 | |
| IL-33R (ST2) | IL-18 | |
| GM-CSFR (CD116/CD131) | IL-25 (IL-17E) | |
| IFNγR (CDw119) | IFNγa | |
| SCFR (c-kit, CD117) | GM-CSFa | |
| TNFαR1 and 2 (CD120a, b) | SCFa | |
| TGFβR | TGFαa | |
| TGFβ | ||
| TNFαa | ||
| TNFβ |
R Receptor, OSMRβ oncostatin M receptor β
Cytokines that have been localized to eosinophil specific granules
Data based on functional response following ex vivo stimulation, not yet confirmed at the protein/mRNA level
Table 2.
Chemokines and lipid mediators and their receptors
| Chemokine receptors | Chemokines | Receptors for lipid mediators |
Lipid mediators |
|---|---|---|---|
|
Al-Rabia et al. 2003; Borchers et al. 2002; Hogan et al. 2008; Jung et al. 2008; Liu et al. 2003; Nagase et al. 2001; Dunzendorfer et al. 2001 |
Hogan et al. 2008; Wong et al. 2007 |
Hogan et al. 2008; Spik et al. 2005 |
Hogan et al. 2008; Weller et al. 1999 |
| CCR1 (CD191) | MCP-1 (CCL2) a | CysLT1R | LTC4 |
| CCR2 (CD192) | MIP-1α (CCL3) | CysLT2R | LTD4 |
| CCR3 (CD193) | RANTES (CCL5) | LTB4R | LTE4 |
| CCR4 (CD194) | MCP-3 (CCL7) | DP2/CRTh2 (PGD2R) | PGE1 |
| CCR5 (CD195) | MCP-2 (CCL8) | DP1 (PGD2R) | PGE2 |
| CCR6 (CD196) | MIP1γ (CCL9) a | PGE2 R | 15-HETE |
| CCR7 (CD197)a | Eotaxin-1 (CCL11) | PAFR | PAF |
| CCR8 (CD198) | MCP-4 (CCL13) | fMLPR | |
| CCR9 (CDw199) | Gro α (CXCL1) | ||
| CXCR3 (CD182 or CD183) | SDF-1 (CXCL12) | ||
| CXCR4 | C10/ CCL6a | ||
| IP-10 (CXCL10) |
Not yet confirmed in primary human eosinophils
Table 3.
Adhesion molecules, complement receptors, immunoglobulin receptors and TLRs
| Adhesion molecules | Complement and Fc receptors | Toll-like receptors |
|---|---|---|
| Hogan et al. 2008 |
Giembycz and Lindsay 1999; Hogan et al. 2008 |
Nagase et al. 2003; Wong et al. 2007 |
| LFA-1 (αLβ2, CD11a/CD18) | CR1 (CD35) | TLR1 |
| CR3 (αMβ2, CD11b/CD18) | CR3 (CD11b/CD18) | TLR2 |
| CR4 (αXβ2, CD11c/CD18) | CR4 (CD11c) | TLR4 |
| αDβ2 (CD11d/CD18) | CD103 | TLR5 |
| VLA-4 (α4β1, CD49d/ CD29) | C1qR | TLR6 |
| VLA-6 (α6β1 CD49f/ CD29) | C3aR | TLR7 |
| α4β7 (CD49d/ Ly69) | C5aR (CD88) | TLR8a |
| αEβ7 CD103/Ly69) | FcαR (CD89) | TLR9 |
| CD44 | Receptor for IgDb | TLR10a |
| CD156 | FcγRII (CD32) | |
| L-selectin (CD62L) | Receptor for IgMb | |
| PSGL-1 (CD162) | FcεRII (CD23) | |
| sialyl-Lewis × (CD15 s) | FcεRI | |
| CD162 |
Shown to be expressed at RNA level
Binding of IgD and IgM to eosinophils has been observed, but specific receptors mediating the interactions have not been definedx
Cationic proteins
The main constituents of eosinophil specific granules are the cationic proteins, such as major basic protein (MBP) (Gleich et al. 1973), EPO (eosinophil peroxidase) (Carlson et al. 1985; Olsen and Little 1983) and eosinophil-associated RNases (EARs) such as ECP (eosinophilic cationic protein) (Olsson and Venge 1974) and EDN (eosinophil-derived neurotoxin) (Durack et al. 1981; Gleich and Adolphson 1986). There is evidence for expression of residual amounts of granular proteins such as ECP, MBP and EDN in other granulocytes such as neutrophils, basophils and activated macrophages (Abu-Ghazaleh et al. 1992; Sur et al. 1998).
Murine eosinophils house a similar content of basic proteins in their granules, including MBP-1 and 2 (Larson et al. 1995; Macias et al. 2000), EPO (Horton et al. 1996) and at least 13 murine EARs with 70% sequence identity (Batten et al. 1997; Cormier et al. 2001; Larson et al. 1996; Rosenberg and Domachowske 2001; Singhania et al. 1999). Six of the murine EARs localized to their specific granules (Cormier et al. 2001; Larson et al. 1995; Lee and Lee 2005).
Eosinophil cationic proteins are noted for their anthel-minthic and tissue cytotoxic properties. Importantly, several of these mediators fulfill additional functions through signaling interactions with other cells, including endothelial cells and mast cells. Mechanisms by which eosinophil cationic proteins mediate a multitude of effects are discussed in the following sections in conjunction with their specific functions. For further reading, the biology of eosinophil-derived cationic mediators has been recently reviewed in great detail (Hogan et al. 2008).
Cytokines, chemokines and growth factors
In addition to cationic proteins, human eosinophils harbor a plethora of cytokines, chemokines and growth factors within specific granules, including interleukin (IL)- 4 (Melo et al. 2005; Moqbel et al. 1995), IL-6 (Lacy et al. 1998), tumor necrosis factor (TNF)α (Beil et al. 1993), IL-5 (Dubucquoi et al. 1994; Moller et al. 1996), granulocyte macrophage colony-stimulating factor (GM-CSF) (Levi-Schaffer et al. 1995), IL-2 (Levi-Schaffer et al. 1996), IL-13 (Woerly et al. 2002), IL-10 (Lamkhioued et al. 1995), IL-12 (Grewe et al. 1998), interferon (IFN)γ (Woerly et al. 1999), transforming growth factor (TGF)-β (Wong et al. 1991), IL-25 (Wang et al. 2007b), and eotaxin (Nakajima et al. 1998). For more exhaustive lists of eosinophil-derived cytokines, chemokines and growth factors, one should refer to Hogan et al. (2008) and Lacy and Moqbel (2000) and Table 1 and Table 2. Of note, although eosinophils are generally found in association with Th2 immunity, eosinophils express Th1, Th2 and regulatory cytokines, as well as cytokines with strong pro- or anti-inflammatory properties. We have recently demonstrated that the relative concentrations of cytokines stored preformed within granules of human blood eosinophils are well conserved between individuals, with the exception of preformed TNF-α, for which concentrations varied among donors (Spencer et al. 2009). Although eosinophils secrete low amounts of inflammatory mediators compared to T cells, the fact that those mediators exist as preformed cytokines and are very rapidly secretable may point to the essential role of eosinophils in the immediate innate immune response to regulate the microenvironment at sites of pathogen penetration or injury. Contributions of eosinophil cytokine and chemokine secretion to the inflammatory, immunoregulatory and tissue-remodeling capacities of eosinophils are discussed in the following sections.
Other eosinophil-derived mediators
Eosinophils are a major source of lipid mediators, including cysteinyl leukotrienes (LT) (Bandeira-Melo and Weller 2003), arachidonic-acid based inflammatory mediators (Table 2). Arachidonic acid metabolism and eicosanoid formation occurs in peri-nuclear membranes and in cytoplasmic lipid bodies, lipid-rich cytoplasmic inclusions (Bozza et al. 1997). Lipid bodies are enriched in eicosanoid forming enzymes, including prostaglandin endoperoxide synthases (cyclooxygenases), 5-lipoxygenase (5-LO), and leukotriene C4-synthase (LTC4S), and, therefore, serve as a site for arachidonic acid esterification and eicosanoid formation. Cysteinyl leukotrienes, such as secreted LTC4, and extracellularly formed LTD4 and LTE4, have broad effects on the inflammatory response. In asthma pathogenesis, effects of cys-LTs include provoking bronchoconstriction, inducing mucus production and augmenting venular permeability. Therefore, LT binding blockers are used to control asthma and allergic diseases.
In addition, eosinophils express proteinases and other enzymes. Matrix metalloproteinases (MMPs) are enzymes critical for the degradation of extracellular matrix materials, enabling cell migration through tissues and participating in tissue reorganization. Eosinophils express soluble MMP-9 (Dahlen et al. 1999), and membrane-bound MMP17 (Gauthier et al. 2003). Moreover, eosinophil granules harbor enzymes including acid phosphatase, collagenase (Hibbs et al. 1982), arysulfatase B (Egesten et al. 1986), histaminase (Zeiger et al. 1976), phospholipase D (Egesten et al. 1997), catalase (Iozzo et al. 1982) and non-specific esterases (Li and Glick 1987; Weinstein et al. 1962). How these enzymes contribute to biological processes such as wound healing and tissue remodeling will be discussed in the following sections.
Membrane receptors with relevance to innate immune mechanisms
Receptors involved in inflammatory responses
Eosinophils are equipped with a variety of receptors that enable their recruitment to inflammatory sites, and response to inflammatory mediators, including receptors for chemokines, cytokines, immunoglobulins, lipids, complement fragments, and serine proteases. It is noteworthy that eosinophils express cognate receptors for many of the mediators stored and secreted by eosinophils (including the eosinophilopoietins IL-5 and GM-CSF, and the key chemoattractant CCL11 (eotaxin)), creating the potential for significant eosinophil autocrine regulation (compare cytokine and cytokine receptor columns of Table 1).
Resting eosinophils constitutively express the chemokine receptors CCR1 and CCR3 (Elsner et al. 2005; Phillips et al. 2003; Ponath et al. 1996). CCL11 (eotaxin-1), acting through eosinophil-expressed CCR3, represents the most important chemoattractant signal for eosinophils, as evidenced by a significant deficiency in tissue eosinophilia at baseline and following airway allergen challenge of CCL11- (Rothenberg et al. 1997) or CCR3-deficient (Humbles et al. 2002) mice. Upon stimulation, eosinophils can upregulate expression of CXCR3, CXCR4, CCR5, CCR6 and CCR8 (Nagase et al. 2000; Oliveira et al. 2002; Sullivan et al. 1999), broadening the range of chemokines to which eosinophils respond (see also Table 2).
Eosinophils can respond to the activated complement cascade through binding complement fragments, including C3a, C5a, C3b, C4b, iC3b and C1q, through expression of C3aR, C5aR, CR1, CR3, CR4, CD103 and receptors for C1q (Giembycz and Lindsay 1999; Walsh et al. 1990) (Table 3). Moreover, eosinophils express Fc receptors for IgD, IgM, IgG, and IgA (Giembycz and Lindsay 1999). Of particular significance to the eosinophil’s association with mucosal tissues, IgA-coated particles elicit eosinophil degranulation (Abu-Ghazaleh et al. 1989), and secretory IgA has been reported to promote eosinophil survival in an antigen-independent manner (Bartemes et al. 2005). Although there is some controversy in the literature as to whether eosinophils express surface IgE receptors (Seminario et al. 1999), several compelling studies find human eosinophils from eosinophilic donors to express both high- and low-affinity receptors for IgE (Abdelilah et al. 1998; Gounni et al. 1994; Rajakulasingam et al. 1998), as well as an additional low-affinity IgE-binding entity of the S-type lectin family, Mac-2/ε-binding protein (Capron et al. 1991). In contrast to human eosinophils, murine eosinophils do not appear to express high affinity IgE receptors, due to lack of expression of the receptor α chain.
IL-3, IL-5 and GM-CSF are critical to eosinophil development, and signal through IL-3Rα, IL-5Rα or GM-CSFRα chains, respectively, in association with the common β chain (Lopez et al. 1986, 1988; Rothenberg et al. 1988; Takatsu et al. 1994). Eosinophils express TNFα receptors 1 and 2, IFN-γRα, IL-4Rα, IL-13Rα and the common γ chain, enabling eosinophils to respond to proinflammatory cytokines, as well as products of Th1 or Th2 immunity. Eosinophils also express IL-9Rα and c-kit (Dubois et al. 1998; Hauber et al. 2004; Wallen et al. 1991; Yuan et al. 1997). Using CCL11-mediated secretion of IL-4 from human blood eosinophils as a model of stimulus-dependent PMD, we have demonstrated that IL-4Rα chains chaperone the intracellular trafficking of IL-4 from granules to the cell membrane for secretion (Spencer et al. 2006) (Fig. 1c). Therefore, granule-expressed cognate cytokine receptors expressed in human eosinophils likely contribute to the regulated release of cytokines stored within intracellular granules, in addition to functioning at the cell membrane in their traditional cell signaling capacities. Further studies are underway to determine whether cognate receptor trafficking is a mechanism utilized in the transport of additional cytokines and chemokines from human eosinophil granules.
In addition to protein recognition, eosinophils respond to lipid mediators and endogenous proteases. Eosinophils utilize multiple receptors for lipid recognition, including cysteinyl receptors (CysLT1 and CysLT2 receptors), the high-affinity prostaglandin type 2 (PGD2) receptor, and PAF receptors (Fujii et al. 2005; Wang et al. 1999; Zinchuk et al. 2005). Eosinophils also recognize and respond to prostaglandins (i.e. PGE2) and leukotrienes (i.e. LTB4). Eosinophil-expressed protease-activated receptors (PARs) enable eosinophil responses to proteases, such as trypsin and mast cell tryptase and chymase. PARs are G protein-coupled receptors with 7 transmembrane spanning regions that are stimulated by serine proteases. First recognized for their roles in clotting and platelet activation, the importance of protease-activated receptors (PARs) to additional processes, including wound healing and inflammation, are now widely recognized. Eosinophils express PAR-2 and PAR-3 (Miike et al. 2001), and presumably PAR-1 as well (Tomimori et al. 2002).
Eosinophil-expressed receptors for PAMPs and DAMPs
Of note, several eosinophil-expressed receptors for host inflammatory mediators also serve as pattern recognition molecules (PRMs), contributing to the eosinophil’s capacity to directly recognize pathogen-associated molecular patterns (PAMPs). For example, eosinophils are directly activated by cysteine and serine proteases derived from mites and fungi through activation of eosinophil-expressed PARs. Whether eosinophil activation by pathogen-derived proteases is accomplished by signaling through one of the recognized PARs or a related, as yet unidentified, receptor (s) has not been determined. Similarly, eosinophil-expressed receptors for endogenous lipids also function in the recognition of pathogen- or allergen-associated lipids. Airborne pollens are associated with eicosanoid-like substances that cross-react with LTB4 and PGE2 antibodies in standard ELISA assays. In one study, such pollen-associated lipid mediators (PALMs) mediated eosinophil activation through an LTB4-dependent mechanism (Plotz et al. 2004). Along the same lines, eosinophil-expressed β2 integrins function not only in adherence of eosinophils to vascular endothelium but also in the adherence to β-glucan, a major fungal cell wall component (Yoon et al. 2008).
In addition to endogenous receptors doubling as PRMs, eosinophils express traditional PAMP receptors, including several members of the Toll-like receptor (TLR) family. Among the TLRs reported to be expressed by human eosinophils are TLR 1, 2, 4, 5, 6, 7, and 9 (Wong et al. 2007). Of note, expression of some of the TLRs (TLR2, TLR4) by human eosinophils has been a contentious issue in the literature, which may reflect donor-dependent and/or differential expression of TLRs by eosinophils. In support of this interpretation, Driss et al. found eosinophils from atopic donors to constitutively express TLR2 and 4, while eosinophils from healthy donors did not (Driss et al. 2009). Ligands for TLR2 (peptidoglycan, BCG), TLR5 (flagellin), and TLR7/TLR8 (imiquimod R837, R-848) induced eosinophil secretion and other evidence of eosinophil activation (Driss et al. 2009; Wong et al. 2007).
Eosinophils are also equipped to respond to damage-associated molecular patterns (DAMPs). Eosinophils are attracted by, recognize and respond to products released by necrotic cells or damaged tissues, including high mobility group box 1 (HMGB1) (Lotfi et al. 2009) and crystalline uric acid (Kobayashi et al. 2010).
Receptors associated with antigen presentation
One mechanism by which eosinophils bridge innate and adaptive immunity is through antigen presentation functions, enabled by the stimulated expression of MHC class II molecules and co-stimulatory receptors, including CD80, CD86 and CD40L. Although resting eosinophils from peripheral blood are generally devoid of major histocompatibility complex MHC II expression, eosinophils primed ex vivo or isolated from the tissues of mice (Mawhorter et al. 1993) or humans (Beninati et al. 1993; Hansel et al. 1991; Lucey et al. 1989; Sedgwick et al. 1992) express detectable MHC II. Moreover, we have recently identified eosinophils to constitutively express Notch ligands (Radke et al. 2009), a family of molecules implicated in the polarization of T cells toward Th1, Th2, or regulatory fates. Further studies are underway to determine the significance(s) of eosinophil Notch ligand expression to the development of adaptive immunity.
Innate immune functions of eosinophils
As seen in the previous section, eosinophils are uniquely outfitted with a preformed armamentarium of cytokines, chemokines, cationic proteins, growth factors, matrix metalloproteinases, lipid mediators and RNAses, stored primarily within intracellular granules. Secretion of preformed mediators endow eosinophils with the capacity to rapidly affect the immune microenvironment, cellular recruitment, tissue repair, remodeling and homeostasis, and direct anti-pathogen responses. Moreover, through expression of receptors recognizing a wide range of PAMPs and DAMPs, eosinophils recognize and are activated by pathogens and evidence of tissue damage. Indirectly through secretion of granule-derived mediators, and directly through cell-cell contacts, eosinophils also interact with innate and adaptive immune cells, including mast cells and T cells, connective tissue cells, and cells of the nervous system, to hone and modulate immune and tissue responses. Recent studies have further revealed eosinophils to expel mitochondrial DNA in conjunction with cytoskeletal materials, forming “extracellular traps” capable of immobilizing and neutralizing extracellular bacteria.
Effector functions of eosinophils can be divided into four categories: (1) terminal effector functions; (2) tissue remodeling; (3) immunomodulation; and (4) cellular interactions, and are described here. How these functions specifically contribute to innate immunity within the context of health and disease will be discussed in the last two sections.
Terminal effector functions of eosinophils
The earliest recognized functions of eosinophils related to the cytotoxic properties of their granule-derived cationic proteins. As will be described in the section (“Eosinophils in disease”), the cytotoxic effects of eosinophils are implicated in host defense mechanisms against helminth, viral and bacterial infections, and are responsible for collateral tissue damage (Fig. 2a–d).
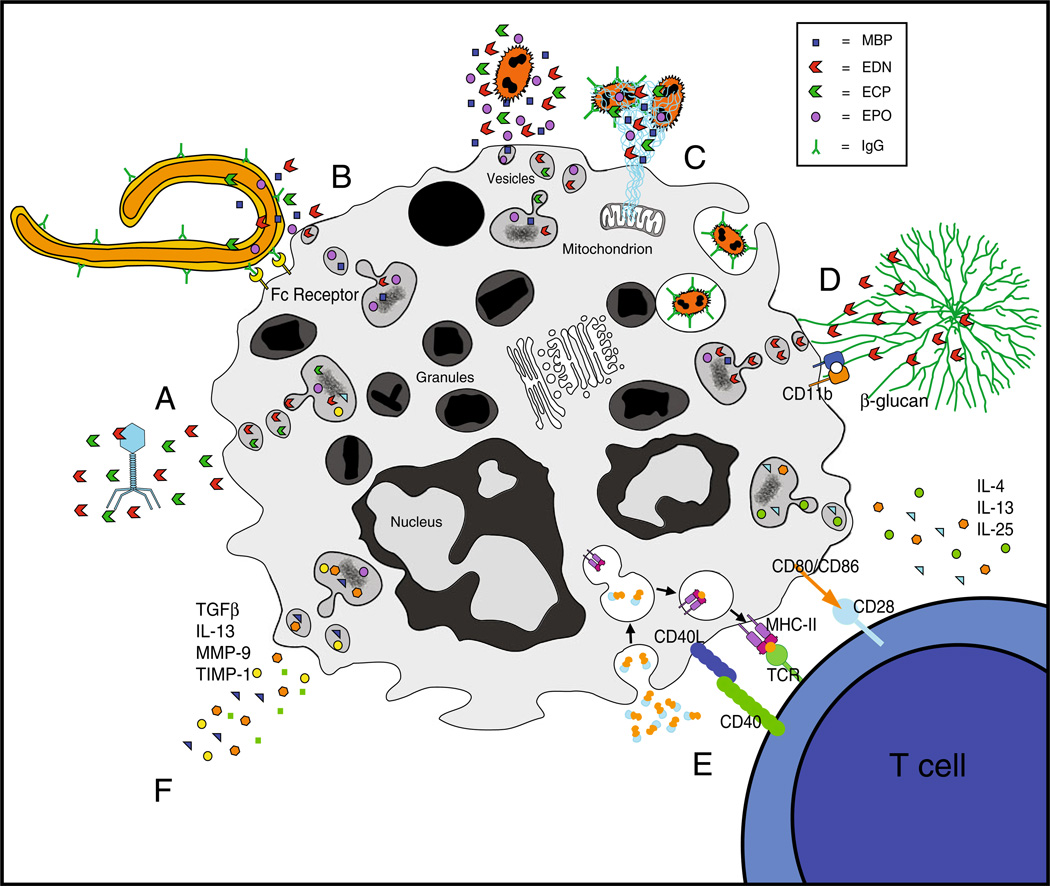
Fig. 2.
Innate immune functions of eosinophils and their contributions to host defense. Innate immune functions of eosinophils include terminal effector functions (a–d), immunomodulation (e) and tissue repair and remodeling (f). a Eosinophilic interactions with extracellular virons result in destruction by eosinophil-associated ribonu-cleases. b Cross-linking of FcγII receptors by helminth-bound IgG1 and IgG3 mediate eosinophil activation and degranulation of helminth-destructive cationic proteins. c LPS-stimulated eosinophils release extracellular traps, containing mitochondrial DNA and granule-derived proteins, to eradicate bacterial infiltration. Bacteria can also be removed by secretion of granule-derived proteins and phagocytosis. d CD11b integrin- mediated adherence of β-glucan induces vesicular release of EDN in response to fungal infestation. e Eosinophils act as immunomodulators and direct T helper cell differentiation through secretion of immune-polarizing cytokines and functioning as professional antigen presenting cells. f Eosinophils secrete numerous mediators with effects on tissue remodeling, including TGFβ, IL-13 and MMPs
Cationic proteins
MBP is a small single peptide (13.8 kD) with a highly basic nature due to a content of 17 arginine residues (Popken-Harris et al. 1998). The suggested cytotoxic mechanism of MBP involves binding of MBP to negatively charged cell membranes due to their very highly basic nature, changing the cell membrane charge, disordering the lipid bilayer and increasing the cell permeability. Among its effects, MBP was found to be toxic to helminths and airway epithelium, and to exert antibacterial effects (Hogan et al. 2008). Both ECP and EDN were shown to have neurotoxic activities and can induce the Gordon phenomenon, characterized by a syndrome of stiffness and ataxia progressing to severe paralysis, when injected intrathecally (Durack et al. 1979; Gleich et al. 1986). ECP exhibits neurotoxicity, and anti- viral, antibacterial and anti helminthic cytotoxicity. However, its RNase activity is essential for neurotoxicity and anti-viral host defense but not for antibacterial and anti helminthic activities (Hogan et al. 2008). As a haloperoxidase, EPO can catalyze oxidation of halide substrates, such as bromide, chloride and iodide, and the pseudohalide thiocyanate into toxic molecules, such as hypochlorous, hypobromous, hypo-iodous acids and hypothiocyanite (Thomas and Fishman 1986), all known for their antibacterial and disinfectant properties.
Antibody- and complement-mediated cytotoxicity
Early in vitro studies of eosinophil cytoxicity against helminths, performed by Butterworth et al. (1975), showed that activated human eosinophils mediated killing of schistosomula of Schistosoma mansoni, in a process requiring heat-inactivated sera from individuals with schis-tosome infection. Later on, IgG1 and IgG3 but not IgM, IgG2, or IgG4 subclasses were found to be effective in mediating this killing by human eosinophils (Khalife et al. 1989). Kaneko et al. (1995) confirmed those results in an elegant in vitro model of ragweed pollen allergen and showed that allergen-specific IgG1 and IgG3, but not IgG4, can induce degranulation and EDN release through FcγRII.
Complement components have also been shown to mediate eosinophil cytotoxicity. Eosinophils express receptors for complement, and complement components, such as C5a, are chemotactic for and activate eosinophils (Kay et al. 1973). Aside from activation, complement has been shown to mediate in vitro killing of helminths, such as Schistosoma mansoni, by rat peritoneal cavity-derived eosinophils (Ramalho-Pinto et al. 1978). Moreover, the adherence of eosinophils to helminths was shown to be due to the activation of complement at the schistosomular surface by the alternative pathway, and was mediated by complement C3 receptor.
Phagocytosis
Phagocytic capabilities of eosinophils have also been recognized. In the early 1960s, Archer and Hirsch (1963) performed in vitro studies on horse eosinophils using cinemicrophotography to demonstrate pahgocytosis by eosinophils. Eosinophils were attracted to and readily engulfed diverse materials such as yeast cell walls, foreign erythrocytes, and antigen–antibody precipitates. Specific antibody was required for phagocytosis of red cells, and greatly accelerated the uptake of yeast cell walls. Interestingly, horse eosinophil granules near to the phagocytic vacuole discharged their contents into or alongside the phagocytic vacuole. Granule disruption resulted in a clear zone and deposition of amorphous, phase-dense material. Cline et al. (1968) later showed in vitro phagocytosis of Gram-positive Staphylococcus aureus and Gram-negative Escherichia Coli bacteria, and living and dead Candida albicans, by human eosinophils purified from patients with eosinophilia. Eosinophils have also been shown to phagocytose and destroy parasites such as T. dionisii, but only in the presence of antiserum (Thorne et al. 1979). Eosinophilic phagocytosis is accompanied by degranulation and involves lysosomal enzymes, similarly to neutrophils. However, phagocytosis by eosinophils differs from neutrophilic phagocytosis both in efficacy and in mechanism. Phagocytosis by eosinophils occurs less efficiently than neutrophil phagocytosis. Moreover, as shown by Hatano et al. (2009), while in vitro phagocytosis of opsonized heat-killed Staphylococcus aureus by neutrophils was CD16- and CD32-dependent, phagocytosis by eosinophils was dependent upon CD35.
Eosinophil-expulsed extracellular DNA traps
More recently, a unique mechanism of antibacterial activity was suggested for eosinophils, by releasing mitochondrial DNA-containing “traps” into the extracellular space (Yousefi et al. 2008). Expulsion of extracellular traps from eosinophils involved release of mitochondrial DNA and cytotoxic granule-derived proteins, apparently without effects on eosinophil viability. Similar processes had previously been described for neutrophils and mast cells, but resulted in cell death. Generation of eosinophil-derived extracellular traps is induced by encounter of IL-5- or IFN-γ-primed eosinophils with Gram-negative LPS, by a mechanism dependent upon reactive oxygen species (Yousefi et al. 2008).
Tissue homeostasis, repair and remodeling
In addition to the direct pathogen-killing strategies described above, eosinophils contribute to many other aspects of innate immunity, including tissue homeostasis. The integrity and composition of tissues is an important part of innate immunity. For example, maintaining the integrity of epithelial barriers is essential to prohibiting pathogen entry, and the density and composition of connective tissue may impact the infectious potential of invading pathogens, as well as the effectiveness of innate immune defensive strategies. In addition to the tissue destructive effects of some eosinophil-derived mediators, eosinophils express cytokines and growth factors with tissue repair properties. Under a variety of physiological and pathological conditions, eosinophils interact with tissue components, maintaining tissue homeostasis, or mediating repair and remodeling. Eosinophil-mediated tissue repair may be beneficial, as in the case of eosinophil interactions with gastrointestinal epithelium (see “Eosinophils in health”, and Fig. 3). However, excessive eosinophil infiltration can be associated with fibrotic consequences, as evidenced by airway remodeling in the lungs of patients with severe asthma, and in the development of endomyocardial fibrosis in patients with tropical pulmonary eosinophilia. Wound healing and tissue remodeling functions of eosinophils are also involved in immunity to helminths.
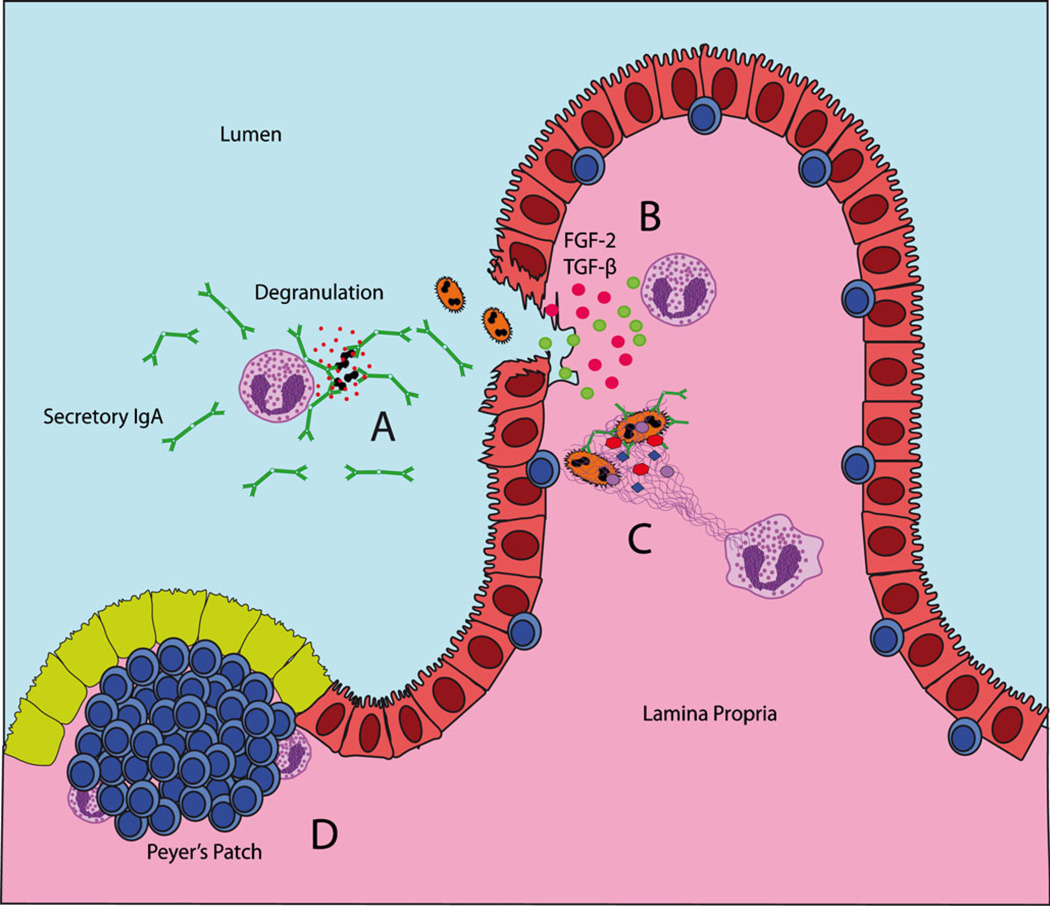
Fig. 3.
Innate immune functions of gastrointestinal eosinophils. a Cross-linking of membrane bound IgA receptors by secretory IgA linked to bacterial microbes elicits secretion of granule-derived proteins by eosinophils. b In response to epithelial damage, eosinophils secrete multiple mediators (including FGF-2 and TGF-β) to promote tissue repair and remodeling, maintaining the integrity of the epithelial barrier. c Eosinophils may be stimulated to secrete mitochondrial DNA and granule derived proteins in the form of an extracellular trap for infiltrating bacteria. d Eosinophils can be found in association with lymphocytes within gastrointestinal Peyer’s patches
Defining mechanisms by which eosinophils accomplish tissue homeostasis, repair and remodeling events is currently an area of active research. Several common mechanisms have emerged from studies across multiple systems that implicate the eosinophil’s vast supply of mediators with distinct effects on connective tissues and the vascular endothelium, including TGF-β, basic fibroblast growth factor (bFGF), Th2 cytokines (namely IL-4, IL-6, IL-9, IL-11, IL-13 and IL-17), matrix metalloproteinases (MMPs), and tissue inhibitors of MMPs (TIMPs) (Fig. 2f). Within tissues, eosinophils may interact with epithelial cells, smooth muscle cells, fibroblasts and endothelial cells to affect epithelial barrier functions, epithelial and/or smooth muscle cell hyperplasia, myofibroblast differentiation, deposition of extracellular matrix materials, and angiogenesis.
Eosinophil-derived mediators with effects on tissue repair and remodeling
Although by no means an exhaustive list, several key eosinophil-derived mediators with demonstrated roles in tissue repair and remodeling are described here.
TGF-β1 TGF-β1, an immunosuppressive cytokine that also serves as a key regulator of tissue fibrosis, has received the most attention as an eosinophil-derived profibrotic factor. Eosinophils are a major source of TGF-β1, and are the predominant cellular source of TGF-β1 in asthmatic airways (Aceves and Broide 2008) and in the esophagus of pediatric eosinophilic esophagitis patients (Aceves et al. 2007). TGF-β1 promotes myofibroblast differentiation of both fibroblasts (Desmouliere et al. 1993; Mattey et al. 1997) and epithelial cells (Willis et al. 2005), resulting in an over-abundance of extracellular matrix proteins, through enhanced production of fibronectin, proteoglycans and collagens I and III, and a decreased expression of collagenases (Aceves and Broide 2008). Moreover, TGF-β1 promotes proliferation of smooth muscle cells (Doherty and Broide 2007). In murine models, treatment with exogenous TGF-β1 or targeted overexpression of TGF-β1 mimics a fibrotic phenotype (Roberts et al. 1986; Sime et al. 1997) and neutralization of this cytokine abrogates bleomycin-induced fibrosis (Giri et al. 1993).
Th2 cytokines Many of the cytokines expressed by eosinophils have pro-fibrotic properties. Second to TGF-β1, the cytokine most clearly associated with fibrotic activities of eosinophils in vivo is IL-13. Inhibition of IL-13 attenuates airway inflammation and responsiveness in murine asthma models (Wills-Karp 2004), while overexpression of IL-13 in the lung induces multiple aspects of airway remodeling, including subepithelial fibrosis, goblet cell metaplasia, and smooth muscle cell hyperplasia (Fulkerson et al. 2006b). Importantly, when fibrosis-prone IL-13 transgenic mice were crossed to CCR3-deficient mice (exhibiting a 98% reduction in pulmonary eosinophils), the IL-13-mediated mucus cell metaplasia and collagen deposition were attenuated (Fulkerson et al. 2006b), demonstrating that eosinophil-derived IL-13 may play a central role in tissue remodeling, at least in the airways.
MMPs MMPs exist in soluble forms or as membrane-anchored proteinases, and function in the degradation of collagen, enabling the migration of inflammatory cells and contributing to ECM reorganization in connective tissues and basement membranes. TIMPs inhibit MMP functions, thus preventing collagen degradation and reorganization. Eosinophils store and secrete soluble MMP-9 and TIMP-1 (Dahlen et al. 1999), and constitutively express membrane-bound MMP17 (Gauthier et al. 2003). Increased expression of activated MMP9 is detected following allergen challenge, and in the airways (Han and Junxu 2003), sputum and bronchoalveolar lavage fluid (BALF) (Ko et al. 2005; Simpson et al. 2005) of asthmatics, and correlates with eosinophil infiltration (Dahlen et al. 1999).
Cationic proteins In addition to their tissue destructive properties, eosinophil-derived MBP and EDN can participate in tissue remodeling. EDN promotes fibroblast proliferation (Puxeddu et al. 2005b), and MBP synergizes with IL-5 and TGF-β to enhance fibroblast expression and secretion of IL-6 and IL-11 (Gomes et al. 2005; Le et al. 2007).
Eosinophils promote angiogenesis
Although blood vessels in adult mammals are generally quiescent, endothelial cells retain their capacity for rapid proliferation in response to physiologic stimuli such as hypoxia and inflammation, enabling the generation of new vessels and remodeling of the vascular tree. Angiogenesis is the process by which new vessels grow from existing ones, and is controlled by the balance of angiogenic inhibitors and pro-angiogenic factors within the vessel microenvironment. Among the most potent pro-angiogenic factors are vascular endothelial cell growth factor (VEGF) family members (previously termed vascular permeability factor, or VPF) (Senger et al. 1983). Thrombospondin and endostatin are major inhibitors of angiogenesis (Gupta et al. 1999; O’Reilly et al. 1997), and antagonize the functions of VEGF (Yamaguchi et al. 1999). Included within the preformed content of eosinophils are several pro-angiogenic factors, including VEGF, bFGF, IL-6, IL-8, GM-CSF, platelet derived growth factor (PDGF), TGF-β and angiogenin (reviewed in Aceves and Broide 2008; Detoraki et al. 2010). Eosinophils are stimulated to release pro-angiogenic factors upon encountering TNF-α (Ohno et al. 1997) or IL-5 and GM-CSF (Horiuchi and Weller 1997) in culture. In vitro, eosinophils promoted endothelial cell proliferation and new vessel formation in the chick embryo chorioallantoic membrane model, and neutralization of eosinophil-derived VEGF inhibited angiogenesis by 55% (Puxeddu et al. 2005a). Angiogenic processes are prominent in several pathological conditions associated with eosinophilia, including asthma. An increase in peribronchial vessels and leaky vessels is seen in asthmatic airways, accompanied by an increase in pro-angiogenic angiogenin, VEGF-A and bFGF expression (Hoshino et al. 2001b), co-localizing with eosinophils (Hoshino et al. 2001a).
Eosinophils as immunomodulators
Eosinophils have long been associated with effector functions of Th2 immune responses in allergic diseases and infections with helminth parasites. In the classic paradigm, IL-5-secreting Th2 lymphocytes recruit eosinophils to tissue sites, where terminal effector functions mediated by eosinophil cationic proteins contribute to parasite killing and tissue damage. However, it is now clear that eosinophils are associated not only with the effector arm of adaptive immunity but also in the elicitation of polarized adaptive responses (Fig. 2e). Evidence to date implicates eosinophils primarily in the initiation and elaboration of Th2-type responses. However, it is yet to be determined whether this reflects an artificial skewing by the specific antigens utilized in experimental studies, or represents a true proclivity of eosinophils toward Th2 immunity. Eosinophils provide immunoregulatory signals very early in an immune response, and continue to function at later time points to engage and regulate T cells in a dynamic interplay with the adaptive immune system. We have recently reviewed this topic in greater detail, specifically with respect to the induction of Th2 immunity (Spencer and Weller 2010).
Early immunoregulatory signals
Early immunoregulatory signals from eosinophils include: (1) secretion of immunomodulatory cytokines; (2) modulation of immature dendritic cells; (3) inhibition of Th1 development through expression of indoleamine 2,3-dioxygenase; and (4) activation of naïve T cells through direct antigen presentation functions. Most in vivo studies investigating early immunoregulatory functions of eosinophils to date have been performed using murine systems.
As detailed in the previous section, eosinophils store and rapidly secrete a vast array of cytokines and chemokines with distinct immunomodulatory capacities. Circulating murine eosinophils express a constitutively active IL-4 gene locus (Shinkai et al. 2002; Voehringer et al. 2004), produce early IL-4 and IL-13 mRNA and proteins (Gessner et al. 2005), and are among the first cells recruited in response to allergens or Th2-eliciting pathogens, independently of adaptive immunity (Sabin and Pearce 1995; Shinkai et al. 2002). Eosinophils are the predominant source of IL-4 within the lungs following infection with Nippostrongylus brasiliensis or intranasal installation of OVA (Shinkai et al. 2002; Voehringer et al. 2006), and within the peritoneal cavity following i.p. inoculation with Schistosoma mansoni eggs (Sabin et al. 1996). Thus, eosinophils can establish the cytokine milieu within a tissue microenvironment very early in infection, influencing subsequent cellular recruitment and the immune polarizing phenotype of immature dendritic cells. In addition to signaling dendritic cells through immune-polarizing cytokines, eosinophils secrete EDN, which acts as an alarmin, inducing differentiation of immature dendritic cells to a Th2-promoting phenotype (Yang et al. 2008).
In murine models, eosinophils migrate from tissue to draining lymph nodes, where they co-localize with T cells (Duez et al. 2004; MacKenzie et al. 2001; Shi et al. 2000, 2004; van Rijt et al. 2003). Within secondary lymphoid tissues, eosinophils may instruct the developing immune response through eosinophil-expressed mediators, and through direct antigen presentation functions. Human eosinophils express indoleamine 2,3-dioxygenase (IDO) (Odemuyiwa et al. 2004), an enzyme that catalyzes the catabolism of tryptophans to kynurenines. Metabolites of tryptophan catabolism specifically induce apoptosis in Th1 cells (Fallarino et al. 2002; Gurtner et al. 2003; Terness et al. 2002), thereby favoring the generation of Th2 lymphocytes.
Eosinophils are antigen presenting cells
Studies in mice and humans also support a role for eosinophils as professional antigen presenting cells (APCs) (reviewed in Akuthota et al. 2008). In vivo, eosinophils isolated from the BAL fluid of a patient with eosinophilic pneumonia (Beninati et al. 1993), from the sputum of asthmatics (Hansel et al. 1991), or from allergic rhinitis patients after undergoing segmental lung allergen challenge (Sedgwick et al. 1992), all express surface MHC II. Peripheral blood eosinophils exposed to superantigen or tetanus toxoid were sufficient to induce antigen-specific T cell proliferation, and prior fixation of eosinophils before (but not following) antigen exposure abrogated the response, demonstrating that active antigen processing by eosinophils was required for the observed T cell activation (Weller et al. 1993).
Likewise, mouse eosinophils isolated from draining lymph nodes following airway challenge (Duez et al. 2004), or from mice infected with the parasitic nematode Brugia malayi (Mawhorter et al. 1993), expressed surface MHC II and co-stimulatory molecules, including CD86 and CD40. Using murine models, eosinophils activated T cell clones and hybridomas (Del Pozo et al. 1992) as well as naïve or antigen-primed primary T cells (Padigel et al. 2006) in an antigen-specific manner. In vivo, eosinophils pulsed with antigen and adoptively transferred into naïve or immunized mice successfully primed (or boosted) recipients against Strongyloides stercoralis (Padigel et al. 2007), and antigen-loaded eosinophils instilled into the trachea of recipient mice induced T cell expansion in endobronchial lymph nodes (Shi et al. 2004; Wang et al. 2007a).
In contrast to significant evidence in favor of the capacity of eosinophils for antigen presentation, one study failed to observe eosinophil-dependent T cell activation. Van Rijt et al. isolated eosinophils from the bronchoalveolar lavage fluid of OVA- challenged mice, and upon adoptive transfer of eosinophils, were unable to detect T cell expansion in the draining lymph nodes of recipient mice (van Rijt et al. 2003). Importantly, prior to transfer of eosinophils to recipient mice, the authors utilized a standard method of red blood cell lysis using an ammonium chloride solution. However, an unfortunate side effect of ammonium chloride is that it interferes with lysosomal antigen processing and MHC II-dependent peptide presentation (Loss and Sant 1993; Ziegler and Unanue 1982). Therefore, technical issues are likely the explanation for the discrepant results. In support of this interpretation, in a follow-up study, Wang et al. 2007a demonstrated that antigen-exposed eosinophils adoptively transferred into recipient mice successfully primed naïve T cells, and that prior exposure of eosinophils to ammonium chloride was sufficient to abrogate this effect.
Eosinophil-T cell interactions throughout an immune response
Th2 cell-secreted IL-5 and CCL11 (eotaxin) promote eosinophil differentiation and recruitment, respectively, and are responsible for tissue eosinophilia. However, recent studies in murine models of Th2-eliciting parasitic infections reveal that, in some systems, an early wave of IL-4-producing tissue eosinophils is observed prior to the arrival of Th2 cells (Sabin and Pearce 1995; Sabin et al. 1996; Shinkai et al. 2002; Voehringer et al. 2004, 2006). Interestingly, T cell recruitment and accumulation was impaired following airway allergen challenge in mice deficient in eosinophils, whether the eosinophil defect was due to genetic deficiency (Fulkerson et al. 2006a; Jacobsen et al. 2008; Lee et al. 2004; Walsh et al. 2008) or through depletion of the CCL11 receptor, CCR3 (Fulkerson et al. 2006a), accompanied by decreases in Th2-associated cytokines (Fulkerson et al. 2006a; Jacobsen et al. 2008; Lee et al. 2004; Walsh et al. 2008) and diminished pathologic outcomes, including mucus production (Fulkerson et al. 2006a; Lee et al. 2004). Full restoration of the Th2 phenotype in eosinophil-deficient hosts required adoptive transfer of both T cells and eosinophils (Jacobsen et al. 2008; Walsh et al. 2008); transfer of Th2 cells alone was insufficient (Jacobsen et al. 2008), suggesting the contributions of eosinophils to T cell accumulation included more than immunoregulatory functions promoting Th2 differentiation. Rather, in both studies, the T cell deficiencies were attributed to the absence of eosinophil-derived chemokines (Jacobsen et al. 2008; Walsh et al. 2008).
In addition to promoting the specific tissue recruitment of Th2 lymphocytes, eosinophils may enhance the function of T cells within the tissues. Eosinophils and basophils have been shown to be sources of IL-25, a cytokine with multiple effects, including increasing Th2 polarization, proliferation and cytokine production of T cells (Wang et al. 2007b). Enhanced IL-25-induced production of T cell-derived IL-5 may in turn support further eosinophilic differentiation and tissue recruitment. Both naïve T cells and Th2 memory cells respond to IL-25. Due to enhanced expression of IL-25 receptors specifically by Th2 memory cells, these cells are especially susceptible to IL-25 signaling. As Th2 memory cells have been shown to be the subset of critical importance in acute allergic responses following allergen re-exposure, eosinophil-derived IL-25 may represent an important axis in modulating T cell activities in allergic responses.
Eosinophil interactions with other immune cells
Eosinophil-mast cell axis
Eosinophils and mast cells are major players in Th2-mediated allergies and parasite infections. An “eosinophil-mast cell axis” has been described whereby the two innate immune leukocytes interact to enhance their respective capabilities. The mast cell-specific protease chymase recruits eosinophils into tissue sites, suppresses eosinophil apoptosis, and promotes the secretion of eosinophil-derived cytokines and chemokines (Wong et al. 2009). Mast cells also secrete eosinophilopoietins including GM-CSF, IL-3, IL-5 and TNF-α (Levi-Schaffer et al. 1998; Shakoory et al. 2004).
Reciprocally, eosinophil-derived stem cell factor (SCF) induces activation, differentiation, maturation and survival of mast cells. A recent study suggests mediator exchange between mast cells and eosinophils may occur through direct cell-cell contacts (Matsuba-Kitamura et al. 2010).
Eosinophils and dendritic cells
Eosinophil-derived mediators are implicated in dendritic cell (DC) recruitment, activation and modulation of function. In a mouse model of allergic lung inflammation, eosinophils were found to be the most prominent source of the CCR1 ligands C10 and macrophage inflammatory protein (MIP)-1γ. Lung infiltration by CCR1-expressing CD11bhigh DCs occurred concurrently with the dominant eosinophil production of CCR1 ligands (Rose et al. 2010). Therefore, activated lung eosinophils are likely key players in attracting CCR1+ dendritic cells to inflammatory sites. Moreover, eosinophils are implicated in the promotion of a Th2-inducing phenotype in immature DCs. While mechanisms of DC maturation leading to Th1 immunity are defined, mechanisms promoting Th2 immune responses remain enigmatic. Eosinophils contribute to the induction of Th2 immune responses in multiple ways, as discussed above. One function relates to their capacity to activate local DCs specifically to adopt a Th2-promoting phenotype. Eosinophil-derived EDN acts as an innate immune alarmin, with the capacity to recruit (Yang et al. 2003) and activate (Yang et al. 2004) DCs in a TLR-2-dependent manner (Yang et al. 2008). Of note, 2 µg/mL of EDN induced the upregulation of co-stimulatory molecule expression and cytokine production from monocyte-derived DCs as robustly as 1 µg/mL of LPS, although with a skewing toward Th2 immunity, as evidenced by decreased IL-12 production in EDN-activated DCs compared with LPS-activated DCs (Yang et al. 2008).
Eosinophils and B lymphocytes
Although eosinophil–T cell cross-talk is now well established (see above), perhaps less well recognized are potential interplays between eosinophils and B lymphocytes. One example of such an interplay is seen in studies utilizing alum. Aluminum-containing compounds (e.g., alum) are routinely used to elicit an effective immune response to vaccines. Although the precise mechanism(s) by which alum successfully awakens the immune system is still unclear, antigen-free alum is known to elicit early priming of splenic B cells in a process relying upon a myeloid population of IL-4-producing Gr-1+ cells (Jordan et al. 2004), now identified to be eosinophils (Wang and Weller 2008). In a murine model of i.p. alum injection, Wang and Weller demonstrated alum-induced eosinophil recruitment to the spleen, in conjunction with eosinophil-dependent early priming of MHC II-mediated Ca2+ fluxes in splenic B cells and antigen-specific IgM production (Wang and Weller 2008). These findings carry important implications, both in the recognition of eosinophils as participants in adjuvant-elicited immune responses and in the identification of immunomodulatory functions of eosinophils affecting early stages of B cell activation and IgM production. Earlier studies from Abraham et al. (1995) suggested B cell activatory functions of eosinophils in immunity of the murine host to larval Strongyloides stercoralis, where the eosinophil deficiency of IL-5 knockout mice corresponded to a loss of IgM-mediated protective immunity. IgM-mediated host protection was restored upon reconstitution of eosinophils at the time of immunization (Galioto et al. 2006; Herbert et al. 2000).
Eosinophils in disease
Eosinophils are best known for their roles in anti-helminth immunity and allergic diseases. However, eosinophils are also effective combatants in some anti-bacterial and antiviral responses. How the innate immune functions of eosinophils (i.e. terminal effector functions, immunomodulation, and tissue remodeling) participate in specific disease processes are discussed here. Figure 2 summarizes multiple immune functions of eosinophils in relation to specific pathogens.
Eosinophils and host defense against helminths
Eosinophil-mediated host defense against helminths was originally ascribed to terminal effector functions of eosinophils. It is now becoming evident that additional aspects of the immunobiology of eosinophils also contribute to host immunity to parasites, including eosinophil-dependent effects on immunomodulation. Eosinophils and host defenses against helminths have been reviewed in greater detail by Klion and Nutman (2004).
Eosinophil terminal effector functions in anti-helminth immunity
Eosinophils are perhaps best recognized for their association with parasitic helminth infections. The presence of eosinophils in association with dead or dying parasites has been noted for over 70 years (Taliaferro and Sarles 1939), and led to the hypothesis that eosinophils were directly cytotoxic to helminths. Numerous in vitro studies give credence to this hypothesis, and implicate eosinophil granule-derived cationic proteins (e.g. MBP, ECP, EDN, EPO) in eosinophil-mediated parasite killing (Butterworth et al. 1979; Hamann et al. 1990) in the presence of antibody and/or complement (David et al. 1980; Haque et al. 1981; Kazura and Grove 1978) (Fig. 2b).
However, despite substantial data from in vitro assays, convincing evidence for direct eosinophil-dependent killing of parasites in vivo has been less forthcoming. In support of a non-redundant effector role for eosinophils, in vivo studies applying different methods to block eosinophil accumulation at sites of parasite infection demonstrated host protective roles for eosinophils against infections with Angiostrongylus cantonensis, Brugia malayi, Strongyloides stercoralis and Litomosoides sigmodontis (Sasaki et al. 1993; Yoshida et al. 1996; Simons et al. 2005; Ramalingam et al. 2003). For example, when S. stercoralis larvae, encased in diffusion chambers, were implanted into the subcutaneous tissue of mice, eosinophils accumulated within the chambers concurrently with larval killing (Abraham et al. 1995; Rotman et al. 1996), and prevention of eosinophil accumulation through treatment with anti-IL-5 antibodies abrogated both eosinophil infiltration and parasite death (Abraham et al. 1995; Herbert et al. 2000; Rotman et al. 1996).
The most prominent indication that eosinophils, and specifically their granular proteins, are a hallmark of the anti-helminth response came from Specht et al. (2006). This study showed that mice deficient for either EPO or MBP developed significantly higher worm burdens than wild-type mice after infection with the rodent filaria Litomosoides sigmodontis.
In contrast, studies of murine infections with Schistosoma mansoni and Toxocara canis failed to support a non-redundant function for eosinophils in helminth infections (Sher et al. 1990; Swartz et al. 2006), and infections with Trichinella spiralis have generated conflicting results. Unlike most helminths, Trichinella exists in its mammalian host in two developmental states. Ingested larvae pass into the small intestines, where they mature to adulthood. Newborn larvae are then shed by adult females into the lymph or blood, and are carried to skeletal muscle, where they form nurse cells. Therefore, immune responses are generated in the gastrointestinal system in response to adult parasites, and in skeletal muscle in response to nurse cell-larval complexes. Mice depleted of eosinophils through CCR3 deficiency exhibited an increase in cyst number in the skeletal muscle, and an increase in the frequency of live encysted larvae, suggesting eosinophils might be involved in limiting larval cysts (Gurish et al. 2002). However, infection of two strains of mice genetically engineered to be entirely deficient in eosinophils exhibited decreased larval survival, suggesting that the presence of eosinophils was actually beneficial to the survival of encysted larvae. In this study, the authors proposed that the presence of eosinophils, even at very low numbers (as may be seen in CCR3 −/− mice), is sufficient to maintain a larval-protective Th2 milieu, while in the complete absence of eosinophils, the ensuing Th1 response is destructive to larval cysts (Fabre et al. 2009).
Eosinophils contribute to anti-helminth immunity through non-cytotoxic methods
Highlighting the potential of eosinophils to function in the modulation of the immune microenvironment, eosinophils were found to be the most prevalent IL-4-producing cell within the lungs of Nippostrongylus brasiliensis infected mice (IL-4-expressing basophils and Th2 cells were also present in lower numbers) (Voehringer et al. 2004), and were rapidly recruited to the lungs of Rag −/− hosts, indicating that eosinophil recruitment does not depend upon an intact adaptive immune system (Shinkai et al. 2002). Of note, despite their quantitative dominance within the lungs, eosinophil-derived IL-4 is dispensable to development of systemic Th2 immunity in this model (Voehringer et al. 2006). Of note, both MBP- and EPO-deficient mice, lack IL-4, suggesting that MBP and EPO, aside from cytotoxic effects, may affect the cytokine milieu and modulate the Th2 response towards helminths (Specht et al. 2006).
Eosinophil antigen presentation functions have been implicated in host immunity to parasites, both in vitro and in vivo. For example, Padigel and colleagues injected antigen-loaded eosinophils intraperitoneally into naïve recipient mice and demonstrated induction of an antigen-specific immune response against Strongyloides stercoralis antigens (Padigel et al. 2007).
In addition, it was previously shown that eosinophils contribute to secondary but not primary-acute infections with some helminthes (Korenaga et al. 1991; Voehringer et al. 2006). This idea of eosinophils contributing to host immunity upon reinfection is strengthened by observations within populations by Hagan et al. (1985) showing that Gambian children with high eosinophil counts were less likely to be reinfected than those with lower counts at all levels of exposure categories. In the case of secondary infections with S. stercoralis in mice, eosinophils function through enhancing B lymphocyte activity. Herbert et al. (2000) noted that, in contrast to wild-type mice, IL-5 deficient mice failed to mount a protective immune response against a challenge infection with S. stercoralis, and exhibited a defect in parasite-specific IgM. Reconstitution of IL-5 knock-out mice with eosinophils at the time of immunization partially restored antigen-specific IgM to a level sufficient to fully restore a protective phenotype.
Collectively, these studies suggest that non-redundant functions of eosinophils in vivo tend to target those helminths with tissue migrating stages. Importantly, anti-helminthic roles of eosinophils involve immunomodulatory capacities in addition to terminal effector functions of eosinophils.
Eosinophils and allergic diseases
Eosinophils are well-known for their association with allergic diseases, including those affecting the skin (e.g., atopic dermatitis), respiratory system (e.g., rhinosinusitis, atopic asthma) and gastrointestinal tract (e.g., eosinophilic esophagitis, eosinophilic gastroenteritis). Defined and proposed roles of eosinophils in many of these conditions have been extensively reviewed elsewhere, and will not be addressed in depth here. However, it is pertinent to the current discussion to view the eosinophil activities of terminal effector functions, modulation of adaptive immunity and tissue repair and remodeling processes within the context of allergic diseases.
Granule-derived cationic proteins, such as MBP, are found in bronchoalveolar lavage fluid of asthmatics, within the skin of atopic dermatitis lesions, and within intestinal tissues of patients with eosinophilic infiltrates, causing damage to epithelial and nerve cells. Eosinophil-derived cationic proteins, including MBP, also stimulate degranulation of mast cells and basophils, further perpetuating the allergic milieu. Eosinophil-derived lipid mediators (i.e. cysteinyl leukotrienes) increase vascular permeability, promote mucus secretion, and cause smooth muscle cell constriction, while reactive oxygen species elaborated from eosinophils may injure cells of the mucosa. Thus, eosinophil terminal effector functions can exacerbate inflammation and clinical outcomes in response to allergens.
In addition to these cytotoxic effector mechanisms, eosinophils affect adaptive immunity generated in response to allergenic provocation. Perhaps the most well-studied example is found in murine models of asthma. In wild-type mice, allergen-induced airway inflammation is characterized by a Th2 immune bias that is diminished in association with impaired eosinophil recruitment in CCR3-deficient, CCL11/CCL24 double-deficient and IL-5/CCL11 double-deficient mice (Fulkerson et al. 2006a; Mattes et al. 2002), and in two distinct strains of mice on the C57BL/6 background genetically engineered to be devoid of eosinophils (Jacobsen et al. 2008; Lee et al. 2004; Walsh et al. 2008). In the latter cases, a defect in Th2 effector cell recruitment was also observed that was restored upon adoptive transfer of eosinophils (Jacobsen et al. 2008; Walsh et al. 2008). Of note, in contrast to their C57BL/6 counterparts, eosinophil-deficient ΔdblGATA1−/− mice on the BALB/c background exhibited no obvious defects in Th2 cell induction in response to airway allergen challenge (Humbles et al. 2004).
Tissue remodeling events are also critical components of allergic diseases, including asthma and eosinophilic esophagitis. Mouse models accomplishing a complete absence of eosinophils support non-redundant roles for eosinophils in the extensive airway remodeling accompanying allergic airways inflammation (Humbles et al. 2004). Confirmation in human subjects is lacking, likely due to inefficiencies in eosinophil depletion strategies (reviewed in Kay et al. 2004). One study administering a single dose of anti-IL-5 antibody failed to detect any improvement in asthma symptoms (Leckie et al. 2000). However, despite success in reducing blood and sputum eosinophilia, anti-IL-5 treatments only partially deplete eosinophils from lung tissue. For example, three infusions of a humanized anti-IL-5 antibody (mepolizumab), although robustly reducing peripheral and BAL eosinophils, achieved only a 55% reduction in eosinophil numbers within the bronchial mucosa. This partial reduction in the bronchial mucosa was sufficient to attenuate some aspects of tissue remodeling, evidenced by decreased deposition of matrix materials, including lumican, tenascin and procollagen III within the reticular basement membrane in comparison to placebo-treated patients. This study was not powered to investigate clinical outcomes (Flood-Page et al. 2003a, b). Thus, in both murine and human studies, eosinophils are implicated in tissue remodeling events associated with asthma. However, the extent to which eosinophil-mediated remodeling contributes to clinical outcomes in humans remains controversial, and further studies are underway to assess the efficacy of eosinophil depletion strategies in asthma.
Eosinophils and host defense against bacteria
During the last two decades, several reports have shown anti bacterial activity of eosinophils toward ingested bacteria, or through the release of eosinophil-derived bactericidal mediators toward extracellular bacteria. Lehrer et al. (1989) have shown that MBP and ECP cause permeabilization of outer and inner membranes of Escherichia coli. In addition, this study suggested that eosinophil cytoplasmic granules translocate their content of ECP and MBP into phagocytic vacuoles in order to kill ingested bacteria. A different killing mechanism was suggested by Nakajima et al. (2001) showing that eosinophil RNases have bactericidal activity against E. coli and Staphylococcus aureus in vitro. In addition, eosinophils demonstrated a rapid and efficient killing of E. coli in vitro through superoxide production in a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase- and EPO- (but not nitric oxide) dependent manner (Persson et al. 2001). Collectively, all cationic granule proteins have been shown to elicit in vitro bactericidal activity. Interestingly, Gram-negative and Gram-positive bacteria have been shown to activate eosinophils, especially E. coli that can induce eosinophil chemotaxis, degranulation and respiratory burst. MBP and EPO were readily released from eosinophils, even in the presence of only low numbers of bacteria, whereas higher bacterial loads were required for the release of ECP (Svensson and Wenneras 2005). Likewise, it was recently shown that isolated mouse eosinophils possess anti-pseudomonal properties in vitro through the release of cationic secondary granule proteins. In addition, hypereosinophilic IL-5 transgenic mice exhibited improved clearance of Pseudomonas aeruginosa introduced into the peritoneal cavity, while eosinophil-deficient mice showed impaired bacterial clearance that improved after adoptive transfer of eosinophils. Administration of eosinophil granule extracts significantly enhanced bacterial clearance in vivo (Linch et al. 2009). As described in the Innate immune functions of eosinophils section above, eosinophils also release extracellular nets, comprised of mitochondrial DNA and granule-derived proteins, that effectively trap and kill bacteria (Yousefi et al. 2008).
Eosinophils and host defense against viruses
In the last decade, several studies have revealed roles for eosinophils in anti-viral defense. In vivo, hypereosinophilic mice (IL-5 Tg) were shown to be more efficient at clearing human respiratory syncytial virus (RSV) from the lungs compared to wild-type mice (Phipps et al. 2007). Likewise, in a rodent model of pneumonia virus of mice (PVM) infection, eosinophil infiltration into the airways was correlated with improved recovery and associated with local expression of mouse eosinophil-associated RNase 11 (Domachowske et al. 2000a, b; Garvey et al. 2005).
In vitro studies suggest that anti-viral defense mechanisms of eosinophils utilize eosinophil-derived ribonuclease activity and other terminal effector functions, as well as immune modulatory capacities of eosinophils. Domachowske et al. (1998) showed that co-culture with intact eosinophils or recombinant human EDN alone can decrease human respiratory syncytial virus group B (RSV-B) infectivity. This antiviral effect was reversed by a ribonuclease inhibitor, suggesting a role for the eosinophil secretory ribonucleases in the clearance of RNA viruses. Indeed, recently, a targeted human EDN construct showed promising results in inhibiting hepatitis B virus (HBV) replication in hepatic cells through EDN RNase activity (Li et al. 2010). In addition to RNase activity, eosinophils have at their disposal alternative mechanisms for killing ssRNA viruses, such as production of nitric oxide, as shown by a murine model of hRSV infection. ssRNA binding to TLR7 on murine eosinophils mediated RSV clearance from the lungs in a MyD88 signaling-dependent manner. TLR7-MyD88 signaling induces expression of IFN regulatory factor (IRF)-7, NOS-2, and IFNβ in addition to eosinophil-associated ribonucleases EAR-1 and EAR-2, all molecules known to be involved in anti-viral host defense mechanisms (Phipps et al. 2007). Moreover, GM-CSF-stimulated human eosinophils were shown to bind rhinovirus RV16 ex vivo through ICAM-1. Eosinophils in co-culture with RV16 and T cells presented RV16 peptides to the antigen-specific T cells, inducing T cell proliferation and IFN-γ production, suggesting eosinophils may function as antigen presenting cells in viral infections (Handzel et al. 1998).
In addition to anti-viral functions of eosinophils, viral infections are correlated with allergic responses. Early-life respiratory viral infection in mice and humans is associated with recurrent respiratory sensitivities, including asthma, in later life. Furthermore, viral infections often trigger acute onset or exacerbations of asthma. The link between exacerbation of lung inflammation and viral pathogenesis may involve eosinophils. Mansson and Cardell (2009) showed that virus-induced activation of eosinophils, through TLR7 and TLR9, affects several eosinophil functions, such as release of EDN, promotion of airway epithelial cell death, and cytokine release, all effects known to participate in asthma and allergy pathology. Likewise, stimulation through TLR7/8 induced superoxide anion production by eosinophils (Nagase et al. 2003). Conversely, IL-5 primed eosinophils (phenotypically similar to eosinophils from atopic individuals) exhibited enhanced TLR7-and TLR9-mediated release of IL-8 and EDN, suggesting the presence of atopy may augment an eosinophil-mediated antiviral response.
Eosinophils and host defense against fungi
Eosinophils accumulate in the bronchoalveolar lavage of allergic bronchopulmonary aspergillosis patients, albeit to a lesser extent than neutrophils (Greenberger et al. 1988). Nevertheless, extracts from Alternaria and Penicillium induce calcium-dependent EDN release from eosinophils. In addition, Alternaria extract increases intracellular calcium concentrations, cell surface expression of CD63 and CD11b, and production of IL-8 from eosinophils in a pertusis toxin, PAF and formyl-methionyl-leucyl-phenylalanine (fMLP) receptor-dependent manner. In the same study, Alternaria extract failed to activate neutrophils, showing the response to be eosinophil-specific (Inoue et al. 2005). In a follow-up study, the same group found that Alternaria alternata produces aspartate proteases that cleave PAR-2 on eosinophils, exposing neo-ligands. These neo-ligands activate eosinophil degranulation and EDN release from eosinophils (Matsuwaki et al. 2009). Interestingly, although eosinophils do not express common fungal receptors, such as dectin-1 and lactosylceramide, human eosinophils were found to adhere to Alternaria alternara through binding to the major cell wall component, beta-glucan, through eosinophil-expressed CD11b integrin (Yoon et al. 2008). CD11b integrin-mediated adherence was required for eosinophil release of cytotoxic granule proteins, such as EDN and MBP, and chemokines such as MCP-1, IL-8, and MIP-1α, into the extracellular milieu and onto the surface of fungal organisms, mediating fungi killing in a contact-dependent manner (Fig. 2d).
Eosinophils and tumors
Many types of human cancer are associated with extensive eosinophilia, either within the tumor itself, in the peripheral blood, or in both locations. CCL11 (eotaxin) is detected in the stroma associated with some tumors, suggesting eosinophils may be actively recruited. Alternatively, since eosinophils are attracted to sites of cellular necrosis, eosinophil accumulation may be a byproduct of tumor cell death. In support of the latter, a recent study by Cormier et al. (2006) using a B16-F10 melanoma cell injection model demonstrated early CD4+ T cell-independent recruitment of eosinophils specifically into the necrotic regions of the tumor mass. That eosinophil accumulation did not depend upon Th2 cells suggests an innate, inflammatory mechanism of eosinophil recruitment driven by chemotactic effects of the necrotic tissue (Cormier et al. 2006). Consequences of eosinophils within tumor sites remain enigmatic. Relatively few studies to date have specifically investigated the participation of eosinophils in cancer. Those studies that have addressed effects of eosinophilic infiltration on tumor growth and regression have arrived at conflicting conclusions, finding the presence of eosinophils to be beneficial in some cancer models, and predictive of a less favorable outcome in others. These conflicting results may not be surprising when one considers the varied innate immune functions of eosinophils as detailed throughout this text.
In favor of anti-tumor roles for eosinophils, high numbers of infiltrating eosinophils have been associated with increased survival in patients with esophageal squamous cell carcinoma, gastric cancer, head and neck cancer and colorectal carcinoma (Ellyard et al. 2007), and several studies have shown that efficacious IL-2 treatments were associated with hyper-eosinophilia (Lotze et al. 1986; West et al. 1987). Some studies using eosinophil-overexpressing or -ablated mice further support a role for eosinophils in tumor regression. Tumor incidence and growth of methylcholanthrene (MCA)-induced fibrosarcomas was significantly attenuated in IL-5 transgenic mice compared to wild-type mice, and the diminished tumors were associated with massive infiltration of eosinophils within and surrounding tumors. Likewise, a higher tumor incidence was observed in CCL11-deficient, IL-5/CCL11-double-deficient, and ΔdblGATA mouse strains. Subsequent in vitro studies found that eosinophils could directly kill MCA-induced fibrosarcoma cells, suggesting a potential role for the eosinophil as an effector cell in tumor immune surveillance (Simson et al. 2007).
One suggested anti-tumor mechanism of eosinophils is direct cytotoxicity, either through granule-derived toxic mediators (Huland and Huland 1992) or antibody-dependent cell lysis (Porta et al. 1998).These mechanisms are supported by in vitro observations in human tumors showing effective tumor cytotoxicy by intact human eosinophils, or by purified granule proteins, such as MBP (Kubo et al. 1999). Furbert-Harris et al. (2003b) showed direct cytotoxicity of eosinophils towards MCF-7 multicellular tumor spheroids (MTS) by in vitro co-culture of tumor cells with eosinophils in the presence of exogenous IL-4. In addition, peripheral blood eosinophils from allergic and asthmatic individuals, or eosinophil conditioned media, have been shown to inhibit prostate tumor cell growth in vitro (Furbert-Harris et al. 2003a). Moreover, Legrand et al. demonstrated expression of functional gamma-delta T cell receptors on the surface of eosinophils, stimulation through which led to release of reactive oxygen species, EPO and EDN granule proteins and cytokines, and was implicated in eosinophil-mediated cytotoxicity against tumor cells (Legrand et al. 2009).
Another potential anti-tumor function of eosinophils may reside in their capacities for immunomodulation. Eosinophils are traditionally associated with Th2 immune responses, and therefore were suggested to take part in IL-4-mediated antitumor responses as demonstrated by several studies (Modesti et al. 1993; Pericle et al. 1994; Tepper et al. 1992). Accordingly, Mattes et al. have shown that Th2 responses are capable of clearing established lung and visceral metastases of a cytotoxic T lymphocyte (CTL)-resistant melanoma. Clearance of lung metastases by the Th2 cells was found to be totally dependent on eotaxin and STAT6, and to be mediated by eosinophils infiltrating into and degranulating within the tumor mass, inducing tumor regression. In contrast, tumor-specific CD4+ Th1 cells, that recruited macrophages into the tumors, had no effect on tumor growth in this model (Mattes et al. 2003).
In contrast to evidence suggesting eosinophils might promote tumor regression, additional studies support the contention that the presence of eosinophils is either inconsequential, or indicative of a poor prognosis. Injection of plasmacytoma J558L cells and the mammary adenocarcinoma together with IL-5-producing cells into mice failed to alter tumor growth kinetics, despite achieving local IL-5 secretion and rapid infiltration of the tumor by eosinophils (Kruger-Krasagakes et al. 1993). Likewise, although eosinophil cationic proteins ECP, EPO and EDN were significantly elevated in patients with renal cell adenocarcinoma before and during treatment with IL-2 and IFN-α (Moroni et al. 1997; Trulson et al. 1997), cationic protein-rich supernatants of cultured eosinophils from IL-2-treated patients with human renal cell carcinoma and melanoma failed to show direct cytotoxic activity, and elevated numbers of infiltrating eosinophils predicts the failure of IL-2 treatment against these kinds of tumors (Moroni et al. 2000; Porta et al. 1998). In addition, eosinophil-ablated mice, using anti-IL-5 monoclonal antibody, showed smaller tumor burdens and delayed onset of tumor development in a rodent model of squamous cell carcinoma (Wong et al. 1999), suggesting eosinophils may have a role in promoting tumors.
Other potential eosinophil-dependent functions that are likely to contribute involve tissue remodeling capacities of eosinophils. As detailed in “Innate immune functions of eosinophils” above, eosinophils are active in wound healing and tissue remodeling responses, through secretion of growth factors and tissue factors, direct interactions with tissue structural cells, and secretion of pro-angiogenic mediators. Under hypoxic conditions eosinophils exhibited increased survival, and increased secretion of VEGF (Nissim Ben Efraim et al. 2010). Eosinophils exposed to the hypoxic environment of a necrotic tumor core may therefore promote angiogenesis within the vicinity of the tumor, promoting tumor growth. A role for eosinophils in promoting angiogenesis and connective tissue formation adjacent to tumors has been proposed (Samoszuk 1997). Relative contributions of the multifarious innate immune functions of eosinophils to tumor biology in response to specific tumors is an area of ongoing research.
Eosinophils in health
Despite the heavy emphasis on blood-derived eosinophils as effector cells in allergic diseases and helminth infections, the often overlooked majority of eosinophils under healthy conditions are found within tissues of the gastrointestinal tract, genitourinary tract, and the thymus. Tissue recruitment of eosinophils at baseline relies predominantly upon CCL11 chemotactic gradients, and is mediated through eosinophil expression of CCR3. While a full understanding of the specific roles of baseline tissue eosinophils remains enigmatic, it is clear that eosinophils promote tissue differentiation and development, and contribute to tissue homeostasis. This is especially apparent through developmental functions of eosinophils in the uterus and mammary glands, and proposed tissue homeostatic roles of gastrointestinal eosinophils in the maintenance of the mucosal epithelial barrier. The full extent to which eosinophils contribute to innate immunity at baseline is yet to be determined. However, when one considers how intertwined innate immunity is with tissue homeostasis, it is appropriate to broaden our current perspective to include a brief discussion of developmental and homeostatic roles of eosinophils in health.
Directly pertinent to innate immunity, baseline tissue eosinophils: (1) may serve as sentinels in the tissues to which they naturally home, which interface with the external environment; and(2) are likely directly involved in the generation of mature adaptive immune cells. As expounded upon below, sentinel functions have been proposed for gastrointestinal eosinophils, and thymic eosinophils are implicated in negative T cell selection, and a predisposition toward Th2 immunity in children.
Eosinophils in uterine and mammary gland development
Eosinophil numbers in the uterine endometrium of rodents fluctuates, with peak values seen at estrous. Numbers of infiltrating eosinophils, and their proximity to the epithelial surface, further increase when exposed to semen during mating. By embryo implantation, eosinophil numbers have decreased, and remain low or absent throughout embryonic development. Reproductive studies in CCL11- or IL-5-deficient mice suggest that the overall capacity for implantation, embryo development and successful delivery remain intact when endometrial eosinophil numbers are absent or diminished. However, CCL11-deficient mice exhibit a delay in the age of initial onset of estrus (Gouon-Evans and Pollard 2001), and IL-5-deficient mice experience a slightly prolonged estrous cycle (Robertson et al. 2000). These findings may suggest a role for eosinophils in uterus maturation, and in uterine responsiveness to estrogen.
Similarly, in rodents, eotaxin expression in the stroma surrounding terminal end buds induces eosinophil infiltration into post-natal mammary tissue in conjunction with adolescent mammary gland development. Although the precise mechanisms by which eosinophils promote ductal branching has not been fully elucidated, it is clear that, in the absence of eosinophils, ductal branching is diminished (Gouon-Evans et al. 2000, 2002). Also critically required for ductal morphogenesis are macrophages. Mammary gland eosinophils secrete the macrophage chemoattractant C10, suggesting that macrophage recruitment may be one mechanism by which eosinophils participate in mammary gland branching. Moreover, the growing literature on the contributions of eosinophils to tissue remodeling in multiple organs suggests eosinophils may also play more direct roles in modulating the extracellular matrix required for mammary gland remodeling.
Gastrointestinal eosinophils
Eosinophils are found at baseline within the lamina propria throughout the intestinal tract, from the stomach through the colon. Although the functional significances of gastrointestinal eosinophils have yet to be fully delineated, eosinophils within the gastrointestinal tract exhibit an activated phenotype in contrast to blood eosinophils (Kato et al. 1998; Straumann et al. 2005), and are proposed to be involved in at least four aspects of innate immunity within the gastrointestinal tract, including: (1) homeostasis of the epithelial barrier; (2) immunity to luminal pathogens; (3) interactions with the enteric nervous system; and (4) bridging innate and adaptive immunity. Functions of gastrointestinal eosinophils are summarized in Fig. 3.
Eosinophil interactions with the epithelial barrier
Eosinophil cross-talk with gastrointestinal epithelium was recently reviewed in greater detail by Fillon et al. (2009). Eosinophils secrete TGF-β in response to co-culture with an epithelial cell line derived from human intestinal tissues. Epithelial cell damage or cell death served as a potent chemoattractant for eosinophils, and induced secretion of eosinophil-derived FGF-2 and TGF-β (Stenfeldt and Wenneras 2004), likely promoting epithelial repair and remodeling. Supportive of this interpretation, Fillon et al. reported epithelial barrier function to be enhanced in the presence of eosinophils at very low eosinophil to epithelial cell ratios (Fillon et al. 2009).
In contrast, other eosinophil granule-derived proteins, such as MBP, ECP and EDN, exert more deleterious effects on the epithelium, as suggested by the findings of Furuta et al. where MBP-deficient mice exhibited less epithelial dysfunction than their wild-type counterparts, and MBP added directly to the basolateral epithelial surface resulted in barrier dysfunction (Furuta et al. 2005). These findings highlight the yin and yang of eosinophil secretory responses, with secretion of granule-derived cationic proteins mediating tissue damage, while eosinophil granule-derived cytokines and growth factors promote tissue repair and remodeling. This dichotomy may explain how beneficial physiological roles of eosinophils at baseline may become detrimental under conditions of excessive eosinophilia.
Interactions with the enteric nervous system
In addition to interactions with the gastrointestinal epithelial barrier, eosinophils can modulate the enteric nervous system. Eosinophil-derived mediators (including MBP and SCF) stimulate mast cells to release mediators such as tryptase, histamine, PGD2, vasoactive intestinal peptide, substance P, serotonin, and leukotrienes, which have effects on nerve cells. Moreover, eosinophils themselves secrete neuroactive chemicals, and bind nerve cells directly, promoting nerve remodeling. Reciprocally, nerve cell-derived substance P induces eosinophil degranulation (Evans et al. 2000). In addition, MBP, and to a lesser extent EPO, bind and inhibit muscarinic M2, but not M3, receptors (Jacoby et al. 1993). Allosteric inhibition of M2 receptors causes an increase in acetylcholine, resulting in hyperresponsiveness and broncho-constriction in a guinea pig model of asthma.
Immunity to luminal pathogens
Within the GI mucosa, eosinophils are well-poised to contribute to anti-microbial immunity. Secretory IgA is found in abundance in association with the gastrointestinal mucosa, and is critical in host anti-microbial defenses. Eosinophils express receptors for IgA, and crosslinking of IgA receptors elicits eosinophil degranulation (Abu-Ghazaleh et al. 1989; Monteiro et al. 1993). As described above, eosinophil granules contain proteins with antimicrobial properties, including ECP and EDN. Moreover, as described in greater detail above, eosinophils share with neutrophils and mast cells the capacity to form “extracellular traps”, effective in sequestering and neutralizing extracellular bacteria. Following exposure to LPS from gram negative bacteria in vitro, eosinophils rapidly extruded granule-derived proteins and mitochondrial DNA in an extracellular structure. In vivo, ECP-expressing cells (which are likely eosinophils) with DNA-containing extrusions are found in the colons of patients with Crohn’s disease, and GI sections from a patient with spirochetosis revealed eosinophils in association with extruded extracellular traps and trapped bacteria (Yousefi et al. 2008).
Bridging innate and adaptive immunity
Although studies investigating eosinophil-mediated antigen-specific responses in the gastrointestinal tract are sparse, eosinophils have been observed in association with Peyer’s patches in wild-type mice. Interestingly, accumulation of eosinophils within Peyer’s patches was observed following airway allergen exposure (Mishra et al. 2000). Functions of eosinophils bridging innate and adaptive immunity within gastrointestinal tissues are currently underway.
Thymic eosinophils
Eosinophils naturally home to the neonatal thymus in humans (Lee et al. 1995). Although physiological significances of thymic eosinophils remain to be fully elucidated, studies in mice and humans reveal functions for thymic eosinophils in modulation of T cell selection and differentiation.
Murine studies reveal eosinophil infiltration into neonatal thymi, peaking at day 14, at which point numbers of thymic eosinophils and thymic dendritic cells have reached parity. Subsequently, numbers of eosinophils decrease over time, while dendritic cells continue to increase in prevalence. Reminiscent of tissue remodeling and homeostatic associations of eosinophils, at 16 weeks of age, thymic eosinophil numbers again rise, corresponding with thymic involution (Throsby et al. 2000).
Thymic eosinophils exhibit an activated phenotype, and express CD11c and antigen presentation machinery, including MHC II and CD86. Of note, thymic eosinophils localize primarily to the cortico-medullary regions in neonates, an area rich in double-positive thymocytes, and a site of negative selection, suggesting eosinophils may participate in T cell selection. In support of this theory, Throsby et al, utilizing a model of cognate peptide injection into TCR transgenic mice, demonstrated a strong eosinophilic influx into the thymus within 6 h following peptide injection, accompanied by the appearance of apoptotic thymocytes surrounding thymic eosinophils (Throsby et al. 2000). Thus, although not yet confirmed in humans, murine thymic eosinophils are implicated in thymocyte negative selection.
A recent elegant study addressed functional significances of thymic eosinophils in humans. Tulic et al. 2009 analyzed thymi from 22 children aged 7 days through 12 years (Tulic et al. 2009). Similar to the murine studies, human thymic eosinophils decrease in number with age. This study also demonstrated human thymic eosinophils to constitutively express active indoleamine 2,3-dioxygenase (IDO), the bi-product of which is known to promote a Th2 milieu through inducing the selective apoptosis of Th1 cells. These findings suggest a mechanistic explanation for the observed Th2 immune bias in young children.
Conclusions
Throughout the past decade, studies probing the immunobiology of eosinophils have begun to uncover an evolving story in which eosinophils participate in innate immune processes as more than terminal effector cells. An appreciation of the stores of granule-derived chemokines, cytokines and growth factors, packaged intracellularly within circulating and tissue eosinophils provides the basis for many newly-recognized functions of eosinophils, including regulation of the immune microenvironment, inflammatory response and tissue homeostasis and remo deling. The demonstration of direct antigen presentation capacities in eosinophils from mice and humans, identify eosinophils as cells that bridge innate and adaptive immunity. Through direct and indirect interactions with innate and adaptive immune cells, and with tissue cells, eosinophils participate in complex networks affecting a multitude of processes, under physiological and pathological conditions, that remain to be fully defined, laying a foundation for the next decade of eosinophil studies.
Acknowledgments
This work was supported by NIH grant HL095699 and an AHA Grant-in-Aid to L.A.S.
References
- Abdelilah SG, Bouchaib L, Morita M, Delphine A, Marika S, Andre C, Monique C. Molecular characterization of the low-affinity IgE receptor Fc epsilonRII/CD23 expressed by human eosinophils. Int Immunol. 1998;10:395–404. doi: 10.1093/intimm/10.4.395. [DOI] [PubMed] [Google Scholar]
- Abraham D, Rotman HL, Haberstroh HF, Yutanawiboonchai W, Brigandi RA, Leon O, Nolan TJ, Schad GA. Strongyloides stercoralis: protective immunity to third-stage larvae inBALB/ cByJ mice. Exp Parasitol. 1995;80:297–307. doi: 10.1006/expr.1995.1036. [DOI] [PubMed] [Google Scholar]
- Abu-Ghazaleh RI, Dunnette SL, Loegering DA, Checkel JL, Kita H, Thomas LL, Gleich GJ. Eosinophil granule proteins in peripheral blood granulocytes. J Leukoc Biol. 1992;52:611–618. doi: 10.1002/jlb.52.6.611. [DOI] [PubMed] [Google Scholar]
- Abu-Ghazaleh RI, Fujisawa T, Mestecky J, Kyle RA, Gleich GJ. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–2400. [PubMed] [Google Scholar]
- Aceves SS, Broide DH. Airway fibrosis and angiogenesis due to eosinophil trafficking in chronic asthma. Curr Mol Med. 2008;8:350–358. doi: 10.2174/156652408785161023. [DOI] [PubMed] [Google Scholar]
- Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Ahlstrom-Emanuelsson CA, Greiff L, Andersson M, Persson CG, Erjefalt JS. Eosinophil degranulation status in allergic rhinitis: observations before and during seasonal allergen exposure. Eur Respir J. 2004;24:750–757. doi: 10.1183/09031936.04.00133603. [DOI] [PubMed] [Google Scholar]
- Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rabia MW, Blaylock MG, Sexton DW, Thomson L, Walsh GM. Granule protein changes and membrane receptor pheno-type in maturing human eosinophils cultured from CD34+ progenitors. Clin Exp Allergy. 2003;33:640–648. doi: 10.1046/j.1365-2222.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- Archer GT, Hirsch JG. Motion picture studies on degranulation of horse eosinophils during phagocytosis. J Exp Med. 1963;118:287–294. doi: 10.1084/jem.118.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira-Melo C, Weller PF. Eosinophils and cysteinyl leuko-trienes. Prostaglandins Leukot Essent Fatty Acids. 2003;69:135–143. doi: 10.1016/s0952-3278(03)00074-7. [DOI] [PubMed] [Google Scholar]
- Bartemes KR, Cooper KM, Drain KL, Kita H. Secretory IgA induces antigen-independent eosinophil survival and cytokine production without inducing effector functions. J Allergy Clin Immunol. 2005;116:827–835. doi: 10.1016/j.jaci.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Batten D, Dyer KD, Domachowske JB, Rosenberg HF. Molecular cloning of four novel murine ribonuclease genes: unusual expansion within the ribonuclease A gene family. Nucleic Acids Res. 1997;25:4235–4239. doi: 10.1093/nar/25.21.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beil WJ, Weller PF, Tzizik DM, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of tumor necrosis factor-alpha to the matrix compartment of eosinophil secondary granules in patients with idiopathic hypereosinophilic syndrome. J Histochem Cytochem. 1993;41:1611–1615. doi: 10.1177/41.11.8409368. [DOI] [PubMed] [Google Scholar]
- Beninati W, Derdak S, Dixon PF, Grider DJ, Strollo DC, Hensley RE, Lucey DR. Pulmonary eosinophils express HLA-DR in chronic eosinophilic pneumonia. J Allergy Clin Immunol. 1993;92:442–449. doi: 10.1016/0091-6749(93)90123-w. [DOI] [PubMed] [Google Scholar]
- Borchers MT, Ansay T, DeSalle R, Daugherty BL, Shen H, Metzger M, Lee NA, Lee JJ. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J Leukoc Biol. 2002;71:1033–1041. [PubMed] [Google Scholar]
- Bozza PT, Yu W, Penrose JF, Morgan ES, Dvorak AM, Weller PF. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J Exp Med. 1997;186:909–920. doi: 10.1084/jem.186.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth AE, Sturrock RF, Houba V, Mahmoud AA, Sher A, Rees PH. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975;256:727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- Butterworth AE, Wassom DL, Gleich GJ, Loegering DA, David JR. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979;122:221–229. [PubMed] [Google Scholar]
- Capron M, Truong MJ, Aldebert D, Gruart V, Suemura M, Delespesse G, Tourvieille B, Capron A. Heterogeneous expression of CD23 epitopes by eosinophils from patients. Relationships with IgE-mediated functions. Eur J Immunol. 1991;21:2423–2429. doi: 10.1002/eji.1830211019. [DOI] [PubMed] [Google Scholar]
- Carlson MG, Peterson CG, Venge P. Human eosinophil peroxidase: purification and characterization. J Immunol. 1985;134:1875–1879. [PubMed] [Google Scholar]
- Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J Immunol. 2008;180:5625–5635. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]
- Cheung PF, Wong CK, Ho AW, Hu S, Chen DP, Lam CW. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol. 2010;22:453–467. doi: 10.1093/intimm/dxq027. [DOI] [PubMed] [Google Scholar]
- Cline MJ, Hanifin J, Lehrer RI. Phagocytosis by human eosinophils. Blood. 1968;32:922–934. [PubMed] [Google Scholar]
- Cormier SA, Larson KA, Yuan S, Mitchell TL, Lindenberger K, Carrigan P, Lee NA, Lee JJ. Mouse eosinophil-associated ribonucleases: a unique subfamily expressed during hematopoiesis. Mamm Genome. 2001;12:352–361. doi: 10.1007/s003350020007. [DOI] [PubMed] [Google Scholar]
- Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O’Neill K, Colbert D, Lombari TR, Constant S, McGarry MP, Lee JJ, Lee NA. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen B, Shute J, Howarth P. Immunohistochemical localisation of the matrix metalloproteinases MMP-3 and MMP-9 within the airways in asthma. Thorax. 1999;54:590–596. doi: 10.1136/thx.54.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JR, Butterworth AE, Vadas MA. Mechanism of the interaction mediating killing of Schistosoma mansoni by human eosinophils. Am J Trop Med Hyg. 1980;29:842–848. doi: 10.4269/ajtmh.1980.29.842. [DOI] [PubMed] [Google Scholar]
- Del Pozo V, De Andres B, Martin E, Cardaba B, Fernandez JC, Gallardo S, Tramon P, Leyva-Cobian F, Palomino P, Lahoz C. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. Eur J Immunol. 1992;22:1919–1925. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- Dent LA, Munro GH, Piper KP, Sanderson CJ, Finlay DA, Dempster RK, Bignold LP, Harkin DG, Hagan P. Eosinophilic interleukin 5 (IL-5) transgenic mice: eosinophil activity and impaired clearance of Schistosoma mansoni . Parasite Immunol. 1997;19:291–300. doi: 10.1046/j.1365-3024.1997.d01-210.x. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy. 2010;65:946–958. doi: 10.1111/j.1398-9995.2010.02372.x. [DOI] [PubMed] [Google Scholar]
- Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19:676–680. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- Domachowske JB, Bonville CA, Dyer KD, Easton AJ, Rosenberg HF. Pulmonary eosinophilia and production of MIP-1alpha are prominent responses to infection with pneumonia virus of mice. Cell Immunol. 2000a;200:98–104. doi: 10.1006/cimm.2000.1620. [DOI] [PubMed] [Google Scholar]
- Domachowske JB, Bonville CA, Gao JL, Murphy PM, Easton AJ, Rosenberg HF. The chemokine macrophage-inflammatory protein-1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxo-virus infection. J Immunol. 2000b;165:2677–2682. doi: 10.4049/jimmunol.165.5.2677. [DOI] [PubMed] [Google Scholar]
- Driss V, Legrand F, Hermann E, Loiseau S, Guerardel Y, Kremer L, Adam E, Woerly G, Dombrowicz D, Capron M. TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood. 2009;113:3235–3244. doi: 10.1182/blood-2008-07-166595. [DOI] [PubMed] [Google Scholar]
- Dubois GR, Schweizer RC, Versluis C, Bruijnzeel-Koomen CA, Bruijnzeel PL. Human eosinophils constitutively express a functional interleukin-4 receptor: interleukin-4 -induced priming of chemotactic responses and induction of PI-3 kinase activity. Am J Respir Cell Mol Biol. 1998;19:691–699. doi: 10.1165/ajrcmb.19.4.3208. [DOI] [PubMed] [Google Scholar]
- Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994;179:703–708. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez C, Dakhama A, Tomkinson A, Marquillies P, Balhorn A, Tonnel AB, Bratton DL, Gelfand EW. Migration and accumulation of eosinophils toward regional lymph nodes after airway allergen challenge. J Allergy Clin Immunol. 2004;114:820–825. doi: 10.1016/j.jaci.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Dunzendorfer S, Kaneider NC, Kaser A, Woell E, Frade JM, Mellado M, Martinez-Alonso C, Wiedermann CJ. Functional expression of chemokine receptor 2 by normal human eosinophils. J Allergy Clin Immunol. 2001;108:581–587. doi: 10.1067/mai.2001.118518. [DOI] [PubMed] [Google Scholar]
- Durack DT, Sumi SM, Klebanoff SJ. Neurotoxicity of human eosinophils. Proc Natl Acad Sci USA. 1979;76:1443–1447. doi: 10.1073/pnas.76.3.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack DT, Ackerman SJ, Loegering DA, Gleich GJ. Purification of human eosinophil-derived neurotoxin. Proc Natl Acad Sci USA. 1981;78:5165–5169. doi: 10.1073/pnas.78.8.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood. 2009;114:2649–2656. doi: 10.1182/blood-2009-01-199497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egesten A, Alumets J, von Mecklenburg C, Palmegren M, Olsson I. Localization of eosinophil cationic protein, major basic protein, and eosinophil peroxidase in human eosinophils by immunoelectron microscopic technique. J Histochem Cytochem. 1986;34:1399–1403. doi: 10.1177/34.11.3772075. [DOI] [PubMed] [Google Scholar]
- Egesten A, Calafat J, Weller PF, Knol EF, Janssen H, Walz TM, Olsson I. Localization of granule proteins in human eosinophil bone marrow progenitors. Int Arch Allergy Immunol. 1997;114:130–138. doi: 10.1159/000237657. [DOI] [PubMed] [Google Scholar]
- Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- Elsner J, Dulkys Y, Gupta S, Escher SE, Forssmann WG, Kapp A, Forssmann U. Differential pattern of CCR1 internalization in human eosinophils: prolonged internalization by CCL5 in contrast to CCL3. Allergy. 2005;60:1386–1393. doi: 10.1111/j.1398-9995.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- Erjefalt JS, Andersson M, Greiff L, Korsgren M, Gizycki M, Jeffery PK, Persson CGA. Cytolysis and piecemeal degranulation as distinct modes of activation of airway mucosal eosinophils. J Allergy Clin Immunol. 1998;102:286–294. doi: 10.1016/s0091-6749(98)70098-3. [DOI] [PubMed] [Google Scholar]
- Evans CM, Belmonte KE, Costello RW, Jacoby DB, Gleich GJ, Fryer AD. Substance P-induced airway hyperreactivity is mediated by neuronal M(2) receptor dysfunction. Am J Physiol Lung Cell Mol Physiol. 2000;279:L477–L486. doi: 10.1152/ajplung.2000.279.3.L477. [DOI] [PubMed] [Google Scholar]
- Fabre V, Beiting DP, Bliss SK, Gebreselassie NG, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182:1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- Fillon S, Robinson ZD, Colgan SP, Furuta GT. Epithelial function in eosinophilic gastrointestinal diseases. Immunol Allergy Clin North Am. 2009;29:171–178. doi: 10.1016/j.iac.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003a;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig M, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003b;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Tanaka H, Abe S. Interferon-gamma up-regulates expression of cysteinyl leukotriene type 2 receptors on eosinophils in asthmatic patients. Chest. 2005;128:3148–3155. doi: 10.1378/chest.128.5.3148. [DOI] [PubMed] [Google Scholar]
- Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci USA. 2006a;103:16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulkerson PC, Fischetti CA, Rothenberg ME. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. Am J Pathol. 2006b;169:2117–2126. doi: 10.2353/ajpath.2006.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbert-Harris P, Parish-Gause D, Laniyan I, Hunter KA, Okomo-Awich J, Vaughn TR, Forrest KC, Howland C, Abdelnaby A, Oredipe OA. Inhibition of prostate cancer cell growth by activated eosinophils. Prostate. 2003a;57:165–175. doi: 10.1002/pros.10286. [DOI] [PubMed] [Google Scholar]
- Furbert-Harris PM, Laniyan I, Harris D, Dunston GM, Vaughn T, Abdelnaby A, Parish-Gause D, Oredipe OA. Activated eosinophils infiltrate MCF-7 breast multicellular tumor spheroids. Anticancer Res. 2003b;23:71–78. [PubMed] [Google Scholar]
- Furuta GT, Nieuwenhuis EE, Karhausen J, Gleich G, Blumberg RS, Lee JJ, Ackerman SJ. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G890–G897. doi: 10.1152/ajpgi.00015.2005. [DOI] [PubMed] [Google Scholar]
- Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, Abraham D. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect Immun. 2006;74:5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey TL, Dyer KD, Ellis JA, Bonville CA, Foster B, Prussin C, Easton AJ, Domachowske JB, Rosenberg HF. Inflammatory responses to pneumovirus infection in IFN-alpha beta R gene-deleted mice. J Immunol. 2005;175:4735–4744. doi: 10.4049/jimmunol.175.7.4735. [DOI] [PubMed] [Google Scholar]
- Gauthier MC, Racine C, Ferland C, Flamand N, Chakir J, Tremblay GM, Laviolette M. Expression of membrane type-4 matrix metalloproteinase (metalloproteinase-17) by human eosinophils. Int J Biochem Cell Biol. 2003;35:1667–1673. doi: 10.1016/s1357-2725(03)00136-5. [DOI] [PubMed] [Google Scholar]
- Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Gleich GJ, Loegering DA, Maldonado JE. Identification of a major basic protein in guinea pig eosinophil granules. J Exp Med. 1973;137:1459–1471. doi: 10.1084/jem.137.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich GJ, Loegering DA, Bell MP, Checkel JL, Ackerman SJ, McKean DJ. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic protein: homology with ribonuclease. Proc Natl Acad Sci USA. 1986;83:3146–3150. doi: 10.1073/pnas.83.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005;116:796–804. doi: 10.1016/j.jaci.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Gounni AS, Lamkhioued B, Ochiai K, Tanaka Y, Delaporte E, Capron A, Kinet JP, Capron M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 2001;142:4515–4521. doi: 10.1210/endo.142.10.8459. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger PA, Smith LJ, Hsu CC, Roberts M, Liotta JL. Analysis of bronchoalveolar lavage in allergic bronchopulmonary aspergillosis: divergent responses of antigen-specific antibodies and total IgE. J Allergy Clin Immunol. 1988;82:164–170. doi: 10.1016/0091-6749(88)90995-5. [DOI] [PubMed] [Google Scholar]
- Grewe M, Czech W, Morita A, Werfel T, Klammer M, Kapp A, Ruzicka T, Schopf E, Krutmann J. Human eosinophils produce biologically active IL-12: implications for control of T cell responses. J Immunol. 1998;161:415–420. [PubMed] [Google Scholar]
- Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3:147–158. doi: 10.1023/a:1009018702832. [DOI] [PubMed] [Google Scholar]
- Gurish MF, Humbles A, Tao H, Finkelstein S, Boyce JA, Gerard C, Friend DS, Austen KF. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis . J Immunol. 2002;168:5730–5736. doi: 10.4049/jimmunol.168.11.5730. [DOI] [PubMed] [Google Scholar]
- Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of indoleamine 2, 3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Hagan P, Wilkins HA, Blumenthal UJ, Hayes RJ, Greenwood BM. Eosinophilia and resistance to Schistosoma haematobium in man. Parasite Immunol. 1985;7:625–632. doi: 10.1111/j.1365-3024.1985.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Hamann KJ, Gleich GJ, Checkel JL, Loegering DA, McCall JW, Barker RL. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol. 1990;144:3166–3173. [PubMed] [Google Scholar]
- Han Z, Junxu ZN. Expression of matrix metalloproteinases MMP-9 within the airways in asthma. Respir Med. 2003;97:563–567. doi: 10.1053/rmed.2001.1162. [DOI] [PubMed] [Google Scholar]
- Handzel ZT, Busse WW, Sedgwick JB, Vrtis R, Lee WM, Kelly EA, Gern JE. Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol. 1998;160:1279–1284. [PubMed] [Google Scholar]
- Hansel TT, Braunstein JB, Walker C, Blaser K, Bruijnzeel PL, Virchow JC, Jr, Virchow C., Sr Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. 1991;86:271–277. doi: 10.1111/j.1365-2249.1991.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Ouaissi A, Joseph M, Capron M, Capron A. IgE antibody in eosinophil- and macrophage-mediated in vitro killing of Dipetalonema viteae microfilariae. J Immunol. 1981;127:716–725. [PubMed] [Google Scholar]
- Hatano Y, Taniuchi S, Masuda M, Tsuji S, Ito T, Hasui M, Kobayashi Y, Kaneko K. Phagocytosis of heat-killed Staphylococcus aureus by eosinophils: comparison with neutrophils. APMIS. 2009;117:115–123. doi: 10.1111/j.1600-0463.2008.00022.x. [DOI] [PubMed] [Google Scholar]
- Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- Herbert DR, Lee JJ, Lee NA, Nolan TJ, Schad GA, Abraham D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J Immunol. 2000;165:4544–4551. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- Hibbs MS, Mainardi CL, Kang AH. Type-specific collagen degradation by eosinophils. Biochem J. 1982;207:621–624. doi: 10.1042/bj2070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- Hokibara S, Takamoto M, Tominaga A, Takatsu K, Sugane K. Marked eosinophilia in interleukin-5 transgenic mice fails to prevent Trichinella spiralis infection. J Parasitol. 1997;83:1186–1189. [PubMed] [Google Scholar]
- Horiuchi T, Weller PF. Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol. 1997;17:70–77. doi: 10.1165/ajrcmb.17.1.2796. [DOI] [PubMed] [Google Scholar]
- Horton MA, Larson KA, Lee JJ, Lee NA. Cloning of the murine eosinophil peroxidase gene (mEPO): characterization of a conserved subgroup of mammalian hematopoietic peroxidases. J Leukoc Biol. 1996;60:285–294. doi: 10.1002/jlb.60.2.285. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angio-genesis in bronchial asthma. J Allergy Clin Immunol. 2001a;107:1034–1038. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001b;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- Hu S, Wong CK, Lam CW. Activation of eosinophils by IL-12 family cytokine IL-27: Implications of the pleiotropic roles of IL-27 in allergic responses. Immunobiology. 2010 doi: 10.1016/j.imbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Huland E, Huland H. Tumor-associated eosinophilia in interleukin-2-treated patients: evidence of toxic eosinophil degranulation on bladder cancer cells. J Cancer Res Clin Oncol. 1992;118:463–467. doi: 10.1007/BF01629431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, Martin TR, Gerard NP, Gerard C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci USA. 2002;99:1479–1484. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1726–1729. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Matsuwaki Y, Shin SH, Ponikau JU, Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005;175:5439–5447. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, MacDonald GH, Wight TN. Immunoelectron microscopic localization of catalase in human eosinophilic leukocytes. J Histochem Cytochem. 1982;30:697–701. doi: 10.1177/30.7.6809811. [DOI] [PubMed] [Google Scholar]
- Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993;91:1314–1318. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–1810. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Woo SY, Jang MH, Miyasaka M, Ryu KH, Park HK, Seoh JY. Human eosinophils show chemotaxis to lymphoid chemokines and exhibit antigen-presenting-cell-like properties upon stimulation with IFN-gamma, IL-3 and GM-CSF. Int Arch Allergy Immunol. 2008;146:227–234. doi: 10.1159/000115891. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Swanson MC, Gleich GJ, Kita H. Allergen-specific IgG1 and IgG3 through Fc gamma RII induce eosinophil degranulation. J Clin Invest. 1995;95:2813–2821. doi: 10.1172/JCI117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Kephart GM, Talley NJ, Wagner JM, Sarr MG, Bonno M, McGovern TW, Gleich GJ. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998;252:418–425. doi: 10.1002/(SICI)1097-0185(199811)252:3<418::AID-AR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kay AB, Shin HS, Austen KF. Selective attraction of eosinophils and synergism between eosinophil chemotactic factor of anaphylaxis (ECF-A) and a fragment cleaved from the fifth component of complement (C5a) Immunology. 1973;24:969–976. [PMC free article] [PubMed] [Google Scholar]
- Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodeling in asthma. Trends in Immunology. 2004;26:477–482. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kazura JW, Grove DI. Stage-specific antibody-dependent eosinophil-mediated destruction of Trichinella spiralis . Nature. 1978;274:588–589. doi: 10.1038/274588a0. [DOI] [PubMed] [Google Scholar]
- Khalife J, Dunne DW, Richardson BA, Mazza G, Thorne KJ, Capron A, Butterworth AE. Functional role of human IgG subclasses in eosinophil-mediated killing of schistosomula of Schistosoma mansoni . J Immunol. 1989;142:4422–4427. [PubMed] [Google Scholar]
- Kierszenbaum F, Ackerman SJ, Gleich GJ. Destruction of bloodstream forms of Trypanosoma cruzi by eosinophil granule major basic protein. Am J Trop Med Hyg. 1981;30:775–779. doi: 10.4269/ajtmh.1981.30.775. [DOI] [PubMed] [Google Scholar]
- Kleine TJ, Gleich GJ, Lewis SA. Eosinophil peroxidase increases membrane permeability in mammalian urinary bladder epithelium. Am J Physiol. 1999;276:C638–C647. doi: 10.1152/ajpcell.1999.276.3.C638. [DOI] [PubMed] [Google Scholar]
- Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- Ko FW, Diba C, Roth M, McKay K, Johnson PR, Salome C, King GG. A comparison of airway and serum matrix metalloproteinase-9 activity among normal subjects, asthmatic patients, and patients with asthmatic mucus hypersecretion. Chest. 2005;127:1919–1927. doi: 10.1378/chest.127.6.1919. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kouzaki H, Kita H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol. 2010;184:6350–6358. doi: 10.4049/jimmunol.0902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenaga M, Hitoshi Y, Yamaguchi N, Sato Y, Takatsu K, Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology. 1991;72:502–507. [PMC free article] [PubMed] [Google Scholar]
- Kruger-Krasagakes S, Li W, Richter G, Diamantstein T, Blankenstein T. Eosinophils infiltrating interleukin-5 gene-transfected tumors do not suppress tumor growth. Eur J Immunol. 1993;23:992–995. doi: 10.1002/eji.1830230438. [DOI] [PubMed] [Google Scholar]
- Kubo H, Loegering DA, Adolphson CR, Gleich GJ. Cytotoxic properties of eosinophil granule major basic protein for tumor cells. Int Arch Allergy Immunol. 1999;118:426–428. doi: 10.1159/000024154. [DOI] [PubMed] [Google Scholar]
- Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–155. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- Lacy P, Levi-Schaffer F, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez J, Kay AB, Moqbel R. Intracellular localization of interleukin-6 in eosinophils from atopic asthmatics and effects of interferon gamma. Blood. 1998;91:2508–2516. [PubMed] [Google Scholar]
- Lamkhioued B, Aldebert D, Gounni AS, Delaporte E, Goldman M, Capron A, Capron M. Synthesis of cytokines by eosinophils and their regulation. Int Arch Allergy Immunol. 1995;107:122–123. doi: 10.1159/000236949. [DOI] [PubMed] [Google Scholar]
- Larson KA, Horton MA, Madden BJ, Gleich GJ, Lee NA, Lee JJ. The identification and cloning of a murine major basic protein gene expressed in eosinophils. J Immunol. 1995;155:3002–3012. [PubMed] [Google Scholar]
- Larson KA, Olson EV, Madden BJ, Gleich GJ, Lee NA, Lee JJ. Two highly homologous ribonuclease genes expressed in mouse eosinophils identify a larger subgroup of the mammalian ribonuclease superfamily. Proc Natl Acad Sci USA. 1996;93:12370–12375. doi: 10.1073/pnas.93.22.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AV, Cho JY, Miller M, McElwain S, Golgotiu K, Broide DH. Inhibition of allergen-induced airway remodeling in Smad 3-deficient mice. J Immunol. 2007;178:7310–7316. doi: 10.4049/jimmunol.178.11.7310. [DOI] [PubMed] [Google Scholar]
- Leckie MJ, ten Brinke A, Khan J, Diamant Z, O’Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- Lee I, Yu E, Good RA, Ikehara S. Presence of eosinophilic precursors in the human thymus: evidence for intra-thymic differentiation of cells in eosinophilic lineage. Pathol Int. 1995;45:655–662. doi: 10.1111/j.1440-1827.1995.tb03518.x. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- Legrand F, Driss V, Woerly G, Loiseau S, Hermann E, Fournie JJ, Heliot L, Mattot V, Soncin F, Gougeon ML, Dombrowicz D, Capron M. A functional gammadeltaTCR/CD3 complex distinct from gammadeltaT cells is expressed by human eosinophils. PLoS ONE. 2009;4:e5926. doi: 10.1371/journal.pone.0005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989;142:4428–4434. [PubMed] [Google Scholar]
- Levi-Schaffer F, Lacy P, Severs NJ, Newman TM, North J, Gomperts B, Kay AB, Moqbel R. Association of granulocyte-macrophage colony-stimulating factor with the crystalloid granules of human eosinophils. Blood. 1995;85:2579–2586. [PubMed] [Google Scholar]
- Levi-Schaffer F, Barkans J, Newman TM, Ying S, Wakelin M, Hohenstein R, Barak V, Lacy P, Kay AB, Moqbel R. Identification of interleukin-2 in human peripheral blood eosinophils. Immunology. 1996;87:155–161. [PMC free article] [PubMed] [Google Scholar]
- Levi-Schaffer F, Temkin V, Malamud V, Feld S, Zilberman Y. Mast cells enhance eosinophil survival in vitro: role of TNF-alpha and granulocyte-macrophage colony-stimulating factor. J Immunol. 1998;160:5554–5562. [PubMed] [Google Scholar]
- Li HL, Glick AD. Ultrastructural localization of 2-naphthylthiol acetate nonspecific esterase in human blood cells and leukemic cells. Exp Mol Pathol. 1987;46:321–330. doi: 10.1016/0014-4800(87)90053-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao Y, Liu J, Huang Y, Liu Z, Xue C. A promising alternative anti-HBV agent: the targeted ribonuclease. Int J Mol Med. 2010;26:51–56. doi: 10.3892/ijmm_00000434. [DOI] [PubMed] [Google Scholar]
- Linch SN, Kelly AM, Danielson ET, Pero R, Lee JJ, Gold JA. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun. 2009;77:4976–4982. doi: 10.1128/IAI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid after segmental antigen challenge. J Allergy Clin Immunol. 2003;112:556–562. doi: 10.1016/s0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- Logan MR, Lacy P, Odemuyiwa SO, Steward M, Davoine F, Kita H, Moqbel R. A critical role for vesicle-associated membrane protein-7 in exocytosis from human eosinophils and neutrophils. Allergy. 2006;61:777–784. doi: 10.1111/j.1398-9995.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- Lopez AF, Begley CG, Williamson DJ, Warren DJ, Vadas MA, Sanderson CJ. Murine eosinophil differentiation factor. An eosinophil-specific colony-stimulating factor with activity for human cells. J Exp Med. 1986;163:1085–1099. doi: 10.1084/jem.163.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss GE, Jr, Sant AJ. Invariant chain retains MHC class II molecules in the endocytic pathway. J Immunol. 1993;150:3187–3197. [PubMed] [Google Scholar]
- Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A, Schrezenmeier H, Lotze MT. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J Immunol. 2009;183:5023–5031. doi: 10.4049/jimmunol.0900504. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Chang AE, Seipp CA, Simpson C, Vetto JT, Rosenberg SA. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. Jama. 1986;256:3117–3124. [PubMed] [Google Scholar]
- Lucey DR, Nicholson-Weller A, Weller PF. Mature human eosinophils have the capacity to express HLA-DR. Proc Natl Acad Sci USA. 1989;86:1348–1351. doi: 10.1073/pnas.86.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias MP, Welch KC, Denzler KL, Larson KA, Lee NA, Lee JJ. Identification of a new murine eosinophil major basic protein (mMBP) gene: cloning and characterization of mMBP-2. J Leukoc Biol. 2000;67:567–576. doi: 10.1002/jlb.67.4.567. [DOI] [PubMed] [Google Scholar]
- MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001;167:3146–3155. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- Mansson A, Cardell LO. Role of atopic status in Toll-like receptor (TLR)7- and TLR9-mediated activation of human eosinophils. J Leukoc Biol. 2009;85:719–727. doi: 10.1189/jlb.0808494. [DOI] [PubMed] [Google Scholar]
- Matsuba-Kitamura S, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Taki Y, Muto T, Ikeda T, Mimura O, Nakanishi K. Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int Immunol. 2010;22:479–489. doi: 10.1093/intimm/dxq035. [DOI] [PubMed] [Google Scholar]
- Matsuwaki Y, Wada K, White TA, Benson LM, Charlesworth MC, Checkel JL, Inoue Y, Hotta K, Ponikau JU, Lawrence CB, Kita H. Recognition of fungal protease activities induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils. J Immunol. 2009;183:6708–6716. doi: 10.4049/jimmunol.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. Intrinsic defect in T cell production of interleukine (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, Parish C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattey DL, Dawes PT, Nixon NB, Slater H. Transforming growth factor beta 1 and interleukin 4 induced alpha smooth muscle actin expression and myofibroblast-like differentiation in human synovial fibroblasts in vitro: modulation by basic fibroblast growth factor. Ann Rheum Dis. 1997;56:426–431. doi: 10.1136/ard.56.7.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhorter SD, Pearlman E, Kazura JW, Boom WH. Class II major histocompatibility complex molecule expression on murine eosinophils activated in vivo by Brugia malayi . Infect Immun. 1993;61:5410–5412. doi: 10.1128/iai.61.12.5410-5412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DJ, McKean JR, Olsson I, Venges P, Kay AB. Morphological studies on the killing of schistosomula of Schistosoma mansoni by human eosinophil and neutrophil cationic proteins in vitro. Parasite Immunol. 1981;3:359–373. doi: 10.1111/j.1365-3024.1981.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Melo RC, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RC, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol. 2008;83:229–236. doi: 10.1189/jlb.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miike S, McWilliam AS, Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol. 2001;167:6615–6622. doi: 10.4049/jimmunol.167.11.6615. [DOI] [PubMed] [Google Scholar]
- Minshall E, Chakir J, Laviolette M, Molet S, Zhu Z, Olivenstein R, Elias JA, Hamid Q. IL-11 expression is increased in severe asthma: association with epithelial cells and eosinophils. J Allergy Clin Immunol. 2000;105:232–238. doi: 10.1016/s0091-6749(00)90070-8. [DOI] [PubMed] [Google Scholar]
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. Peyer’s patch eosinophils: identification, characterization, and regulation by mucosal allergen exposure, interleukin-5, and eotaxin. Blood. 2000;96:1538–1544. [PubMed] [Google Scholar]
- Modesti A, Masuelli L, Modica A, D’Orazi G, Scarpa S, Bosco MC, Forni G. Ultrastructural evidence of the mechanisms responsible for interleukin-4-activated rejection of a spontaneous murine adenocarcinoma. Int J Cancer. 1993;53:988–993. doi: 10.1002/ijc.2910530622. [DOI] [PubMed] [Google Scholar]
- Moller GM, de Jong TA, Overbeek SE, van der Kwast TH, Postma DS, Hoogsteden HC. Ultrastructural immunogold localization of interleukin 5 to the crystalloid core compartment of eosinophil secondary granules in patients with atopic asthma. J Histochem Cytochem. 1996;44:67–69. doi: 10.1177/44.1.8543784. [DOI] [PubMed] [Google Scholar]
- Monteiro RC, Hostoffer RW, Cooper MD, Bonner JR, Gartland GL, Kubagawa H. Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. J Clin Invest. 1993;92:1681–1685. doi: 10.1172/JCI116754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqbel R, Coughlin JJ. Differential secretion of cytokines. Sci STKE 2006. 2006 doi: 10.1126/stke.3382006pe26. pe26. [DOI] [PubMed] [Google Scholar]
- Moqbel R, Lacy P. Exocytotic events in eosinophils and mast cells. Clin Exp Allergy. 1999;29:1017–1022. doi: 10.1046/j.1365-2222.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- Moqbel R, Ying S, Barkans J, Newman TM, Kimmitt P, Wakelin M, Taborda-Barata L, Meng Q, Corrigan CJ, Durham SR, Kay AB. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J Immunol. 1995;155:4939–4947. [PubMed] [Google Scholar]
- Moroni M, Porta C, Gritti D, Di Amici M, Giacobbe O, Bobbio-Pallavicini E, Notario A. Cationic protein-rich supernatants of cultured eosinophils from IL-2-treated patients have no cytotoxic activity on human renal cell carcinoma and melanoma cells: a preliminary report. Ann NY Acad Sci. 1997;832:295–303. doi: 10.1111/j.1749-6632.1997.tb46257.x. [DOI] [PubMed] [Google Scholar]
- Moroni M, Porta C, De Amici M, Quaglini S, Cattabiani MA, Buzio C. Eosinophils and C4 predict clinical failure of combination immunotherapy with very low dose subcutaneous interleukin-2 and interferon in renal cell carcinoma patients. Haematologica. 2000;85:298–303. [PubMed] [Google Scholar]
- Motojima S, Frigas E, Loegering DA, Gleich GJ. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis. 1989;139:801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- Nagase H, Miyamasu M, Yamaguchi M, Kawasaki H, Ohta K, Yamamoto K, Morita Y, Hirai K. Glucocorticoids preferentially upregulate functional CXCR4 expression in eosinophils. J Allergy Clin Immunol. 2000;106:1132–1139. doi: 10.1067/mai.2000.110923. [DOI] [PubMed] [Google Scholar]
- Nagase H, Kudo K, Izumi S, Ohta K, Kobayashi N, Yamaguchi M, Matsushima K, Morita Y, Yamamoto K, Hirai K. Chemokine receptor expression profile of eosinophils at inflamed tissue sites: decreased CCR3 and increased CXCR4 expression by lung eosinophils. J Allergy Clin Immunol. 2001;108:563–569. doi: 10.1067/mai.2001.118292. [DOI] [PubMed] [Google Scholar]
- Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Yamada H, Iikura M, Miyamasu M, Izumi S, Shida H, Ohta K, Imai T, Yoshie O, Mochizuki M, Schroder JM, Morita Y, Yamamoto K, Hirai K. Intracellular localization and release of eotaxin from normal eosinophils. FEBS Lett. 1998;434:226–230. doi: 10.1016/s0014-5793(98)00863-1. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Hirakata M, Nittoh T, Ishihara K, Ohuchi K. Expression and purification of recombinant rat eosinophil-associated ribonucleases, homologues of human eosinophil cationic protein and eosinophil-derived neurotoxin, and their characterization. Int Arch Allergy Immunol. 2001;125:241–249. doi: 10.1159/000053822. [DOI] [PubMed] [Google Scholar]
- Neves JS, Weller PF. Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr Opin Immunol. 2009;6:694–699. doi: 10.1016/j.coi.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves JS, Perez SA, Spencer LA, Melo RC, Reynolds L, Ghiran I, Mahmudi-Azer S, Odemuyiwa SO, Dvorak AM, Moqbel R, Weller PF. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci USA. 2008;105:18478–18483. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissim Ben Efraim AH, Eliashar R, Levi-Schaffer F. Hypoxia modulates human eosinophil function. Clin Mol Allergy. 2010;8:10. doi: 10.1186/1476-7961-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Moqbel R. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2, 3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno Y, Tamura G, Yamauchi K, Nagura H, Shirato K. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997;16:212–219. doi: 10.1165/ajrcmb.16.3.9070604. [DOI] [PubMed] [Google Scholar]
- Oliveira SH, Lira S, Martinez AC, Wiekowski M, Sullivan L, Lukacs NW. Increased responsiveness of murine eosinophils to MIP-1beta (CCL4) and TCA-3 (CCL1) is mediated by their specific receptors, CCR5 and CCR8. J Leukoc Biol. 2002;71:1019–1025. [PubMed] [Google Scholar]
- Olsen RL, Little C. Purification and some properties of myeloperoxidase and eosinophil peroxidase from human blood. Biochem J. 1983;209:781–787. doi: 10.1042/bj2090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I, Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood. 1974;44:235–246. [PubMed] [Google Scholar]
- Padigel UM, Lee JJ, Nolan TJ, Schad GA, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis . Infect Immun. 2006;74:3232–3238. doi: 10.1128/IAI.02067-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, Abraham D. Eosinophils act as antigen presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis. 2007;196:1844–1851. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericle F, Giovarelli M, Colombo MP, Ferrari G, Musiani P, Modesti A, Cavallo F, Di Pierro F, Novelli F, Forni G. An efficient Th2-type memory follows CD8+ lymphocyte-driven and eosinophil-mediated rejection of a spontaneous mouse mammary adenocarcinoma engineered to release IL-4. J Immunol. 1994;153:5659–5673. [PubMed] [Google Scholar]
- Persson T, Andersson P, Bodelsson M, Laurell M, Malm J, Egesten A. Bactericidal activity of human eosinophilic granulocytes against Escherichia coli . Infect Immun. 2001;69:3591–3596. doi: 10.1128/IAI.69.6.3591-3596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RM, Stubbs VE, Henson MR, Williams TJ, Pease JE, Sabroe I. Variations in eosinophil chemokine responses: an investigation of CCR1 and CCR3 function, expression in atopy, and identification of a functional CCR1 promoter. J Immunol. 2003;170:6190–6201. doi: 10.4049/jimmunol.170.12.6190. [DOI] [PubMed] [Google Scholar]
- Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- Plotz SG, Traidl-Hoffmann C, Feussner I, Kasche A, Feser A, Ring J, Jakob T, Behrendt H. Chemotaxis and activation of human peripheral blood eosinophils induced by pollen-associated lipid mediators. J Allergy Clin Immunol. 2004;113:1152–1160. doi: 10.1016/j.jaci.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popken-Harris P, Checkel J, Loegering D, Madden B, Springett M, Kephart G, Gleich GJ. Regulation and processing of a precursor form of eosinophil granule major basic protein (ProMBP) in differentiating eosinophils. Blood. 1998;92:623–631. [PubMed] [Google Scholar]
- Porta C, Moroni M, De Amici M. Eosinophils and serum eosinophilic cationic proteins in interleukin-2-based immunotherapy for cancer. Br J Haematol. 1998;100:607–609. doi: 10.1046/j.1365-2141.1998.0636d.x. [DOI] [PubMed] [Google Scholar]
- Puxeddu I, Alian A, Piliponsky AM, Ribatti D, Panet A, Levi-Schaffer F. Human peripheral blood eosinophils induce angiogenesis. Int J Biochem Cell Biol. 2005a;37:628–636. doi: 10.1016/j.biocel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Puxeddu I, Ribatti D, Crivellato E, Levi-Schaffer F. Mast cells and eosinophils: a novel link between inflammation and angiogenesis in allergic diseases. J Allergy Clin Immunol. 2005b;116:531–536. doi: 10.1016/j.jaci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Radke A, Reynolds LE, Melo RCN, Dvorak AM, Weller PF, Spencer LA. Mature human eosinophils express functional Notch ligands mediating eosinophil autocrine regulation. Blood. 2009;113:3092–3101. doi: 10.1182/blood-2008-05-155937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulasingam K, Till S, Ying S, Humbert M, Barkans J, Sullivan M, Meng Q, Corrigan CJ, Bungre J, Grant JA, Kay AB, Durham SR. Increased expression of high affinity IgE (FcepsilonRI) receptor-alpha chain mRNA and protein-bearing eosinophils in human allergen-induced atopic asthma. Am J Respir Crit Care Med. 1998;158:233–240. doi: 10.1164/ajrccm.158.1.9708106. [DOI] [PubMed] [Google Scholar]
- Ramalho-Pinto FJ, McLaren DJ, Smithers SR. Complement-mediated killing of schistosomula of Schistosoma mansoni by rat eosinophils in vitro. J Exp Med. 1978;147:147–156. doi: 10.1084/jem.147.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam T, Ganley-Leal L, Porte P, Rajan TV. Impaired clearance of primary but not secondary Brugia infections in IL-5 deficient mice. Exp Parasitol. 2003;105:131–139. doi: 10.1016/j.exppara.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Mau VJ, Young IG, Matthaei KI. Uterine eosinophils and reproductive performance in interleukin 5-deficient mice. J Reprod Fertil. 2000;120:423–432. doi: 10.1530/jrf.0.1200423. [DOI] [PubMed] [Google Scholar]
- Rose CE, Jr, Lannigan JA, Kim P, Lee JJ, Fu SM, Sung SS. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell Mol Immunol. 2010 doi: 10.1038/cmi.2010.31. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME, Owen WF, Jr, Silberstein DS, Woods J, Soberman RJ, Austen KF, Stevens RL. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988;81:1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman HL, Yutanawiboonchai W, Brigandi RA, Leon O, Gleich GJ, Nolan TJ, Schad GA, Abraham D. Strongyloides stercoralis: eosinophil-dependent immune-mediated killing of third stage larvae in BALB/cByJ mice. Exp Parasitol. 1996;82:267–278. doi: 10.1006/expr.1996.0034. [DOI] [PubMed] [Google Scholar]
- Sabin EA, Pearce EJ. Early IL-4 production by non-CD4+ cells at the site of antigen deposition predicts the development of a T helper 2 cell response to Schistosoma mansoni eggs. J Immunol. 1995;155:4844–4853. [PubMed] [Google Scholar]
- Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoszuk M. Eosinophils and human cancer. Histol Histopathol. 1997;12:807–812. [PubMed] [Google Scholar]
- Sasaki O, Sugaya H, Ishida K, Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 1993;15:349–354. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–3718. [PubMed] [Google Scholar]
- Seminario MC, Saini SS, MacGlashan DW, Jr, Bochner BS. Intracellular expression and release of Fc epsilon RI alpha by human eosinophils. J Immunol. 1999;162:6893–6900. [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res. 2004;24:271–281. doi: 10.1089/107999004323065057. [DOI] [PubMed] [Google Scholar]
- Sher A, Coffman RL, Hieny S, Scott P, Cheever AW. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni . Proc Natl Acad Sci USA. 1990;87:61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Xiao CQ, Li CQ, Mo XY, Yang QL, Leng J, Chen YQ. Endobronchial eosinophils preferentially stimulate T helper cell type 2 responses. Allergy. 2004;59:428–435. doi: 10.1046/j.1398-9995.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JE, Rothenberg ME, Lawrence RA. Eotaxin-1-regulated eosinophils have a critical role in innate immunity against experimental Brugia malayi infection. Eur J Immunol. 2005;35:189–197. doi: 10.1002/eji.200425541. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Scott RJ, Boyle MJ, Gibson PG. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Respir Crit Care Med. 2005;172:559–565. doi: 10.1164/rccm.200503-369OC. [DOI] [PubMed] [Google Scholar]
- Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, Smyth MJ, Parish CR. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- Singhania NA, Dyer KD, Zhang J, Deming MS, Bonville CA, Domachowske JB, Rosenberg HF. Rapid evolution of the ribonuclease A superfamily: adaptive expansion of independent gene clusters in rats and mice. J Mol Evol. 1999;49:721–728. doi: 10.1007/pl00006594. [DOI] [PubMed] [Google Scholar]
- Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee NA, Lee JJ, Hoerauf A. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun. 2006;74:5236–5243. doi: 10.1128/IAI.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer LA, Weller PF. Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol. 2010;88:250–256. doi: 10.1038/icb.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer LA, Melo RC, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci USA. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik I, Brenuchon C, Angeli V, Staumont D, Fleury S, Capron M, Trottein F, Dombrowicz D. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- Stenfeldt AL, Wenneras C. Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunology. 2004;112:605–614. doi: 10.1111/j.1365-2567.2004.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straumann A, Kristl J, Conus S, Vassina E, Spichtin HP, Beglinger C, Simon HU. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm Bowel Dis. 2005;11:720–726. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- Sullivan SK, McGrath DA, Liao F, Boehme SA, Farber JM, Bacon KB. MIP-3alpha induces human eosinophil migration and activation of the mitogen-activated protein kinases (p42/p44 MAPK) J Leukoc Biol. 1999;66:674–682. doi: 10.1002/jlb.66.4.674. [DOI] [PubMed] [Google Scholar]
- Sur S, Glitz DG, Kita H, Kujawa SM, Peterson EA, Weiler DA, Kephart GM, Wagner JM, George TJ, Gleich GJ, Leiferman KM. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J Leukoc Biol. 1998;63:715–722. doi: 10.1002/jlb.63.6.715. [DOI] [PubMed] [Google Scholar]
- Svensson L, Wenneras C. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect. 2005;7:720–728. doi: 10.1016/j.micinf.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K, Takaki S, Hitoshi Y. Interleukin-5 and its receptor system: implications in the immune system and inflammation. Adv Immunol. 1994;57:145–190. doi: 10.1016/s0065-2776(08)60673-2. [DOI] [PubMed] [Google Scholar]
- Taliaferro WH, Sarles MP. The cellular reactions in the skin, lungs, and intestine of normal and immune rats after infection with Nippostrongylus brasiliensis . J Infect Dis. 1939;64:157–192. [Google Scholar]
- Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indole-amine 2, 3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EL, Fishman M. Oxidation of chloride and thiocyanate by isolated leukocytes. J Biol Chem. 1986;261:9694–9702. [PubMed] [Google Scholar]
- Thorne KJ, Glauert AM, Svvennsen RJ, Franks D. Phagocytosis and killing of Trypanosoma dionisii by human neutrophils, eosinophils and monocytes. Parasitology. 1979;79:367–379. doi: 10.1017/s0031182000053762. [DOI] [PubMed] [Google Scholar]
- Throsby M, Herbelin A, Pleau JM, Dardenne M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol. 2000;165:1965–1975. doi: 10.4049/jimmunol.165.4.1965. [DOI] [PubMed] [Google Scholar]
- Tomimori Y, Muto T, Fukami H, Saito K, Horikawa C, Tsuruoka N, Saito M, Sugiura N, Yamashiro K, Sumida M, Kakutani S, Fukuda Y. Chymase participates in chronic dermatitis by inducing eosinophil infiltration. Lab Invest. 2002;82:789–794. doi: 10.1097/01.lab.0000018827.78602.f4. [DOI] [PubMed] [Google Scholar]
- Trulson A, Nilsson S, Venge P. The eosinophil granule proteins in serum, but not the oxidative metabolism of the blood eosinophils, are increased in cancer. Br J Haematol. 1997;98:312–314. doi: 10.1046/j.1365-2141.1997.2203035.x. [DOI] [PubMed] [Google Scholar]
- Tulic MK, Sly PD, Andrews D, Crook M, Davoine F, Odemuyiwa SO, Charles A, Hodder ML, Prescott SL, Holt PG, Moqbel R. Thymic indoleamine 2, 3-dioxygenase-positive eosinophils in young children: potential role in maturation of the naive immune system. Am J Pathol. 2009;175:2043–2052. doi: 10.2353/ajpath.2009.090015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijt LS, Vos N, Hijdra D, de Vries VC, Hoogsteden HC, Lambrecht BN. Airway eosinophils accumulate in the mediastinal lymph nodes but lack antigen-presenting potential for naive T cells. J Immunol. 2003;171:3372–3378. doi: 10.4049/jimmunol.171.7.3372. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen N, Kita H, Weiler D, Gleich GJ. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol. 1991;147:3490–3495. [PubMed] [Google Scholar]
- Walsh GM, Hartnell A, Moqbel R, Cromwell O, Nagy L, Bradley B, Furitsu T, Ishizaka T, Kay AB. Receptor expression and functional status of cultured human eosinophils derived from umbilical cord blood mononuclear cells. Blood. 1990;76:105–111. [PubMed] [Google Scholar]
- Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, August A. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Weller PF. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J Leukoc Biol. 2008;83:817–821. doi: 10.1189/jlb.0607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tan X, Chang H, Huang W, Gonzalez-Crussi F, Hsueh W. Platelet-activating factor receptor mRNA is localized in eosinophils and epithelial cells in rat small intestine: regulation by dexamethasone and gut flora. Immunology. 1999;97:447–454. doi: 10.1046/j.1365-2567.1999.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007a;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007b;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D, Sommer JR, Beard JW. Cytochemical localization of an adenosine triphosphate dephosphorylating enzyme in the granules of human eosinophils. Blood. 1962;19:612–614. [PubMed] [Google Scholar]
- Weller PF, Rand TH, Barrett T, Elovic A, Wong DT, Finberg RW. Accessory cell function of human eosinophils. HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J Immunol. 1993;150:2554–2562. [PubMed] [Google Scholar]
- Weller PF, Bozza PT, Yu W, Dvorak AM. Cytoplasmic lipid bodies in eosinophils: central roles in eicosanoid generation. Int Arch Allergy Immunol. 1999;118:450–452. doi: 10.1159/000024161. [DOI] [PubMed] [Google Scholar]
- West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB, Oldham RK. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987;316:898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, Capron M. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med. 1999;190:487–495. doi: 10.1084/jem.190.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerly G, Lacy P, Younes AB, Roger N, Loiseau S, Moqbel R, Capron M. Human eosinophils express and release IL-13 following CD28-dependent activation. J Leukoc Biol. 2002;72:769–779. [PubMed] [Google Scholar]
- Wong DT, Elovic A, Matossian K, Nagura N, McBride J, Chou MY, Gordon JR, Rand TH, Galli SJ, Weller PF. Eosinophils from patients with blood eosinophilia express transforming growth factor beta 1. Blood. 1991;78:2702–2707. [PubMed] [Google Scholar]
- Wong DT, Bowen SM, Elovic A, Gallagher GT, Weller PF. Eosinophil ablation and tumor development. Oral Oncol. 1999;35:496–501. doi: 10.1016/s1368-8375(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- Wong CK, Ng SS, Lun SW, Cao J, Lam CW. Signalling mechanisms regulating the activation of human eosinophils by mast-cell-derived chymase: implications for mast cell-eosinophil interaction in allergic inflammation. Immunology. 2009;126:579–587. doi: 10.1111/j.1365-2567.2008.02916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Anand-Apte B, Lee M, Sasaki T, Fukai N, Shapiro R, Que I, Lowik C, Timpl R, Olsen BR. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J. 1999;18:4414–4423. doi: 10.1093/emboj/18.16.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Sedgwick JB, Vrtis RF, Busse WW. The effect of transendothelial migration on eosinophil function. Am J Respir Cell Mol Biol. 2000;23:379–388. doi: 10.1165/ajrcmb.23.3.3707. [DOI] [PubMed] [Google Scholar]
- Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–3403. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Rosenberg HF, Rybak SM, Newton DL, Wang ZY, Fu Q, Tchernev VT, Wang M, Schweitzer B, Kingsmore SF, Patel DD, Oppenheim JJ, Howard OM. Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation. J Immunol. 2004;173:6134–6142. doi: 10.4049/jimmunol.173.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh M, Tai PC, Spry CJ, Gleich GJ, Roos D. Synergism between eosinophil cationic protein and oxygen metabolites in killing of schistosomula of Schistosoma mansoni . J Immunol. 1987;138:3443–3447. [PubMed] [Google Scholar]
- Yoon J, Ponikau JU, Lawrence CB, Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ikuta K, Sugaya H, Maki K, Takagi M, Kanazawa H, Sunaga S, Kinashi T, Yoshimura K, Miyazaki J, Takaki S, Takatsu K. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity. 1996;4:483–494. doi: 10.1016/s1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Austen KF, Friend DS, Heidtman M, Boyce JA. Human peripheral blood eosinophils express a functional c-kit receptor for stem cell factor that stimulates very late antigen 4 (VLA-4)-mediated cell adhesion to fibronectin and vascular cell adhesion molecule 1 (VCAM-1) J Exp Med. 1997;186:313–323. doi: 10.1084/jem.186.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger RS, Yurdin DL, Colten HR. Histamine metabolism. II. Cellular and subcellular localization of the catabolic enzymes, histaminase and histamine methyl transferase, in human leukocytes. J Allergy Clin Immunol. 1976;58:172–179. doi: 10.1016/0091-6749(76)90152-4. [DOI] [PubMed] [Google Scholar]
- Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk O, Fukushima A, Zinchuk V, Fukata K, Ueno H. Direct action of platelet activating factor (PAF) induces eosinophil accumulation and enhances expression of PAF receptors in conjunctivitis. Mol Vis. 2005;11:114–123. [PubMed] [Google Scholar]