Abstract
A growing body of evidence indicates that carbon monoxide (CO), once perceived merely as a poisonous gas, exerts antiapoptotic and cytoprotective effects. Using a water-soluble CO-releasing molecule (CORM) tricarbonylchloro(glycinato)ruthenium(II) (CORM-3), we previously reported that CO induces a delayed protection against myocardial infarction similar to that observed in the late phase of ischemic preconditioning (PC). In the current study, we investigated the molecular mechanisms underlying this cardioprotective effect. The impact on apoptotic signaling pathways was first examined in the setting of ischemia/reperfusion injury. Mice were pretreated with CORM-3 or iCORM-3 (which does not release CO) and subjected to coronary occlusion/reperfusion 24 h later. In mice that received CORM-3, there was a significant reduction in markers of apoptosis (cleaved lamin A, cleaved caspase-3, and cleaved PARP-1) after ischemia/reperfusion injury. To elucidate the mechanism of CORM-3-induced cardioprotection we further examined the activation of transcription factors and induction of cardioprotective and apoptosis modulating proteins. Infusion of CORM-3 rapidly activated the stress-responsive transcription factors nuclear factor kappaB (NF-κB), signal transducers and activators of transcription (STAT)1, STAT3, and NF-E2-related factor-2 (Nrf2). This was followed 24 h later by upregulation of cardioprotective proteins (heme oxygenase-1 [HO-1], cyclooxygenase-2 [COX-2], and extracellular superoxide dismutase [Ec-SOD]) and antiapoptotic proteins involving both the mitochondria-mediated (Mcl-1) and the death receptor-mediated (c-FLIPS, and c-FLIPL) apoptosis pathways. We conclude that CO released by CORM-3 triggers a cardioprotective signaling cascade that recruits the transcription factors NF-κB, STAT1/3, and Nrf2 with a subsequent increase in cardioprotective and antiapoptotic molecules in the myocardium leading to the late PC-mimetic infarct-sparing effects.
Keywords: carbon monoxide-releasing molecules, late preconditioning, myocardial infarction, apoptosis, NF-κB, STAT1/3, Nrf2
INTRODUCTION
Although carbon monoxide (CO) has historically been viewed as toxic to biological systems [1], recent studies suggest that this gaseous byproduct of heme oxygenase-1 (HO-1) plays an important regulatory role in many cellular and biological processes. In this regard, CO has been shown to ameliorate inflammation [2, 3], reduce oxidative stress [4], and inhibit apoptosis [5, 6]. We previously reported that administration of a water soluble CO-releasing molecule (CORM) tricarbonylchloro(glycinato)ruthenium(II) (CORM-3), induces a delayed infarct-sparing effect similar to that seen during the late phase of ischemic preconditioning (PC) [7]. However, the molecular mechanism underlying this robust cardioprotection remains unknown.
The late phase of ischemic PC provides sustained cardioprotection, and therefore, exploitation of late PC has potential clinical significance [8]. Importantly, numerous studies have demonstrated that a delayed cardioprotective effect similar to that afforded by the late phase of ischemic PC can be elicited by a variety of pharmacologic agents [9–13]. Unfortunately, most of these interventions are either not clinically applicable or have significant side effects. In our previous work, we demonstrated that CORM-3 induces robust cardioprotection without significantly increasing carboxy-hemoglobin levels [14]. This suggests that CO administered by CORM-3 may be both clinically applicable and safe.
Mechanistically, cyclooxygenase-2 (COX-2) and HO-1 are two obligatory mediators of late PC [15, 16]. We have previously shown that the late phase of ischemic PC induces antiapoptotic proteins involving both the mitochondria-mediated (Mcl-1) and death receptor-mediated (c-FLIPL and c-FLIPS) apoptosis pathways [17]. Increased expression of these antiapoptotic proteins presumably culminates in the attenuation of mediators of apoptosis common to both pathways (cleaved lamin A, cleaved caspase-3 and cleaved PARP-1) and reduces apoptosis in response to myocardial ischemia/reperfusion injury [17]. Similarly, the cytoprotective effects of CO have also been associated with inhibition of apoptosis and upregulation of antiapoptotic proteins [5, 18, 19]. The stress-responsive transcription factors signal transducers and activators of transcription (STAT)1, STAT3 and nuclear factor kappaB (NF-κB) are known to orchestrate the induction of cardioprotective and antiapoptotic proteins in the heart [20–22]. In addition, recent evidence suggests that exogenous CO induces HO-1 expression via the transcription factor NF-E2-related factor-2 (Nrf2) utilizing the ER stress response pathway and PERK activation in endothelial cells [23].
Since the magnitudes of cardioprotection afforded by ischemic late PC and CORM-3 are similar [7] and both seem to influence antiapoptotic and cardioprotective signaling pathways [5, 17, 18], we postulated that the cardioprotective benefits of CORM-3 may involve modulation of antiapoptotic and cardioprotective molecules. Accordingly, the goals of the present study were: (i) to determine whether CORM-3-induced cardioprotection attenuates the I/R-induced upregulation of the common mediators of apoptosis (cleaved lamin A, cleaved caspase-3, and cleaved PARP-1); (ii) to determine whether CORM-3-induced cardioprotection is associated with increased expression of the antiapoptotic proteins in the mitochondria-mediated (Mcl-1), the death receptor-mediated (c-FLIPS, and c-FLIPL) antiapoptotic pathways; (iii) to determine whether CORM-3 induces upregulation of the cardioprotective proteins (COX-2, HO-1, and EC-SOD); and (iv) to determine whether CORM-3 activates the transcription factors known to influence antipoptotic and cytoprotective signaling (NF-κB, STAT1, STAT3, and Nrf2).
METHODS
The study was performed in accordance with the guidelines of the Animal Care and Use Committee of the University of Louisville School of Medicine and with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, National Institutes of Health, Publication No. 85-23, revised 1996).
Animals
Wild-type male ICR mice (body wt. 35.1±1.2 g, age 9.8±0.4 wk) were used for this study. All mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were maintained in microisolator cages under specific pathogen-free conditions in a room with a temperature of 24°C, 55–65% relative humidity, and a 12-h light-dark cycle.
Experimental protocol
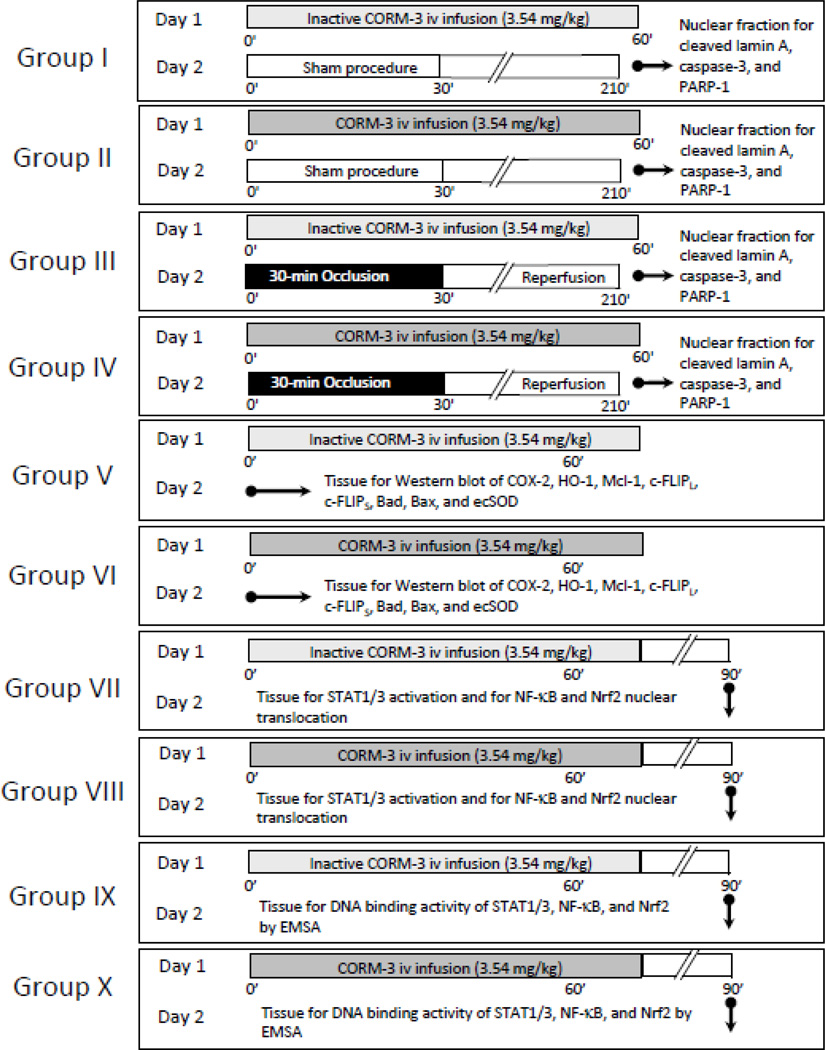
The overall experimental design is summarized in Figure 1. Mice were assigned to ten groups: four groups were used to assess the impact of CORM-3/iCORM-3 pretreatment on I/R-induced expression of the common apoptotic mediators cleaved lamin A, cleaved caspase-3, and cleaved PARP-1 (groups I–IV); two groups were used for the measurement of myocardial protein levels by Western immunoblotting (groups V–VI); and four groups for transcription factor studies (groups VII–X). CORM-3 was inactivated by dissolving it in PBS (0.35 mg/ml) and leaving it at room temperature for 24 h; under these conditions, one mole of CO per mole of compound is released in the solution, and as a result, no additional CO is liberated upon administration of the drug [24]. Active CORM-3 was dissolved in distilled water (pH 7.0). The inactive form iCORM-3, which is unable to liberate CO [24] was used as control. Mice received a 60-min i.v. infusion of inactive CORM-3 (groups I, III, V, VII, and IX; total dose 3.54 mg/kg) or CORM-3 (groups II, IV, VI, VIII, and X; total dose 3.54 mg/kg). This dose of CORM-3 was selected because in our previous study in this model it afforded robust cardioprotection [14]. Twenty-four hours after CORM-3 or iCORM-3 infusion, mice in groups I–IV were subjected to a sham open-chest procedure (groups I and II) or a 30-min coronary occlusion/reperfusion (groups II and IV) followed 3 h later by harvest of myocardial tissue samples [7]. Nuclear extracts were prepared from the ischemic zone, and Western immunoblotting was performed for cleaved lamin A, cleaved caspase-3, and cleaved PARP-1, common mediators of apoptosis. In groups V and VI, mice received iCORM-3 or CORM-3 and were euthanized 24 h later (day 2) and myocardial tissue samples were collected for Western immunoblotting assays of COX-2, Ec-SOD, HO-1, Mcl-1, c-FLIPS, and c-FLIPL. In groups VII–X, mice were sacrificed 30-min after the completion of iCORM-3 or CORM-3 infusion and myocardial tissue samples were harvested for the determination of transcription factor activation and DNA-binding activity. Myocardial samples were rapidly removed from the left ventricular free wall, and frozen immediately in liquid nitrogen.
Figure 1.
Experimental protocol. Ten groups of mice were used. On day 1, mice in groups I and III received 3.54 mg/kg of iCORM-3 i.v. over 60 min, while mice in groups II and IV received 3.54 mg/kg of CORM-3 i.v. over the same period. Twenty-four hours later, mice were subjected to either a sham open-chest procedure (groups I and II) or a 30-min coronary occlusion followed by reperfusion (groups III and IV). Three hours later tissue samples were harvested from the risk region for cleaved lamin A, cleaved caspase-3, and cleaved PARP-1 assays. On day 1, mice in groups V and VI received the same dose of iCORM-3 and CORM-3, respectively, and myocardial tissue samples were harvested for the measurement of Mcl-1, c-FLIPS, c-FLIPL, COX-2, HO-1, Ec-SOD, Bad, and Bax 24 h later. For transcription factor assays, mice in groups VII and IX received the same dose of iCORM-3, while mice in groups VIII and X received the same dose of CORM-3. Thirty min after the completion of infusion, myocardial tissue samples were harvested for the determination of nuclear translocation and/or phosphorylation of p65, pTyr-STAT1, pTyr-STAT3, and Nrf2 (groups VII and VIII) and DNA binding activity using gel shift assays (groups IX and X).
Preparation of cytosolic and membranous proteins
Myocardial tissue samples were homogenized in buffer A (25 mM Tris-HCl pH 7.4], 0.5 mM EDTA, 0.5mM EGTA, 1 mM PMSF, 25 µg/ml leupeptin, 1mM DTT, 25 mM NaF, and 1 mM Na3VO4) and centrifuged at 14,000g for 12 min at 4°C, and the resulting supernatants were collected as cytosolic fractions [25]. The pellets were incubated in a lysis buffer (buffer A+1% Triton X-100) for 2 h and centrifuged at 14,000g for 15 min at 4°C, and the resulting supernatants were collected as membranous fraction [25]. Protein contents were determined by a Bio-Rad protein assay kit.
Preparation of nuclear extracts
Nuclear extracts were prepared using a modification of a previously described method [26]. The samples were homogenized in buffer A (10 mmol/L, HEPES pH 7.9], 1.5 mmol/L MgCl2, 10 mmol/L KCl, 1 mmol/L DTT, 25 mg/mL leupeptin, and 1 mmol/L PMSF). After a 10-min incubation on ice, the samples were centrifuged at 1,850g for 10 min at 4°C. The pellets were dissolved in buffer B (buffer A+0.1% Triton X-100), incubated on ice for 10 min, and then centrifuged as above. The crude nuclear pellets were washed once with buffer A and resuspended in buffer C (20 mmol/L HEPES [pH 7.9], 25% glycerol [vol/vol], 0.42 mol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.5 mmol/L DTT, and 1 mmol/L PMSF) for 30 min at 4°C. Nuclear proteins were recovered after centrifugation at 25,000g for 30 min. The resulting clear supernatants were dialyzed against 100 volumes of buffer D (20 mmol/L HEPES [pH 7.9], 4% glycerol, 50 mmol/L NaCl, 0.5 mmol/L EDTA, 1 mmol/L MgCl2, 0.5 mmol/L PMSF, and 0.5 mmol/L DTT) for 6 h at 4°C. The dialysates were centrifuged again at 25,000g for 30 min. The resulting supernatants were designated as the nuclear protein extracts. The purity of the nuclear extracts was confirmed using lactate dehydrogenase (LDH) as a cytosolic marker.
Western immunoblotting
Protein expression was determined by standard SDS-PAGE immunoblotting techniques [25, 27]. Gel transfer efficiency and was recorded carefully by making photocopies of membranes dyed with reversible Ponceau staining [25, 27]; gel retention was determined by Coomassie blue staining [25, 27]. Polyclonal anti-Mcl-1 (Santa Cruz Biotechnology) and monoclonal anti-rat c-FLIPL and c-FLIPS (Alexis, San Diego, CA), anti-COX-2 (Cayman Chemical), anti-Ec-SOD (Stressgen), anti-Bax and anti-Bad (Santa Cruz), and anti-HO-1 (Stressgen) antibodies were used for respective assays. Antibodies against NF-κB p65 (Santa Cruz Biotechnology), pTyr(701)-STAT1 (Upstate Biotechnology), pTyr(705)-STAT3 (Cell Signaling Technology), pSer(729)-STAT3 (Santa Cruz Biotechnology), and Nrf2 (Santa Cruz Biotechnology) were used for the assays of NF-κB, phosphorylated STAT1/3, and Nrf2. Antibodies against cleaved caspase-3 (Cell Signaling Technology), cleaved PARP-1 (Cell Signaling Technology) and cleaved lamin A (Cell Signaling Technology), were used for the respective assays. In order to ensure equal protein loading in all lanes, the total amounts of proteins transferred from each lane to the nitrocellulose membrane were quantified by Ponceau staining. Given the critical importance of quantitating signal intensity as accurately as possible, each specific signal of the band of interest detected by immunoblotting was normalized to the corresponding Ponceau stain signal determined by densitometric analysis of the Ponceau stain record, as previously described [25, 27, 28]. In all samples, the content of each protein was expressed as a percentage of the corresponding protein in iCORM-3 control samples (100%).
Electrophoretic mobility shift assays
The DNA binding activity of STAT1/3, NF-κB, and Nrf2 was measured with electrophoretic mobility shift assays. Nuclear fraction was isolated as above. A synthetic double-stranded probe containing the STAT1/3 consensus sequence 5'-GATCAGCTTCAATTTCCCGTAAATCCCTA-3' (Gibco, Carlsbad, CA) was used for STAT1/3 DNA binding activity. A double-stranded 22-mer oligonucleotide containing the κB consensus sequence 5'-AGTTGAGGGGACTTTCCCAGGC-3' (Promega Corp) was used for NF-κB DNA binding activity. A double-stranded 27-mer oligonucleotide with the Nrf2 consensus sequence 5'-TGGGGAACCTGTGCTGAGTCACTGGAG-3' (Santa Cruz) was used for Nrf2 DNA binding activity. These probes were end-labeled using [γ-32P] ATP (3000 Ci/mmol, Amersham) and T4 polynucleotide kinase, and purified with a G-25 Sephadex column.
Statistical analysis
Data are reported as means±SEM. Differences among groups with respect to Mcl-1, c-FLIPL, c-FLIPS, COX-2, HO-1, Ec-SOD, Bad, Bax, NF-κB, STAT1, STAT3, and Nrf2 were analyzed using one-way ANOVA. If the ANOVA showed an overall difference, post hoc contrasts were performed with Student t-tests for unpaired data with the Bonferroni correction. P<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS (version 8.0) statistical software (SPSS Inc., Chicago, IL).
RESULTS
A total of 56 mice were used (20 mice for the I/R studies [groups I–IV], 12 mice for the determination of protein levels by Western immunoblotting [groups V and VI], and 24 mice for the studies of transcription factor activation and gel shift assays [groups VII–X]).
CORM-3 attenuates the expression of common mediators of apoptosis in the ischemic/reperfused myocardium
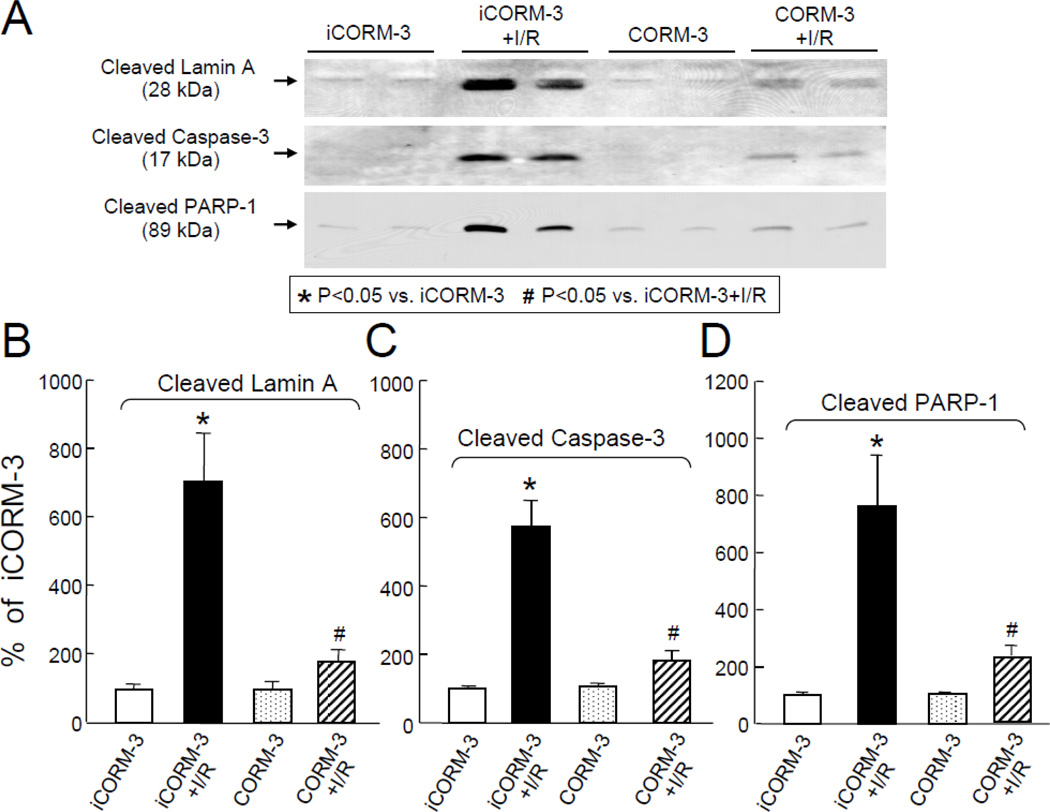
The myocardial expression of molecular indicators of apoptosis were examined in iCORM-3 and CORM-3-treated mice that were subjected to coronary occlusion/reperfusion. Figure 2A shows the representative immunoblots. In sham-operated mice treated with iCORM-3 (group I) or CORM-3 (group III) the levels of cleaved lamin A (100±15% vs. 98±21%, respectively, Figure 2B), cleaved caspase-3 (100±3% vs. 98±12%, respectively, Figure 2C), and cleaved PARP-1 (100±11% vs. 103±8%, respectively, Figure 2D) were similar. However, compared with iCORM-3 (group II), pretreatment with CORM-3 (group IV) significantly attenuated the levels of cleaved lamin A (+179±36% vs. +704±140%, respectively, P<0.05, Figure 2B), cleaved caspase-3 (+183±27% vs. +573±76%, respectively, P<0.05, Figure 2C), and cleaved PARP-1 (+230±34% vs. +761±180%, respectively, P<0.05, Figure 2D) in the ischemic/reperfused myocardium. These data indicate that infusion of CORM-3 attenuates the activation of proapoptotic signaling after myocardial ischemia/reperfusion injury 24 h later.
Figure 2.
Effects of iCORM-3 and CORM-3 pretreatment on ischemia/reperfusion-induced expression of the common mediators of apoptosis. Panel A: Representative Western immunoblots showing attenuation of ischemia/reperfusion-induced increase in levels of common mediators of apoptosis with CORM3 pretreatment. Panels B–D: Densitometric analysis of cleaved lamin A (B), cleaved caspase-3 (C), and cleaved PARP-1 (D) signals. Data are means±SEM. *P<0.05 vs. iCORM-3,#P<0.05 vs. iCORM-3+I/R.
CORM-3 increases myocardial levels of COX-2, HO-1, and Ec-SOD
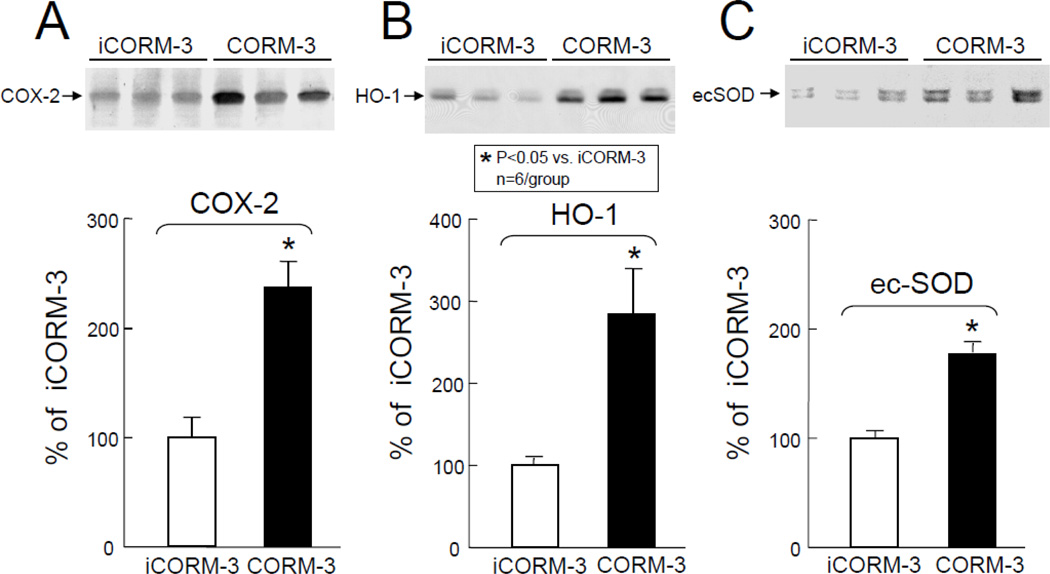
The protein levels of cytoprotective molecules in myocardial tissues samples harvested 24 h after CORM-3 and iCORM-3 administration were determined by Western immunoblotting. In CORM-3-treated mice, the levels of COX-2 (+137±24% above iCORM-3-treated group, P<0.05, Figure 3A), HO-1 (+185±55% above iCORM-3-treated group, P<0.05, Figure 3B), and Ec-SOD (+77±7% above iCORM-3-treated group, P<0.05, Figure 3C) were significantly greater compared with iCORM-3-treated mice. These data indicate that CORM-3 infusion increases myocardial expression of molecules with known cardioprotective and antiapoptotic effects.
Figure 3.
Effects of inactive CORM-3 (iCORM-3, group V) and CORM-3 (group VI) administration on myocardial levels of COX-2, HO-1, and Ec-SOD 24 h later. Upper panels: Representative Western immunoblots showing increased expression of COX-2 (A), HO-1 (B), and Ec-SOD (C) in the hearts of CORM-3-treated mice compared with iCORM-3-treated mice. Lower panels: Densitometric analysis of COX-2, HO-1, and Ec-SOD signals. Data are means±SEM. *P<0.05 vs. iCORM-3.
CORM-3 increases myocardial antiapoptotic protein levels
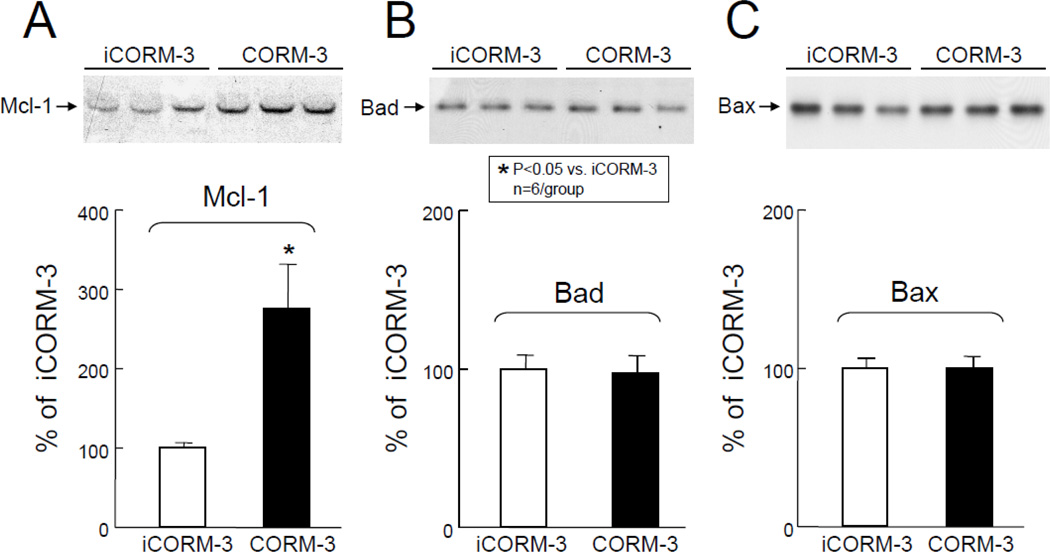
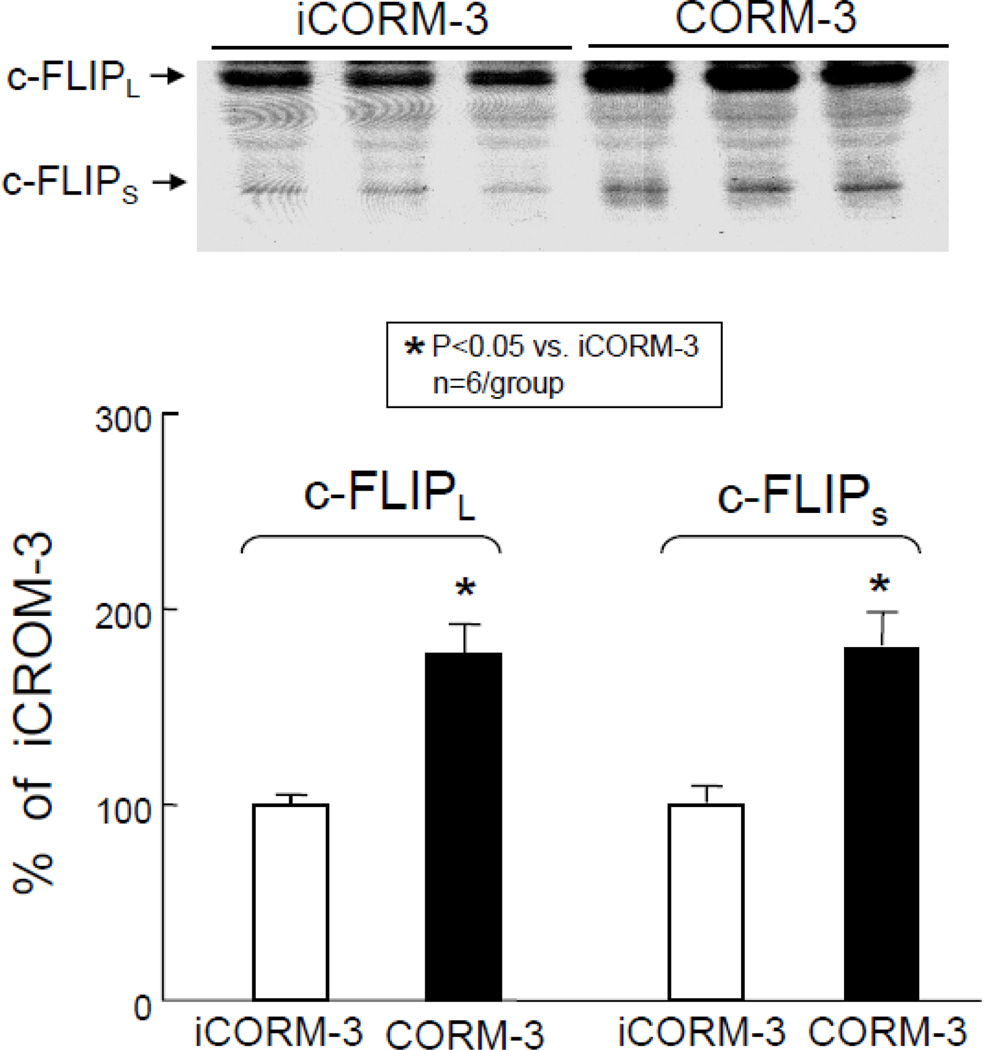
As shown in Figure 4A, Western immunoblotting analysis revealed a marked increase in Mcl-1 protein in the cytosolic fraction at 24 h after CORM-3 administration. Densitometric analysis of the immunoreactive bands showed that in CORM-3-treated mice the expression of Mcl-1 increased by 276±56% (P<0.05 vs. iCORM3, Figure 4A). Similarly, CORM-3 treatment was associated with a significant increase in the expression of c-FLIPL and c-FLIPS (77±16% and 80±17% above iCORM-3-treated mice, respectively, P<0.05 for both, Figure 5) 24 h later, indicating that CORM-3 infusion induces an antiapoptotic milieu in the myocardium.
Figure 4.
Effects of inactive CORM-3 (iCORM-3, group V) and CORM-3 (group VI) administration on myocardial levels of Mcl-1, Bad, and Bax 24 h later. Upper panels: Representative Western immunoblots showing increased expression of antiapoptotic molecule Mcl-1 (A) and no significant change in expression of proapoptotic proteins Bad (B) and Bax (C) in the hearts of CORM-3-treated mice compared with iCORM-3-treated mice. Lower panels: Densitometric analysis of Mcl-1 (A), Bad (B), and Bax (C) signals. Data are means±SEM. *P<0.05 vs. iCORM-3.
Figure 5.
Effects of inactive CORM-3 (iCORM-3, group V) and CORM-3 (group VI) administration on myocardial levels of c-FLIPL and c-FLIPS 24 h later. Upper panels: Representative Western immunoblots showing increased expression of antiapoptotic molecules c-FLIPL and c-FLIPS in the hearts of CORM-3-treated mice compared with iCORM-3-treated mice. Lower panels: Densitometric analysis of c-FLIPL and c-FLIPS signals. Data are means±SEM. *P<0.05 vs. iCORM-3.
The expression of proapoptotic proteins
In contrast to the above, Western immunoblotting analysis of the cytosolic fractions of cardiac homogenates revealed no significant change in Bad (Figure 4B) and Bax (Figure 4C) immunoreactivity 24 h after CORM-3 administration. Densitometric analysis of the immunoreactive bands showed no statistically significant change in CORM-3-treated mice compared with iCORM-3-treated mice.
CORM-3 activates stress-responsive transcription factors in the heart
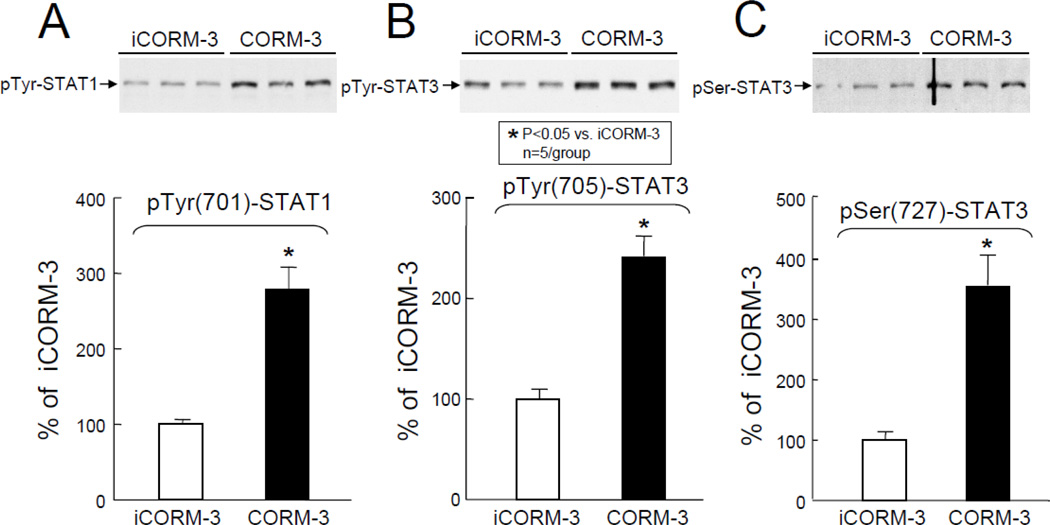
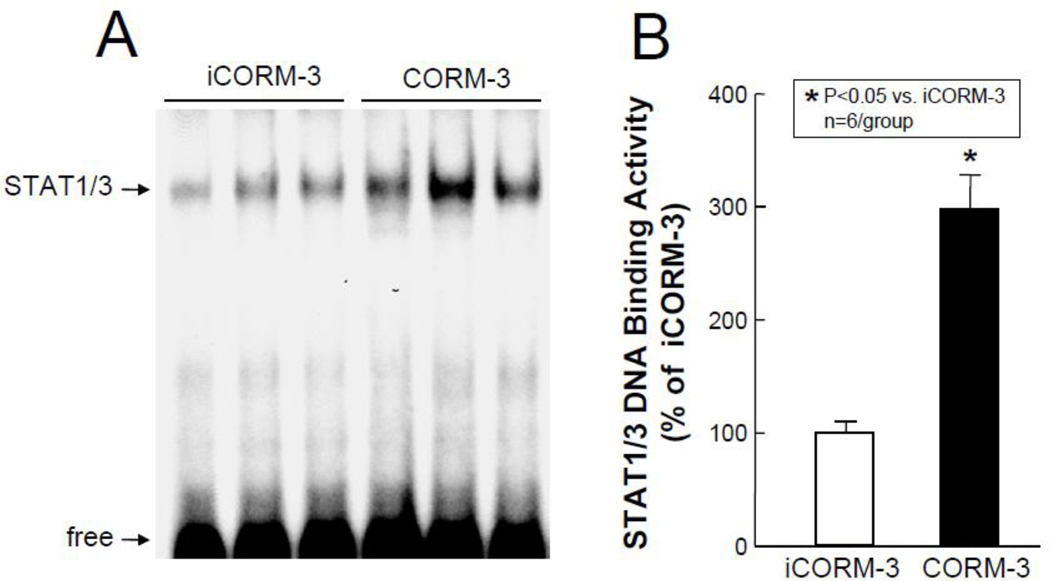
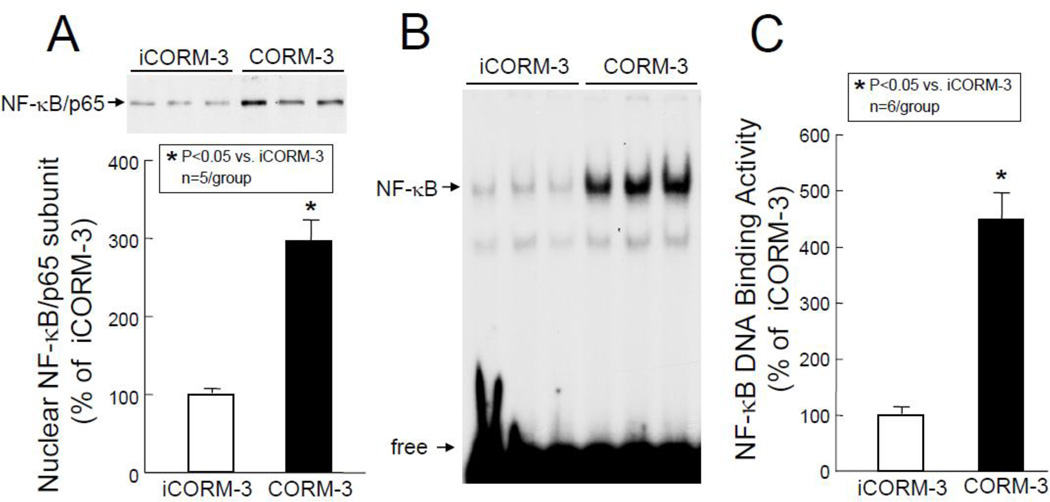
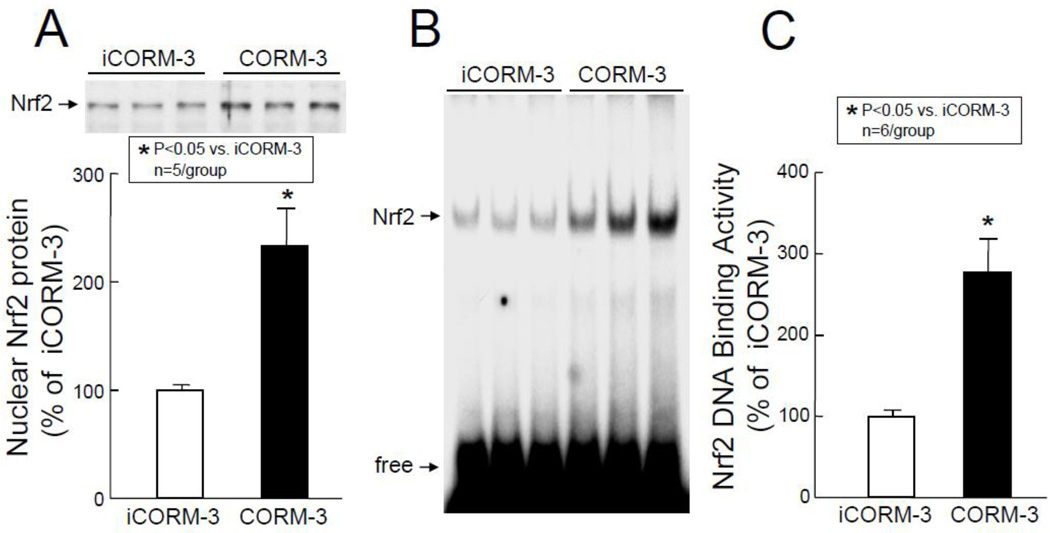
In order to identify the molecular basis of the above observations, transcription factor assays were carried out groups VII–X (Figure 1). As shown in Figure 6, Western immunoblotting of the nuclear fraction for phosphorylated STAT1/3 revealed a marked increase in the activated forms of these transcription factors in the nuclear fraction in CORM-3-treated mice (pTyr[701]-STAT1: +178±29% above iCORM-3-treated mice, P<0.05, Figure 6A; pTyr[705]-STAT3: +141±20% above iCORM-3, P<0.05, Figure 6B; and pSer[727]-STAT3, +254±50% above iCORM-3, P<0.05, Figure 6C). These increases in phosphorylated (activated) forms of STAT1 and STAT3 were consistent with greater STAT1/3 DNA binding activity in nuclear extracts isolated from myocardial tissue samples of CORM-3-treated mice (+198±29% above iCORM-3-treated mice, P<0.05, Figure 7). In addition, the levels of p65 (a subunit of NF-κB) in the nuclear fraction at 30 min after CORM-3 administration showed a striking increase (+196±28% above iCORM-3-treated mice, P<0.05, Figure 8A), indicating translocation of NF-κB from cytosolic to nuclear fraction. Consistently, NF-κB DNA binding activity was greater in CORM-3-treated mice (+349±48% above iCORM-3-treated mice, P<0.05, Figure 8B–C). Finally, CORM-3 administration was associated with increased translocation of Nrf2 to the nuclear fraction (+133±35% above iCORM-3-treated mice, P<0.05; Figure 9A) and greater Nrf2 DNA binding activity (+177±41% above iCORM-3-treated mice, P<0.05; Figure 9B–C) as compared with inactive CORM-3. Together, these data indicate that CORM-3 administration is associated with rapid activation of several stress-responsive transcription factors in the myocardium.
Figure 6.
Nuclear contents of pTyr-STAT1 (A), pTyr-STAT3 (B), and pSer-STAT3 (C) at 30 min after administration of iCORM-3 (group VII) and CORM-3 (group VIII). Upper panels: Representative Western immunoblots showing increased nuclear levels of pTyr(701)-STAT1 (A), pTyr(705)-STAT3 (B), and pSer(727)-STAT3 (C) in myocardial samples from CORM-3-treated mice compared with iCORM-3-treated mice. Lower panels: Densitometric analysis of pTyr(701)-STAT1 (A), pTyr(705)-STAT3 (B), and pSer(727)-STAT3 (C) signals. Data are means±SEM. *P<0.05 vs. iCORM-3.
Figure 7.
STAT1/3 DNA binding activity. Myocardial tissue samples were harvested from mice in groups IX (iCORM-3-treated) and X (CORM-3-treated) 30 min after the completion of infusion. (A) Representative EMSA showing STAT1/3 DNA binding activity. (B) Densitometric analysis shows a striking increase in STAT1/3 DNA binding activity in CORM-3-treated hearts compared with iCORM-3-treated hearts. Data are means±SEM. *P<0.05 vs. iCORM-3.
Figure 8.
Nuclear translocation of p65 subunit and NF-κB DNA binding activity. Myocardial tissue samples were harvested from mice in groups VII and IX (iCORM-3-treated) and VIII and X (CORM-3-treated) 30 min after the completion of infusion. (A) Upper panel: A representative Western immunoblot showing increased nuclear levels of p65 in CORM-3-treated hearts compared with iCORM-3-treated hearts. Lower panel: Densitometric analysis of p65 signals. (B) EMSA showing NF-κB DNA binding activity. (C) Densitometric analysis shows a marked increase in NF-κB DNA binding activity in CORM-3-treated hearts compared with iCORM-3-treated hearts. Data are means±SEM. *P<0.05 vs. iCORM-3.
Figure 9.
Nrf2 nuclear translocation and DNA binding activity. Myocardial tissue samples were harvested from mice in groups VII and IX (iCORM-3-treated) and VIII and X (CORM-3-treated) 30 min after the completion of infusion. (A) Upper panel: A representative Western immunoblot showing increased nuclear levels of Nrf2 in CORM-3-treated hearts compared with iCORM-3-treated hearts. Lower panel: Densitometric analysis of Nrf2 signals. (B) EMSA showing Nrf2 DNA binding activity. (C) Densitometric analysis shows increased Nrf2 DNA binding activity in CORM-3-treated hearts compared with iCORM-3-treated hearts. Data are means±SEM. *P<0.05 vs. iCORM-3.
DISCUSSION
In recent years there has been a remarkable paradigm shift with respect to our understanding of the function of CO in biological systems. Mounting evidence indicates that this gaseous molecule, traditionally regarded as a toxic byproduct of HO-1 activity, exerts an important homeostatic function and plays a cytoprotective role in many pathophysiological conditions. In the heart, CO released by CORM-3 induces infarct-sparing benefits similar in magnitude to that induced by the late phase of ischemic PC [7, 14, 24]. The present study is the first to explore the mechanism of CO-induced cardioprotection.
Salient findings
Our results demonstrate that administration of a CO-releasing molecule (CORM-3): (i) attenuates the expression of common mediators of apoptosis after ischemia/reperfusion (cleaved lamin A, cleaved caspase-3, and cleaved PARP-1); (ii) upregulates several key cardioprotective molecules (COX-2, HO-1, and Ec-SOD) in the myocardium 24 h later; (iii) increases myocardial levels of a battery of antiapoptotic proteins (Mcl-1, c-FLIPS, and c-FLIPL) known to influence both the mitochondria-dependent as well as death receptor-mediated apoptosis pathways; and (iv) induces rapid nuclear translocation of stress-responsive transcription factors (NF-κB, STAT1, STAT3, and Nrf2) with increased DNA binding. Taken together, these results indicate that CO induces a genetic reprogramming of the heart that promotes cell survival in a manner that recapitulates the functional and molecular aspects of late PC. These findings advance our understanding of the role of CO in cardiovascular homeostasis by elucidating its molecular effects.
CORM-3 upregulates the mediators of late PC
Since the magnitudes of infarct-sparing effects afforded by CO and ischemia-induced late PC are similar [7], we sought to investigate whether the mechanism that underlies CO-induced protection recapitulates the mechanism for ischemia-induced late PC. Two of the obligatory mediators of late PC are HO-1 and COX-2 [27, 29]. The current results demonstrate, for the first time, that CO released by CORM-3 upregulates COX-2 and HO-1 in the heart 24 h later. Moreover, CORM-3 infusion also increased myocardial contents of Ec-SOD, viral induction of which has been shown to protect against both myocardial stunning [30] and myocardial infarction [31] in a fashion akin to the late phase of ischemic PC. These results indicate that CO and ischemia share several common mediators of delayed cardioprotection.
CORM-3 induces an antiapoptotic milieu in the myocardium
With regard to programmed cell death, two major pathways have been identified: the extrinsic or death receptor pathway, which is triggered by exogenous signals and leads to caspase-8 activation; and the intrinsic or mitochondrial pathway, which is activated by intrinsic cellular perturbations and leads to caspase-9 activation [32]. In order to beget apoptosis, these pathways utilize common mediators, such as cleaved lamin A, cleaved caspase-3, and cleaved PARP-1. As for antiapoptotic molecules, recent evidence suggests that c-FLIPL and c-FLIPS suppress primarily the extrinsic pathway, whereas Mcl-1 serves to inhibit the intrinsic pathway [32, 33]. To gain insight into the mechanism whereby CORM-3 induces delayed cardioprotection, we examined the effect of this agent on cleaved lamin A, cleaved caspase-3, and cleaved PARP-1, as well as Mcl-1, c-FLIPS, and c-FLIPL. The rationale for these studies stems from the fact that apoptosis contributes importantly to ischemia/reperfusion injury and that ischemic PC reduces apoptosis through these pathways [17]. In addition, CO has been shown to exert powerful anti-apoptotic actions in various noncardiac tissues [4, 5, 18, 34, 35].
Our data demonstrate that CORM-3 attenuates the ischemia/reperfusion-induced expression of the common mediators of apoptosis (cleaved lamin A, cleaved caspase-3, and cleaved PARP-1). Our finding that CORM-3 upregulates the expression of Mcl-1, c-FLIPL and c-FLIPS further indicates that CORM-3-induced cardioprotection involves inhibition of both the intrinsic and the extrinsic pathways. This is congruent with the protective role of FLIP against ischemia/reoxygenation-induced apoptosis in cardiomyocytes [36]. To our knowledge, this is the first indication that CO reprograms the ensemble of apoptosis-related proteins in a manner that inhibits both the intrinsic and the extrinsic pathways.
Activation of stress-responsive transcription factors
Having established that CO upregulates anti-apoptotic and cytoprotective proteins, we sought to elucidate the molecular mechanism responsible for this phenomenon. We focused on NF-κB, STAT1, STAT3, because these stress-responsive transcription factors have been previously implicated in the development of the late PC phenotype [25, 37, 38]. Several additional lines of evidence indicate that NF-kB and STAT1/3 are involved in the transcriptional control of Mcl-1, c-FLIPL, c-FLIPS, COX-2, and HO-1 [39–44]. Unlike NF-κB and STAT1/3, Nrf2 has not been extensively studied in myocardial ischemia/reperfusion injury. Nrf2, a member of the “cap ‘n’ collar” family of basic leucine zipper transcription factors [45], has been shown to mediate several responses to stress [46, 47]. Nrf2 has also been implicated in the transcriptional regulation of HO-1 [48–50], and recently in endothelial cells Nrf2 has been shown to provide a mechanism whereby CO can induce HO-1 and inhibit apoptosis [23].
Our current data demonstrate that CORM-3 induces rapid activation of all of these transcription factors, supporting the concept that the recruitment of one or more of these factors leads to transcriptional activation of anti-apoptotic and cytoprotective genes. To our knowledge, these data constitute the first evidence that CO activates NF-κB, STAT1/3, and Nrf2 in the heart. Further studies will be necessary to firmly establish a causative role of these factors in CO-dependent protection. Nevertheless, the results of this study suggest a paradigm whereby CORM-3 induces delayed cardioprotection by activating NF-κB, STAT1/3, and Nrf2 leading to subsequent activation of Mcl-1, c-FLIPS, and c-FLIPL. Both NF-κB and STAT1/3 are also known to be activated by ischemic PC, leading to the upregulation of various cardioprotective genes, such as COX-2 and HO-1 [25, 37]. Our present data indicate that a similar activation can be elicited by CO, corroborating the notion that CO effectively reproduces the signaling cascade triggered by ischemic PC. An association between CO, Nrf2, and HO-1 has not been previously shown in cardiomyocytes, and thus, our observation is the first suggestion that such a relationship exists.
Clinical relevance of CORM-3
The implicit goal of studying PC is to exploit this phenomenon for the protection of ischemic myocardium in patients with coronary artery disease. Although many pharmacological agents have been shown to induce a late PC phenotype in a variety of animal models [8], many of these agents are not clinically applicable or have significant side effects. Using CORMs it is possible to administer CO in biological systems in a predictable, effective, and safe manner. Moreover, unlike other late PC-mimetic agents, CORM-3 has also been shown to reduce infarct size when given at the time of reperfusion [14]. These results suggest that CO delivered by CORM-3 may offer a feasible and safe method of inducing clinically-relevant cardioprotection.
CONCLUSIONS
Our results indicate that CO induces a delayed cardioprotective phenotype by activating prosurvival signaling pathways that attenuate ischemia/reperfusion injury via the recruitment of a cluster of stress-responsive transcription factors, leading to the upregulation of antiapoptotic molecules that inhibit both extrinsic and intrinsic pathways of apoptosis, and cytoprotective proteins that alleviate myocardial ischemia/reperfusion injury. These observations also indicate that CO induces a cardioprotective and antiapoptotic milieu in the myocardium via molecular mechanisms similar to those involved in the late phase of ischemic PC. Thus, far from being a toxic waste product, CO exerts a fundamental function in protecting the heart against ischemia and apoptosis. Our findings support that CO-releasing agents may be potentially useful for inducing a sustained cardioprotective phenotype in patients at risk for myocardial infarction.
Research Highlights.
CORM-3 activates stress-responsive transcription factors in the heart.
CORM-3 upregulates several cardioprotective molecules.
CORM-3 attenuates the mitochondria-dependent apoptotic pathway
CORM-3 attenuates the death-receptor-mediated apoptotic pathway
CORM-3 induces a late PC-mimetic cardioprotective milieu in the heart.
ACKNOWLEDGMENTS
This study was supported in part by NIH grants R01 HL-65660, and HL-89939, and HL-55757 and P01 HL-78825 and by the National AHA Fellow-to-Faculty Transition Award 0575035N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures. None
REFERENCES
- 1.Chance B, Erecinska M, Wagner M. Mitochondrial responses to carbon monoxide toxicity. Ann N Y Acad Sci. 1970 Oct 5;174(1):193–204. doi: 10.1111/j.1749-6632.1970.tb49786.x. [DOI] [PubMed] [Google Scholar]
- 2.Otterbein LE. Carbon monoxide: innovative anti-inflammatory properties of an age-old gas molecule. Antioxid Redox Signal. 2002 Apr;4(2):309–319. doi: 10.1089/152308602753666361. [DOI] [PubMed] [Google Scholar]
- 3.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000 Apr;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 4.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999 Apr;276(4 Pt 1):L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 5.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000 Oct 2;192(7):1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-alpha-induced apoptosis in cultured fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2000 Feb;278(2):L312–L319. doi: 10.1152/ajplung.2000.278.2.L312. [DOI] [PubMed] [Google Scholar]
- 7.Stein AB, Guo Y, Tan W, Wu WJ, Zhu X, Li Q, et al. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol. 2005 Jan;38(1):127–134. doi: 10.1016/j.yjmcc.2004.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolli R. The late phase of preconditioning. Circ Res. 2000 Nov 24;87(11):972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S, Tang XL, Qiu Y, Takano H, Manchikalapudi S, Dawn B, et al. Nitroglycerin induces late preconditioning against myocardial stunning via a PKC-dependent pathway. Am J Physiol. 1999 Dec;277(6 Pt 2):H2488–H2494. doi: 10.1152/ajpheart.1999.277.6.H2488. [DOI] [PubMed] [Google Scholar]
- 10.Fryer RM, Hsu AK, Eells JT, Nagase H, Gross GJ. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ Res. 1999 Apr 16;84(7):846–851. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- 11.Baxter GF, Marber MS, Patel VC, Yellon DM. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation. 1994 Dec;90(6):2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- 12.Takano H, Bolli R, Black RG, Jr, Kodani E, Tang XL, Yang Z, et al. A(1) or A(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001 Mar 16;88(5):520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchida A, Miura T, Tanno M, Sakamoto J, Miki T, Kuno A, et al. Infarct size limitation by nicorandil: roles of mitochondrial K(ATP) channels, sarcolemmal K(ATP) channels, and protein kinase C. J Am Coll Cardiol. 2002 Oct 16;40(8):1523–1530. doi: 10.1016/s0735-1097(02)02268-4. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, et al. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004 May;286(5):H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, et al. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res. 2002 Aug 15;55(3):506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Guo Y, Ou Q, Cui C, Wu WJ, Tan W, et al. Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-{kappa}B-dependent pathway. Circulation. 2009 Sep 29;120(13):1222–1230. doi: 10.1161/CIRCULATIONAHA.108.778688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein AB, Bolli R, Guo Y, Wang OL, Tan W, Wu WJ, et al. The late phase of ischemic preconditioning induces a prosurvival genetic program that results in marked attenuation of apoptosis. J Mol Cell Cardiol. 2007 Jun;42(6):1075–1085. doi: 10.1016/j.yjmcc.2007.03.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunther L, Berberat PO, Haga M, Brouard S, Smith RN, Soares MP, et al. Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes. 2002 Apr;51(4):994–999. doi: 10.2337/diabetes.51.4.994. [DOI] [PubMed] [Google Scholar]
- 19.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, et al. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001 Mar;166(15)(6):4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 20.Shinmura K, Xuan YT, Tang XL, Kodani E, Han H, Zhu Y, et al. Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ Res. 2002 Mar 22;90(5):602–608. doi: 10.1161/01.res.0000012202.52809.40. [DOI] [PubMed] [Google Scholar]
- 21.Bolli R, Dawn B, Xuan YT. Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc Med. 2003 Feb;13(2):72–79. doi: 10.1016/s1050-1738(02)00230-x. [DOI] [PubMed] [Google Scholar]
- 22.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, et al. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005 Sep 27;112(13):1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KM, Pae HO, Zheng M, Park R, Kim YM, Chung HT. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res. 2007 Oct 26;101(9):919–927. doi: 10.1161/CIRCRESAHA.107.154781. [DOI] [PubMed] [Google Scholar]
- 24.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, et al. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003 Jul 25;93(2):e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 25.Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, et al. Nuclear factor-kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res. 1999 May 14;84(9):1095–1109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 26.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, et al. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci U S A. 2000 Aug 29;97(18):10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, et al. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997 Sep;81(3):404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 29.Bolli R, Stein AB, Guo Y, Wang OL, Rokosh G, Dawn B, et al. A murine model of inducible, cardiac-specific deletion of STAT3: its use to determine the role of STAT3 in the upregulation of cardioprotective proteins by ischemic preconditioning. J Mol Cell Cardiol. 2011 Apr;50(4):589–597. doi: 10.1016/j.yjmcc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Bolli R, Qiu Y, Tang XL, Murphree SS, French BA. Gene therapy with extracellular superoxide dismutase attenuates myocardial stunning in conscious rabbits. Circulation. 1998 Oct 6;98(14):1438–1448. doi: 10.1161/01.cir.98.14.1438. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Bolli R, Qiu Y, Tang XL, Guo Y, French BA. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation. 2001 Apr 10;103(14):1893–1898. doi: 10.1161/01.cir.103.14.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004 Jan 23;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 33.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001 Dec;21(24):8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003 Aug;24(8):449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 35.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003 Feb;9(2):183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 36.Davidson SM, Stephanou A, Latchman DS. FLIP protects cardiomyocytes from apoptosis induced by simulated ischemia/reoxygenation, as demonstrated by short hairpin-induced (shRNA) silencing of FLIP mRNA. J Mol Cell Cardiol. 2003 Nov;35(11):1359–1364. doi: 10.1016/j.yjmcc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci U S A. 2001 Jul 31;98(16):9050–9055. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hattori R, Maulik N, Otani H, Zhu L, Cordis G, Engelman RM, et al. Role of STAT3 in ischemic preconditioning. J Mol Cell Cardiol. 2001 Nov;33(11):1929–1936. doi: 10.1006/jmcc.2001.1456. [DOI] [PubMed] [Google Scholar]
- 39.Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997 Jan 3;272(1):601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 41.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001 Jun;21(12):3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001 Aug;21(16):5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra A, Haudek SB, Knuefermann P, Vallejo JG, Chen ZJ, Michael LH, et al. Nuclear factor-kappaB protects the adult cardiac myocyte against ischemia-induced apoptosis in a murine model of acute myocardial infarction. Circulation. 2003 Dec 23;108(25):3075–3078. doi: 10.1161/01.CIR.0000108929.93074.0B. [DOI] [PubMed] [Google Scholar]
- 44.Leu CM, Wong FH, Chang C, Huang SF, Hu CP. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003 Oct 30;22(49):7809–7818. doi: 10.1038/sj.onc.1207084. [DOI] [PubMed] [Google Scholar]
- 45.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997 Jul 18;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 47.Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol Med. 2001 Feb;7(2):135–145. [PMC free article] [PubMed] [Google Scholar]
- 48.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999 Sep 10;274(37):26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 49.Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, et al. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000 Sep 8;275(36):27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 50.He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, et al. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001 Jun 15;276(24):20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]