Abstract
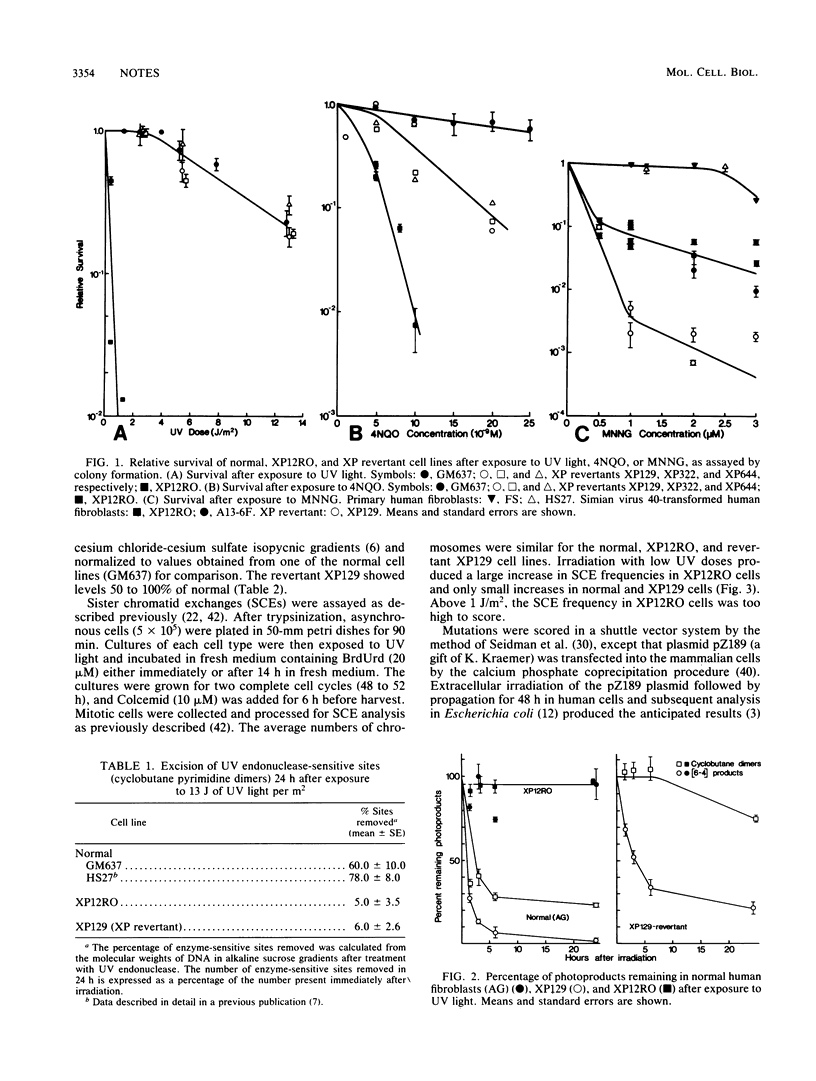
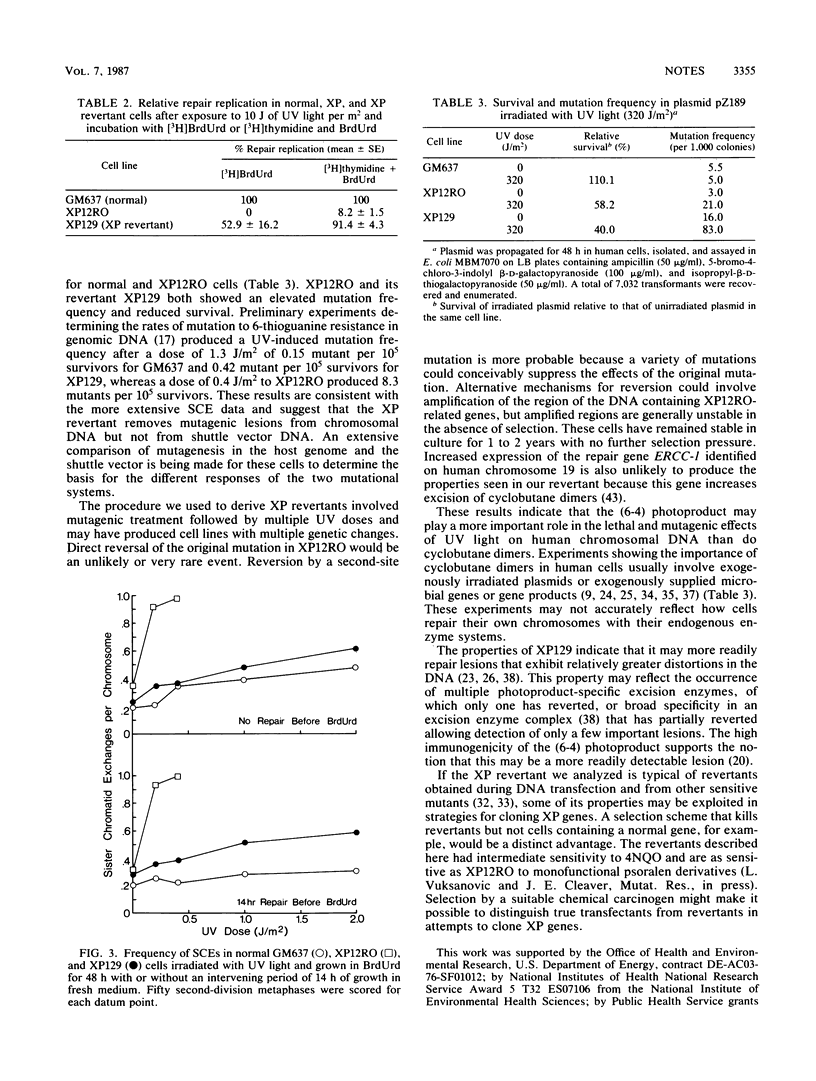
A group A xeroderma pigmentosum revertant with normal sensitivity was created by chemical mutagenesis. It repaired (6-4) photoproducts normally but not pyrimidine dimers and had near normal levels of repair replication, sister chromatid exchange, and mutagenesis from UV light. The rate of UV-induced mutation in a shuttle vector, however, was as high as the rate in the parental xeroderma pigmentosum cell line.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews A. D., Barrett S. F., Robbins J. H. Xeroderma pigmentosum neurological abnormalities correlate with colony-forming ability after ultraviolet radiation. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1984–1988. doi: 10.1073/pnas.75.4.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J. M., Mitchell D. L., Adair G. M. The use of an immunological probe to measure the kinetics of DNA repair in normal and UV-sensitive mammalian cell lines. Mutat Res. 1983 Oct;112(5):287–299. doi: 10.1016/0167-8817(83)90004-4. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair in human xeroderma pigmentosum group C cells involves a different distribution of damaged sites in confluent and growing cells. Nucleic Acids Res. 1986 Oct 24;14(20):8155–8165. doi: 10.1093/nar/14.20.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis A. A., Snyder R. D., Dunn W. C., Regan J. D. Classification of chemical agents as to their ability to induce long- or short-patch DNA repair in human cells. Mutat Res. 1981 Sep;83(2):159–169. doi: 10.1016/0027-5107(81)90001-4. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Tatsumi M. Cross-link repair in human cells and its possible defect in Fanconi's anemia cells. J Mol Biol. 1977 Jul 15;113(4):635–649. doi: 10.1016/0022-2836(77)90227-3. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Karentz D., Cleaver J. E. Excision repair in xeroderma pigmentosum group C but not group D is clustered in a small fraction of the total genome. Mutat Res. 1986 May;165(3):165–174. doi: 10.1016/0167-8817(86)90051-9. [DOI] [PubMed] [Google Scholar]
- Karentz D., Cleaver J. E. Repair-deficient xeroderma pigmentosum cells made UV light resistant by fusion with X-ray-inactivated Chinese hamster cells. Mol Cell Biol. 1986 Oct;6(10):3428–3432. doi: 10.1128/mcb.6.10.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer K. H., Andrews A. D., Barrett S. F., Robbins J. H. Colony-forming ability of ultraviolet-irradiated xeroderma pigmentosum fibroblasts from different DNA repair complementation groups. Biochim Biophys Acta. 1976 Aug 18;442(2):147–153. doi: 10.1016/0005-2787(76)90485-8. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Use of recombinant DNA techniques in cloning DNA repair genes and in the study of mutagenesis in mammalian cells. Mutat Res. 1985 Jun-Jul;150(1-2):61–67. doi: 10.1016/0027-5107(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Clarkson J. M., Chao C. C., Rosenstein B. S. Repair of cyclobutane dimers and (6-4) photoproducts in ICR 2A frog cells. Photochem Photobiol. 1986 May;43(5):595–597. doi: 10.1111/j.1751-1097.1986.tb09539.x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. (6-4)Photoproducts are removed from the DNA of UV-irradiated mammalian cells more efficiently than cyclobutane pyrimidine dimers. Mutat Res. 1985 Jul;143(3):109–112. doi: 10.1016/s0165-7992(85)80018-x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. Further characterisation of a polyclonal antiserum for DNA photoproducts: the use of different labelled antigens to control its specificity. Mutat Res. 1985 Sep;146(2):129–133. doi: 10.1016/0167-8817(85)90002-1. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S., Alvillar J. A., Clarkson J. M. Loss of thymine dimers from mammalian cell DNA. The kinetics for antibody-binding sites are not the same as that for T4 endonuclease V sites. Biochim Biophys Acta. 1982 Jun 30;697(3):270–277. doi: 10.1016/0167-4781(82)90089-6. [DOI] [PubMed] [Google Scholar]
- Morgan W. F., Cleaver J. E. 3-Aminobenzamide synergistically increases sister-chromatid exchanges in cells exposed to methyl methanesulfonate but not to ultraviolet light. Mutat Res. 1982 Jul;104(6):361–366. doi: 10.1016/0165-7992(82)90170-1. [DOI] [PubMed] [Google Scholar]
- Pearlman D. A., Holbrook S. R., Pirkle D. H., Kim S. H. Molecular models for DNA damaged by photoreaction. Science. 1985 Mar 15;227(4692):1304–1308. doi: 10.1126/science.3975615. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. Reduced repair of non-dimer photoproducts in a gene transfected into xeroderma pigmentosum cells. Photochem Photobiol. 1986 May;43(5):509–513. doi: 10.1111/j.1751-1097.1986.tb09528.x. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Tuteja N., Munson P. J., Hauser J., Kraemer K. H., Dixon K. UV light-induced cyclobutane pyrimidine dimers are mutagenic in mammalian cells. Mol Cell Biol. 1986 Oct;6(10):3349–3356. doi: 10.1128/mcb.6.10.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. N., Kollman P. A. Conformations of deoxydodecanucleotides with pyrimidine (6-4)-pyrimidone photoadducts. Photochem Photobiol. 1985 Nov;42(5):465–475. doi: 10.1111/j.1751-1097.1985.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Royer-Pokora B., Haseltine W. A. Isolation of UV-resistant revertants from a xeroderma pigmentosum complementation group A cell line. 1984 Sep 27-Oct 3Nature. 311(5984):390–392. doi: 10.1038/311390a0. [DOI] [PubMed] [Google Scholar]
- Schultz R. A., Barbis D. P., Friedberg E. C. Studies on gene transfer and reversion to UV resistance in xeroderma pigmentosum cells. Somat Cell Mol Genet. 1985 Nov;11(6):617–624. doi: 10.1007/BF01534726. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Dixon K., Razzaque A., Zagursky R. J., Berman M. L. A shuttle vector plasmid for studying carcinogen-induced point mutations in mammalian cells. Gene. 1985;38(1-3):233–237. doi: 10.1016/0378-1119(85)90222-7. [DOI] [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Suzuki N. A UV-resistant mutant without an increased repair synthesis activity, established from a UV-sensitive human clonal cell line. Mutat Res. 1984 Jan;125(1):55–63. doi: 10.1016/0027-5107(84)90031-9. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Nishimaki J., Kuwata T. Characterization of a UV-resistant strain, UVr-10, established from a human clonal cell line, RSb, with high sensitivity to UV, 4NQO, MNNG and interferon. Mutat Res. 1982 Dec;106(2):357–376. doi: 10.1016/0027-5107(82)90116-6. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Hayakawa H., Sekiguchi M., Okada Y. Specific action of T4 endonuclease V on damaged DNA in xeroderma pigmentosum cells in vivo. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2958–2962. doi: 10.1073/pnas.74.7.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Sekiguchi M., Okada Y. Restoration of ultraviolet-induced unscheduled DNA synthesis of xeroderma pigmentosum cells by the concomitant treatment with bacteriophage T4 endonuclease V and HVJ (Sendai virus). Proc Natl Acad Sci U S A. 1975 Oct;72(10):4071–4075. doi: 10.1073/pnas.72.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M. S., Hrncir J., Mitchell D., Ross J., Clarkson J. The relative cytotoxicity and mutagenicity of cyclobutane pyrimidine dimers and (6-4) photoproducts in Escherichia coli cells. Mutat Res. 1986 Jun;161(1):9–17. doi: 10.1016/0027-5107(86)90095-3. [DOI] [PubMed] [Google Scholar]
- Valerie K., de Riel J. K., Henderson E. E. Genetic complementation of UV-induced DNA repair in Chinese hamster ovary cells by the denV gene of phage T4. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7656–7660. doi: 10.1073/pnas.82.22.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J., Mullaart E., van der Schans G. P., Lohman P. H., Knook D. L. Kinetics of ultraviolet induced DNA excision repair in rat and human fibroblasts. Mutat Res. 1984 Sep-Oct;132(3-4):129–138. doi: 10.1016/0167-8817(84)90007-5. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Williams J. I., Cleaver J. E. Excision repair of ultraviolet damage in mammalian cells. Evidence for two steps in the excision of pyrimidine dimers. Biophys J. 1978 May;22(2):265–279. doi: 10.1016/S0006-3495(78)85488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Roza L., Westerveld A., Bootsma D., Simons J. W. Biological and biochemical consequences of the human ERCC-1 repair gene after transfection into a repair-deficient CHO cell line. Mutat Res. 1987 Jan;183(1):69–74. doi: 10.1016/0167-8817(87)90047-2. [DOI] [PubMed] [Google Scholar]
- Zelle B., Lohman P. H. Repair of UV-endonuclease-susceptible sites in the 7 complementation groups of xeroderma pigmentosum A through G. Mutat Res. 1979 Sep;62(2):363–368. doi: 10.1016/0027-5107(79)90091-5. [DOI] [PubMed] [Google Scholar]
- de Jonge A. J., Vermeulen W., Keijzer W., Hoeijmakers J. H., Bootsma D. Microinjection of Micrococcus luteus UV-endonuclease restores UV-induced unscheduled DNA synthesis in cells of 9 xeroderma pigmentosum complementation groups. Mutat Res. 1985 Jun-Jul;150(1-2):99–105. doi: 10.1016/0027-5107(85)90106-x. [DOI] [PubMed] [Google Scholar]


