Abstract
Development of neutralizing Abs to blood coagulation factor VIII (FVIII) provides a major complication in hemophilia care. In this study we explored whether modulation of the uptake of FVIII by APCs can reduce its intrinsic immunogenicity. Endocytosis of FVIII by professional APCs is significantly blocked by mAb KM33, directed toward the C1 domain of FVIII. We created a C1 domain variant (FVIII-R2090A/K2092A/F2093A), which showed only minimal binding to KM33 and retained its activity as measured by chromogenic assay. FVIII-R2090A/K2092A/F2093A displayed a strongly reduced internalization by human monocyte-derived dendritic cells and macrophages, as well as murine BM-derived dendritic cells. We subsequently investigated the ability of this variant to induce an immune response in FVIII-deficient mice. We show that mice treated with FVIII-R2090A/K2092A/F2093A have significantly lower anti-FVIII Ab titers and FVIII-specific CD4+ T-cell responses compared with mice treated with wild-type FVIII. These data show that alanine substitutions at positions 2090, 2092, and 2093 reduce the immunogenicity of FVIII. According to our findings we hypothesize that FVIII variants displaying a reduced uptake by APCs provide a novel therapeutic approach to reduce inhibitor development in hemophilia A.
Introduction
Over the past decades protein therapeutics such as hormones, enzymes, blood coagulation factors, or Abs have provided effective treatment for numerous diseases.1 Treatment commonly requires frequent high-dose administration of protein therapeutics and, although generally considered safe, they often induce immune responses.2 The factors that underlie immunogenicity of biomedical products can be related to the structure of protein, such as the presence of promiscuous T-cell epitopes3 or posttranslational modifications,4 but also to the formulation of the biomolecule.5 Treatment-related parameters such as dosage, frequency, route of administration, and concomitant infections may also contribute to the induction of antidrug immune responses.2 In patients with protein deficiencies administered therapeutics may be recognized by the immune system as non-self, thereby greatly increasing the risk of Ab development.2
Hemophilia A is an X-linked bleeding disorder that is caused by a deficiency in blood coagulation factor VIII (FVIII). Conventional treatment comprising frequent administration of FVIII often results in formation of neutralizing Abs, which inhibit FVIII activity.6,7 Both treatment-related factors, such as intensive treatment episodes,8 and genetic risk factors can contribute to the development of inhibitors. Polymorphic sites in genes involved in the adaptive immune response have been associated with anti-FVIII Ab formation.9–11 Development of high-affinity IgG Abs directed against FVIII is a CD4+ T cell–dependent process.12,13 Endocytosis of FVIII by professional APCs comprises the initial step leading to activation of helper T cells. Uptake and transfer of Ags through the lyso-endosomal pathway results in intracellular processing and presentation of FVIII-derived peptides on MHC II molecules to CD4+ helper T cells.14 Here, we hypothesized that prevention of FVIII uptake by APCs will lead to diminished T- and B-cell responses. Previously, we have shown that endocytosis of FVIII by APCs is mediated via its C1 domain, because administration of a mAb directed toward an antigenic surface in the C1 domain reduced inhibitor titers in FVIII-deficient mice.15 With the use of an Ab-guided mutagenesis strategy we designed a C1 domain variant of FVIII which displayed a strongly reduced internalization by APCs. In vivo studies revealed that this C1 domain variant showed decreased immunogenicity in a murine model for inhibitor development in hemophilia A. Our findings provide a novel paradigm for the reduction of the intrinsic immunogenicity of FVIII by modulating its uptake by APCs.
Methods
Materials
Ficoll-Paque Plus (GE Healthcare), CD14 microbeads (Miltenyi Biotech), and human recombinant GM-CSF and IL-4 (both Cellgenix Technology Transfer) were used for generation of human monocyte–derived dendritic cells (MDDCs); M-CSF (PeproTech) was used to generate human monocyte-derived macrophages (MDMΦs). For culturing murine BM-derived DCs (BMDCs), mouse recombinant GM-CSF was purchased (R&D System). Penicillin/streptomycin, DMEM/F12, RPMI 1640, and serum-free X-VIVO 15 medium were from Lonza; serum-free CellGro DC medium was from CellGenix. FCS was purchased from Thermo Fisher Scientific. Cell factories, culture flasks, and 96-well microtiter plates were purchased from Nunc. Ultrapure methanol-free paraformaldehyde was from Polysciences. Abs used were mouse IgG isotype control Abs conjugated with FITC and PE (Dako); mouse IgG isotype control IgG conjugated with allophycocyanin, anti–human CD80-FITC, anti–human CD83-allophycocyanin, anti–human CD86-allophycocyanin, anti–human CD206-allophycocyanin, anti–murine CD83-allophycocyanin, anti–murine CD86, anti–murine CD11b-FITC, rat IgG isotype control Ab conjugated with FITC, allophycocyanin, or biotin, streptavidin-allophycocyanin, anti–human CD16, anti–human CD32, and anti–human CD64 (BD Biosciences); anti–human CD14-PE; anti–human IgG1-HRP (Sanquin Reagents); anti–human CD209-allophycocyanin (AbD Serotec); anti–mouse CD14-biotin, anti–mouse CD45R-biotin, anti–mouse Gr-1-biotin, and anti–mouse CD8 (eBioscience); and anti–human CD91-PE (Santa Cruz Biotechnology). Anti-CD11c Ab-producing cell line (clone HB-224) was purchased from American Type Culture Collection. mAbs CLB-CAg117 and CLB-CAg9 that target different domains of FVIII have been described previously.16 Recombinant Abs EL-14, KM33, and VK34 have been described previously.15,17 For flow cytometry, Ab CLB-CAg117 was labeled with FITC using the FluoReporter FITC Protein Labeling Kit (Invitrogen).
Preparation of FVIII mutants
Construction of a B domain–deleted FVIII (del 746-1639) has been described previously.18 The remaining linker region (first 5 and last 9 amino acids of the FVIII B domain) was identical for all variants prepared. Arg2090Ala, Lys2092Ala, and Phe2093Ala substitutions were introduced by QuickChange site-directed mutagenesis. (Numbering of substitutions was according to the “legacy” system. If new nomenclature recommendations are taken into account (numbering including signal peptide), the appropriate numbering should be Arg2109Ala, Lys2111Ala, and Phe2112Ala). Coding regions of all constructs were verified by sequence analysis. HEK293 stable cell lines expressing recombinant proteins were prepared as described previously.19 All proteins were purified by immunoaffinity chromatography with the use of anti-FVIII Ab VK34 coupled to CNBr Sepharose 4B as described previously.20 Protein concentration was measured by Bradford protein assay. FVIII concentration was determined by ELISA essentially as described.21 FVIII activity was determined by a chromogenic assay according to the manufacturer's instructions (Chromogenix).
Binding of FVIII to KM33
FVIII binding to KM33 was measured by sandwich ELISA and with surface plasmon resonance analysis. For the ELISA assays, Nunc-Maxisorp 96-well plates were coated with KM33 IgG1 (5 μg/mL) Ab in coating buffer (50mM NaHCO3, pH 9.8) overnight at 4°C. Subsequently, FVIII variants were incubated in binding buffer (50mM Tris pH 7.4, 1M NaCl, 2% human serum albumin) in a concentration up to 1 U/mL on the plate. Bound FVIII was detected with HRP-conjugated mAb CLB-CAg117 (targeting C2 domain of FVIII) or CLB-CAg9 (targeting A2 domain of FVIII). Optical densities were measured at 450 nm with the subtraction of values obtained at 540 nm. Surface plasmon resonance analysis was performed with a BIAcore3000 biosensor system (GE Healthcare) essentially as described.22 Briefly, human mAb EL-14 (27 fmol/mm2) was covalently coupled to the dextran surface of an activated CM5-sensor chip. Subsequently, FVIII variants (6.1 fmol/mm2) were loaded on EL-14 in 20mM HEPES, 150mM NaCl, 5mM CaCl2, and 0.005% Tween 20 (pH 7.4). Association and dissociation of 25nM full-length Ab KM33 (IgG1) were performed in the same buffer at a flow rate of 20 μL/min for 4 minutes at 25°C. Association and dissociation curves were corrected for nonspecific binding to a channel coated with EL-14 only.
Hemophilic E17KO Mice
Hemophilic E17KO mice, characterized by a targeted disruption of exon 17 of the FVIII gene23 were backcrossed into the C57BL/6J background as described previously.24 Mice used in this study were male and aged between 8 and 12 weeks at the beginning of the experiment. The genotypes of hemophilic mice were confirmed by PCR analysis of genomic DNA extracted from ear clippings, as described previously.25
Generation of MDDCs, MDMΦs, and BMDCs
Human MDDCs were prepared as described.15 Blood of healthy individuals was drawn in accordance with Dutch regulations and approval from Sanquin Ethical Advisory Board in accordance with the Declaration of Helsinki. For macrophage culture (MDMΦ), monocytes were resuspended at 2.5 × 106 cells/well in 6-well plates in RPMI 1640 medium supplemented with 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 ng/mL recombinant human M-CSF. Murine BMDCs were essentially prepared as described before.26 Briefly, BM cells were isolated by flushing femurs from E17KO mice with PBS supplemented with 2% FCS. After erythrocyte lysis, cells were resuspended at 1 × 106 cells/mL containing 20 ng/mL mouse recombinant GM-CSF and cultured for 8-10 days in RPMI 1640 medium supplemented with 2.5mM HEPES, 55μM 2-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin, 5mM glutamine, and 10% FCS. Based on MHC class II and CD11c expression, > 95% of the cells generated by this method were DCs.
FVIII endocytosis by flow cytometry
FVIII endocytosis was measured as essentially described before with the use of Ab CLB-CAg117 conjugated with FITC.15 Saponin was used to permeabilize the cells and to visualize internalized FVIII. FACS plots were analyzed with FlowJo Version 7.6 software (TreeStar Inc).
Administration of FVIII in hemophilic E17KO mice
Recombinant B domain–deleted human wild-type FVIII (FVIII WT) or mutants were diluted to 10 μg/mL in sterile PBS and a dose of 1 μg was administered intravenously in E17KO mice (n = 8) 5 times weekly. Endotoxin levels were measured with a Limulus amebocyte lysate chromogenic assay (ToxinSensor; GenScript). One week after the last FVIII injections, animals were killed and blood samples and spleens were collected for further analysis.
Anti-FVIII inhibitory Ab measurements from mouse plasma by ELISA and Bethesda assay
Levels of anti-FVIII Abs in murine plasma were measured by ELISA and functional Bethesda assay essentially as described.15 For the ELISA, 1 AU corresponds to the signal obtained with 1 μg of mouse mAb CLB-CAg9. Data were analyzed with nonparametric Mann-Whitney U test.
Analysis of anti-FVIII ASCs by ELISPOT
Ab-secreting cells (ASCs) present in spleen were analyzed as described.27,28
CD4+ T-cell proliferation assay
Spleens collected after weekly injections of FVIII were processed into single-cell suspensions. Erythrocytes were removed and CD8+ cells were depleted by magnetic bead separation with the use of beads coated with the anti–mouse CD8 Ab. Remaining CD8− cells were cultured in round-bottomed 96-well plates for 72 or 96 hours in X-VIVO 15 medium supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 55μM 2-mercaptoethanol in the presence of increasing concentration of FVIII WT (0, 0.1, 0.5, or 1 μg/mL) to generate Ag-specific T-cell proliferation or concanavalin A (1 μg/mL) to generate nonspecific proliferation. Proliferation was assayed by the addition of 1 μCi (0.037 MBq = 3.7 × 104 Bq)/well of [3H]thymidine for the last 18-20 hours. The results are expressed as the stimulation index defined as counts per minute of cells incubated with FVIII divided by counts per minute of cells with medium only.
Statistical analysis
Unless stated otherwise, data were analyzed by Student t test and differences were considered significant at P < .05 (*P < .05, **P < .01, ***P < .001, and NS indicates not significant).
Results
FVIII with alanine substitutions in positions 2090, 2092, and 2093 shows diminished binding to C1 domain–directed mAb KM33
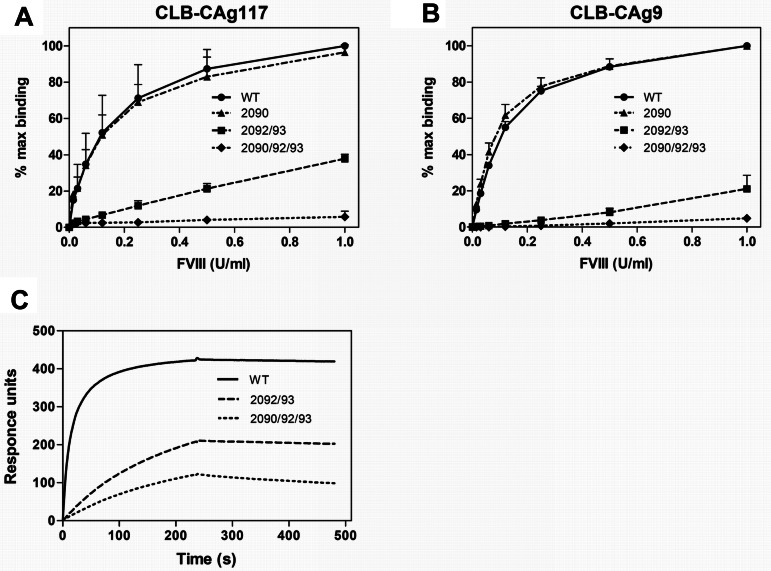
We have previously shown that endocytosis of FVIII by human and murine APCs is blocked by human monoclonal anti-C1 domain Ab KM3315 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Moreover, upon in vivo administration, KM33 was able to delay the immune response to FVIII.15 These findings indicate that Ab KM33 shields a binding site that is crucial for the uptake of FVIII by APCs. We explored which residues in the C1 domain are involved in binding of KM33 to FVIII. Lys2092 and Phe2093 have previously been implicated in KM33 binding.22 However, mutation of these residues to alanines does not completely abolish KM33 binding; therefore, additional C1 domain amino acids might be involved in the interaction between FVIII and KM33. To test this hypothesis, a FVIII variant was prepared in which, besides Lys2092 and Phe2093, we additionally substituted Arg2090 for an alanine (Figure 1). FVIII-R2090A, FVIII-K2092A/F2093A, and FVIII-R2090A/K2092A/F2093A were expressed by stably transfected HEK293 cells and purified by affinity chromatography. Activities of all variants were similar to FVIII WT, as measured by a chromogenic assay (Table 1). To explore the binding of purified variants to KM33, we used a solution-phase sandwich ELISA in which FVIII, bound to immobilized KM33, was detected either via an A2 or C2 domain–targeting Ab (CLB-CAg9 or CLB-CAg117, respectively). As expected, FVIII-K2092A/F2093A showed a significant reduction in ability to bind KM33 as compared to FVIII WT. Introduction of a single alanine substitution at position 2090 did not influence the binding; however, the presence of this mutation additional to those in FVIII-K2092A/F2093A showed more pronounced decrease in binding to KM33 (Figure 2A-B). Moreover, we performed surface plasmon resonance analysis of the FVIII-KM33 interaction, where KM33 was passed over FVIII WT, FVIII-K2092A/F2093A, and FVIII-R2090A/K2092A/F2093A. Similar to previous observations, FVIII-R2090A/K2092A/F2093A showed a greater reduction of binding to KM33 than the variant with alanine substitutions at positions 2092 and 2093 (Figure 2C). In addition, other control mAbs directed toward various FVIII domains bound all 3 FVIII variants tested (supplemental Figure 4). Altogether, these results show that residues Arg2090, Lys2092, and Phe2093 are crucially involved in binding of KM33 to FVIII.
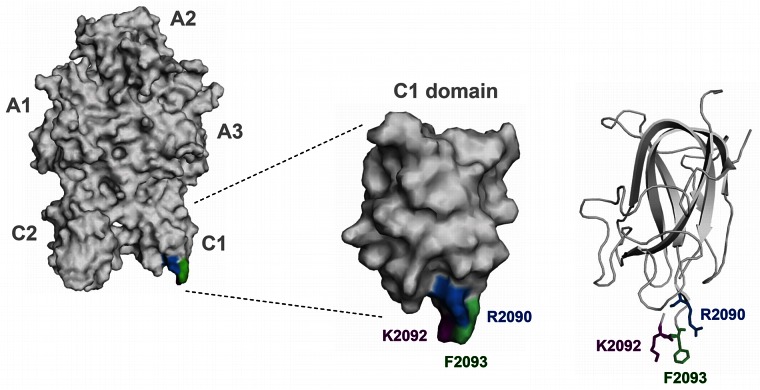
Figure 1.
Three-dimensional structure of FVIII molecule highlighting C1 domain residues Arg2090 (blue), Lys2092 (purple), and Phe2093 (green). Left panel provides an overview of the entire FVIII molecule. The position of the A1, A2, A3, C1, and C2 domains is indicated next to the model. The middle panel provides an overview of the surface coverage of the C1 domain. The right panel shows the secondary structure of the protein backbone of the C1 domain. Residues Arg2090, Lys2092, and Phe2093 are displayed in a ball and stick format. Models were based on FVIII crystal structure (PDB code 3cdz) and prepared with PyMol V0.99 imaging software (Schrodinger).
Table 1.
Specific activity of FVIII variants
| FVIII | Specific activity, U/mg |
|---|---|
| Wild-type | 7.7 ± 1.9 × 103 |
| FVIII-R2090A | 6.2 ± 1.0 × 103 |
| FVIII-K2092A/F2093A | 8.3 ± 1.9 × 103 |
| FVIII-R2090A/K2092A/F2093A | 7.7 ± 0.8 × 103 |
Figure 2.
FVIII with alanine substitutions in positions 2090, 2092, and 2093 shows diminished binding to KM33. (A-B) KM33 was immobilized on microtiter plates and incubated with increasing concentrations of FVIII WT (●, solid line) or mutants FVIII-R2090A (▴, dashed-dotted line), FVIII-K2092A/F2093A (■, dashed line), or FVIII-R2090A/K2092A/F2093A (♦, dotted line). Bound FVIII was detected either with C2 domain–targeting Ab CLB-CAg117 (A) or A2 domain–targeting Ab CLB-CAg9 (B). Data show mean ± SD of 3 experiments and are expressed as percentage of maximum binding, whereby 100% corresponds to signal obtained with 1 U/mL FVIII WT. (C) Surface plasmon resonance analysis of FVIII-KM33 interaction. KM33 IgG1 (25nM) was passed over FVIII WT (solid line), FVIII-K2092A/F2093A (dashed line), or FVIII-R2090A/K2092A/F2093A (dotted line) immobilized on a sensor chip via C2 domain–targeting mAb EL-14. Dissociation was initiated on replacement of ligand solution by buffer. Data are representative of 3 independent experiments. WT indicates wild-type FVIII; 2090, FVIII-R2090A; 2092/93, FVIII-K2092A/F2093A; and 2090/92/93, FVIII-R2090A/K2092A/F2093A.
C1 domain mutations alter FVIII endocytosis by APCs
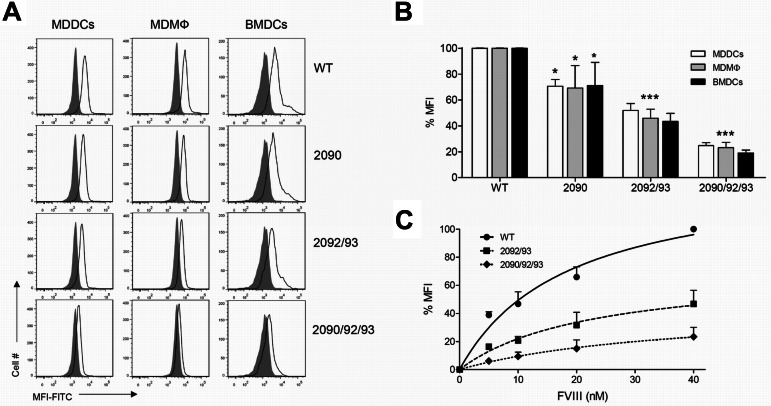
We subsequently explored whether residues 2090, 2092, and 2093 in the C1 domain direct endocytosis of FVIII by APCs. Human MDDCs and MDMΦs were incubated for 30 minutes with 15nM WT or variant FVIII, then fixed and stained with FITC-conjugated CLB-CAg117 Ab in the presence of saponin. Endocytosis of all tested FVIII variants by MDDCs or MDMΦs was significantly reduced compared with FVIII WT (Figure 3A-B white and gray bars, respectively). Interestingly, FVIII-R2090A/K2092A/F2093A showed decreased uptake compared with FVIII-K2092A/F2093A, which indicates the critical importance of the additional mutation in position 2090. Similar findings were observed when endocytosis was tested with the use of murine BMDCs (Figure 3A-B black bars). Moreover, when increased concentrations of variants were tested for endocytosis, uptake of FVIII-R2090A/K2092A/F2093A was significantly lower than that of the FVIII-K2092A/F2093A variant at all concentrations evaluated (Figure 3C). Altogether, these data indicate that Arg2090, Lys2092, and Phe2093 within the C1 domain are essential for the cellular uptake of FVIII by APCs.
Figure 3.
C1 domain mutations alter FVIII endocytosis by APCs. (A-B) Final concentration of 15nM FVIII WT or mutants FVIII-R2090A, FVIII-K2092A/F2093A, or FVIII-R2090A/K2092A/F2093A was added to MDDCs (white bars), MDMΦs (gray bars), or BMDCs (black bars) for 30 minutes at 37°C. Internalized FVIII was detected by adding CLB-CAg117-FITC in the presence of saponin. Representative histograms (gray-filled histograms show untreated cells, open histograms represent cells treated with FVIII; A) and quantification from ≥ 3 independent experiments (B) are shown (mean ± SD). Results are expressed as the percentage of mean fluorescence intensity (MFI), whereby 100% corresponds to MFI obtained with FVIII WT. (C) Increasing concentrations (0-40nM) FVIII WT (●, solid line), FVIII-K2092A/F2093A (■, dashed line), or FVIII-R2090A/K2092A/F2093A (♦, dotted line) were incubated with MDDCs at 37°C. 100% MFI refers to MFI measured for 40nM FVIII WT. Data show mean ± SD from 3 independent experiments. WT indicates wild-type FVIII; 2090, FVIII-R2090A; 2092/93, FVIII-K2092A/F2093A; and 2090/92/93, FVIII-R2090A/K2092A/F2093A.
Role of C1 domain in modulation of immune responses to FVIII in vivo
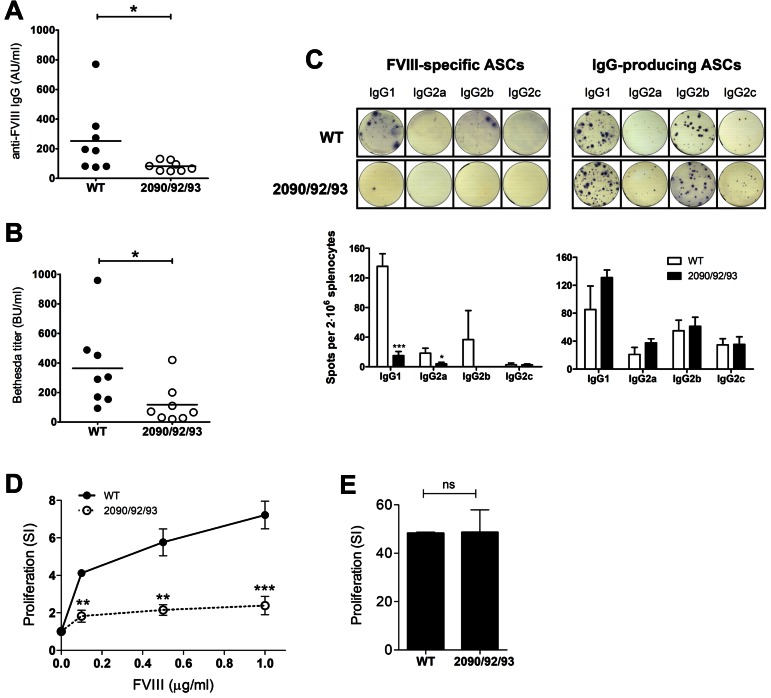
Residues 2090, 2092, and 2093 are important for uptake of FVIII by both human as well as murine APCs. To investigate whether decreased endocytosis of FVIII-R2090A/K2092A/F2093A leads to reduced in vivo immune responses, FVIII−/− mice (E17KO) were injected weekly with 1 μg of either FVIII WT or FVIII-R2090A/K2092A/F2093A. After 5 consecutive injections, the plasma of mice infused with FVIII WT contained high anti-FVIII Ab titers (ELISA: 252 ± 82 AU/mL; Bethesda assay: 364 ± 98 BU/mL), whereas the titer was significantly lower in the group that received FVIII-R2090A/K2092A/F2093A (ELISA: 80 ± 12 AU/mL; Bethesda assay: 118 ± 48 BU/mL; Figure 4A-B). In addition, to analyze the splenic anti-FVIII ASCs, an ELISPOT assay has been performed. In agreement with the Ab titers in the plasma, mice injected with FVIII WT had significant number of anti-FVIII ASCs, producing mainly IgG1 or IgG2b Abs, whereas the group that received FVIII-R2090A/K2092A/F2093A had little or no FVIII-specific ASCs (Figure 4C). Moreover, administration of FVIII-R2090A/K2092A/F2093A led to reduced proliferation of splenic CD4+ T cells upon in vitro restimulation with FVIII (7.2 ± 0.4 vs 2.3 ± 0.3 SI for the highest FVIII concentration used; Figure 4D), whereas nonspecific proliferation in the presence of concanavalin A was similar for both groups (Figure 4E). Together, our results show that both B-cell and CD4+ T-cell responses are greatly reduced in mice that received FVIII-R2090A/K2092A/F2093A compared with mice infused with FVIII WT. Altogether, these findings clearly indicate that the immune recognition of FVIII by APCs is a major determinant for its intrinsic immunogenicity. Therefore, FVIII variants displaying a reduced uptake by APCs could provide a novel therapeutic approach to reduce the risk of inhibitor development in patients with hemophilia A.
Figure 4.
Reduction of immune responses on in vivo administration of FVIII-R2090A/K2092A/F2093A in FVIII−/− mice. Hemophilic E17KO mice (n = 8) were injected intravenously 5 times weekly with 1 μg of FVIII WT or FVIII-R2090A/K2092A/F2093A. One week after the last injections, mice were killed, and blood samples were collected. (A-B) Anti-FVIII Ab titers from the plasma samples were evaluated by ELISA (A) and Bethesda assay (B) as described in detail in “Anti-FVIII inhibitory Ab measurements from mouse plasma by ELISA and Bethesda assay.” *P < .05 (nonparametric Mann-Whitney U test). (C) The presence of splenic ASCs producing FVIII-specific Abs was determined by ELISPOT. As a control, IgG-producing ASCs were detected. Representative wells displaying both ASCs types are shown for both groups. Spots were quantified with the AELvis reader and eli.analyze Version 6.0 software. Group injected with FVIII WT is represented by the white bars and group injected with FVIII-R2090A/K2092A/F2093A is represented by the black bars. (D-E) CD8− splenocytes were assayed in a thymidine (3H) incorporation assay. Proliferation was measured after 72 hours, and thymidine was added for the last 18-20 hours. Results are shown as stimulation index (SI) from triplicate wells (mean ± SD) for both FVIII-specific (D) and nonspecific (E) proliferation. Mice injected with FVIII WT (●), group injected with FVIII-R2090A/K2092A/F2093A (○). WT indicates wild-type FVIII; and 2090/92/93, FVIII-R2090A/K2092A/F2093A.
Discussion
Immune responses generated toward protein therapeutics represent a major complication in drug therapy. Production of high-affinity Abs to protein Ags requires priming of naive CD4+ T cells after recognition of peptide-sequences associated with MHC class II on APCs. Several strategies such as selective modification of B- and T-cell epitopes aim for modulation of adaptive immune responses to biologicals.3,29 Here, we sought to reduce immunogenicity by interfering with recognition and endocytosis of Ag by APCs. A number of studies have shown that enhancement of Ag endocytosis and/or subsequent presentation leads to stronger immune responses.30–32 Therefore, we anticipated that reduction of Ag uptake will result in decreased immunogenicity of biotherapeutics. Indeed, we found that introduction of Ala substitutions in positions 2090, 2092, and 2093 results in a FVIII variant is poorly endocytosed by APCs and therefore yields lower anti-FVIII Ab titers in vivo. Decreased uptake of this FVIII-R2090A/K2092A/F2093A leads to reduced CD4+ T-cell activation as well as diminished numbers of FVIII-specific ASCs. Together our findings suggest that prevention of endocytosis of FVIII by APCs comprises a powerful approach to reduce its immunogenicity.
Binding of FVIII to VWF protects FVIII from endocytosis by human DCs.15,33 Therefore, changes in immunogenicity profile can be potentially explained by lack of or an increased binding of FVIII to VWF. However, binding of FVIII-R2090A/K2092A/F2093A to both human as well as murine VWF was similar to FVIII WT (supplemental Figure 2). In another report, the immunogenicity of FVIII was indirectly linked to its activity.34 Thrombin, the end-product of the coagulation cascade, is capable of acting as a “danger” signal that is a necessary stimulus for APCs to activate CD4+ T cells. Given that the FVIII-R2090A/K2092A/F2093A variant displays similar activity levels as FVIII WT as measured by a chromogenic assay, the reduction of immune response in mice infused with this variant is unlikely to be caused by a reduction in the amount of thrombin generated.
At present, it is not clear which endocytic receptors promote uptake of FVIII by APCs.15,35 Macrophage mannose receptor has been proposed as an important interaction partner of FVIII.36 FVIII contains multiple potential glycosylation sites37 with 2 major residues described to expose mannose-ending glycosylations located on the heavy (N239) and the light (N2118) chain.38,39 However, none of the FVIII variants tested in this study has modified glycans, yet their endocytosis is significantly impaired. This argues against mannose receptor being a major receptor for FVIII endocytosis by APCs. Broadly expressed scavenger receptor, low-density lipoprotein receptor-related protein (LRP), and other low-density lipoprotein receptor family members have been reported as important receptors for FVIII clearance.40 Lysine 2092 and phenylalanine 2093 have been shown to play a role in FVIII binding to LRP.20 FVIII-R2090A/K2092A/F2093A displays reduced affinity to LRP1 cluster II compared with FVIII WT (supplemental Figure 3). Moreover, it has been suggested that disruption of FVIII-LRP binding could potentially lead to prolonged half-life of FVIII in vivo.41 However, despite the importance of these residues in endocytosis of FVIII by APCs, LRP has not been implicated in the uptake of FVIII by DCs.15,42 Moreover, we and others have previously shown that FVIII-K2092A/F2093A has reduced affinity to phospholipid membranes containing a low percentage of phosphatidyl-serine.22,43 However, because the phosphatidylserine–binding protein lactadherin does not compete for FVIII uptake by DCs,15 it is unlikely that this interaction plays a major role in the endocytosis of FVIII by APCs. All in all, despite the identification of a crucial determinant for endocytosis of FVIII, the precise mechanism by which FVIII is internalized by APCs remains to be established.
A number of other approaches have been used to induce tolerance or to diminish inhibitor development in hemophilia A. Qian et al reported that disruption of the interaction between costimulatory molecules B7 and CD28 resulted in lower anti-FVIII Ab titers.25 In addition, blockade of CD40/CD40L interactions has been shown to transiently suppress inhibitor development in murine hemophilia A.44,45 Other studies showed that using anti-CD3 Ab46 or coinjecting FVIII with anti-inflammatory drugs such as rapamycin47 can induce tolerance or reduce inhibitor development. Despite their promising outcome, these approaches are limited by their lack of Ag-specificity. Suppression of protective immune responses against incoming pathogens may present a potential side effect of such treatment. B-cell blasts expressing Ig fusions of FVIII A2 and C2 domains have been successfully used to restore tolerance in hemophilic mice with preexisting inhibitors.48 More recently, FVIII-expressing foamy virus–transduced tolerogenic DCs have been used to reduce inhibitor titers in mice with hemophilia A.49 However, concerns for possible genotoxicity of viral vectors potentially limit the clinical application of these Ag-specific approaches to induce tolerance in hemophilia A.50
Immune responses to intravenously administered FVIII are heterogeneous.6 Abs to FVIII develop in ∼ 25% of patients with severe hemophilia who lack circulating endogenous levels of FVIII.6 The threshold for immune activation depends on FVIII genotype, but it is also determined by the efficiency of immune recognition and processing of FVIII by APCs and polymorphism in genes encoding proteins involved in adaptive immune responses.11 Our current findings document that the FVIII-R2090A/K2092A/F2093A variant is less efficiently endocytosed and displays lower immunogenicity compared with FVIII WT in the mouse model of hemophilia A.
Supplementary Material
Acknowledgments
The authors thank Prof Dr K. Mertens for helpful discussions.
This work was supported by the Joghem van Loghem Foundation.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.W. and S.D.v.H. designed the research, performed the experiments, analyzed the data, made the figures, and wrote the paper; E.H. designed the research and analyzed the data; P.K. and A.R. performed the experiments; S.-Y.J. and X.L.Z. provided reagents; M.v.d.B., A.t.B., and A.B.M designed the research and analyzed the data; and J.V. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Voorberg, Department of Plasma Proteins, Sanquin Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: j.voorberg@sanquin.nl.
References
- 1.Buttel IC, Chamberlain P, Chowers Y, et al. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals. 2011;39(2):100–109. doi: 10.1016/j.biologicals.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Scott DW, De Groot AS. Can we prevent immunogenicity of human protein drugs? Ann Rheum Dis. 2010;69:i72–i76. doi: 10.1136/ard.2009.117564. [DOI] [PubMed] [Google Scholar]
- 3.Tangri S, Mothe BR, Eisenbraun J, et al. Rationally engineered therapeutic proteins with reduced immunogenicity. J Immunol. 2005;174(6):3187–3196. doi: 10.4049/jimmunol.174.6.3187. [DOI] [PubMed] [Google Scholar]
- 4.Brooks SA. Strategies for analysis of the glycosylation of proteins: current status and future perspectives. Mol Biotechnol. 2009;43(1):76–88. doi: 10.1007/s12033-009-9184-6. [DOI] [PubMed] [Google Scholar]
- 5.Villalobos AP, Gunturi SR, Heavner GA. Interaction of polysorbate 80 with erythropoietin: a case study in protein-surfactant interactions. Pharm Res. 2005;22(7):1186–1194. doi: 10.1007/s11095-005-5356-7. [DOI] [PubMed] [Google Scholar]
- 6.Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 7.Lollar P. Pathogenic antibodies to coagulation factors. Part one: factor VIII and factor IX. J Thromb Haemost. 2004;2(7):1082–1095. doi: 10.1111/j.1538-7836.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 8.Gouw SC, van den Berg HM, le Cessie S, van der Bom JG. Treatment characteristics and the risk of inhibitor development: a multicenter cohort study among previously untreated patients with severe hemophilia A. J Thromb Haemost. 2007;5(7):1383–1390. doi: 10.1111/j.1538-7836.2007.02595.x. [DOI] [PubMed] [Google Scholar]
- 9.Astermark J, Oldenburg J, Carlson J, et al. Polymorphisms in the TNFA gene and the risk of inhibitor development in patients with hemophilia A. Blood. 2006;108(12):3739–3745. doi: 10.1182/blood-2006-05-024711. [DOI] [PubMed] [Google Scholar]
- 10.Astermark J, Oldenburg J, Pavlova A, Berntorp E, Lefvert AK. Polymorphisms in the IL10 but not in the IL1beta and IL4 genes are associated with inhibitor development in patients with hemophilia A. Blood. 2006;107(8):3167–3172. doi: 10.1182/blood-2005-09-3918. [DOI] [PubMed] [Google Scholar]
- 11.Pavlova A, Delev D, Lacroix-Desmazes S, et al. Impact of polymorphisms of the major histocompatibility complex class II, interleukin-10, tumor necrosis factor-alpha and cytotoxic T-lymphocyte antigen-4 genes on inhibitor development in severe hemophilia A. J Thromb Haemost. 2009;7(12):2006–2015. doi: 10.1111/j.1538-7836.2009.03636.x. [DOI] [PubMed] [Google Scholar]
- 12.Qian J, Burkly LC, Smith EP, et al. Role of CD154 in the secondary immune response: the reduction of pre-existing splenic germinal centers and anti-factor VIII inhibitor titer. Eur J Immunol. 2000;30(9):2548–2554. doi: 10.1002/1521-4141(200009)30:9<2548::AID-IMMU2548>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Jones TD, Phillips WJ, Smith BJ, et al. Identification and removal of a promiscuous CD4+ T cell epitope from the C1 domain of factor VIII. J Thromb Haemost. 2005;3(5):991–1000. doi: 10.1111/j.1538-7836.2005.01309.x. [DOI] [PubMed] [Google Scholar]
- 14.van Haren SD, Herczenik E, ten Brinke A, Mertens K, Voorberg J, Meijer AB. HLA-DR-presented peptide repertoires derived from human monocyte-derived dendritic cells pulsed with blood coagulation factor VIII. Mol Cell Proteomics. 2011;10(6) doi: 10.1074/mcp.M110.002246. M110.002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herczenik E, van Haren SD, Wroblewska A, et al. Uptake of blood coagulation factor VIII by dendritic cells is mediated via its C1 domain. J Allergy Clin Immunol. 2012;129(2):501–9. doi: 10.1016/j.jaci.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Brinkman HJ, Mertens K, van Mourik JA. Phospholipid-binding domain of factor VIII is involved in endothelial cell-mediated activation of factor X by factor IXa. Arterioscler Thromb Vasc Biol. 2002;22(3):511–516. doi: 10.1161/hq0302.105359. [DOI] [PubMed] [Google Scholar]
- 17.van Helden PM, van den Berg HM, Gouw SC, et al. IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A. Br J Haematol. 2008;142(4):644–652. doi: 10.1111/j.1365-2141.2008.07232.x. [DOI] [PubMed] [Google Scholar]
- 18.van den Biggelaar M, Bouwens EA, Voorberg J, Mertens K. Storage of factor VIII variants with impaired von Willebrand factor binding in Weibel-Palade bodies in endothelial cells. PLoS One. 2011;6(8):e24163. doi: 10.1371/journal.pone.0024163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fribourg C, Meijer AB, Mertens K. The interface between the EGF2 domain and the protease domain in blood coagulation factor IX contributes to factor VIII binding and factor X activation. Biochemistry. 2006;45(35):10777–10785. doi: 10.1021/bi060451h. [DOI] [PubMed] [Google Scholar]
- 20.Meems H, van den Biggelaar M, Rondaij M, van der Zwaan C, Mertens K, Meijer AB. C1 domain residues Lys 2092 and Phe 2093 are of major importance for the endocytic uptake of coagulation factor VIII. Int J Biochem Cell Biol. 2011;43(8):1114–1121. doi: 10.1016/j.biocel.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Donath MS, Lenting PJ, Van Mourik JA, Mertens K. The role of cleavage of the light chain at positions Arg1689 or Arg1721 in subunit interaction and activation of human blood coagulation factor VIII. J Biol Chem. 1995;270(8):3648–3655. doi: 10.1074/jbc.270.8.3648. [DOI] [PubMed] [Google Scholar]
- 22.Meems H, Meijer AB, Cullinan DB, Mertens K, Gilbert GE. Factor VIII C1 domain residues Lys 2092 and Phe 2093 contribute to membrane binding and cofactor activity. Blood. 2009;114(18):3938–3946. doi: 10.1182/blood-2009-01-197707. [DOI] [PubMed] [Google Scholar]
- 23.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10(1):119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 24.Reipert BM, Ahmad RU, Turecek PL, Schwarz HP. Characterization of antibodies induced by human factor VIII in a murine knockout model of hemophilia A. Thromb Haemost. 2000;84(5):826–832. [PubMed] [Google Scholar]
- 25.Qian J, Collins M, Sharpe AH, Hoyer LW. Prevention and treatment of factor VIII inhibitors in murine hemophilia A. Blood. 2000;95(4):1324–1329. [PubMed] [Google Scholar]
- 26.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 27.Hausl C, Maier E, Schwarz HP, et al. Long-term persistence of anti-factor VIII antibody-secreting cells in hemophilic mice after treatment with human factor VIII. Thromb Haemost. 2002;87(5):840–845. [PubMed] [Google Scholar]
- 28.Bril WS, van Helden PM, Hausl C, et al. Tolerance to factor VIII in a transgenic mouse expressing human factor VIII cDNA carrying an Arg(593) to Cys substitution. Thromb Haemost. 2006;95(2):341–347. doi: 10.1160/TH05-08-0559. [DOI] [PubMed] [Google Scholar]
- 29.Nagata S, Pastan I. Removal of B cell epitopes as a practical approach for reducing the immunogenicity of foreign protein-based therapeutics. Adv Drug Deliv Rev. 2009;61(11):977–985. doi: 10.1016/j.addr.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts C, West MA, Zaru R. TLR signalling regulated antigen presentation in dendritic cells. Curr Opin Immunol. 2010;22(1):124–130. doi: 10.1016/j.coi.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 31.van Luijn MM, Chamuleau ME, Thompson JA, et al. Class II-associated invariant chain peptide down-modulation enhances the immunogenicity of myeloid leukemic blasts resulting in increased CD4+ T-cell responses. Haematologica. 2010;95(3):485–493. doi: 10.3324/haematol.2009.010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh SK, Streng-Ouwehand I, Litjens M, et al. Design of neo-glycoconjugates that target the mannose receptor and enhance TLR-independent cross-presentation and Th1 polarization. Eur J Immunol. 2011;41(4):916–925. doi: 10.1002/eji.201040762. [DOI] [PubMed] [Google Scholar]
- 33.Dasgupta S, Repesse Y, Bayry J, et al. VWF protects FVIII from endocytosis by dendritic cells and subsequent presentation to immune effectors. Blood. 2007;109(2):610–612. doi: 10.1182/blood-2006-05-022756. [DOI] [PubMed] [Google Scholar]
- 34.Skupsky J, Zhang AH, Su Y, Scott DW. A role for thrombin in the initiation of the immune response to therapeutic factor VIII. Blood. 2009;114(21):4741–4748. doi: 10.1182/blood-2008-10-186452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Haren SD, Wroblewska A, Fischer K, Voorberg J, Herczenik E. Requirements for immune recognition and processing of factor VIII by antigen-presenting cells. Blood Rev. 2012;26(1):43–49. doi: 10.1016/j.blre.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Dasgupta S, Navarrete AM, Bayry J, et al. A role for exposed mannosylations in presentation of human therapeutic self-proteins to CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 2007;104(21):8965–8970. doi: 10.1073/pnas.0702120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenting PJ, Christophe OD, Gueguen P. The disappearing act of factor VIII. Haemophilia. 2010;16(102):6–15. doi: 10.1111/j.1365-2516.2008.01864.x. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman RJ, Wasley LC, Dorner AJ. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988;263(13):6352–6362. [PubMed] [Google Scholar]
- 39.Medzihradszky KF, Besman MJ, Burlingame AL. Structural characterization of site-specific N-glycosylation of recombinant human factor VIII by reversed-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal Chem. 1997;69(19):3986–3994. doi: 10.1021/ac970372z. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham N, Laffan MA, Manning RA, O'Donnell JS. Low-density lipoprotein receptor-related protein polymorphisms in patients with elevated factor VIII coagulant activity and venous thrombosis. Blood Coagul Fibrinolysis. 2005;16(7):465–468. doi: 10.1097/01.mbc.0000178831.45049.aa. [DOI] [PubMed] [Google Scholar]
- 41.Bovenschen N, van Dijk KW, Havekes LM, Mertens K, van Vlijmen BJ. Clearance of coagulation factor VIII in very low-density lipoprotein receptor knockout mice. Br J Haematol. 2004;126(5):722–725. doi: 10.1111/j.1365-2141.2004.05093.x. [DOI] [PubMed] [Google Scholar]
- 42.Dasgupta S, Navarrete AM, Andre S, et al. Factor VIII bypasses CD91/LRP for endocytosis by dendritic cells leading to T-cell activation. Haematologica. 2008;93(1):83–89. doi: 10.3324/haematol.11535. [DOI] [PubMed] [Google Scholar]
- 43.Lu J, Pipe SW, Miao H, Jacquemin M, Gilbert GE. A membrane-interactive surface on the factor VIII C1 domain cooperates with the C2 domain for cofactor function. Blood. 2011;117(11):3181–3189. doi: 10.1182/blood-2010-08-301663. [DOI] [PubMed] [Google Scholar]
- 44.Rossi G, Sarkar J, Scandella D. Long-term induction of immune tolerance after blockade of CD40-CD40L interaction in a mouse model of hemophilia A. Blood. 2001;97(9):2750–2757. doi: 10.1182/blood.v97.9.2750. [DOI] [PubMed] [Google Scholar]
- 45.Reipert BM, Sasgary M, Ahmad RU, Auer W, Turecek PL, Schwarz HP. Blockade of CD40/CD40 ligand interactions prevents induction of factor VIII inhibitors in hemophilic mice but does not induce lasting immune tolerance. Thromb Haemost. 2001;86(6):1345–1352. [PubMed] [Google Scholar]
- 46.Waters B, Qadura M, Burnett E, et al. Anti-CD3 prevents factor VIII inhibitor development in hemophilia A mice by a regulatory CD4+CD25+-dependent mechanism and by shifting cytokine production to favor a Th1 response. Blood. 2009;113(1):193–203. doi: 10.1182/blood-2008-04-151597. [DOI] [PubMed] [Google Scholar]
- 47.Moghimi B, Sack BK, Nayak S, Markusic DM, Mah CS, Herzog RW. Induction of tolerance to factor VIII by transient co-administration with rapamycin. J Thromb Haemost. 2011;9(8):1524–1533. doi: 10.1111/j.1538-7836.2011.04351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lei TC, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105(12):4865–4870. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su RJ, Epp A, Feng J, et al. Suppression of the immune response to FVIII in hemophilia A mice by transgene modified tolerogenic dendritic cells. Mol Ther. 2011;19(10):1896–1904. doi: 10.1038/mt.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barese CN, Dunbar CE. Contributions of gene marking to cell and gene therapies. Hum Gene Ther. 2011;22(6):659–668. doi: 10.1089/hum.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.