Abstract
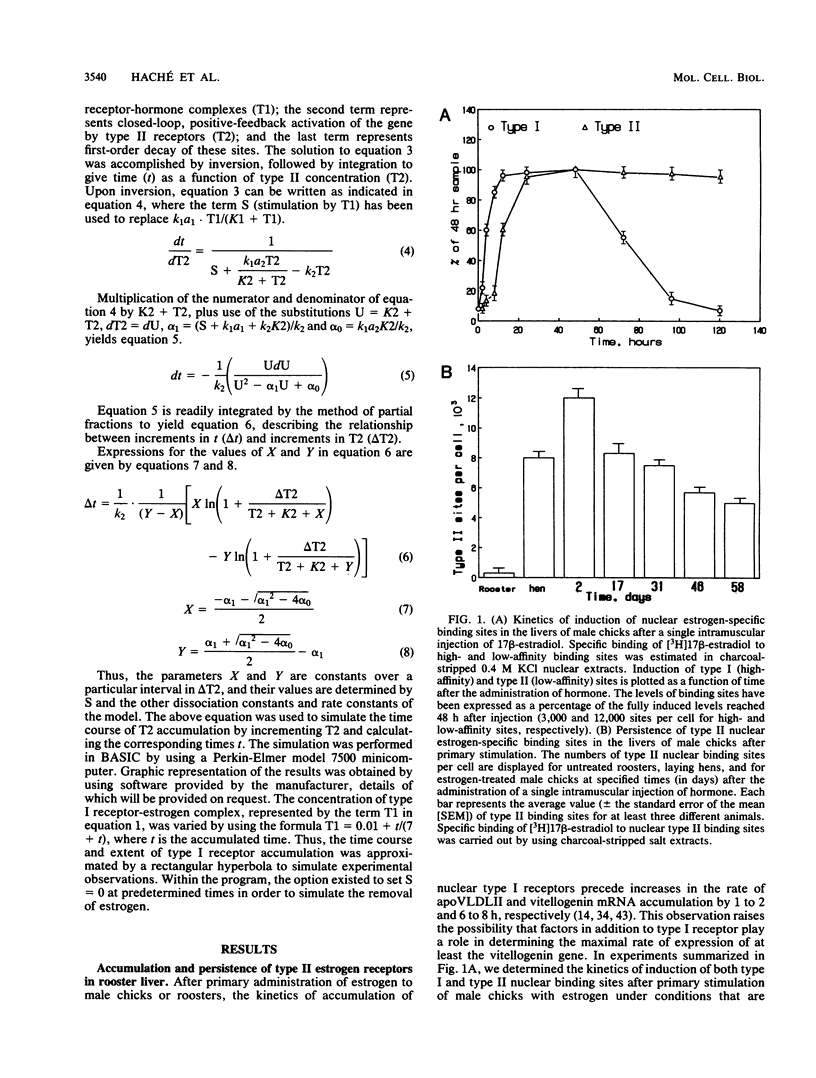
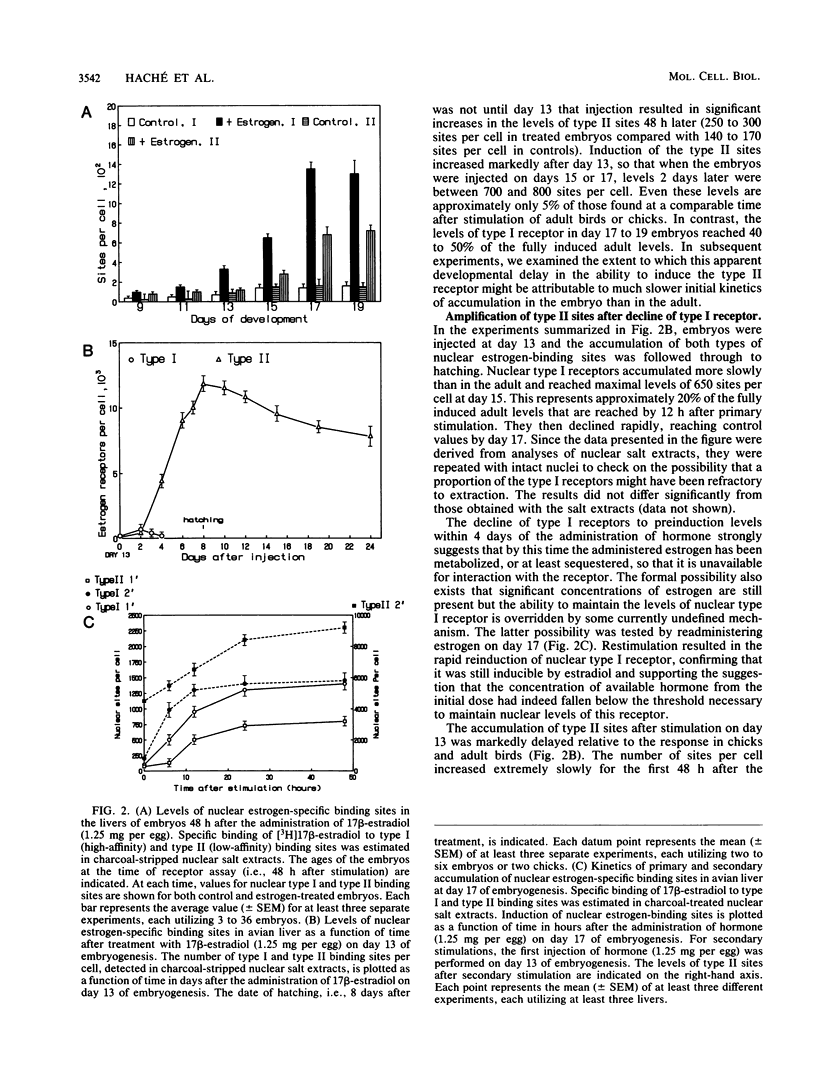
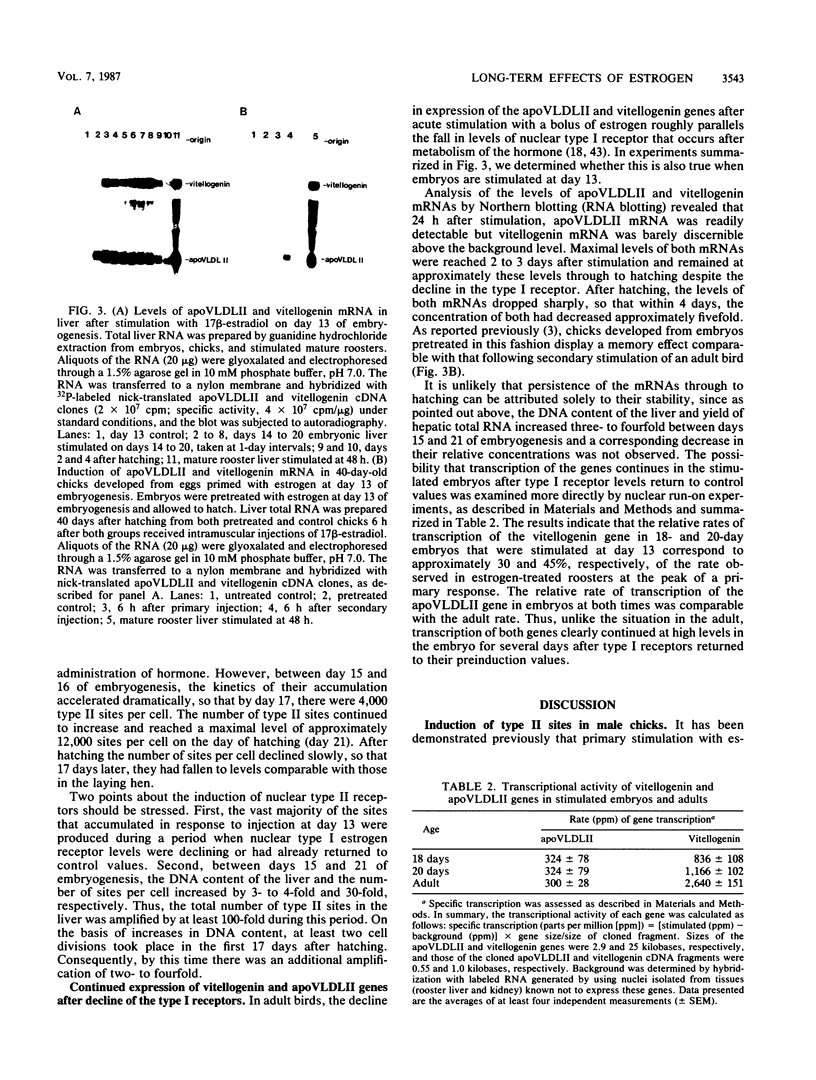
The stimulation of chicks or embryos with estrogen results in transient, hepatic expression of the vitellogenin gene, as well as long-term, propagatable alterations in the rapidity with which the gene can be reactivated. We examined the possibility that nuclear, type II estrogen-binding sites are involved in this long-term change in response characteristics. We demonstrate that the primary induction kinetics of type II sites in embryos and chicks correlated with the expression of the vitellogenin gene and that once their induction was triggered by estrogen, they accumulated, were propagated, and persisted for months after withdrawal of the hormone. We also show that their accumulation in the embryo was accompanied by prolonged expression of both the vitellogenin and very low-density apolipoprotein II genes, in the absence of elevated levels of type I receptor, and that the type II sites, like the classical receptor, appear to be preferentially associated with active or potentially active chromatin. Finally, we describe a regulatory mechanism, tested by computer modelling, that simulated the behavioral characteristics of these nuclear estrogen-binding sites and which may explain their role in mediating the long-term effects of estrogen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burch J. B., Evans M. I. Chromatin structural transitions and the phenomenon of vitellogenin gene memory in chickens. Mol Cell Biol. 1986 Jun;6(6):1886–1893. doi: 10.1128/mcb.6.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B. Identification and sequence analysis of the 5' end of the major chicken vitellogenin gene. Nucleic Acids Res. 1984 Jan 25;12(2):1117–1135. doi: 10.1093/nar/12.2.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Upchurch S., Markaverich B. M. Oestrogenic stimulation of uterine growth: relation to oestrogen receptor binding and the stimulation of nuclear type II oestradiol binding sites. J Endocrinol. 1981;89 (Suppl):47P–57P. [PubMed] [Google Scholar]
- Clark J. H., Watson C. S., Markaverich B. M., Syne J. S., Panko W. B. Heterogeneity of estrogen binding sites in mammary tumors. Breast Cancer Res Treat. 1983;3(1):61–65. doi: 10.1007/BF01806234. [DOI] [PubMed] [Google Scholar]
- Codina-Salada J., Moore J. P., Chan L. Kinetics of primary and secondary stimulation of the mRNA for APOVLDL-II, a major yolk protein, in the cockerel liver by estrogen. Endocrinology. 1983 Sep;113(3):1158–1160. doi: 10.1210/endo-113-3-1158. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Deeley R. G., Udell D. S., Burns A. T., Gordon J. I., Goldberger R. F. Kinetics of avian vitellogenin messenger RNA induction. Comparison between primary and secondary response to estrogen. J Biol Chem. 1977 Nov 25;252(22):7913–7915. [PubMed] [Google Scholar]
- Ekman P., Barrack E. R., Greene G. L., Jensen E. V., Walsh P. C. Estrogen receptors in human prostate: evidence for multiple binding sites. J Clin Endocrinol Metab. 1983 Jul;57(1):166–176. doi: 10.1210/jcem-57-1-166. [DOI] [PubMed] [Google Scholar]
- Elbrecht A., Lazier C. B., Protter A. A., Williams D. L. Independent developmental programs for two estrogen-regulated genes. Science. 1984 Aug 10;225(4662):639–641. doi: 10.1126/science.6740331. [DOI] [PubMed] [Google Scholar]
- Gorski J., Welshons W. V., Sakai D., Hansen J., Walent J., Kassis J., Shull J., Stack G., Campen C. Evolution of a model of estrogen action. Recent Prog Horm Res. 1986;42:297–329. doi: 10.1016/b978-0-12-571142-5.50011-2. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Press M. F. Structure and dynamics of the estrogen receptor. J Steroid Biochem. 1986 Jan;24(1):1–7. doi: 10.1016/0022-4731(86)90024-5. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W. Specific binding of estradiol to the liver chromatin of estrogenized roosters. Biochim Biophys Acta. 1974 Aug 15;361(1):84–96. doi: 10.1016/0005-2787(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Ohno T., Panyim S., Schuerch A. R. Appearance of vitellogenin mRNA sequences and rate of vitellogenin synthesis in chicken liver following primary and secondary stimulation by 17 beta-estradiol. Eur J Biochem. 1978 Mar 15;84(2):355–361. doi: 10.1111/j.1432-1033.1978.tb12175.x. [DOI] [PubMed] [Google Scholar]
- Knowler J. T., Beaumont J. M. The mechanism of action of oestrogens. Essays Biochem. 1985;20:1–39. [PubMed] [Google Scholar]
- Kok K., Snippe L., Ab G., Gruber M. Nuclease-hypersensitive sites in chromatin of the estrogen-inducible apoVLDL II gene of chicken. Nucleic Acids Res. 1985 Jul 25;13(14):5189–5202. doi: 10.1093/nar/13.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazier C. B., Jordan V. C. High affinity binding of anti-oestrogen to the chick liver nuclear oestrogen receptor. Biochem J. 1982 Aug 15;206(2):387–394. doi: 10.1042/bj2060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazier C. (3H)-estradiol binding by chick liver nuclear extracts: mechanism of increase in binding following estradiol injection. Steroids. 1975 Sep;26(3):281–298. doi: 10.1016/0039-128x(75)90075-6. [DOI] [PubMed] [Google Scholar]
- Lewis J., Slack J. M., Wolpert L. Thresholds in development. J Theor Biol. 1977 Apr 7;65(3):579–590. doi: 10.1016/0022-5193(77)90216-8. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Clark J. H. Two binding sites for estradiol in rat uterine nuclei: relationship to uterotropic response. Endocrinology. 1979 Dec;105(6):1458–1462. doi: 10.1210/endo-105-6-1458. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Roberts R. R., Alejandro M., Clark J. H. The effect of low dose continuous exposure to estradiol on the estrogen receptor (type I) and nuclear type II sites. Endocrinology. 1984 Mar;114(3):814–820. doi: 10.1210/endo-114-3-814. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Williams M., Upchurch S., Clark J. H. Heterogeneity of nuclear estrogen-binding sites in the rat uterus: a simple method for the quantitation of type I and type II sites by [3H]estradiol exchange. Endocrinology. 1981 Jul;109(1):62–69. doi: 10.1210/endo-109-1-62. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Saluz H. P., Jiricny J., Jost J. P. Genomic sequencing reveals a positive correlation between the kinetics of strand-specific DNA demethylation of the overlapping estradiol/glucocorticoid-receptor binding sites and the rate of avian vitellogenin mRNA synthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7167–7171. doi: 10.1073/pnas.83.19.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Krauter P., von der Ahe D., Janich S., Rabenau O., Cato A. C., Suske G., Westphal H. M., Beato M. Mechanism of gene regulation by steroid hormones. J Steroid Biochem. 1986 Jan;24(1):19–24. doi: 10.1016/0022-4731(86)90026-9. [DOI] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Schoenberg D. R., Clark J. H. Nuclear association states of rat uterine oestrogen receptors as probed by nuclease digestion. Biochem J. 1981 May 15;196(2):423–432. doi: 10.1042/bj1960423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen R. C., Means A. R., Clark J. H. Estrogen modulation of nuclear matrix-associated steroid hormone binding. Endocrinology. 1984 Sep;115(3):1197–1202. doi: 10.1210/endo-115-3-1197. [DOI] [PubMed] [Google Scholar]
- Snow L. D., Eriksson H., Hardin J. W., Chan L., Jackson R. L., Clark J. H., Means A. R. Nuclear estrogen receptor in the avian liver: correlation with biologic response. J Steroid Biochem. 1978 Nov;9(11):1017–1026. doi: 10.1016/0022-4731(78)90026-2. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Williams J. G. Quantitative analysis of specific labelled RNA'S using DNA covalently linked to diazobenzyloxymethyl-paper. Nucleic Acids Res. 1979 Jan;6(1):195–203. doi: 10.1093/nar/6.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syne J. S., Markaverich B. M., Clark J. H., Panko W. B. Estrogen binding sites in the nucleus of normal and malignant human tissue: optimization of an exchange assay for the measurement of specific binding. Cancer Res. 1982 Nov;42(11):4443–4448. [PubMed] [Google Scholar]
- Tam S. P., Archer T. K., Deeley R. G. Biphasic effects of estrogen on apolipoprotein synthesis in human hepatoma cells: mechanism of antagonism by testosterone. Proc Natl Acad Sci U S A. 1986 May;83(10):3111–3115. doi: 10.1073/pnas.83.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S. P., Haché R. J., Deeley R. G. Estrogen memory effect in human hepatocytes during repeated cell division without hormone. Science. 1986 Dec 5;234(4781):1234–1237. doi: 10.1126/science.3022381. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle B., Peyrat J. P., Bonneterre J., Hecquet B., Dewailly D., Lefebvre J. Nuclear estradiol-binding sites in human breast cancer. Cancer Res. 1983 Sep;43(9):4497–4503. [PubMed] [Google Scholar]
- Watson C. S., Medina D., Clark J. H. Characterization of progesterone receptors, estrogen receptors, and estrogen (type II)-binding sites in the hormone-independent variant of the MXT-3590 mouse mammary tumor. Endocrinology. 1980 Nov;107(5):1432–1437. doi: 10.1210/endo-107-5-1432. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L., Lewis J. H. Towards a theory of development. Fed Proc. 1975 Jan;34(1):14–20. [PubMed] [Google Scholar]