Abstract
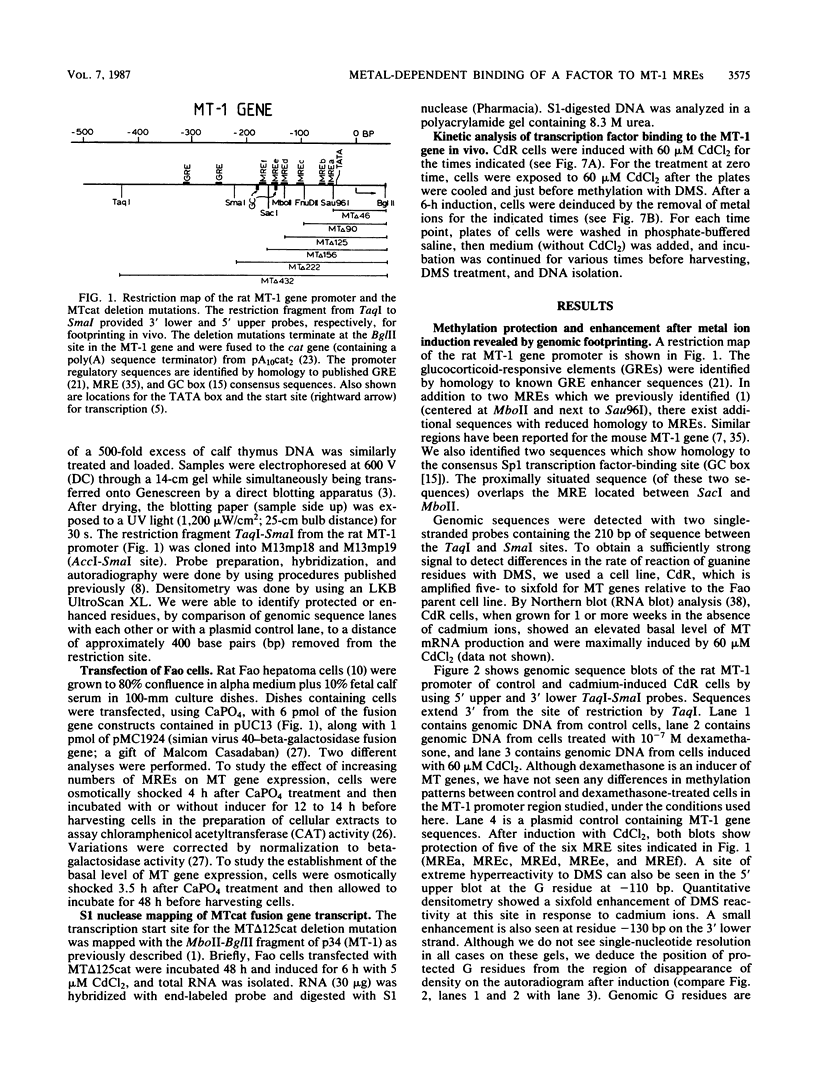
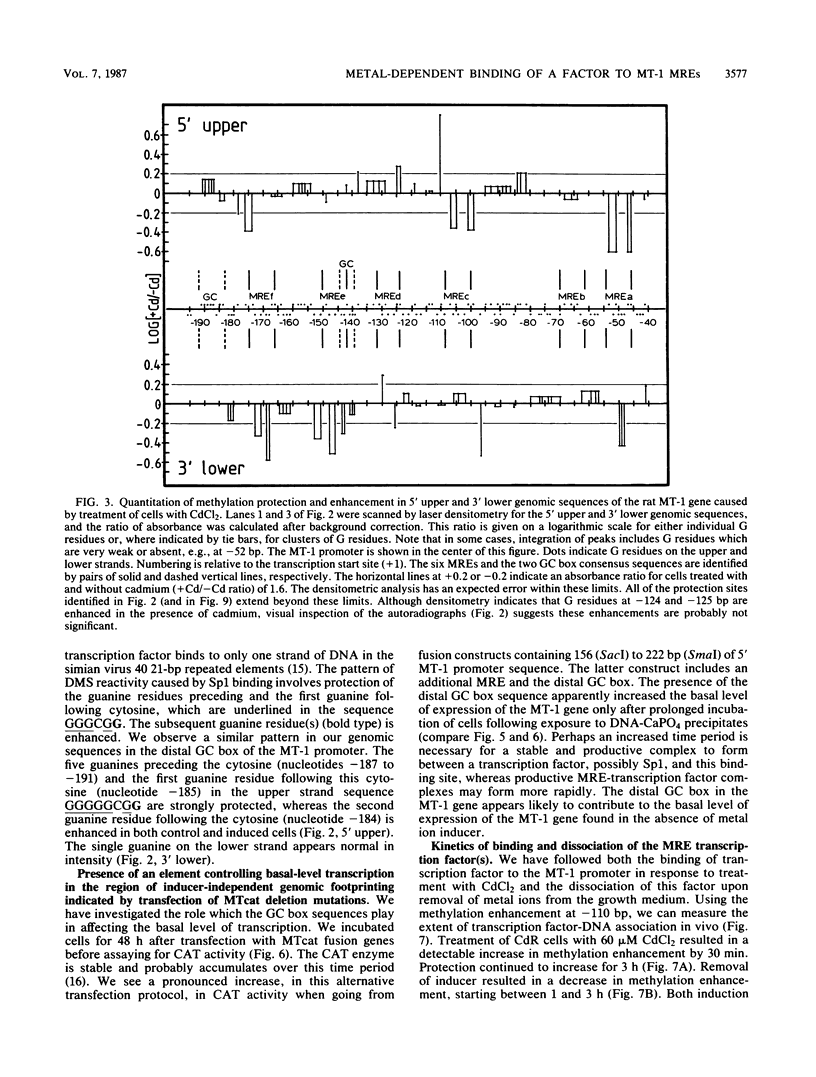
Using the technique of genomic footprinting, we demonstrate cadmium-inducible protection from dimethyl sulfate (DMS) modification of guanine residues in vivo in five metal-responsive elements (MREs) in the promoter of the rat metallothionein 1 (MT-1) gene. We also identify a site of extreme DMS hyperreactivity which, like the MRE protection, occurs only after metal ion induction. With this hyperreactive site as an indicator, we can measure the kinetics of induction and deinduction. Changes in the intracellular metal ion concentrations are reflected in alterations in the reactivity with DMS of guanine residues in the MT-1 gene promoter. Lastly, for both control and metal-induced cells, we observe DMS protection and enhancement of a binding site (located 5' of the distal MRE) which is a consensus sequence for the Sp1 transcription factor. Transfection experiments with deletion mutations of a fusion gene construct indicate both that a sequence region which includes this GC box regulates the basal level of expression of the MT-1 gene and that increasing the number of MREs in the promoter increases the induced level of transcription. Our genomic footprinting and transfection data together suggest that (i) a transcription factor, possibly Sp1, plays an important role in regulating the basal level of expression of the MT-1 gene and (ii) metal induction involves the metal-dependent binding to a sequence-specific binding factor which responds to changes in intracellular metal ion levels.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Birren B. W., Taplitz S. J., Herschman H. R. Rat metallothionein-1 structural gene and three pseudogenes, one of which contains 5'-regulatory sequences. Mol Cell Biol. 1986 Jan;6(1):302–314. doi: 10.1128/mcb.6.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Pöting A., Mallick U., Rahmsdorf H. J., Schorpp M., Herrlich P. Induction of metallothionein and other mRNA species by carcinogens and tumor promoters in primary human skin fibroblasts. Mol Cell Biol. 1986 May;6(5):1760–1766. doi: 10.1128/mcb.6.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S., Pohl F. M. DNA sequencing with direct blotting electrophoresis. EMBO J. 1984 Dec 1;3(12):2905–2909. doi: 10.1002/j.1460-2075.1984.tb02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. B., Gloss B., Schmid W., Strähle U., Schütz G. In vivo protein-DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature. 1986 Dec 18;324(6098):686–688. doi: 10.1038/324686a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Carter A. D., Felber B. K., Walling M. J., Jubier M. F., Schmidt C. J., Hamer D. H. Duplicated heavy metal control sequences of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7392–7396. doi: 10.1073/pnas.81.23.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. The genomic sequencing technique. Prog Clin Biol Res. 1985;177:17–21. [PubMed] [Google Scholar]
- Deschatrette J., Weiss M. C. Characterization of differentiated and dedifferentiated clones from a rat hepatoma. Biochimie. 1974;56(11-12):1603–1611. doi: 10.1016/s0300-9084(75)80286-0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983 Mar;32(3):669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hager L. J., Palmiter R. D. Transcriptional regulation of mouse liver metallothionein-I gene by glucocorticoids. Nature. 1981 May 28;291(5813):340–342. doi: 10.1038/291340a0. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Irwin N., Ptashne M. Repressor structure and the mechanism of positive control. Cell. 1983 Feb;32(2):319–325. doi: 10.1016/0092-8674(83)90451-8. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Ptashne M. Cooperative binding of lambda repressors to sites separated by integral turns of the DNA helix. Cell. 1986 Mar 14;44(5):681–687. doi: 10.1016/0092-8674(86)90833-0. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Imbra R. J., Heguy A., Wong G. Interleukin 1 regulates human metallothionein gene expression. Mol Cell Biol. 1985 Oct;5(10):2866–2869. doi: 10.1128/mcb.5.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mercola M., Goverman J., Mirell C., Calame K. Immunoglobulin heavy-chain enhancer requires one or more tissue-specific factors. Science. 1985 Jan 18;227(4684):266–270. doi: 10.1126/science.3917575. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Chou J., MacKrell A. J., Casadaban M. J., Steiner D. F. Expression of a preproinsulin-beta-galactosidase gene fusion in mammalian cells. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5198–5202. doi: 10.1073/pnas.80.17.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Scholer H., Haslinger A., Heguy A., Holtgreve H., Karin M. In vivo competition between a metallothionein regulatory element and the SV40 enhancer. Science. 1986 Apr 4;232(4746):76–80. doi: 10.1126/science.3006253. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Stuart G. W., Palmiter R. D. Building a metal-responsive promoter with synthetic regulatory elements. Mol Cell Biol. 1985 Jun;5(6):1480–1489. doi: 10.1128/mcb.5.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin C., Hamer D. H. Regulation in vitro of metallothionein gene binding factors. Science. 1987 Mar 13;235(4794):1383–1387. doi: 10.1126/science.3103216. [DOI] [PubMed] [Google Scholar]
- Shuey D. J., Parker C. S. Bending of promoter DNA on binding of heat shock transcription factor. Nature. 1986 Oct 2;323(6087):459–461. doi: 10.1038/323459a0. [DOI] [PubMed] [Google Scholar]
- Storb U., Wilson R., Selsing E., Walfield A. Rearranged and germline immunoglobulin kappa genes: different states of DNase I sensitivity of constant kappa genes in immunocompetent and nonimmune cells. Biochemistry. 1981 Feb 17;20(4):990–996. doi: 10.1021/bi00507a053. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Chen H. Y., Brinster R. L., Palmiter R. D. A 12-base-pair DNA motif that is repeated several times in metallothionein gene promoters confers metal regulation to a heterologous gene. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7318–7322. doi: 10.1073/pnas.81.23.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Palmiter R. D. Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. 1985 Oct 31-Nov 6Nature. 317(6040):828–831. doi: 10.1038/317828a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Vigneron M., Matthes H., Wildeman A., Zenke M., Chambon P. Requirement of stereospecific alignments for initiation from the simian virus 40 early promoter. Nature. 1986 Jan 9;319(6049):121–126. doi: 10.1038/319121a0. [DOI] [PubMed] [Google Scholar]
- Taplitz S. J., Calame K. L., Herschman H. R. Alternative inducers of the rat metallothionein I gene cause distinct changes in chromatin structure in the 5' region of the gene. Mol Cell Biol. 1986 Jul;6(7):2576–2581. doi: 10.1128/mcb.6.7.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle W. B., Tate C. A., Bick R. J., Entman M. L. Nucleotide triphosphate utilization by cardiac and skeletal muscle sarcoplasmic reticulum. Evidence for a hydrolysis cycle not coupled to intermediate acyl phosphate formation and calcium translocation. J Biol Chem. 1981 Mar 10;256(5):2268–2274. [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]






