Abstract
Background and purpose
Determining the role of vascular receptors in vivo is difficult and not readily accomplished by systemic application of antagonists or genetic manipulations. Here we used intravital microscopy to measure the contributions of sympathetic receptors, particularly α1-adrenoceptor subtypes, to contractile activation of femoral artery in vivo.
Experimental approach
Diameter and intracellular calcium ([Ca2+]i) in femoral arteries were determined by intravital fluorescence microscopy in mice expressing a Myosin Light Chain Kinase (MLCK) based calcium-calmodulin biosensor. Pharmacological agents were applied locally to the femoral artery to determine the contributions of vascular receptors to tonic contraction and [Ca2+]i,.
Key results
In the anesthetized animal, femoral arteries were constricted to a diameter equal to 54% of their passive diameter (i.e. tone = 46%). Of this total basal tone, 16% was blocked by RS79948 (0.1 µM) and thus attributable to α2-adrenoceptors. A further 46% was blocked by prazosin (0.1 µM) and thus attributable to α1-adrenoceptors. Blockade of P2X and NPY1 receptors with suramin (0.5 mM) and BIBP3226 (1.0 µM) respectively, reduced tone by a further 22%, leaving 16% of basal tone unaffected at these concentrations of antagonists. Application of RS100329 (α1A-selective antagonist) and BMY7378 (α1D-selective) decreased tone by 29% and 26%, respectively, and reduced [Ca2+]i. Chloroethylclonidine (1 µM preferential for α1B-) had no effect. Abolition of sympathetic nerve activity (hexamethonium, i.p.) reduced basal tone by 90%.
Conclusion and Implications
Tone of mouse femoral arteries in vivo is almost entirely sympathetic in origin. Activation of α1A- and α1D-adrenoceptors elevates [Ca2+]i and accounts for at least 55% of the tone.
Introduction
The sympathetic nervous system (SNS) plays a major role in maintaining arterial blood pressure, through its effects on the heart, blood vessels, kidneys and adrenal glands. In rats, total block of autonomic ganglionic transmission results in a rapid fall in arterial blood pressure, due acutely to a decrease in total peripheral vascular resistance [1]. Pathologically, SNS hyperactivity is involved in heart failure, hypertension, and metabolic syndrome [2], [3]. Sympathetic nerves release three neurotransmitters onto arterial smooth muscle; noradrenaline (NA), ATP, and neuropeptide Y (NPY). Each binds to several types of pre-and post-junctional receptors that activate several distinct intracellular signaling pathways [4]. The physiological role of each receptor type in a particular blood vessel in vivo is difficult to determine however; receptors are present in different amounts in different blood vessels, the amount of SNA varies, and relative amounts of NA, ATP and NPY released vary with the frequency and pattern of nerve action potentials [5], [6]. Here, we sought to define the roles of SNA and of the α1-adrenoceptor subtypes in particular, to maintenance of vascular tone in femoral arteries in vivo. α1-adrenoceptors are classified into 3 functional subtypes α1A, α1B-, and α1D-, corresponding to the cloned α1-adrenoceptors, α1a-,α1b- and α1d- respectively [7], [8]. α1-Adrenoceptors with low affinity for prazosin (pA2<9) have also been identified in functional studies and classified as the α1L-subtype [9]–[11]. The α1L-adrenoceptor subtype has not been defined and evidence suggests that it is not a separate gene product but a low-affinity state of the α1A-adrenoceptor [12], [13]. Many studies have shown the relative importance of these subtypes in the maintenance of arterial blood pressure [14]–[17] or in response to exogenously introduced agonists and/or electrical nerve stimulation in mouse arterial beds in vitro [16], [18]–[22]. Nevertheless, these studies have not shown directly the relative importance of the vascular α1-adrenoceptor subtypes in vascular tone in vivo. Systemic application of receptor blockers or agonists or genetic ablation of receptor subtypes inevitably involve receptors located elsewhere than the vasculature. Similarly, many of the factors potentially influencing vascular tone in vivo will be lost when arteries are removed from the animal for study.
In the present study therefore, we utilized a new experimental model, the exMLCK optical biosensor mouse [23]–[26] to determine for the first time the functional roles of the α1-adrenoceptor subtypes in basal-state tone of femoral arteries of anesthetized mice in vivo. These mice express a FRET-based genetically encoded Ca2+/Calmodulin biosensor molecule, based on smooth muscle myosin light chain kinase (MLCK) specifically on smooth muscle cells. The fluorescence in smooth muscle cells of the artery walls provides 1) quantitative measurement of smooth muscle cell [Ca2+], and 2) a precise measurement of artery diameter. The preparation we developed [26] provides the ability to apply receptor blockers and agonists locally, to a segment of artery, and thus isolate effects to vascular receptors only in that region of the artery. We have shown previously that systemic effects are avoided through the use of this approach [25], [26]. In this study we determined the functional contributions of α1-adrenoceptor subtype to femoral artery tone in vivo by using the α1-adrenoceptor selective antagonist prazosin [27], the α1A-adrenoceptor selective antagonist RS100329 [28], the α1D-adrenoceptor selective antagonist BMY7378 [29], [30] and the preferential α1B-adrenoceptor alkylating agent, chloroethylclonidine [31].
Methods
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Maryland, School of Medicine, MD. The transgenic mouse line (ICR, inbred Charles River) was the same as used previously [23]–[26], that expresses a MLCK biosensor that monitors the binding of Ca2+-calmodulin through changes in FRET (Forster Resonance Energy Transfer) between cyan (CFP) and yellow (YFP) fluorescent proteins. All mice were maintained on 12∶12-h light/dark schedule at 22–25°C and 45–65% humidity and fed ad libitum on a standard rodent diet and tap water. A total of 29 (12 male, 17 female) mice were used, ages of 16–20 weeks, weights 28–32 grams.
Preparation of Mice
Anesthesia was induced with 1–5% isoflurane (Baxter Pharmaceutical Products Inc., Deerfield, IL) in O2. During the surgical procedure and the subsequent experiment anesthesia was maintained with 1.5% isoflurane in O2. After induction of anesthesia, mice were placed in a supine position on a custom made temperature-controlled platform set to maintain core temperature of animals at 37–38°C.
Preparation of Arteries for Recording in vivo
Hair from the hind limb region was removed using a depilatory agent. Under microscopic observation, the femoral artery was exposed via a cutaneous incision in the upper thigh. The underlying connective tissue above the artery was lightly dissected, taking care to avoid severing nerves. After exposing the femoral artery in this way, the animal was moved to the stage of a fluorescence microscope and superfusion of the artery was begun, with the standard physiological salt solution containing (PSS, in mmol/l) 112.0 NaCl, 25.7 NaHCO3, 4.9 KCl, 2.0 CaCl2, 2.0 MgSO4, 1.2 KHPO4, 11.5 glucose, and 10.0 HEPES (pH 7.4, equilibrated with gas of 12% O2, 5% CO2, 83% N2). Solutions containing elevated KCl were made by replacing the NaCl with KCl on an equimolar basis. Experiments in which a “zero”-calcium solution was used, the solution had the same composition as the standard PSS with the omission of CaCl2 and the addition of Na2EGTA (2 mM). Superfusion was at 2 ml/min, 35°C monitored continuously by a temperature probe (Sensortek, Clifton, NJ). The arteries were continuously exposed to antagonist for 10 minutes before arterial diameter was recorded using fluorescent edges of the blood vessel. For CEC treatment, arterial segments were incubated with CEC for 10 min at 35°C followed by washing in PSS for 30 min [19], [32]. For recording of arterial blood pressure, methods were as described previously [26] a small incision is made in the femoral artery and a mouse femoral artery catheter (PE-10) (Braintree Scientific, Braintree, MA) was inserted into the artery. The catheter was connected to a fluid-filled pressure transducer (SP 844, Memscap, Skoppum, Norway). Arterial diameter was recorded from the femoral artery of the other leg. Arterial BP measurements were sampled at 1 kHz with a PowerLab data acquisition system and Lab-Chart Pro (ADInstruments, Colorado Springs, CO). Systemic administration of the autonomic ganglion blocker, hexamethonium, was by intra-peritoneal (i.p) injection (30 µg/g body weight).
Fluorescence Recording
For imaging femoral arteries in vivo, an Olympus MVX10 MacroView microscope (Olympus America, Center Valley, PA) (objective lens: 2X Plan Apochromat, 0.5 NA) was used. Excitation illumination was via a xenon arc lamp (Lambda LS, Sutter Instrument, Novato, CA). For measurements of arterial diameter, the biosensor was excited at 426–446 nm. Emission was collected at 455–485 nm (CFP) and 520–550 nm (YFP) with a charge-coupled device (ORCA ER, Hamamatsu, Bridgewater, NJ). Total optical zoom was set such that an effective imaging of 1.0–2.0 µm/pixel was established. Acquisition of images was set at 1.0 Hz. The microscope was equipped with an image splitter equipped with the appropriate filters for CFP/YFP FRET microscopy (DualView, Photometrics, AZ) and a sensitive CCD video camera (ORCA ER, Hamamatsu Photonics, K.K., Japan). The camera was controlled and images acquired using HCImage (Hamamatsu, Japan).
Myography
Wire myography
Arterial segments of 2 mm length (normalized internal diameter, IC0.9, c. 310 µm) were mounted in a four-channel wire myograph (Danish Myotech, Aarhus, Denmark) for isometric tension measurement and were maintained in gassed PSS at 35°C. After incubation for 1 hr, the vessels were then normalized, that is, the resting tension–internal circumference (IC) relation was determined for each vessel segment [19], [33], [34]. The resting tension was set to a normal IC of IC0.9, where IC0.9 = 0.9IC100 and IC100 is the internal circumference of the vessel under an effective resting transmural pressure (ERTP) of 100 mmHg (13.3 kPa). ERTP was calculated from the Laplace equation (ERTP = wall tension/(IC/2π)). Lab View software was used for acquisition. At 30 min after normalization, the vessels were exposed to 60 mM KCl solution twice followed by 10 µM noradrenaline in the presence of 60 mM KCl solution. The arteries were considered viable if the equivalent transmural pressure produced by 60 mM KCl was >100 mmHg (13.3 kPa) [32], [35]. Vessels were allowed to equilibrate for a further 30 min before beginning experiments.
Functional studies using bath applied phenylephrine in vitro (isolated femoral arteries)
After equilibration, three to four concentration–response curves (CRC) to PE were obtained in each femoral arterial segment (30 min between each CRC). Preliminary experiments showed no significant time-dependent changes in sensitivity. The first CRC was taken as control and subsequent curves were obtained after incubating the vessels with increasing concentrations of the same antagonist for 30 min. To characterize α1-adrenoceptors in femoral arteries, RS79948 (0.1 µM, α2-adrenoceptor blocker), desmethylimipramine (50 nM, neuronal uptake blocker) and corticosterone acetate (3 µM, non-neuronal uptake inhibitor) were added to the PSS before each CRC. Results are expressed as mean±s.e.m., n being the number of animals.
Calcium calibration
Third order mesenteric arterial segments from exMLCK FRET biosensor mice, 2–3 mm in length were dissected [24]. Arteries were mounted on a single channel wire myograph similar to the one described above. Calcium calibration was done on isolated blood vessels because even if a segment of artery could be permeabilized successfully in vivo, there is no way to clamp the [Ca2+]i concentration reliably due to blood flow. For calcium calibration curves arteries were allowed to stabilize in Krebs solution, followed by incubation of the vessel in a high relaxing (HR) solution for 10–20 min before permeabilization. The high relaxing (HR) and pCa solutions were used during and following permeabilization of the mesenteric vessels were designed to maintain a desired free Ca2+ concentration, using a Ca2+-EGTA buffering system. The composition of the solutions was calculated with the MAXC Computer Program for calculating free Ca2+ concentrations. The HR solution (pCa >9) was composed of the following chemicals (in mM): 53.28 KCl, 6.81 MgCl2, 0.025 CaCl2, 10.0 EGTA, 5.4 Na2ATP, and 12.0 creatine phosphate. The composition of the pCa 4.5 solution was similar to HR, except for the following differences (in mM): 33.74 KCl, 6.48 MgCl2, and 9.96 CaCl2. The pH of the HR and pCa 4.5 solutions were adjusted to 7.1 with KOH, and ionic strength was held constant (0.15). Solutions containing a desired free Ca2+ concentration between pCa 9 and 4.5 were achieved by mixing appropriate volumes of the HR and pCa 4.5 solutions based on the Bathe algorithm. All solutions contained the protease inhibitors leupeptin (1 µg/ml), pepstatin A (2.5 µg/ml), and PMSF (50 µM). Mesenteric arteries were permeabilized with α-toxin from staphylococcus aureus by incubating the vessel segment with 1000 U/ml α-toxin in HR for 1 hour at room temperature. After permeabilization, segments were washed with HR solution and force was allowed to stabilize. The pCa-tension relationship was then determined by bathing the permeabilized vessels in solutions of sequentially increasing Ca2+ concentrations, ranging from pCa 8.5 to 4.5, while recording force for 5 min in each solution or until stabilized. The Ca2+ induced alteration in tension was expressed as a percent relative to the basal tension at pCa 9. The changes in FRET ratios and force, measured with [Ca2+] ranging from 1 nM to 50 µM at excitation wavelength of 426–446 nm, is characterized by a normalized sigmoid curve fit. The calcium calibration graph was plotted with the normalized FRET ratio on the y-axis and calcium concentration on the x-axis. The EC50 and Hill Coefficient extracted from the curve were 6.05 (pCa) and 1.4 respectively. Rmin and Rmax were calculated by exposing the artery to 0Ca2+ (2 mM EGTA and 1 µM acetylcholine) and KCl (60 mM) respectively.
Pressure myography
Dissected segments of femoral artery, 1–2 mm in length, were transferred to a recording chamber, where their ends were mounted on glass pipettes (tip diameter 60–100 µm) and secured by 10-0 Ethilon ophthalmic nylon sutures (Ethicon, Somerville, NJ). One pipette was attached to a servo-controlled pressure-regulating device (Living Systems, Burlington, VT), whereas the other was attached to a closed stopcock to study the pressure-dependent effects in the absence of intraluminal flow. The intraluminal pressure was set to 70 mmHg and was continuously superfused with gassed PSS at 35°C. During the entire process, the arteries that developed significant leaks were discarded. Measurements of arterial wall position from transmitted light images were recorded at 2 images/sec with a Nikon ×20 objective.
Data Analysis
Agonist potency is expressed as the pEC50 (the negative logarithm of the concentration required to produce 50% of the maximum response, Emax). The pEC50 and Emax values were calculated using the Graphpad Prism software program, which fits CRCs to the four parameter logistic equation below:
Y = Bottom+[top-bottom)/(1+10(logEC50-X)P)], where X is the logarithm of the molar concentration of agonist, Y is the response and P is the Hill slope. Antagonist affinity was expressed either as pKB or pIC50 values. When three different concentrations of the antagonist were used, pKB values were obtained from the x-intercept of the plot of log (r−1) vs. log(B), where r is the ratio of the agonist EC50 in the presence and absence of antagonist and B is the molar concentration of antagonist [36]. If the antagonism met the criteria of competition (Schild slope of unity), then affinity was expressed as pKB. When one concentration of antagonist was used to obtain the affinity, estimated pKB values were calculated from the Schild equation [37]: pKB = -log[(B)/(r-1)]. The change in arterial diameter (in vivo) was calculated and expressed as a fractional diameter based on full passive diameter with 0[Ca2+]. Antagonist potencies were also expressed as mean pIC50 values (the negative logarithm of the concentration of antagonist producing 50% inhibition of the prazosin-sensitive component of the vascular tone). Best fit pEC50 and Emax values obtained from nonlinear regression of CRC (described above) and other mean values were compared by an unpaired t-test for two groups or by repeated measures one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test (three or more groups) after checking for normality (Kolmogorov–Smirnov test).
Calculating [Ca2+]i
The change in FRET ratios obtained from cumulative addition of antagonist was plotted against the [Ca2+] calibration curve to give [Ca2+]i. Image processing was via custom software, written using Interactive Data Language (IDL) v8.1 (ITT Systems, Inc. USA). To obtain correct FRET ratios with a ‘wide-field’ imaging system, several methodological issues were addressed: 1) spectral overlap, 2) image alignment for ratioing, 3) accounting for ‘background’ fluorescence (i.e. that arising from sources other than the artery being studied, and 4) artery intrinsic fluorescence.
Drugs Used
The following drugs were used: Phenylephrine, hexamethonium bromide, (−)- noradrenaline bitartrate, acetylcholine chloride, corticosterone acetate, suramin sodium salt, desmethylimipramine, prazosin hydrochloride, α-toxin from staphylococcus aureus, protease inhibitors, creatine phosphate, chloroethylclonidine and (8-[2-[4-(2-methoxyphenyl)-1piperazinyl]ethyl]-8-azaspiro[4.5]decane-7,9-dione (BMY7378) (Sigma, USA). (8aR,12aS,13aS)-5,8,8a,9,10,11,12,12a,13,13a-dechydro-3-methoxy-12-(ethylsulfonyl)-6H-isoquino[2,1-g] [1], [6]naphthyridine hydrochloride (RS79948), 5-Methyl-3-[3-[3-[4-[2-(2,2,2,-trifluroethoxy)phenyl]-1-piperazinyl]propyl]-2,4-(1H,3H)-pyrimidinedione hydrochloride (RS100329), N-[(1R)]-4-[(Aminoiminomethyl)amino -1-[[[(4-hydroxyphenyl)methyl]amino]carbonyl]butyl-α-phenylbenzeneacetamide trifluoroacetate (BIBP3226) (Tocris, USA). Prazosin was dissolved in 30% methanol, hexamethomium in sterile saline for injection, BIBP3226 in DMSO and corticosterone in 20% methanol. Stock solutions of all other drugs were prepared in distilled water. All drug dilutions were made using PSS.
Results
Sources of Vascular Tone in Murine Femoral Arteries in vivo
In the anesthetized animal, femoral arteries were constricted to a diameter equal to 54±1% (n = 29) of their passive diameter (PD, in local 0 mM external [Ca2+] and 2 mM EGTA). Basal ‘tone’ was thus 46±1% (100%–54%). We found no significant difference in femoral artery tone in vivo between female and male mice (16–30 weeks old). Basal tone in females = 46.48±1.49% (n = 15) and males = 46.63±1.25% (n = 11), P>0.05 (un-paired t-test). To determine the component of this tone that might be due to autonomic nervous system activity, we blocked autonomic ganglionic transmission with systemically applied hexamethonium (i.p, 30 µg/gm body weight). This caused a vasodilation of femoral arteries nearly to PD, and a reduction of arterial blood pressure, as we have reported previously [26]. Thus, a major component of the vascular tone of these arteries in vivo is revealed as neurogenic. Myogenic tone [38] was absent in these arteries; isolated, pressurized (70 mm Hg) femoral arteries did not develop any vascular tone (data not shown, n = 4) nor myogenic responses to step changes in pressure (30–110 mm Hg).
Sympathetic Neurotransmitter Receptors
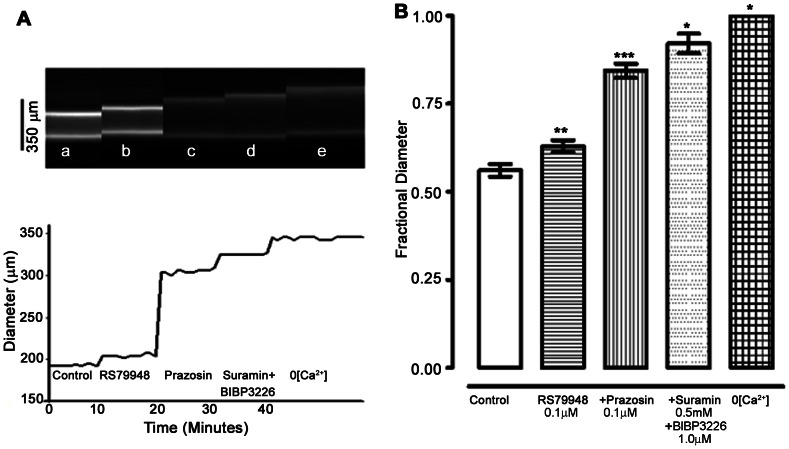
The contribution to vascular tone of several neurotransmitter receptors that might be involved in sympathetic neurogenic tone in vivo was examined ( Fig. 1 ). Local application of RS79948 (0.1 µM, α2-adrenoceptor antagonist) and prazosin (0.1 µM, α1-adrenoceptor antagonist) reduced vascular tone by 16±3% (n = 12) and 46±4% (n = 9), respectively. Subsequent addition of both BIBP 3226 (1 µM, NPY1 blocker) and Suramin (0.5 mM, Purinergic-P2X blocker) also had significant effect on vascular tone (P<0.001, % of tone 22±3, n = 6). A summary of the effects on femoral artery tone in vivo of combined block of adrenergic, P2X, and NPY1 receptors is shown in Fig. 1B . Except for prazosin, the concentrations of all drugs used were maximally effective.
Figure 1. Receptors for sympathetic neurotransmitters activate at least 80% of contractile tone in mouse femoral arteries in vivo.
(A) Upper panel: 5 (a–e) successive transverse line-scan images of a femoral artery in vivo. Artery diameter was obtained as the distance between the two peaks in fluorescence intensity, representing the fluorescence within the walls of the artery. Artery diameter was measured only during the final 10 seconds of a 10 min duration control period or exposure to an antagonist. There is thus a 10 minute gap between each image (a–e) and between each corresponding diameter trace in the lower panel. Lower panel: Artery diameter under each condition (a–e). (a) Control, represents artery in basal state of anesthetized animal. (b–e) Arterial diameter upon on cumulative exposure to RS79948 (0.1 µM) (b), Prazosin (0.1 µM) (c), Suramin (0.5 mM)+BIBP3226 (1 µM) and (e). 0[Ca2+]. (B) Average data from 6 arteries. Fractional diameter is the measured diameter divided by the passive diameter for each artery, measured in the presence of 0[Ca2+] solution. In the combined presence of α1-, α2- adrenoceptor, NPY1 and P2X receptor blockers, active vascular tone was reduced to less than 20% of the control level. As shown later, α1-adrenoceptors are not fully blocked at the concentration of prazosin used (0.1 µM), and thus the tone activated by noradrenaline is greater than shown here. Significance of difference from previous drug treatment, * P<0.05, ** P<0.01, *** P<0.001 (ANOVA followed by Newman-Keuls multiple comparison test).
α1L- Adrenoceptors Contribute to Vascular Tone
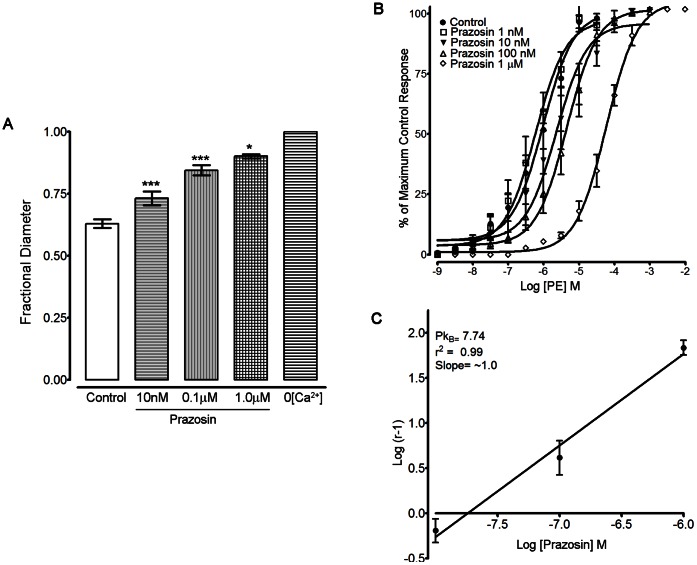
Since pre- and post-junctional α2-adrenoceptors activated by neurally released NA could affect the contribution of α1-adrenoceptors to vascular tone in vivo, the experiments to determine the α1-adrenoceptor subtypes involved were all carried out in the presence of RS79948 (0.1 µM). In vivo, local prazosin (10 nM-1 µM, n = 8, Fig. 2A , supplementary table. 1) produced concentration dependent inhibition of vascular tone (pIC50 value of 8.0±0.1). To determine the α1-adrenoceptor subtype based on affinity to prazosin, femoral arteries were isolated and mounted on a wire myograph for isometric force recording and the effect of prazosin on the concentration-response curves to phenylephrine (PE) was determined. Prazosin (10 nM–1 µM, n = 5, Fig. 2B ) produced a rightward shift of the concentration response curve; the Schild plot gave a pKB value of 7.74 with a slope of 1.01±0.11, not significantly different from 1.0 ( Fig. 2C ). Based on affinity to prazosin, the α1-adrenoceptors in the mouse femoral artery are of α1L-subtype.
Figure 2. The α1-adrenoceptors in the mouse femoral arterial bed are of the α1L-subtype.
(A) Fractional diameter in femoral arteries in vivo. Prazosin (10.0 nM to 1.0 µM) produced successive increases in fractional diameter (i.e. decreased active vascular tone). These arteries were isolated and mounted on a wire myograph for force measurement (B) Ex vivo characterization of α1-receptors in femoral artery: Prazosin (10 nM–1 µM, n = 5) produced concentration-dependent parallel rightward shifts in the potency of phenylephrine (PE) without significantly affecting the maximum responses. (C) The Schild regression analysis gave a pKB value of 7.74 with a slope of ∼1.0. All experiments were carried in the presence of RS79948 (0.1 µM). Significance of difference from previous drug treatment, ** P<0.01, *** P<0.001 (ANOVA followed by Newman-Keuls multiple comparison test).
α1A-, α1B-, α1D- Adrenoceptor Subtypes
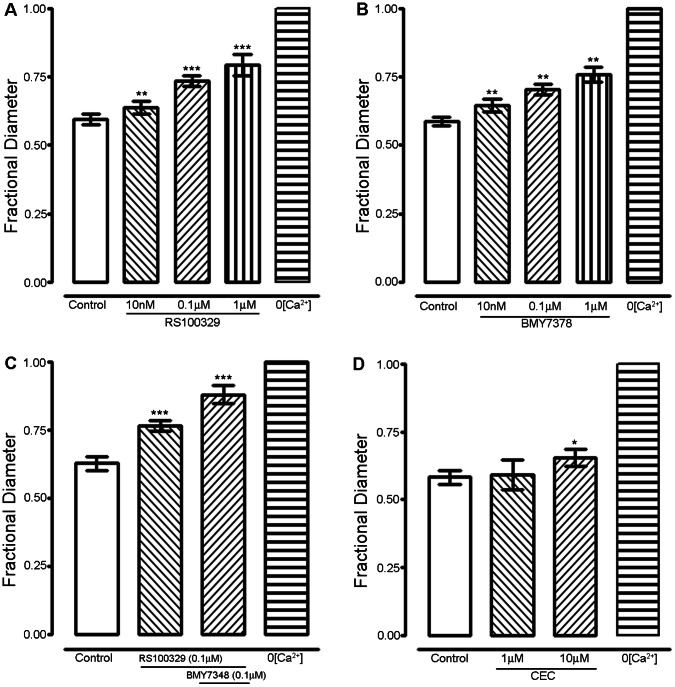
We examined the contributions of α1-adrenoceptors by using subtype specific antagonists. RS 100329, specific for α1A-adrenoceptors, (10 nM-1 µM, n = 5, Fig. 3A , supplementary table. 1) significantly inhibited vascular tone with a pIC50 value of 7.4±0.1. The fractional diameter increased by 0.14±0.01 (P<0.001) with 0.1 µM RS 100329. BMY 7378, specific for α1D-adrenoceptors (10 nM-1 µM, n = 5, Fig. 3B , supplementary table. 1) also inhibited vascular tone significantly with a pIC50 value of 7.2±0.2 and an increase in fractional diameter by 0.12±0.01 (P<0.001) with 0.1 µM BMY7378. The combination of RS100329 (0.1 µM) and BMY7378 (0.1 µM) effected a change in fractional diameter of 0.25±0.03 (n = 5, Fig. 3C ) similar to prazosin (0.1 µM, 0.21±0.02 Fig. 2A ). At 1 µM, CEC, preferentially selective for α1B-adrenoceptors, (1 µM, n = 3) had no significant effect on vascular tone, but higher doses of CEC (10 µM, n = 4) did have a small effect, increasing fractional diameter by 0.07±0.01 (P<0.01, Fig. 3D , supplementary table. 1).
Figure 3. α1A- and α1D- adrenoceptor subtypes are the major adrenoceptor subtypes that activate vascular tone in femoral artery in vivo.
All experiments were carried in the presence of the α2-adrenoceptor blocker, RS 79948 (0.1 µM), the effects of which are represented by the ‘control’ bars. (A) Effect of RS100329 (10 nM-1 µM, n = 5) and (B) BMY7378 (10 nM-1 µM, n = 5) on active vascular tone of mouse femoral artery. (C) Combined effect of RS 100329(0.1 µM) and BMY7378 (0.1 µM). (D) Effect of alkylating agent CEC (1 and 10 µM, n = 3). Significance of difference from previous drug treatment, * P<0.05, ** P<0.01, *** P<0.001 (ANOVA followed by Newman-Keuls multiple comparison test).
α1-Adrenoceptors and [Ca2+]i
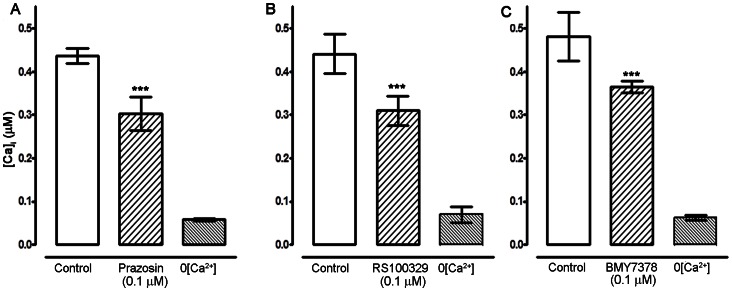
The presence of the genetically encoded biosensor in the arterial smooth muscle provided not only a convenient way to measure artery diameter, but also provided a measurement of smooth muscle intracellular [Ca2+]. The inhibition of vascular tone using α1-adrenoceptor antagonists was also evident in the changes in intracellular calcium calculated by the changes in CFP and YFP ratios in ex-MLCK biosensor animals. Prazosin (0.1 µM) decreased [Ca2+]i by 0.18±0.03 µM ( Fig. 4A, P<0.001). RS100329 (0.1 µM) significantly decreased [Ca2+]i by 0.15±0.02 µM ( Fig. 4B, P<0.001). Similarly, BMY7378 significantly reduced [Ca2+]i by 0.11±0.03 µM ( Fig. 4C, P<0.001). CEC (10 µM) did not significantly change [Ca2+]i. (not shown).
Figure 4. Block of α1A/1D-adrenoceptors reduces intracellular [Ca2+] in femoral artery in vivo.
All experiments were carried in the presence of RS 79948 (0.1 µM) represented by the ‘control’ bar. (A) Change in [Ca2+]i on local application of prazosin (0.1 µM, n = 8) on mouse femoral artery in vivo (n = 8). (B) Decrease in [Ca2+]i in the presence of RS100329 (0.1 µM, n = 5) and (C) Decrease in [Ca2+]i in the presence of BMY7378 (0.1 µM, n = 5) in mouse femoral artery in vivo. Significance of difference from previous drug treatment, *** P<0.001 (ANOVA followed by Newman-Keuls multiple comparison test).
Discussion and Conclusions
Vasoconstriction (‘tone’) of femoral artery of living anesthetized mice is substantial (∼ 50%) and activated mostly (∼90%) by the sympathetic nervous system, through receptors for NA, ATP and NPY. Our previously published work demonstrated gender differences in myogenic reactivity in cochlear arteries [39] and others have noted gender differences in rat cerebral vessels [40], [41]. However, no significant difference in femoral artery tone in vivo was recorded between female and male mice in the present study. Combined block of the adrenoceptors α2-, α1A-, and α1D- reduced vascular tone by ∼71% (Fig. 3C). Additional block of NPY1 and P2X receptors typically reduced tone by a further ∼ 22% (of the original amount, Fig. 1B), for a total reduction of ∼90%. As might be predicted therefore, abolition of SNA by block of autonomic ganglion transmission also reduced vasoconstriction to ∼90% of passive diameter. Thus we conclude that at least ∼ 90% of the tone of femoral arteries in vivo is attributable to neurally released NA, ATP and NPY. A small (∼10%) component of the femoral artery vasoconstriction did not arise from sympathetic nerve activity. Neither did this component arise from the myogenic mechanism [38], as isolated femoral arteries lacked completely any active response to intra-luminal pressure changes. It seems likely that the remaining component of tone is activated by circulating substances (e.g. Angiotensin II), endothelial vasoconstrictors (e.g. endothelin) and/or many other vasoactive substances present in the normal circulation and arterial wall.
α2-Adrenoceptors
A small but significant contribution to tone from activation of post-synaptic α2-adrenoceptors was evident. Post-synaptic α2-adrenoceptors are known to play a small but significant role in vasoconstriction in isolated (ex vivo) murine tail, first order cremaster, and femoral small arteries [19], [21], [42]. The net effect of α2-adrenoceptor inhibition was dilation, in these experiments. We expect the α2-adrenoceptor selective inhibitor RS79948 to block both pre and post junctional α2-adrenoceptors. A net vasodilation could have resulted if the antagonist produced a greater inhibition of post-junctional α2-adrenoceptor mediated contraction than of pre-junctional α2-adrenoceptor mediated inhibition of neurotransmitter release.
α1L-Adrenoceptors
α1-adrenergic receptors with low affinity for prazosin, viz. α1L- adrenoceptors, have not been found previously in mouse vasculature. Rather, the high-affinity type, α1H-, occurs in mouse first order mesenteric, aorta, carotid, caudal, and femoral small arteries [18], [19], [22]. The pIC50 value we measured for the effect of prazosin on femoral arteries in vivo was ∼8.0. Clearly however, that value is not a direct measure of affinity for prazosin of the α1-adrenoceptors activated by neuronally released NA, since equilibrium conditions do not apply in vivo. Under equilibrium conditions of the wire myograph organ bath, we measured a pKB for prazosin antagonism of phenylephrine of 7.74, consistent with the presence of α1L-adrenoceptors [9], [11], [12]. α1L-adrenoceptor is a pharmacological phenotype of α1A-subtype and is derived from the same gene [12], [13], [43]. Although not found previously in mouse, α1L-adrenoceptors are present in rat femoral arteries [44], [45], and small mesenteric arteries [46].
α1A-Adrenoceptor
RS100329, a selective α1A-adrenoceptor antagonist, inhibited vascular tone in a concentration dependent manner. The pIC50 value for RS100329 (7.4) was significantly lower than that of prazosin (8.0). Since RS100329 and prazosin have similar affinity for α1A-adrenoceptors, but RS100329 has significantly lower affinity for α1D-adrenoceptors than prazosin, this might suggest that not all of the prazosin-sensitive α1-adrenoceptors activated by neurogenically released noradrenaline are of the α1A-subtype. This possibility was explored with selective α1D-adrenoceptor antagonists (below).
α1D-Adrenoceptor
The α1D-adrenoceptor antagonist BMY7378 also inhibited vascular tone in a concentration dependent manner. BMY7378 does bind also to α1A/B-adrenoceptors, but with lower affinity than it does to α1D-adrenoceptor and than prazosin does [29]. On femoral artery, similar to the case with RS100329, the pIC50 values of BMY 7378 (7.2) were less than that of prazosin. Thus, the presence of α1D-adrenoceptors was confirmed and the existence of other α1-adrenoceptor subtype.
α1B-Adrenoceptor
Finally, α1B-adrenoceptors appeared to contribute negligibly to femoral vascular tone, since CEC at 1 µM had no effect on vascular tone or [Ca2+]i. The small effect on vascular tone with CEC at 10 µM that was observe may be attributable to α1A/D-adrenoceptors since at that concentration, CEC, also alkylates α1A/D-adrenoceptors [47]–[49].
Summary
When RS100329 (0.1 µM) and BMY7378 (0.1 µM) were used in combination, the prazosin (0.1 µM) sensitive response was completely eliminated. This suggests the major role from α1A- and α1D-adrenoceptors is maintenance of sympathetic neurogenic tone in mouse femoral artery in vivo. Consistent with this, α1A-adrenoceptors play a major role in tonic maintenance of mouse blood pressure [14], [50] and vasoconstriction in resistance vasculature [14], [18], [19]. The role of α1D-adrenoceptors in mediating nerve mediated responses are well documented in rat caudal artery where the authors suggested that α1D-adrenoceptors are restricted to the junctional region by neuronal activity, but if the nerves are lost, these receptor subtypes spread from the postsynaptic region along the smooth muscle [51]. Pharmacological and immuno-histochemical studies have shown the presence of α1D-adrenoceptors in rat femoral arteries [52]. α1D-adrenoceptors are known to play a modulatory role which is constitutively active in contractile tone in rat conductance arteries [53]. α1D-adrenoceptors are also involved in nerve mediated responses in rat [35] and mouse [19] femoral resistance arteries. Studies in α1D-adrenoceptor knock out models have directly shown that α1D-adrenoceptor participates in the regulation of systemic blood pressure [15]. In conclusion, the present study has shown a dominant role of adrenoceptor subtypes α1A- and α1D- in neurogenic tone of mouse femoral arteries in vivo.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)
Funding Statement
This work was supported by National Heart, Lung, and Blood Institute Grants 1RO1 HL091969 to WGW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kuroki MT, Guzman PA, Fink GD, Osborn JW (2012) Time-dependent changes in autonomic control of splanchnic vascular resistance and heart rate in ANG II-salt hypertension. Am J Physiol Heart Circ Physiol 302: H763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huggett RJ, Burns J, Mackintosh AF, Mary DA (2004) Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension 44: 847–852. [DOI] [PubMed] [Google Scholar]
- 3. Parati G, Esler M (2012) The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J 33: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 4. Wier WG, Zang WJ, Lamont C, Raina H (2009) Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y. Exp Physiol. 94: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rummery NM, Brock JA, Pakdeechote P, Ralevic V, Dunn WR (2007) ATP is the predominant sympathetic neurotransmitter in rat mesenteric arteries at high pressure. J Physiol 582: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tarasova O, Sjoblom-Widfeldt N, Nilsson H (2003) Transmitter characteristics of cutaneous, renal and skeletal muscle small arteries in the rat. Acta Physiol Scand 177: 157–166. [DOI] [PubMed] [Google Scholar]
- 7. Docherty JR (2010) Subtypes of functional α1-adrenoceptor. Cell Mol Life Sci 67: 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hieble JP, Ruffolo RR Jr (1996) Subclassification and nomenclature of α1- and α2-adrenoceptors. Prog Drug Res 47: 81–130. [PubMed] [Google Scholar]
- 9. Flavahan NA, Vanhoutte PM (1986) α1-Adrenoceptor subclassification in vascular smooth muscle. Trends in Pharmacological Sciences 7: 347–349. [Google Scholar]
- 10. Muramatsu I, Kigoshi S, Oshita M (1990a) Two distinct α1-adrenoceptor subtypes involved in noradrenaline contraction of the rabbit thoracic aorta. Br J Pharmacol 101: 662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muramatsu I, Ohmura T, Kigoshi S, Hashimoto S, Oshita M (1990b) Pharmacological subclassification of α1-adrenoceptors in vascular smooth muscle. Br J Pharmacol 99: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, et al. (1997) Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1-adrenoceptor classification. Br J Pharmacol 121: 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muramatsu I, Morishima S, Suzuki F, Yoshiki H, Anisuzzaman AS, et al. (2008) Identification of α1L-adrenoceptor in mice and its abolition by α1A-adrenoceptor gene knockout. Br J Pharmacol 155: 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rokosh DG, Simpson PC (2002) Knockout of the α1A/C-adrenergic receptor subtype: the α1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci U S A 99: 9474–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, et al. (2002) The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest 109: 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hosoda C, Hiroyama M, Sanbe A, Birumachi J, Kitamura T, et al. (2007) Blockade of both α1A- and α1B-adrenergic receptor subtype signaling is required to inhibit neointimal formation in the mouse femoral artery. Am J Physiol Heart Circ Physiol 293: H514–519. [DOI] [PubMed] [Google Scholar]
- 17. Lyssand JS, DeFino MC, Tang XB, Hertz AL, Feller DB, et al. (2008) Blood pressure is regulated by an α1D-adrenergic receptor/dystrophin signalosome. J Biol Chem 283: 18792–18800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, et al. (2002) A knockout approach indicates a minor vasoconstrictor role for vascular α1B-adrenoceptors in mouse. Physiol Genomics 9: 85–91. [DOI] [PubMed] [Google Scholar]
- 19. Zacharia J, Hillier C, Tanoue A, Tsujimoto G, Daly CJ, et al. (2005) Evidence for involvement of α1D-adrenoceptors in contraction of femoral resistance arteries using knockout mice. Br J Pharmacol 146: 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Townsend SA, Jung AS, Hoe YS, Lefkowitz RY, Khan SA, et al. (2004) Critical role for the α1B-adrenergic receptor at the sympathetic neuroeffector junction. Hypertension 44: 776–782. [DOI] [PubMed] [Google Scholar]
- 21. Moore AW, Jackson WF, Segal SS (2010) Regional heterogeneity of α-adrenoreceptor subtypes in arteriolar networks of mouse skeletal muscle. J Physiol 588: 4261–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Methven L, Simpson PC, McGrath JC (2009) α1A/B-knockout mice explain the native α1D-adrenoceptor’s role in vasoconstriction and show that its location is independent of the other α1-subtypes. Br J Pharmacol 158: 1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Isotani E, Zhi G, Lau KS, Huang J, Mizuno Y, et al. (2004) Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc Natl Acad Sci U S A 101: 6279–6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wier WG, Rizzo MA, Raina H, Zacharia J (2008) A technique for simultaneous measurement of Ca2+, FRET fluorescence and force in intact mouse small arteries. J Physiol 586: 2437–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Chen L, Raina H, Blaustein MP, Wier WG (2010) In vivo assessment of artery smooth muscle [Ca2+]i and MLCK activation in FRET-based biosensor mice. Am J Physiol Heart Circ Physiol 299: H946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mauban JR, Zacharia J, Zhang J, Wier WG (2013) Vascular tone and Ca2+ signaling in murine cremaster muscle arterioles in vivo . Microcirculation 20: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cambridge D, Davey MJ, Massingham R (1977) Prazosin, a selective antagonist of post-synaptic α-adrenoceptors [proceedings]. Br J Pharmacol 59: 514P–515P. [PMC free article] [PubMed] [Google Scholar]
- 28. Williams TJ, Blue DR, Daniels DV, Davis B, Elworthy T, et al. (1999) In vitro α1-adrenoceptor pharmacology of Ro 70–0004 and RS-100329, novel α1A-adrenoceptor selective antagonists. Br J Pharmacol 127: 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goetz AS, King HK, Ward SD, True TA, Rimele TJ, et al. (1995) BMY 7378 is a selective antagonist of the D subtype of α1-adrenoceptors. Eur J Pharmacol 272: R5–6. [DOI] [PubMed] [Google Scholar]
- 30. Bautista DL, Morris DH, Stein L, Asher W, Hammitt T (2006) A two model receptor system of the α1D adrenergic receptor to describe interactions with epinephrine and BMY7378. J Chem Inf Model 46: 334–344. [DOI] [PubMed] [Google Scholar]
- 31. Han C, Abel PW, Minneman KP (1987) α1-adrenoceptor subtypes linked to different mechanisms for increasing intracellular Ca2+ in smooth muscle. Nature 329: 333–335. [DOI] [PubMed] [Google Scholar]
- 32. Jarajapu YP, Hillier C, MacDonald A (2001) The α1A-adrenoceptor subtype mediates contraction in rat femoral resistance arteries. Eur J Pharmacol 422: 127–135. [DOI] [PubMed] [Google Scholar]
- 33. Mulvany MJ, Halpern W (1977) Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26. [DOI] [PubMed] [Google Scholar]
- 34. Zhang H, Fisher SA (2007) Conditioning effect of blood flow on resistance artery smooth muscle myosin phosphatase. Circ Res 100: 730–737. [DOI] [PubMed] [Google Scholar]
- 35. Zacharia J, Hillier C, MacDonald A (2004) α1-adrenoceptor subtypes involved in vasoconstrictor responses to exogenous and neurally released noradrenaline in rat femoral resistance arteries. Br J Pharmacol 141: 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arunlakshana O, Schild HO (1959) Some quantitative uses of drug antagonists. Br J Pharmacol Chemother 14: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schild HO (1949) pAx and competitive drug antagonism. Br J Pharmacol Chemother 4: 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davis MJ, Hill MA (1999) Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423. [DOI] [PubMed] [Google Scholar]
- 39. Reimann K, Krishnamoorthy G, Wier WG, Wangemann P (2011) Gender differences in myogenic regulation along the vascular tree of the gerbil cochlea. PLoS One 6: e25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geary GG, Krause DN, Duckles SP (1998) Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol 275: H292–300. [DOI] [PubMed] [Google Scholar]
- 41. Ibrahim J, McGee A, Graham D, McGrath JC, Dominiczak AF (2006) Sex-specific differences in cerebral arterial myogenic tone in hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 290: H1081–1089. [DOI] [PubMed] [Google Scholar]
- 42. Crassous PA, Flavahan S, Flavahan NA (2009) Acute dilation to α2-adrenoceptor antagonists uncovers dual constriction and dilation mediated by arterial α2-adrenoceptors. Br J Pharmacol 158: 1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marti D, Miquel R, Ziani K, Gisbert R, Ivorra MD, et al. (2005) Correlation between mRNA levels and functional role of α1-adrenoceptor subtypes in arteries: evidence of α1L as a functional isoform of the α1A-adrenoceptor. Am J Physiol Heart Circ Physiol 289: H1923–1932. [DOI] [PubMed] [Google Scholar]
- 44. Fujimoto S (1994) α1-adrenoceptor subtypes mediating contraction of the femoral artery in spontaneously hypertensive rats. Can J Physiol Pharmacol 72: 862–869. [DOI] [PubMed] [Google Scholar]
- 45. Tsurumaki T, Honglan P, Higuchi H (2003) Neuropeptide Y selectively potentiates α1-adrenoceptor-mediated contraction through Y1 receptor subtype in rat femoral artery. J Cardiovasc Pharmacol 42 Suppl 1S33–37. [DOI] [PubMed] [Google Scholar]
- 46. Stam WB, Van der Graaf PH, Saxena PR (1999) Analysis of α1L-adrenoceptor pharmacology in rat small mesenteric artery. Br J Pharmacol 127: 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwinn DA, Page SO, Middleton JP, Lorenz W, Liggett SB, et al. (1991) The α1C-adrenergic receptor: characterization of signal transduction pathways and mammalian tissue heterogeneity. Mol Pharmacol 40: 619–626. [PubMed] [Google Scholar]
- 48. Yang M, Reese J, Cotecchia S, Michel MC (1998) Murine α1-adrenoceptor subtypes. I. Radioligand binding studies. J Pharmacol Exp Ther 286: 841–847. [PubMed] [Google Scholar]
- 49. Hirasawa A, Sugawara T, Awaji T, Tsumaya K, Ito H, et al. (1997) Subtype-specific differences in subcellular localization of α1-adrenoceptors: chlorethylclonidine preferentially alkylates the accessible cell surface α1-adrenoceptors irrespective of the subtype. Mol Pharmacol 52: 764–770. [DOI] [PubMed] [Google Scholar]
- 50. Lopez-Guerrero JJ, Ibarra M, Villalobos-Molina R (2005) Postjunctional α1-adrenoceptors in the vasculature of the pithed mouse are of the α1A-subtype. Auton Autacoid Pharmacol 25: 101–103. [DOI] [PubMed] [Google Scholar]
- 51. Taki N, Tanaka T, Zhang L, Suzuki F, Israilova M, et al. (2004) α1D adrenoceptors are involved in reserpine-induced supersensitivity of rat tail artery. Br J Pharmacol 142: 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Piascik MT, Hrometz SL, Edelmann SE, Guarino RD, Hadley RW, et al. (1997) Immunocytochemical localization of the α1B-adrenergic receptor and the contribution of this and the other subtypes to vascular smooth muscle contraction: analysis with selective ligands and antisense oligonucleotides. J Pharmacol Exp Ther 283: 854–868. [PubMed] [Google Scholar]
- 53. Ziani K, Gisbert R, Noguera MA, Ivorra MD, D’Ocon P (2002) Modulatory role of a constitutively active population of α1D-adrenoceptors in conductance arteries. Am J Physiol Heart Circ Physiol 282: H475–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)