Abstract
p53 is a bona fide tumor suppressor gene whose loss of function marks the most common genetic alteration in human malignancy. Although the causal link between loss of p53 function and tumorigenesis has been clearly demonstrated, the mechanistic links by which loss of p53 potentiates oncogenic signaling are not fully understood. Recent evidence indicates that the microRNA-34 (miR-34) family, a transcriptional target of the p53, directly suppresses a set of canonical Wnt genes and Snail, resulting in p53-mediated suppression of Wnt signaling and the EMT process. In this study, we report that p53 regulates GSK-3β nuclear localization via miR-34-mediated suppression of Axin2 in colorectal cancer. Exogenous miR-34a decreases Axin2 UTR-reporter activity through multiple binding sites within the 5′ and 3′ UTR of Axin2. Suppression of Axin2 by p53 or miR-34 increases nuclear GSK-3β abundance and leads to decreased Snail expression in colorectal cancer cells. Conversely, expression of the non-coding UTR of Axin2 causes depletion of endogenous miR-34 via the miR-sponge effect together with increased Axin2 function, supporting that the RNA-RNA interactions with Axin2 transcripts act as an endogenous decoy for miR-34. Further, RNA transcripts of miR-34 target were correlated with Axin2 in clinical data set of colorectal cancer patients. Although the biological relevance of nuclear GSK-3 level has not been fully studied, our results demonstrate that the tumor suppressor p53/miR-34 axis plays a role in regulating nuclear GSK-3 levels and Wnt signaling through the non-coding UTR of Axin2 in colorectal cancer.
Keywords: Axin2, GSK-3, Snail, epithelial-mesenchymal transition (EMT), microRNA-34 (miRNA-34, miR-34), p53
Introduction
p53 is a well-known tumor suppressor whose loss of function is the most frequent genetic alteration in human cancer. Although most of its functional inactivation arises from somatic mutations observed in 50% of human cancers, the p53 pathway is also inactivated through indirect mechanisms such as MDM2 amplification or expression of viral oncoprotein. The p53 functions mainly as a transcriptional factor that directly binds DNA through a domain localized in responsive elements.1 Among the functions of p53 on hundreds of downstream targets, transcriptional activation of miRs sheds new light on the p53 tumor suppressor network,2,3 as tumor-suppressive miRs directly link the loss of tumor suppressor function with sustained activation of oncogenic signaling pathways. Indeed, it has recently been determined that p53 suppresses canonical Wnt and the Snail-mediated EMT program through transactivation of the miR-34 family.4-7
The canonical Wnt signaling plays pivotal roles in cell fate determination during development and adult tissue homeostasis.8 Mutations of APC or β-catenin resulting in constitutive activation of Wnt signaling, especially in colorectal tumor, are implicated in the development of human cancer as well as in its progression.9 Intracellular signaling of the canonical Wnt pathway largely depends on the regulation of glycogen synthase kinase-3 (GSK-3).10 Axin, a key scaffolding protein of GSK-3, not only regulates its kinase activity but also shuttles it from the cell membrane into the nucleus.11 Although Axin was first identified as a β-catenin degradation complex with APC in cytoplasm, its function is also critical to transduction of the intracellular Wnt cascade in the presence of an extracellular Wnt signal. For example, the GSK-3 shuttling function of Axin promotes phosphorylation of the membranous LRP6 co-receptor, resulting in activation of the intracellular canonical Wnt signaling cascade,12 while the GSK-3 nuclear export function of Axin participates in the EMT program of breast as well as colon cancer by stabilizing E-cadherin repressor Snail, thus inhibiting serial phosphorylation and subsequent proteasomal degradation of Snail.13,14 Whereas transcriptional regulation of Axin by TCF/LEF has been clearly shown,15,16 post-transcriptional regulation of Axin and nuclear GSK-3 trafficking, especially in colorectal cancer, wherein Axin2 is highly expressed, has been less well studied.
Although identification of miR targets relies mainly on sequences at the 5′ end of the miR, known as the “seed match,”17,18 we have reported miR interaction sites not only on the 3′ UTR but also on the 5′ UTR.19 In this molecular model, a miR can interact with both end regions of an mRNA through combinatory interactions of the 3′- and 5′-end of one miR with the 5′-UTR and 3′-UTR of the target mRNA, respectively.19 In such reciprocal miR-mRNA interactions, non-coding UTRs of mRNA can conversely modulate endogenous miRs, as in the “sponge” effect,20 and non-coding regions of mRNA transcripts can regulate other mRNA transcripts (so called “competing endogenous RNA, ceRNA”) through the competition and titration of endogenous miRs.4,21-23 Following on recent reports of the functional and clinical relevance of the p53/miR-34 axis and Wnt on EMT and cancer progression,5-7 we show here that p53 and miR-34 directly control Axin2 post-transcriptionally in colorectal cancer cells, thereby regulating Axin2-dependent nuclear GSK-3 levels. We also demonstrate that expression of the non-coding 5′ UTR as well as of the 3′ UTR of Axin2 leads to depletion of endogenous miR-34 together with increased endogenous Axin2 expression, and the non-coding UTR of Axin2 transcripts and miR-34 participate in the genome wide RNA-RNA network of Wnt signaling in colorectal cancer.
Results
Axin2 regulates nuclear GSK-3 localization and Snail-mediated E-cadherin promoter activity in colorectal cancer cells
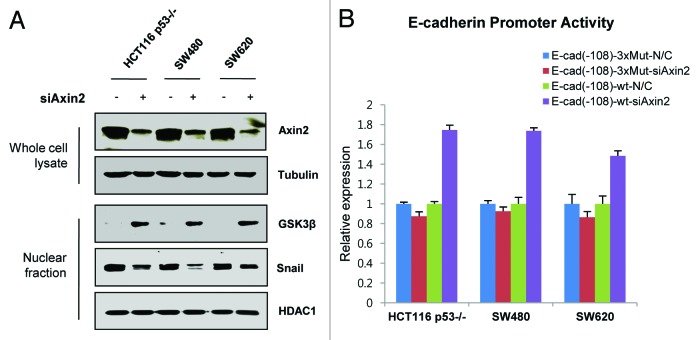
We chose several colorectal cancer cells having high levels of Axin2 to examine its function on nuclear GSK-3 and its direct substrate Snail level. Similar to the case in breast cancer cells,14 downregulation of endogenous Axin2 promotes increased nuclear GSK-3 in conjunction with lowered nuclear Snail level (Fig. 1A). Consistent with a well-known function of Snail,13,24,25 E-cadherin proximal promoter activity was increased by knockdown of Axin2 (Fig. 1B), supporting the notion that Axin2 plays an important role regulating Snail-mediated EMT programs via a nuclear GSK-3 shuttling function in colorectal cancer cells.14,26
Figure 1. Nuclear GSK-3 level regulated by Axin2 expression in colorectal cancer cell lines. (A) Immunoblot analysis of Axin2 in whole-cell lysate, GSK-3 and Snail in nuclear fraction after siRNA-mediated knockdown of Axin2 in HCT116-p53−/−, SW480 and SW620 cells. Tubulin and HDAC were used as the loading control of whole-cell lysate and nuclear fraction, respectively. (B) E-cadherin proximal promoter activities of firefly luciferase reporter constructs were determined in HCT116-p53−/−, SW480 and SW620 cells. Increases in E-cadherin promoter activity were observed with siRNAs against Axin2 compared with scrambled siRNA control. Activity of the E-cadherin proximal promoter constructs was normalized to that of a cotransfected SV-40-driven Renilla luciferase construct. Results are expressed as the mean ± SD of triplicate.
p53/miR-34a axis suppresses Axin2 expression in human colon cancer cells
Recently, we have reported that p53 directly suppresses a set of GSK-3-related canonical Wnt genes including Wnt1, Wnt3, LRP6, β-catenin, LEF1 and Snail1.5,6 As Axin2 is a key regulator of GSK-3 in canonical Wnt signaling and the Snail-mediated EMT program, we examined whether Axin2 is also regulated by the p53/miR-34 axis in colorectal cancer cells. In a panel of characterized colorectal cancer cell lines, HCT116 and RKO cells having wild type (wt) p53 function showed lowered expression of Axin2 protein level, while SW480 and SW620 cells having mutant p53 protein exhibited increased Axin2 expression (Fig. 2A). Notably, isogenic knockout of p53 in HCT116 cells showed increased Axin2 protein level compared with wild type (wt) cells, suggesting that the p53 tumor suppressor function regulates Axin2 expression in colorectal cancer cells. Because the miR-34 level is mainly regulated via the transcriptional function of p53,2,3,27,28 we next examined the quantitative expression of miR-34 and Axin2 transcripts. In these cells, Axin2 mRNA expressions were consistent with protein expression level and inversely correlated with pri-miR-34a as well as mature miR-34a expression (Fig. 2B). As with protein abundance, Axin2 transcript level was significantly increased, whereas miR-34 was downregulated in p53-null isogenic HCT116 cells compared with wt HCT116. To further assess the functionality of p53 on Axin2 expression, we administered the genotoxic agent adriamycin to induce p53 and then analyzed the relationship between the p53/miR-34a axis and Axin2 in these cells. As expected, Axin2 protein abundance in wt HCT116 and RKO cells was significantly suppressed by induction of wt p53 but was unchanged in p53-null HCT116 cells or p53 mutant colorectal cancer cells (Fig. 2C). An inverse correlation between Axin2 protein and miR-34 transcript abundance was identified by induction of wt p53 (Fig. 2D), supporting that p53 and miR-34 negatively regulate Axin2 in colorectal cancer cells.
Figure 2. p53 and miR-34 inversely correlated with Axin2 expression. (A) Endogenous Axin2 level was determined by immunoblot analysis in a panel of colorectal cancer cell lines according to the p53 functional status. Tubulin was used as the loading control. (B) The transcript levels of Axin2 and pri-miR-34a (upper panel) and mature miR-34a (lower panel) were determined by quantitative RT-PCR analysis in a panel of colorectal cancer cells. Results are expressed as the mean ± SD of triplicate experiment. (C) Immunoblot analysis of endogenous p53 and Axin2 protein levels after treatment of 0.1 mg/ml adriamycin (ADR) for 8 h in colorectal cancer cells. (D) Quantitative RT-PCR analysis of pri-miR-34a transcript levels after treatment of ADR. Results are expressed as the mean ± SD of triplicate experiment.
miR-34 target sites on Axin2 UTRs
Compared with that of lower vertebrates, human Axin2 mRNA possesses a relatively longer 5′ UTR (NM_004655.3), which potentially interacts with the 3′-end of miR-34.19 Potential target sites of miR-34 on the Axin2 3′ UTR as well as on the 5′ UTR were predicted by the miBridge algorithm (Table S1), as shown in Figure 3A. While miR-34b/c as well as miR-34a is transcribed by p53, we focused on miR-34a function, since it is most abundant in cells.2,3,28 To examine the functionality of miR-34 on Axin2 UTRs, we performed the reporter assay of Axin2 UTR with miR-34a in p53-null or p53 mutant colon cancer cells. The Axin2 reporter activities of the 5′ UTR as well as of the 3′ UTR were downregulated by exogenous miR-34a in these colorectal cancer cells (Fig. 3B), and the putative overlapping target sites on the 5′ UTR were functional in HCT116 cells (Fig. 3C).19 Consistent with reporter activities, endogenous Axin2 expression level was significantly suppressed by miR-34, whereas nuclear GSK-3 increased (Fig. 3D) in these cells. For clear evidence of miR-34 targeting of Axin2 transcripts, we chose and analyzed a data set of Dicer−/− colorectal cancer cells that would allow us to observe effects of such direct targeting.2 Indeed, Axin2 transcript abundances were decreased by the miR-34 family in Dicer−/− colorectal cancer cells (Fig. 3E), supporting that p53 and miR-34 modulate nuclear GSK-3 levels through direct targeting of Axin2 transcripts in colorectal cancer cells.
Figure 3. miR-34 directly targets Axin2 UTRs. (A) Predicted Axin2 target sites (NM_004655.3) of miR-34a-5p, miR-34b-5p and miR-34c-5p are indicated by the arrows on the 5′ UTR and 3′ UTR. (B) Inhibition of 5′ and 3′ UTR reporter activities of Axin2 by miR-34a. Relative luciferase activity by miR-34 in comparison to the non-UTR control luciferase reporter is shown; the error bar represents the SD of triplicate experiment. Relative reporter activity = (ULuc/URenilla)/(CLuc/CRenilla); U, UTR vector; C, luciferase vector control; Luc, raw firefly luciferase activity; Renilla, internal transfection control renilla activity. (C) Effect of mutation on the 5′ UTR. The luciferase reporter having 3′ UTR of Axin with wild type 5′ UTR or mutant 5′ UTR was transfected into the HCT116 cells and the relative 5′ UTR mutant reporter activity compared with wild type UTR after transduction with a negative control oligo (N/C) or miR-34a (*p < 0.01 compared with control, t-test). (D) Immunoblot analysis of Axin2 and nuclear GSK-3 levels after transfection of control (−) or miR-34a in p53-null or p53-mutant colorectal cancer cells. (E) An unsupervised hierarchical clustering based on Axin2 transcript expression profiles in Dicer−/− HCT116 and DLD1 colorectal cancer cells with the miR-34 transduction. In the heatmap, red denotes higher relative expression, whereas green indicates lower relative expression, with degree of color saturation reflecting the magnitude of the log expression signal.
p53 and endogenous miR-34 regulate nuclear GSK-3 levels via Axin2 UTR in HCT116 cells
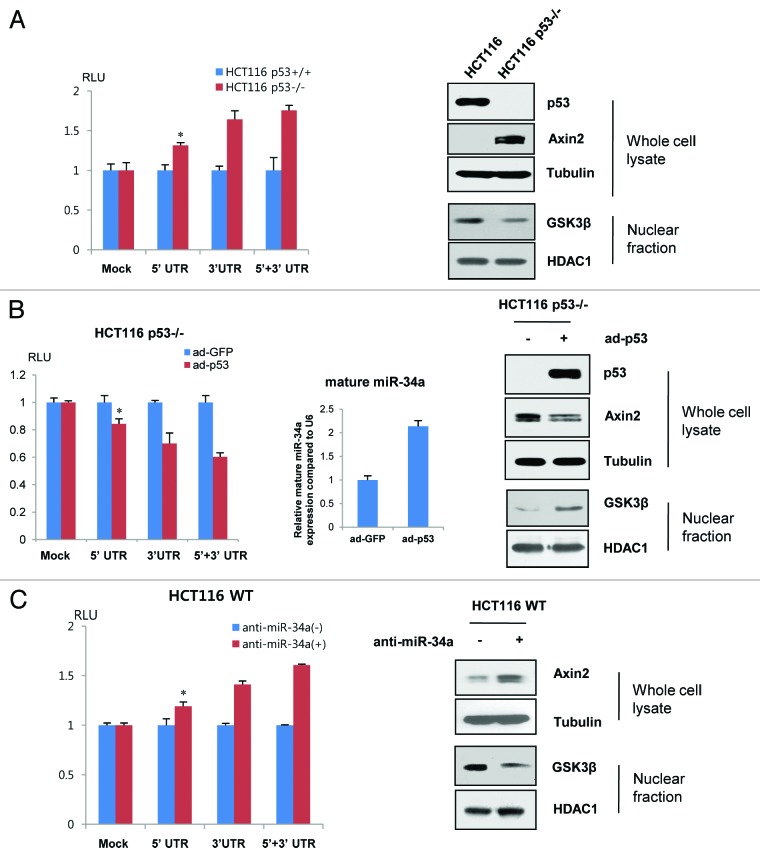
Given the mature miR-34a level and Axin UTR activity in HCT116 cells and isogenic p53−/− HCT116 cells, we next compared UTR reporter activities on these cells to further assess the functional effect of p53 on Axin2 expression and nuclear GSK-3β level. Axin2 UTR reporter activities and endogenous Axin2 protein level consistently increased in p53-null HCT116 cells, while loss of wt p53 decreased nuclear GSK-3 (Fig. 4A). Separately, transduction of wt p53 into p53-null HCT116 cells reversed the consequences of p53 knockout in terms of UTR activities, mature miR-34, Axin2 expression and nuclear GSK-3 expression level (Fig. 4B). Functional depletion in wt HCT116 cells of endogenous miR-34a, the most abundant member of the miR-34 family, by complementary RNA showed effects similar to p53-loss on reporter activities and nuclear GSK-3 (Fig. 4C), suggesting that endogenous miR-34 in HCT116 is functional for p53-mediated Axin2 repression.
Figure 4. p53 and endogenous miR-34 are functional for Axin2 suppression and nuclear GSK 3 levels. (A) UTR reporter assay and immunoblot analysis in wt vs. p53-null HCT116 cells. UTR reporter activities of Axin2 5′ UTR or/and 3′ UTR constructs were compared with wt p53 and p53−/− isogenic HCT116 cells (left). The UTR reporter activities were significantly increased by loss of p53 function (*p < 0.01 compared with control, t-test). Immunoblot analysis of Axin2 and nuclear GKS3 levels in wt vs. p53-null HCT116 cells. Tubulin and HDCA1 are used as the loading control of whole-cell lysates and nuclear fraction, respectively. (B) UTR reporter (left), mature miR-34a (middle) and immunoblot analyses (right) after adenoviral transduction with a control (ad-GFP) or wt p53 (ad-p53) expression vector in p53-null HCT116 cells (*p < 0.01 compared with control, t-test). The endogenous Axin2 and nuclear GSK-3 levels were determined after transduction of adenovirus. (C) Inhibition of endogenous miR-34a increased activity of Axin2 UTR reporter in wt HCT116 cells (left, *p < 0.05 compared with control, t-test). Immunoblot analysis of Axin2 and nuclear GSK-3 level after transduction of negative control oligo (−) or anti-miR-34a (+) in HCT116 cells.
miR-34 is inversely correlated with Axin2 in colorectal cancer samples
Loss of wt-p53 function tends to occur prior to invasive colorectal carcinoma.29 To confirm the loss of miR-34 accompanying increased Axin2 transcripts, we obtained 10 cases of colon cancer tissues paired with adjacent normal mucosa and measured the mRNA expression of Axin2 and pri-miR-34a using quantitative real-time PCR. Compared with paired normal tissues, miR-34a mRNA levels were significantly lower in human colon cancer samples, whereas Axin2 expression was higher (Fig. 5), supporting that the miR-34 and Axin2 transcripts were inversely correlated in colorectal cancer progression.

Figure 5. Expression of Axin2 in matched human colon cancer samples. Quantitative real-time PCR analysis of miR-34a and Axin2 was performed on DNA isolated from either colon cancer or paired counterpart normal tissues; results are expressed as the expression levels in cancer tissue relative to normal tissue. Experiments were performed in triplicate and the log expression values in all panels normalized to the levels of GAPDH.
Non-coding Axin2 UTR depletes endogenous miR-34 and suppresses Axin2 function
Because miRs mediate networks of RNA-RNA interaction,22,23 non-coding Axin2 UTR functions as a cis regulatory element that increases expression of endogenous Axin2 transcripts and protein expression.4 To determine the role of the non-coding Axin2 UTR in RNA interactions, we overexpressed luciferase expression construct having the 3′ and/or 5′ UTR of Axin2 in HCT116 cells and in Dicer mutant HCT116 cells (HCT116 DicerEX5). The expression of the non-coding UTR increased the abundance of Axin2 protein with decreased nuclear GSK-3 and Snail expression, whereas the effects were minimal in Dicer mutant cells (Fig. 6A). Indeed, the endogenous mature miR-34a was depleted by expression on the non-coding UTR of Axin2 via the miR-sponge effect in HCT116 cells, whereas it was undetected in Dicer mutant cells (Fig. 6B), revealing that the non-coding UTR can regulate endogenous Axin2 via titration of miR-34. Interestingly, expression of both the 5′ UTR and 3′ UTR affects the depletion of miR-34 and functionality of endogenous Axin2. Taking into account the Axin2 transcript function as a ceRNA (competing endogenous RNA), our previous observations predict that transcripts targeted by miR-34 are co-regulated through RNA-RNA interactions.4,5 To understand the genome-wide context of such interactions, we chose a publicly available colorectal cancer data set of 177 patients from the Moffitt cancer center (GSE17536) and analyzed the co-expression of RNA transcripts of miR-34 target genes and Axin2. Significantly, LEF1, WNT3 and LRP6 transcripts revealed differential expression when the samples were subcategorized according to Axin2 expression levels (Fig. 6C). Calculating the Pearson correlation coefficients between each transcript and Axin2 expression levels, we found these transcripts to be significantly correlated with Axin2 in each sample (Fig. 6D). The ceRNA function in these samples suggests that miR-34 acts as a trans modulator in co-expression of the target genes.
Figure 6. RNA-RNA interaction of non-coding Axin2 UTRs in colorectal cancer cells and clinical data set. (A) Immunoblot analysis of Axin2, nuclear GSK-3 and Snail levels was performed following transfection of the firefly luciferase expression vectors without UTR (mock) or having UTR in HCT116 (left panels) and DicerEX5 (right panels) cells. (B) The relative expression levels of mature miR-34a were determined by TaqMan quantitative RT-PCR after transfection in HCT116 cells of the firefly luciferase expression vectors without UTR (mock) or having UTR (*p < 0.01 compared with control, t-test). (C) Comparison of miR-34 target genes of LEF1, LRP6 and WNT3 transcript levels between two subsets of AXIN2 in 177 cases of colorectal cancer patient samples (GSE17536). (D) Pearson correlation scatter plots of AXIN2 ceRNA transcripts in GSE17536. The p values for correlation coefficients of LEF1, LRP6 and WNT3 were 4.79e-05, 2.97e-08 and 1.97e-02, respectively. See the “Materials and Methods” section for data processing and statistical analysis.
Discussion
The tumor suppressor function of p53 in various oncogenic signaling events such as increased β-catenin expression resulting from loss of p53 function has long been observed.1,30 Axin and GSK-3 dynamics have been shown to be regulated by p53, although the mechanistic links between p53 and Axin were not identified.31 Recently, the miR-34 family, which is directly transcribed by the p53 tumor suppressor, has emerged as a mechanistic link between the p53 and canonical Wnt signaling and EMT program.4-6,32 In this process, a set of GSK-3-related Wnt genes, including EMT inducer Snail, are directly targeted by p53 and miR-34, demonstrating that the p53/miR-34 axis endogenously suppresses canonical Wnt signaling and the Snail-mediated EMT program.4-6 In this study, we report that the p53 tumor suppressor axis also modulates nuclear GSK-3 level by targeting the Axin2 UTRs in colorectal cancer. Taken together with the well-known post-translational and post-transcriptional regulation of Wnt and the EMT program by the p53/miR-34 axis,4-6 these observations demonstrate the close connection between the tumor suppressor function and oncogenic signaling in human cancer.
GSK-3 is a widely expressed and multifunctional Ser/Thr kinase involved in a broad range of biological processes, from glycogen metabolism to transcriptional program and cell cycle regulation.33,34 GSK-3 is constitutively active under resting conditions and acts as a core regulator in pathways implicated in diseases such as Alzheimer, diabetes, bipolar disorder and cancer. In these distinct biological contexts, scaffolding proteins insulating the kinase activity confer signaling selectivity and substrate specificity.33,34 Together with its kinase activity, GSK-3’s subcellular localization and sequestration by a number of binding partners are key elements in cellular signaling, especially with regard to β–catenin and Snail activity.35 In canonical Wnt signaling, a membrane-localized form of GSK-3 recruited with Axin is essential to LRP6 phosphoryation and subsquent activation for signal transduction.12 In this process, membranous GSK-3, unlike cytosolic GSK-3, stimulates canonical Wnt signaling. Further, Axin promotes EMT by acting as a shuttling chaperone for nuclear GSK-3, which is responsible for controlling Snail protein activity through post-translational modification.14,26,36 Interestingly, nuclear GSK-3 is highly active compared with its major cytosolic fraction, its kinase activity in colon cancer cells being enhanced by silencing of Axin2,26,37 indicating that Axin functions as a key regulator of GSK-3 in Wnt signal transduction not only in terms of kinase activity, but also with respect to intracellular compartmentalization. Considering the large amount of GSK-3 substrate in nuclear transcription factors such as Snail, the p53/miR-34 axis regulating nuclear GSK level comprises a wide range of gene functions beyond β-catenin-mediated canonical Wnt signaling and the Snail-mediated EMT program.
A key component in canonical Wnt signaling, Axin serves as a multimeric scaffold for a regulatory complex including β-catenin, GSK-3, APC (adenomatous polyposis coli), CK1, Dishevelled, MEKK4 and protein phosphatase 2.38-41 While Axin1 is expressed ubiquitously during embryogenesis, Axin2 expression is restricted to specific tissues. Interestingly, Axin1 and Axin2 proteins are functionally equivalent in mouse embryogenesis and development,42 suggesting that Axin function is mainly regulated by transcriptional activation or post-transcriptional regulation of Axin2. β-catenin directly induces transcription of Axin2 through TCF/LEF consensus binding sites on its promoter,15,16 and Axin2 expression level is elevated in colorectal cancer as a result of β-catenin/TCF activation. In this study, we show that p53 suppresses Axin2 expression in colorectal cancer cells, and that loss of p53 function induces sustained activation of Axin2 via loss of transcriptional regulation of the miR-34 family. As TCF/LEF transcriptional activity is directly suppressed by the p53/miR-34 axis,5 this study suggests that p53 governs Wnt genes by direct targeting of individual genes in tandem with post-translational regulation of the signaling cascade.4
miRs are small non-coding RNAs which post-transcriptionally regulate mRNA stability and protein translation. Although 7–8 nt seed-matched sites on the 5′-ends of miRs are known to preferentially bind to the 3′-UTRs of mRNAs,18,43 some genes of higher mammals have a 5′ UTR site that interacts with the 3′-end of the miR, allowing combinatory interactions between a single miR and both ends of the mRNA. For example, the 3′ end of an individual miR-34 can directly target the 5′ UTR of Axin2 transcripts, leading to mRNA deadenylation and inhibiting protein translation in a cap-dependent fashion.19,44 In this study, we show that overexpression of the Axin2 5′ UTR as well as of the 3′ UTR is sufficient to deplete endogenous miR-34 and to induce Axin2 protein expression as a cis regulator. We also showed co-expression of Axin2 and other miR-34 target genes in colorectal cancer samples, consistent with Axin2 functioning as a miR-sponge competing with other endogenous RNAs through non-coding UTRs,22,23 suggesting that increased expression of the endogenous Axin2 transcript in colorectal cancer comprises a range of RNA-RNA interactions beyond the protein coding function of the transcripts. Conversely, endogenous miR-34 dynamically reduces peaks in canonical Wnt genes to maintain balanced signaling by means of dynamic range compression,4 whereas endogenous depletion of miR-34 due to loss of p53 function during later-stage colorectal adenoma contributes to sustained activation of the canonical Wnt signaling RNA network and the EMT program.
Materials and Methods
Cell lines and expression vectors
Colorectal cancer cells SW480, SW620 and RKO cell lines were obtained from ATCC and maintained according to the manufacturer’s instructions. HCT116, Dicer mutant EX5 cells and p53−/− isogenic cells were kindly provided from Dr Vogelstein’s lab as described previously.6,45 The functional status of p53 in colorectal cancer cell lines was confirmed through the TP53 database (www-p53.iarc.fr).
Constructs and viral transduction
Adenovirus expressing GFP (ad-GFP, #1060) and GFP-p53 (ad-GFP-p53, #1260) were purchased from Vector Biolabs. p53−/− HCT116 was transduced with adenoviral vectors by directly applying the diluted viruses to the culture medium at 100 multiplicity of infections as described previously.5 The transduction efficiency was determined by direct visualization using fluorescent microscopy of GFP-expressing cells. Wild type and 3× E-box mutated E-cadherin proximal reporter constructs pGL3-Ecad(-108)-wt-Luc and pGL3-Ecad(-108)-3× Mut-Luc were used as described previously.13,14 The activity of E-cadherin reporter constructs was normalized to the activity of the co-transfected SV40-renilla luciferase construct (2 ng, Promega). Cells were lysed at 48 h after transfection, and the relative ratio of renilla luciferase to firefly luciferase activity was measured in a dual luciferase assay from triplicate experiment (Promega).
Axin2 UTR reporter and miR experiments
The luciferase reporter of pcDNA3.1-Hyg(+), a mammalian expression vector, was used as described previously.5,19 Briefly, the 3′ UTR of Axin2 (NM_004655.3; + 1 ~+ 1,059) was amplified from genomic DNA of MCF-7 cells and cloned into the BamH1 and NotI sites downstream of luciferase. The recent version of miR-34 family annotations and sequences were obtained from miRBase (www.mirbase.org). The wild type and mutant synthetic oligonucleotide containing 59-bp 5′ UTR sequences putatively targeted by miR-34a (wt; 5′- GCCCGGGGGAGTCGGCTGGAGCCGGCTGCGCTTTGA; + 141 ~ +179, putative overlapped miR-34 target region underlined, mut; GCCCGGGGGAGggatagTGGtcgGGCTGCGCTTTGA, mutation of predicted target sites lowercase letters) was inserted into NheI and BamH1 sites upstream of luciferase as described previously (GR 2009). The UTR reporter assays were performed as described previously.5,6 Briefly, the cells were co-transfected with each of the Axin2 UTR reporter constructs (5 ng) and synthetic miRNA (Ambion) as indicated. As a transfection control, 1 ng of SV40-promoter driven Renilla construct (Promega) was co-transfected with reporter vectors. For functional analysis of miRNA precursor, 20 nM of miR-34 (Ambion, PM11030) and negative control (Ambion, AM17110) were transduced with lipofectamine 2000 (Invitrogen). For functional inhibition of endogenous miRNA, anti-miR-34a inhibitor (Ambion, AM11030) and negative control (Ambion, AM17110) were used. Cells were lysed at 48 h after transfection and the relative ratio of Renilla to firefly luciferase was measured with dual luciferase assay (Promega). For ceRNA experiment, the Axin2 UTR reporter constructs were transiently transfected into the wt-HCT116 or isogenic Dicer mutant EX5 HCT116 cells, and subjected for immunoblot and quantitative RT-PCR assay as described previously.5
Microarray data analysis
Publicly available gene expression data sets of Dicer −/− colorectal cancer cells (GSE7864) and of 177 patients from the Moffitt cancer center (GSE17536) were downloaded and processed as described previously.4,5 In GSE7864, the transcript expression levels of AXIN2 (10023806666 AXIN2, 10025909737 AXIN2) were compared according to miR-34 transduction. The transcript levels of LEF1 (221558_s_at), LRP6 (34697_at) and WNT3 (229103_at) were compared according to high and low subsets of AXIN2 (222696_at). The subsets were determined based on the median expression level of Axin2 in the Moffitt cohort and their p-values obtained from student t-test. The scatter plots were obtained using the Correl function for Pearson correlation between LEF1 or LRP6 or WNT3 and AXIN2 transcripts. The p-values of correlation coefficients were obtained from 10,000 permutations.
Quantitative real-time PCR analysis and clinical samples of colorectal cancer tissue
The relative expression levels of Axin2 and pri-miR-34a transcript were determined by real time quantitative PCR analysis as described previously.5 Briefly, total RNA of the cells was isolated with Trizol (Invitrogen) and cDNA was synthesized using random hexamer reverse transcription primer (Intron). Axin2 and miR-34a transcripts levels were detected using the 7300 Real-Time PCR System and SYBR Green (Applied Biosystems) and both normalized to the levels of GAPDH; the relative log2 expression levels in cancer tissue compared with paired normal tissue were then calculated. Primers that amplify human Axin2, pri-miR-34a or GAPDH were designed using Primer3 software, the oligonucleotide sequences of the primers having been described previously.5 For quantitative analysis of mature miR-34a levels, human TaqMan miRNA assay kits (Applied Biosystems, assay ID 000426 for miR-34a and ID001973 for U6 snRNA) were used for reverse transcription with specific primers and qPCR was performed with corresponding probes (n = 3). Ten cases of colorectal adenocarcinoma patient tissues paired with normal mucosa (IRB exempted) were obtained from Severance Hospital Gene Bank, total RNA being isolated with Trizol.
Western blot analysis
The protein levels of p53 and Axin2 were detected by western blot analysis of whole-cell lysate with Triton X-100 as described previously.13 The expression levels of endogenous GSK-3 and Snail were detected from nuclear fraction of protein lysates with hypotonic buffer as described previously.14 Briefly, the cells (0.5 × 106) were collected in microcentrifuge tubes after trypsinization and the PBS washed cells treated with 400 μl of hypotonic buffer (10 mM HEPES, pH 7.9; 10 mM KCl; 1 mM DTT with protease inhibitors) on ice for 5 min. The cell membrane was ruptured by adding 10% NP-40 solution to a final concentration of 0.6%, then vigorously vortexed for 10 sec followed by high-speed centrifuge (12,000 rpm of microcentrifuge) for 30 sec. After removal of supernatant cytosolic fraction, the nuclear pellets were washed with ice-cold PBS twice and subjected to nuclear protein extraction with 40 μl of hypertonic buffer (20 mM HEPES, pH 7.9; 0.4 M NaCl; 1 mM DTT with protease inhibitor cocktail) for 15 min on ice followed by maximum speed microcentrifuge to remove insoluble precipitate. Antibodies against Axin2 (Cell Signaling Technology), Snail (Cell Signaling Technology), GSK-3β (BD bioscience), p53 (DO-1, Santa Cruz), HDAC1 (Santa Cruz) and Tubulin (Labfrontier) were commercially available.
Statistical analysis
Statistical significance of reporter assays was determined by the Student’s t-test.
Supplementary Material
Acknowledgments
We thank E. Tunkle for preparation of the manuscript. This work was supported by grants from the National Research Foundation of Korea (2012-0000128, 2011-0031396, 2012M3A9B2052523), a grant from the National R&D Program for Cancer Control (1020110).
Disclosure of Potential Conflicts of Interest
Inhan Lee is the founder of the nonprofit organization miRcore. The other authors declare that they have no competing financial interests.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/24739
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24739
References
- 1.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 2.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–4. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Cha YH, Kim NH, Park C, Lee I, Kim HS, Yook JI. MiRNA-34 intrinsically links p53 tumor suppressor and Wnt signaling. Cell Cycle. 2012;11:1273–81. doi: 10.4161/cc.19618. [DOI] [PubMed] [Google Scholar]
- 5.Kim NH, Kim HS, Kim NG, Lee I, Choi HS, Li XY, et al. p53 and microRNA-34 are suppressors of canonical Wnt signaling. Sci Signal. 2011;4:ra71. doi: 10.1126/scisignal.2001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim NH, Kim HS, Li XY, Lee I, Choi HS, Kang SE, et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol. 2011;195:417–33. doi: 10.1083/jcb.201103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siemens H, Jackstadt R, Hünten S, Kaller M, Menssen A, Götz U, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10:4256–71. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 8.Fossat N, Jones V, Garcia-Garcia MJ, Tam PP. Modulation of WNT signaling activity is key to the formation of the embryonic head. Cell Cycle. 2012;11:26–32. doi: 10.4161/cc.11.1.18700. [DOI] [PubMed] [Google Scholar]
- 9.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 10.Tauriello DV, Maurice MM. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle. 2010;9:3700–9. doi: 10.4161/cc.9.18.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong F, Varmus H. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin. Proc Natl Acad Sci USA. 2004;101:2882–7. doi: 10.1073/pnas.0307344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–7. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280:11740–8. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 14.Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8:1398–406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 15.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, et al. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–65. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 17.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–11. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee I, Ajay SS, Yook JI, Kim HS, Hong SH, Kim NH, et al. New class of microRNA targets containing simultaneous 5′-UTR and 3′-UTR interaction sites. Genome Res. 2009;19:1175–83. doi: 10.1101/gr.089367.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–6. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–8. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer A, Llobet-Navas D, et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147:370–81. doi: 10.1016/j.cell.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011;147:344–57. doi: 10.1016/j.cell.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 25.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 26.Wu ZQ, Brabletz T, Fearon E, Willis AL, Hu CY, Li XY, et al. Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity. Proc Natl Acad Sci USA. 2012;109:11312–7. doi: 10.1073/pnas.1203015109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008;105:13421–6. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamakuchi M, Lowenstein CJ. MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle. 2009;8:712–5. doi: 10.4161/cc.8.5.7753. [DOI] [PubMed] [Google Scholar]
- 29.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 30.Cagatay T, Ozturk M. P53 mutation as a source of aberrant beta-catenin accumulation in cancer cells. Oncogene. 2002;21:7971–80. doi: 10.1038/sj.onc.1205919. [DOI] [PubMed] [Google Scholar]
- 31.Levina E, Oren M, Ben-Ze’ev A. Downregulation of beta-catenin by p53 involves changes in the rate of beta-catenin phosphorylation and Axin dynamics. Oncogene. 2004;23:4444–53. doi: 10.1038/sj.onc.1207587. [DOI] [PubMed] [Google Scholar]
- 32.Ory B, Ellisen LW. A microRNA-dependent circuit controlling p63/p73 homeostasis: p53 family cross-talk meets therapeutic opportunity. Oncotarget. 2011;2:259–64. doi: 10.18632/oncotarget.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;2001:re12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- 35.Wiechens N, Heinle K, Englmeier L, Schohl A, Fagotto F. Nucleo-cytoplasmic shuttling of Axin, a negative regulator of the Wnt-beta-catenin Pathway. J Biol Chem. 2004;279:5263–7. doi: 10.1074/jbc.M307253200. [DOI] [PubMed] [Google Scholar]
- 36.Park SY, Kim HS, Kim NH, Ji S, Cha SY, Kang JG, et al. Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition. EMBO J. 2010;29:3787–96. doi: 10.1038/emboj.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bijur GN, Jope RS. Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3 beta. J Biol Chem. 2001;276:37436–42. doi: 10.1074/jbc.M105725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo W, Ng WW, Jin LH, Ye Z, Han J, Lin SC. Axin utilizes distinct regions for competitive MEKK1 and MEKK4 binding and JNK activation. J Biol Chem. 2003;278:37451–8. doi: 10.1074/jbc.M305277200. [DOI] [PubMed] [Google Scholar]
- 39.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, et al. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273:10823–6. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 40.Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–22. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–45. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- 42.Chia IV, Costantini F. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–6. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mavrakis KJ, Wendel HG. TargetScreen: an unbiased approach to identify functionally important microRNA targets. Cell Cycle. 2010;9:2080–4. doi: 10.4161/cc.9.11.11807. [DOI] [PubMed] [Google Scholar]
- 44.Moretti F, Thermann R, Hentze MW. Mechanism of translational regulation by miR-2 from sites in the 5′ untranslated region or the open reading frame. RNA. 2010;16:2493–502. doi: 10.1261/rna.2384610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA, Jr., Sjoblom T, et al. The colorectal microRNAome. Proc Natl Acad Sci USA. 2006;103:3687–92. doi: 10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.