Abstract
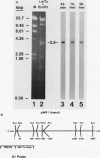
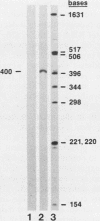
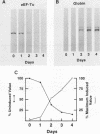
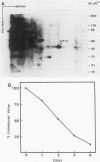
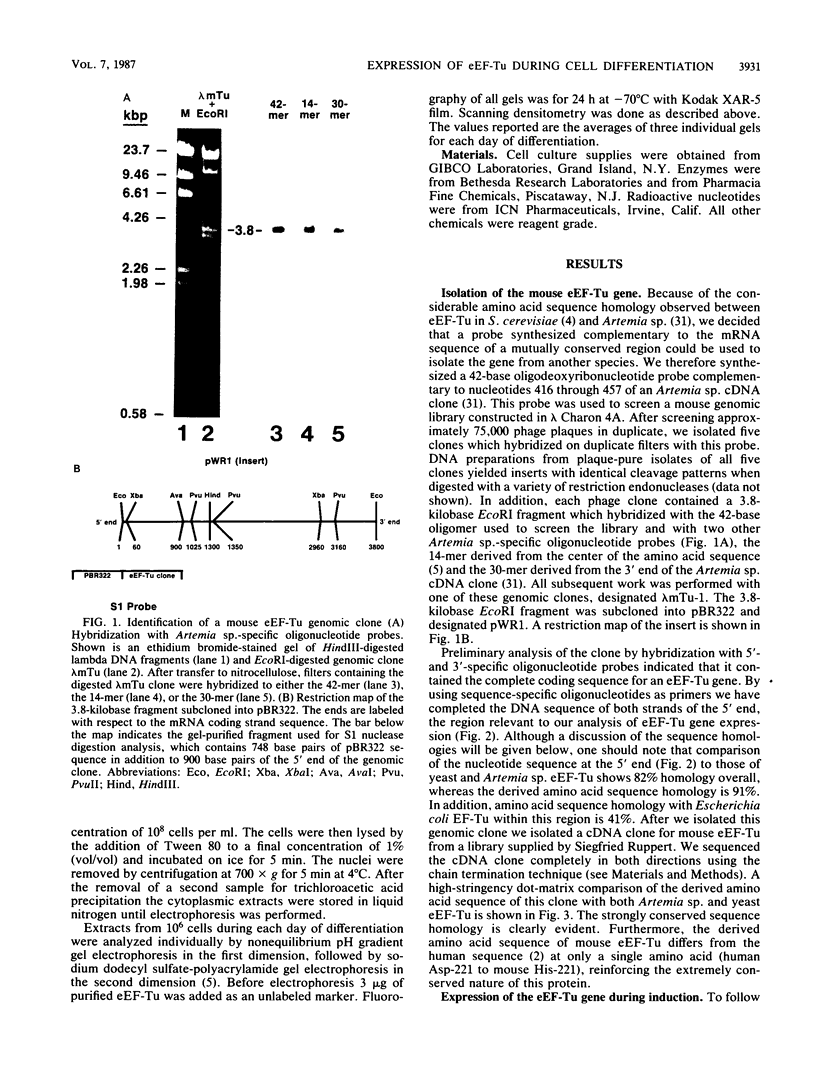
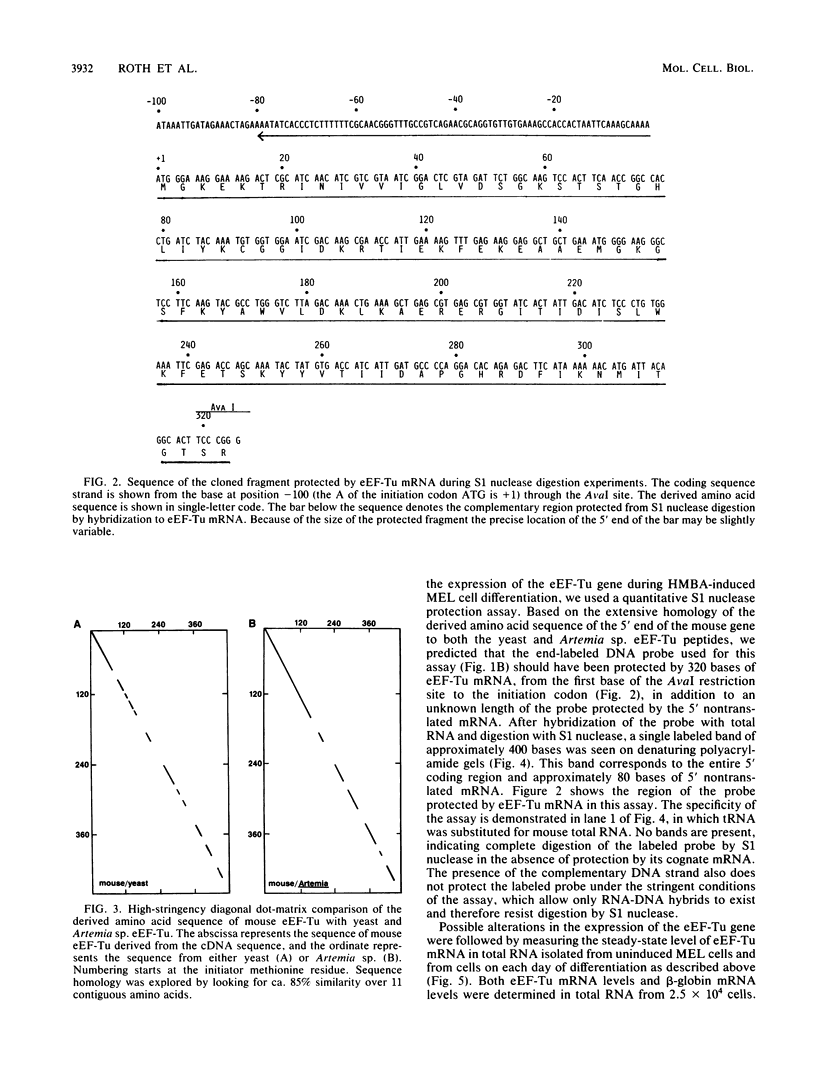
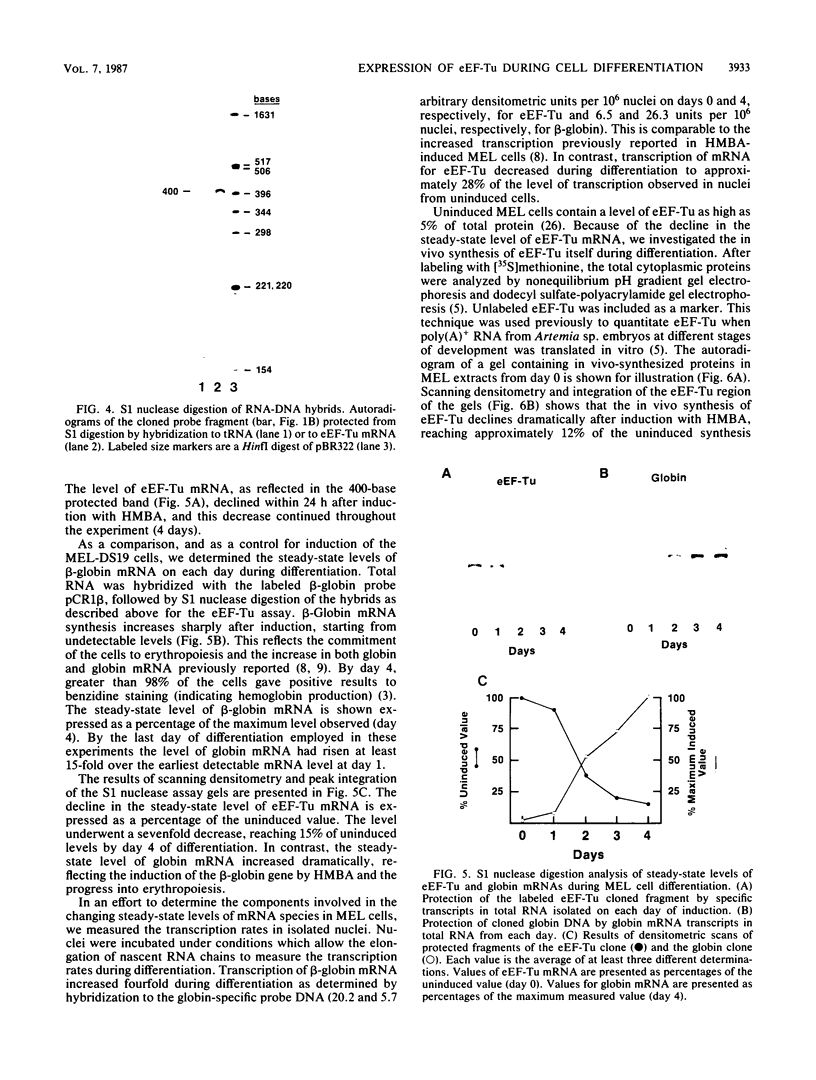
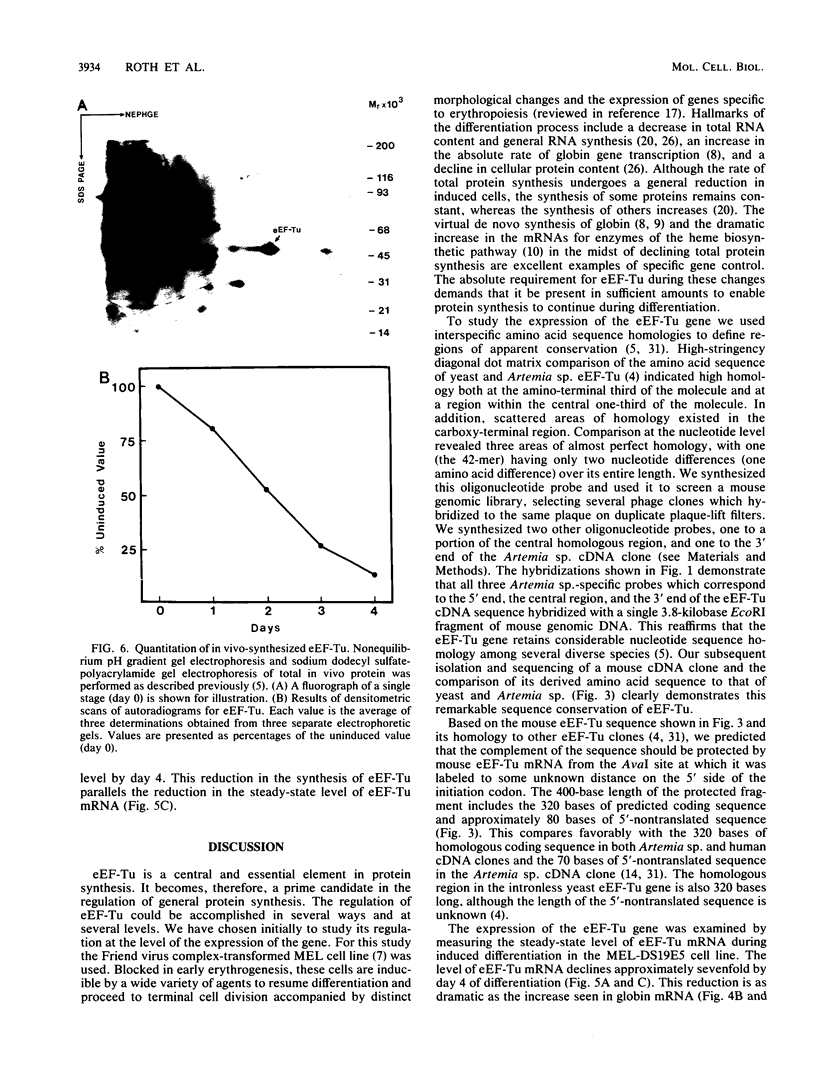
The eucaryotic elongation factor Tu (eEF-Tu) is a single polypeptide with an approximate Mr of 53,000. During protein synthesis eEF-Tu promotes the binding of aminoacyl-tRNA to the ribosome. To study the expression of the gene(s) for this factor, a genomic clone was isolated that contains a mouse eEF-Tu gene. We screened a phage genomic library with a synthetic oligonucleotide probe complementary to a region of the Saccharomyces cerevisiae and Artemia sp. eEF-Tu genes which codes for an area that is highly conserved between both yeast and Artemia sp. eEF-Tu. From approximately 75,000 phage plaques we obtained five isolates with apparently identical inserts. All five clones contained a 3.8-kilobase EcoRI fragment that hybridized to additional oligonucleotide probes corresponding to different conserved regions of eEF-Tu. We sequenced the 5' end of one genomic clone and determined the length of the cloned fragment that was protected by eEF-Tu mRNA in S1 nuclease protection assays. A quantitative S1 nuclease protection assay was used to compare the relative steady-state levels of eEF-Tu mRNA in total mRNA in total RNA isolated from hexamethylene-bisacetamide-induced murine erythroleukemia cells. The results show a dramatic reduction in the steady-state level of eEF-Tu mRNA as differentiation proceeds. A similar reduction in transcription of eEF-Tu mRNA was observed in isolated nuclei. Finally, we examined the in vivo synthesis of eEF-Tu during differentiation and found that it declined in a manner parallel to the decline in the steady-state level of eEF-Tu mRNA. In addition, we have isolated and sequenced a cDNA clone for mouse eEF-Tu. The derived amino acid sequence is compared with sequences from other eucaryotes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brands J. H., Maassen J. A., van Hemert F. J., Amons R., Möller W. The primary structure of the alpha subunit of human elongation factor 1. Structural aspects of guanine-nucleotide-binding sites. Eur J Biochem. 1986 Feb 17;155(1):167–171. doi: 10.1111/j.1432-1033.1986.tb09472.x. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Wellman S. E., Sittman D. B. Changes in the levels of three different classes of histone mRNA during murine erythroleukemia cell differentiation. Mol Cell Biol. 1985 Nov;5(11):2879–2886. doi: 10.1128/mcb.5.11.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrelle P., Thiele D., Price V. L., Memet S., Micouin J. Y., Marck C., Buhler J. M., Sentenac A., Fromageot P. Cloning, nucleotide sequence, and expression of one of two genes coding for yeast elongation factor 1 alpha. J Biol Chem. 1985 Mar 10;260(5):3090–3096. [PubMed] [Google Scholar]
- Daum H. A., 3rd, Bragg P. W., Sittman D. B., Dholakia J. N., Woodley C. L., Wahba A. J. The expression of a gene for eukaryotic elongation factor Tu in Artemia during development. Translation of poly(A)+ RNA and the use of a synthetic oligonucleotide to detect the presence of eukaryotic elongation factor Tu-specific mRNA. J Biol Chem. 1985 Dec 25;260(30):16347–16353. [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S., Skoultchi A. I. Absolute rates of globin gene transcription and mRNA formation during differentiation of cultured mouse erythroleukemia cells. J Biol Chem. 1985 Oct 5;260(22):12167–12173. [PubMed] [Google Scholar]
- Garrett C., Kredich N. M. Induction of hemoglobin synthesis by xylosyladenine in murine erythroleukemia cells. Metabolism of xylosyladenine and effects on transmethylation. J Biol Chem. 1981 Dec 25;256(24):12705–12709. [PubMed] [Google Scholar]
- Grandchamp B., Beaumont C., de Verneuil H., Nordmann Y. Accumulation of porphobilinogen deaminase, uroporphyrinogen decarboxylase, and alpha- and beta-globin mRNAs during differentiation of mouse erythroleukemic cells. Effects of succinylacetone. J Biol Chem. 1985 Aug 15;260(17):9630–9635. [PubMed] [Google Scholar]
- Ilan J., Ilan J. Translation of maternal messenger ribonucleoprotein particles from sea urchin in a cell-free system from unfertilized eggs and product analysis. Dev Biol. 1978 Oct;66(2):375–385. doi: 10.1016/0012-1606(78)90246-4. [DOI] [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Lenstra J. A., Van Vliet A., Arnberg A. C., Van Hemert F. J., Möller W. Genes coding for the elongation factor EF-1 alpha in Artemia. Eur J Biochem. 1986 Mar 17;155(3):475–483. doi: 10.1111/j.1432-1033.1986.tb09514.x. [DOI] [PubMed] [Google Scholar]
- Maassen J. A., Schop E. N., Brands J. H., van Hemert F. J., Lenstra J. A., Möller W. Molecular cloning and analysis of cDNA sequences for two ribosomal proteins from Artemia. The coordinate expression of genes for ribosomal proteins and elongation factor 1 during embryogenesis of Artemia. Eur J Biochem. 1985 Jun 18;149(3):609–616. doi: 10.1111/j.1432-1033.1985.tb08968.x. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Nagata S., Nagashima K., Tsunetsugu-Yokota Y., Fujimura K., Miyazaki M., Kaziro Y. Polypeptide chain elongation factor 1 alpha (EF-1 alpha) from yeast: nucleotide sequence of one of the two genes for EF-1 alpha from Saccharomyces cerevisiae. EMBO J. 1984 Aug;3(8):1825–1830. doi: 10.1002/j.1460-2075.1984.tb02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Tanaka M., Terada M., Miller O. J., Bank A., Marks P., Rifkind R. A. Erythroid cell differentiation: murine erythroleukemia cell variant with unique pattern of induction by polar compounds. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1232–1236. doi: 10.1073/pnas.73.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D., Housman D. Regulation of protein synthesis and accumulation during murine erythroleukemia cell differentiation. J Biol Chem. 1985 Jan 10;260(1):604–609. [PubMed] [Google Scholar]
- Peterson J. L., McConkey E. H. Proteins of Friend leukemia cells. Comparison of hemoglobin-synthesizing and noninduced populations. J Biol Chem. 1976 Jan 25;251(2):555–558. [PubMed] [Google Scholar]
- Rougeon F., Mach B. Cloning and amplification of alpha and beta mouse globin gene sequences synthesised in vitro. Gene. 1977 May;1(3-4):229–239. doi: 10.1016/0378-1119(77)90047-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmaier F., Philippsen P. Identification of two genes coding for the translation elongation factor EF-1 alpha of S. cerevisiae. EMBO J. 1984 Dec 20;3(13):3311–3315. doi: 10.1002/j.1460-2075.1984.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherton C. C., Kabat D. Changes in RNA and protein metabolism preceding onset of hemoglobin synthesis in cultured Friend leukemia cells. Dev Biol. 1976 Jan;48(1):118–131. doi: 10.1016/0012-1606(76)90051-8. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobin L. I. The role of eucaryotic factor Tu in protein synthesis. The measurement of the elongation factor Tu content of rabbit reticulocytes and other mammalian cells by a sensitive radioimmunoassay. Eur J Biochem. 1980 Sep;110(2):555–563. doi: 10.1111/j.1432-1033.1980.tb04898.x. [DOI] [PubMed] [Google Scholar]
- Sreedharan S. P., Beck C. M., Spremulli L. L. Euglena gracilis chloroplast elongation factor Tu. Purification and initial characterization. J Biol Chem. 1985 Mar 10;260(5):3126–3131. [PubMed] [Google Scholar]
- Thiele D., Cottrelle P., Iborra F., Buhler J. M., Sentenac A., Fromageot P. Elongation factor 1 alpha from Saccharomyces cerevisiae. Rapid large-scale purification and molecular characterization. J Biol Chem. 1985 Mar 10;260(5):3084–3089. [PubMed] [Google Scholar]
- Van Noort J. M., Kraal B., Bosch L., La Cour T. F., Nyborg J., Clark B. F. Cross-linking of tRNA at two different sites of the elongation factor Tu. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3969–3972. doi: 10.1073/pnas.81.13.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Goldenberg S., Scherrer K. Comparisons of proteins associated with duck-globin mRNA and its polyadenylated segment in polyribosomal and repressed free messenger ribonucleoprotein complexes. Eur J Biochem. 1981 Feb;114(2):179–193. doi: 10.1111/j.1432-1033.1981.tb05135.x. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Woodley C. L. Molecular aspects of development in the brine shrimp Artemia. Prog Nucleic Acid Res Mol Biol. 1984;31:221–265. doi: 10.1016/s0079-6603(08)60379-7. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley C. L., Roychowdhury M., MacRae T. H., Olsen K. W., Wahba A. J. Protein synthesis in brine shrimp embryos. Regulation of the formation of the ternary complex (Met-tRNAf X eIF-2 X GTP) by two purified protein factors and phosphorylation of Artemia eIF-2. Eur J Biochem. 1981 Jul;117(3):543–551. [PubMed] [Google Scholar]
- van Hemert F. J., Amons R., Pluijms W. J., van Ormondt H., Möller W. The primary structure of elongation factor EF-1 alpha from the brine shrimp Artemia. EMBO J. 1984 May;3(5):1109–1113. doi: 10.1002/j.1460-2075.1984.tb01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert F. J., Lenstra J. A., Möller W. Genes for elongation factor EF-1 alpha in the brine shrimp Artemia. FEBS Lett. 1983 Jul 4;157(2):295–299. doi: 10.1016/0014-5793(83)80564-x. [DOI] [PubMed] [Google Scholar]