Abstract
Right ventricular failure (RVF) is the most frequent cause of death in patients with pulmonary arterial hypertension (PAH); however, specific therapies targeted to treat RVF have not been developed. Chronic treatment with carvedilol has been shown to reduce established maladaptive right ventricle (RV) hypertrophy and to improve RV function in experimental PAH. However, the mechanisms by which carvedilol improves RVF are unknown. We have previously demonstrated by microarray analysis that RVF is characterized by a distinct gene expression profile when compared with functional, compensatory hypertrophy. We next sought to identify the effects of carvedilol on gene expression on a genome-wide basis. PAH and RVF were induced in male Sprague-Dawley rats by the combination of VEGF-receptor blockade and chronic hypoxia. After RVF was established, rats were treated with carvedilol or vehicle for 4 wk. RNA was isolated from RV tissue and hybridized for microarray analysis. An initial prediction analysis of carvedilol-treated RVs showed that the gene expression profile resembled the RVF prediction set. However, a more extensive analysis revealed a small group of genes differentially expressed after carvedilol treatment. Further analysis categorized these genes in pathways involved in cardiac hypertrophy, mitochondrial dysfunction, and protein ubiquitination. Genes encoding proteins in the cardiac hypertrophy and protein ubiquitination pathways were downregulated in the RV by carvedilol, while genes encoding proteins in the mitochondrial dysfunction pathway were upregulated by carvedilol. These gene expression changes may explain some of the mechanisms that underlie the functional improvement of the RV after carvedilol treatment.
Keywords: microarray, right ventricle, right ventricular failure
right ventricular failure (RVF) is a common cause of death of patients with severe pulmonary arterial hypertension (PAH) (53), and RV dysfunction has also been associated with increased mortality in patients with left ventricular failure (11, 29). However, despite its prognostic importance, therapies targeted to treat RVF are still lacking. We have reported that treatment with the nonselective adrenergic receptor antagonist carvedilol reverses established RVF in two different rat models of PAH (SU5416/hypoxia- and monocrotaline-induced PAH), and that this improvement in RV function was associated with reduced hypertrophy and improved capillary density of the myocardium (3). These changes were partially explained by an effect of carvedilol on the expression of genes which are likely potentially relevant for the adaptive response of the right ventricle (RV) to chronic pressure overload (3). However, the mechanisms by which carvedilol reverses maladaptive RV hypertrophy remain largely incomplete. Carvedilol has been shown to have pleiotrophic cardioprotective effects beyond heart-rate control. Indeed, a spectrum of anti-inflammatory, antioxidant, and antiapoptotic actions of carvedilol has been reported in vitro and in vivo (27, 51).
Based on microarray analysis, we have previously reported that RVF, in contrast to functional, compensated RV hypertrophy, is characterized by a distinct gene expression profile (6). The set of differentially expressed genes was designated the “RVF prediction set,” and we speculated that these genes might play a critical role in the transition between adaptive hypertrophy and failure (6). Based on a similar approach, we provide an in-depth assessment to show that carvedilol treatment affects the expression of a number of genes contained in the RVF prediction set (6). An initial analysis of the carvedilol-treated RVs showed that the gene expression profile resembled mostly that of the RVF tissue. However, an analysis beyond the boundaries of the prediction set revealed a small group of genes that were differentially expressed after carvedilol treatment.
METHODS
Justification of animal model.
The SuHx model of severe angio-obliterative PAH and RVF reproduces multiple salient features of human PAH and RVF. As previously described, the RV in the SuHx rat model responds to pulmonary hypertension with a robust degree of hypertrophy followed by dysfunction and failure (33). Furthermore, the RV of these rats is characterized by fibrosis, capillary rarefaction, and cardiomyocyte apoptosis (2), which results in decreased cardiac output and exercise capacity, as well as a marked RV dilation (3). Although the monocrotaline model of PAH also develops significant RV dysfunction, we do not use this model because it presents with multiple features that do not resemble the human disease (13); pertinently, treatment with monocrotaline generates liver damage and myocarditis (reviewed in Ref. 13).
Animal models.
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 200 g were injected subcutaneously with SU5416 suspended in 0.5% (wt/vol) carboxymethylcellulose sodium, 0.9% (wt/vol) sodium chloride, 0.4% (wt/vol) polysorbate 80, and 0.9% (vol/vol) benzyl alcohol in deionized water. Rats were given a single injection of SU5416 (20 mg/kg) at the beginning of the 6 wk experiment. The animals were then exposed to chronic hypoxia (simulated altitude of 5,000 m in a nitrogen dilution chamber) for 4 wk; thereafter the animals were kept at the altitude of Richmond, VA (45.7 m), for another 2 wk. Carvedilol (15 mg/kg; Sigma-Aldrich, St. Louis, MO) was dissolved in 20% dimethyl sulfoxide and water and administered once daily per oral gavage for 4 wk, beginning after return to room air breathing. All research was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Microarray analysis.
Total RNA was isolated from ∼30 mg of snap-frozen rat heart tissue using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). Hearts were homogenized with Buffer RLT and β-mercaptoethanol in an MP FastPrep-24 Lysing Matrix D tube (MP Biomedicals, Solon, OH), and then RNA was isolated and purified following the manufacturer's protocol. RNA concentration was determined with a NanoDrop ND-1000 (Thermo Fisher Scientific, Wilmington, DE). All samples had an A260/A280 ratio between 1.9 and 2.1.
The amplification and hybridization process is as follows: 500 ng of total RNA was amplified and labeled with Cyanine-5, and 500 ng of universal rat reference RNA (Stratagene, Santa Clara, CA) was amplified and labeled with Cyanine-3 using the Agilent QuickAmp Labeling kit (Agilent Technologies, Santa Clara, CA) to produce labeled cRNA following the manufacturer's protocol. After amplification and labeling, the dye incorporation was determined with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). All ratios were >8.0 pmol dye/μg cRNA per the manufacturer's recommendation. We combined 825 ng of sample and 825 ng of reference RNA and incubated that with an Agilent whole rat genome 4 × 44k microarray slide (Agilent Technologies, Wilmington, DE) for 17 h at 65°C. Following hybridization, slides were washed following the manufacturer's protocol and scanned with an Axon GenePix 4200A scanner (Axon Instruments, Union City, CA) at a resolution of 5 μM. The raw data were generated using GenePix Pro 5.0 software (Axon Instruments) and submitted to the Ramhorn Array Database, a VCU-specific implementation of the Longhorn Array Database (19).
Microarray data were retrieved from Ramhorn after filtering the data using a set of spot-quality metrics designed to ensure the reliability of the data. Features, individual spots on the microarray, were included in the dataset if they have a signal ≥1.5-fold above background in both the red and green channels. Those features with <50% good data, defined as passing aforementioned filtering conditions across the set of arrays, were eliminated. Technical replicates were averaged together by a best-effort averaging procedure.
Statistical analysis of biological replicates was performed using the Significance Analysis of Microarrays (SAM) algorithm (46) with a two-class paired unpaired design (SuHx + Carv RV vs. SuHx RV) with median centering to identify differentially expressed genes. Briefly, the SAM algorithm for a two-class comparison is as follows. First, the relative difference in expression between two sets of samples is calculated for each gene; this is the observed relative difference. To calculate the expected relative difference, data labels are permuted and the relative difference is calculated for each gene. The expected relative difference for a gene will be the average relative difference across all permutations. The observed relative difference is plotted against the expected relative difference. Genes that fall outside of a threshold value, Δ, will be called significant. Δ-Values were chosen to give an acceptable false discovery rate (FDR) of < 5%. For each gene, we report the FDR-equivalent P value, the q value as a percentage. The q value is defined as the minimum FDR of a given individual test at which a gene may be called significant.
The gene list was clustered by hierarchical clustering methods in Cluster 3.0 (9) and visualized in Java Tree View (39). Based on literature search results and pathway analysis results obtained using Ingenuity Pathway Analysis [(IPA); Ingenuity Systems, Redwood City, CA] and the PANTHER classification system (30, 44), we selected genes for confirmation by quantitative RT-PCR.
For prediction analyses, raw expression data files were uploaded into R (36) and normalized with the marray package (50) by the Lowess normalization algorithm and then exported to BRB Array Tools. Lowess intensity-dependent normalization was used to adjust for differences in labeling intensities of the Cy3 and Cy5 dyes. The adjusting factor varied over intensity levels (40). We identified genes that were differentially expressed between the three classes by an F test, using an α-level of 0.0001 for univariate testing, and performing 1,000 permutations for multivariate analysis.
The class prediction model was developed using diagonal linear discriminant analysis (37), nearest neighbor classification (7), and nearest centroid classification. The models incorporated genes that were previously deemed significant. We estimated the prediction error of each model using leave-one-out cross-validation (LOOCV) as described by Simon et al. (42). For each LOOCV training set, the entire model building process was repeated, including the gene selection process. We also evaluated whether the cross-validated error rate estimate for a model was significantly less than one would expect from random prediction. The class labels were randomly permuted, and the entire LOOCV process was repeated. The significance level is the proportion of the random permutations that gave a cross-validated error rate no greater than the cross-validated error rate obtained with the real data. We used 2,000 random permutations.
We have previously described a set of 450 genes that were most discriminatory between normal RVs, adaptive nondysfunctional hypertrophic RVs (induced by chronic hypoxia PH), and failing RVs (induced by SU5416/hypoxia), and we designated this group of genes the RVF prediction set (6). By comparing the expression of carvedilol-treated failing RVs to the RVF prediction dataset, we determined to which expression pattern carvedilol-treated RV tissues samples most resembled and identified the effects that carvedilol treatment had among this relatively small subset of genes. The RVF prediction set, derived from normal, chronic hypoxia (nondysfunctional RV hypertrophy) and RVF (SuHx RV) microarrays, acted as a training data set against which the SuHx rats treated with carvedilol (hereafter SuHx + Carv) data set (predictor data set) was compared.
We employed a class comparison algorithm that uses a t-test to compare the expression of each gene between the SuHx and SuHx + Carv microarrays data sets (41). Those genes with a P value below a given threshold value were accepted as significantly changed. In addition, multivariate permutation testing was performed to control for the FDR (21, 40). For each permutation in the multivariate permutation testing algorithm, the data labels were changed and the P value was recalculated for each gene. The P value threshold was set at 0.001 to allow no more than 10 false-positive genes and a proportion of false-positive genes <5%.
All microarray data discussed have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO)(8) database and are accessible through GEO Series accession number GSE42579 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE42579).
Gene expression studies.
Quantitative real-time PCR (qRT-PCR) was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) and Power SYBR Green PCR Master Mix (Applied Biosystems) along with rat-specific primers (Invitrogen, Carlsbad, CA). A dissociation profile was generated after each run to verify specificity of amplification. α-Actinin was used as a housekeeping gene.
Isolation of mitochondria and mitochondrial oxidative phosphorylation.
Isolation of a single population of cardiac mitochondria was conducted as described previously (12). Oxygen consumption by intact mitochondria was measured using a Clark-type oxygen electrode (Strathkelvin Instruments, North Lanarkshire, UK) as previously reported (12). Briefly, substrates for complex I (20 mM glutamate), complex II (20 mM succinate with 7.5 μM rotenone), and complex IV (1 mM N,N,N′,N′-tetramethyl-p-phenylenediamine/20 mM l-ascorbate with 7.5 μM rotenone) were used, and state 3 (0.2 mM ADP-stimulated), state 4 (ADP-limited) respiration, respiratory control ratio, maximal rate of state 3 respiration (2 mM ADP), and ADP/O ratio were determined.
Protein isolation.
Whole cell lysates were isolated and used for enzyme-linked immune sorbent assays (ELISAs). We homogenized 50 mg of tissue in radioimmunoprecipitation assay (Sigma-Aldrich) buffer containing 1 mM Na3VO4 (New England Biolabs, Ipswich, MA), 10 μM PMSF (Sigma-Aldrich), 10 μg/ml aprotinin (Sigma-Aldrich), 50 μM PR-619 (LifeSensors, Malvern, PA), 1% phosphatase inhibitor cocktail (Sigma-Aldrich), and 1% protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was calculated with the DC protein assay kit (Bio-Rad, Hercules, CA).
Western blot analysis.
Western blot analysis was performed using whole cell lysate as described previously (12) using the NuPAGE system (Invitrogen, Carlsbad, CA). Immunodetection was performed with commercially available antibodies (PGC-1α #2178, α-actinin #3134; Cell Signaling Technology, Danvers, MA).
20S proteasome assay.
20S proteasome activity was measured from 50 μg whole cell lysate using the 20S proteasome assay kit (Chemicon International, Temecula, CA) following the manufacturer's protocol. The assay is based on detection of the fluorophore 7-amino-4-methylcoumarin (AMC) after cleavage from the labeled substrate LLVY-AMC. Free AMC fluorescence was measured using a 380/460-nm filter set on a SpectraMax GeminiXS fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) and quantified with SOFTMax PRO software (Molecular Devices).
Ubiquitin ELISA.
Concentration of total ubiquitinated (poly- and monoubiquitinated) was measured with the UbiQuant ELISA kit (LifeSensors) from whole cell lysates following the manufacturer's protocol. Chemiluminescence was measured on a Synergy HT multimode microplate reader (BioTek Instruments, Winooski, VT) and quantified in Gen5 data analysis software (BioTek Instruments).
Statistical analysis.
Results are reported as means ± SE or as fold-changes ± SE unless otherwise specified. Differences between groups were assessed with one-way ANOVA and Newman-Keuls post hoc tests using GraphPad Prism (La Jolla, CA). A P value < 0.05 was accepted as significant.
RESULTS
Prediction analysis.
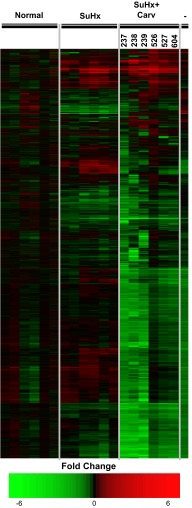
Using the one-nearest neighbor, three-nearest neighbors, nearest centroid, and linear discriminant analysis algorithms (7), we assigned each of the arrays in the SuHx + Carv data set a label of either mostly resembling “normal,” “hypertrophy,” and “failure” based on the similarity between the 450 genes from the training data set and the 450 genes in the predictor (SuHx + Carv) data set (Table 1). As can be seen in Fig. 1 and Table 1, each of the RV SuHx + Carv arrays was classified as most closely resembling failure by all of the algorithms. In other words, it was apparent that the expression of the genes in the SuHx + Carv dataset had a great deal of similarity to the RVF prediction set but also that there were a set of genes that were differentially expressed, significantly. Although the SuHx + Carv data were classified as most closely resembling RVF, the result encouraged us to search for pathways that could explain the improvement in RV function that has been reported after carvedilol treatment (3). We hypothesized that a relatively small number of differently expressed genes between the RVF and carvedilol-treated RVs were indeed responsible for the improvement in RV function.
Table 1.
Summary of prediction analysis results
| Array ID | Genes in Classifier, n | 1-Nearest Neighbor | 3-Nearest Neighbors | Nearest Centroid | Linear Discriminant Analysis |
|---|---|---|---|---|---|
| 237 | 435 | failure | failure | failure | failure |
| 238 | 435 | failure | failure | failure | failure |
| 239 | 435 | failure | failure | failure | failure |
| 526 | 435 | failure | failure | failure | failure |
| 527 | 435 | failure | failure | failure | failure |
| 604 | 435 | failure | failure | failure | failure |
Fig. 1.
Dendrogram for clustering experiments using centered correlation and average linkage of the 450 probes that showed 100% agreement across leave-one-out cross-validation (LOOCV). The first 3 major columns on the left, labeled “normal,” “SuHx,” and “SuHxCarv,” denote expression in the normal, SU5416/hypoxia, and SU5416/hypoxia + carvedilol right ventricles (RVs). Red coloration indicates increased expression compared with the average normal RV expression level, while green indicates reduced expression. The small columns represent different animals investigated under the same condition. Each row represents a gene from the prediction set. The last major column to the right of the gray line labeled “-” denotes the difference in expression between the SU5416/hypoxia + carvedilol and SU5416/hypoxia RVs. Red coloration indicates increased expression in the SU5416/hypoxia RV with carvedilol treatment compared with an untreated SU5416/hypoxia RV, and green indicates reduced expression in the SU5416/hypoxia + carvedilol RV compared with the SU5416/hypoxia RV.
Multivariate analysis.
To test this hypothesis, we used a class comparison algorithm, which identified 489 genes as significant given the chosen analysis conditions Of the 43,379 probes compared between SuHx and SuHx + Carv overall gene expression data sets. The probability of selecting at least 489 probes by chance at the threshold P value of 0.001 (if there was no difference between the classes) was 0.2%.
Cardiac hypertrophy, ceramide signaling, and apoptosis.
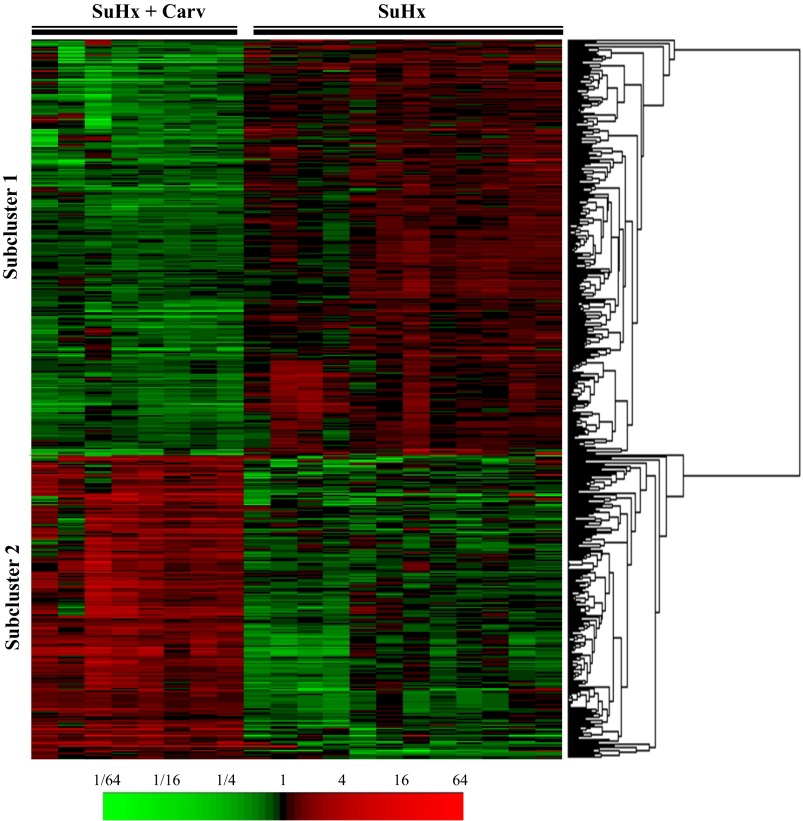
We found two clearly defined subclusters, which are illustrated in Fig. 2. Subcluster 1 was composed of 282 probes that corresponded to 275 different genes. The top five canonical pathways for genes in this cluster are listed in Table 2. Two of the top canonical pathways, regulation of eukaryotic initiation factor (eIF)4, p70S6k signaling and eIF2 signaling, were also categorized as cellular processes. eIF-2 and 4 are both required for the initiation of translation, and it is known that cardiac hypertrophy requires an increase in protein synthesis by individual cardiomyocytes (43). Thus, cardiac hypertrophy development likely requires greater activity of the eIFs, and indeed in a model of cardiac hypertrophy caused by chronic alcohol intake, eIF2 levels were shown to be increased (24). In our microarray dataset, several of the eIF genes were decreased in expression after carvedilol treatment.
Fig. 2.
Cluster for class comparison analysis between SU5416/hypoxia RV (failure) and SU5416/hypoxia + carvedilol RV microarray data sets. 489 probes were called significant at an α < 0.001 level and multivariate permutation testing gives a false discovery rate < 5%. Red represents greater relative expression than reference RNA, and green represents less relative expression than reference RNA.
Table 2.
Top five canonical pathways of subcluster 1
| Pathway Name | P Value |
|---|---|
| Regulation of eIF4 and p70S6k signaling | 1.98 × 10−4 |
| Glucocorticoid receptor signaling | 2.61 × 10−4 |
| Ceramide signaling | 4.04 × 10−4 |
| EGF signaling | 4.94 × 10−4 |
| eIF2 signaling | 5.68 × 10−4 |
eIF, eukaryotic initiation factor.
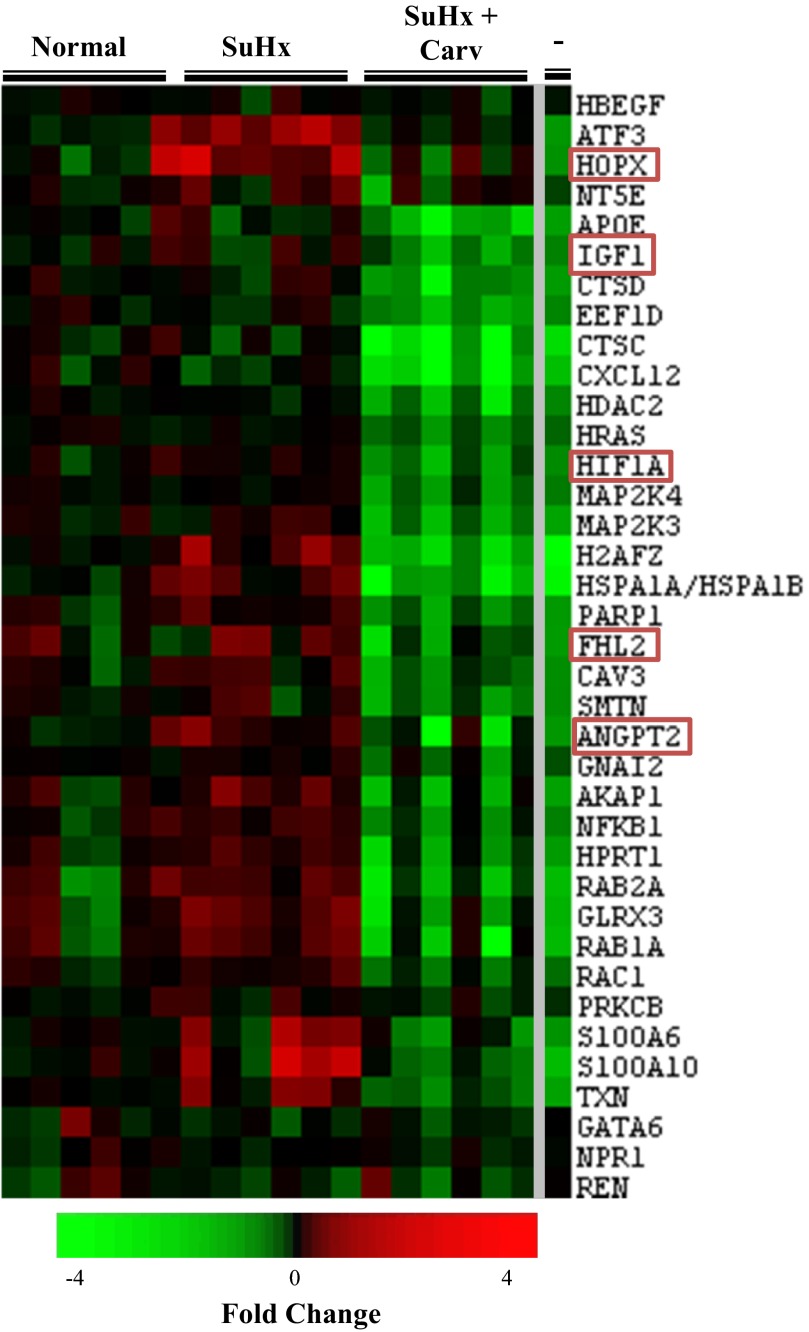
A reduction in genes encoding proteins involved in the development of cardiac hypertrophy (Fig. 2, subcluster 1, and Fig. 3) is reflected in the experimental data, which show that carvedilol reduces hypertrophy in the SuHx RV (3). Of particular importance to pulmonary hypertension are the genes hypoxia-inducible factor 1 alpha (HIF-1α), and angiopoietin-2 (Ang2). HIF-1α expression was decreased 2.1-fold in the SuHx + Carv RV compared with the SuHx RV. Increased expression of HIF-1a was found in the hearts of patients with PAH (26). Four and a half LIM domains 2 (FHL2) was decreased 2.3-fold in the SuHx RV. A decrease in FHL2 expression occurs in human left ventricular heart failure and is associated with a reduction in activity of phosphofructokinase 2 and adenylate cyclase (4). Ang2 is also known to play a role in PAH. Ang2 has proangiogenic properties when expressed in high concentrations (31) or in combination with VEGF (25, 52). Patients with idiopathic PAH have increased plasma levels of Ang2, and these levels correlate with a low cardiac index and high pulmonary vascular resistance (22). Ang2 expression decreased 2.3-fold in the SuHx + Carv RV compared with the SuHx RV. A reduction in the expression of these genes, as seen in the SuHx + Carv RV, could lead to reduced cardiac hypertrophy and improved myocardial function.
Fig. 3.
Microarray gene expression of genes involved in cardiac hypertrophy functions in subcluster 2. The first 3 major columns on the left labeled “normal,” “SuHx,” and “SuHxCarv” denote expression in the normal, SU5416/hypoxia, and SU5416/hypoxia + carvedilol RVs. Red coloration indicates increased expression compared with the average normal RV expression level, while green indicates reduced expression. The small columns represent different animals treated with the same condition. The last major column to the right of the gray line labeled “-” denotes the difference in expression between the SU5416/hypoxia + carvedilol and SU5416/hypoxia RVs. Red coloration indicates increased expression in the SU5416/hypoxia RV with carvedilol treatment compared with an untreated SU5416/hypoxia RV, and green indicates reduced expression in the SU5416/hypoxia + carvedilol RV compared with the SU5416/hypoxia RV.
We found that the ceramide signaling and glucocorticoid receptor signaling pathways were two of the top five canonical pathways in this subcluster (Table 2). Ceramide is considered injurious to the heart (34), while glucocorticoid receptors have been shown to play a role in postmyocardial infarction heart remodeling (23). Several genes of these pathways were decreased in expression in the SuHx + Carv RV relative to the SuHx RV. To a lesser extent, the sphingosine-1-phosphate signaling pathway was affected by carvedilol treatment as several member genes of this signaling pathway were decreased in expression after carvedilol treatment.
Metabolism, mitochondrial function, and oxidative stress response.
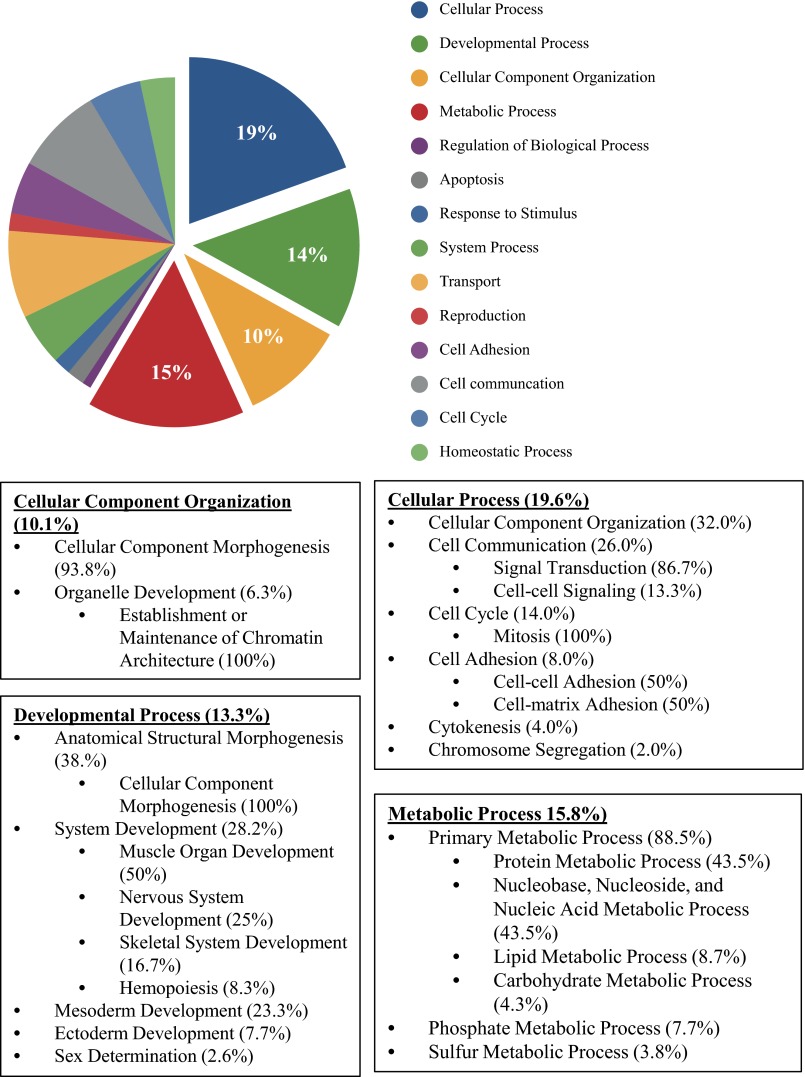
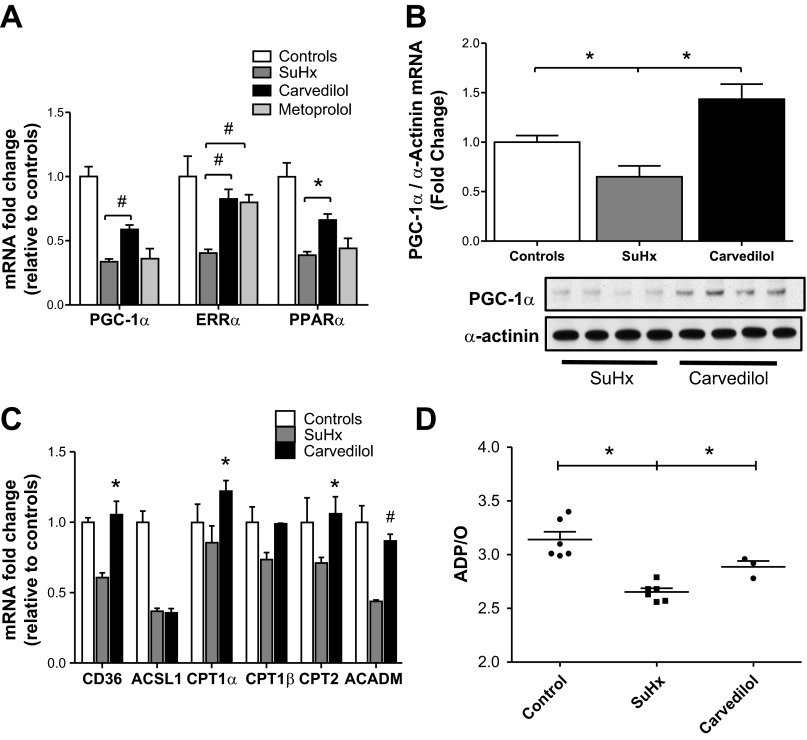
Subcluster 2 was made up of 207 probes that represented 192 different genes (Fig. 2). Figure 4 illustrates the different biological processes, of which cellular, metabolic, and developmental processes, as well as cellular component organization pathways, were the most abundant. The top five canonical pathways for the genes in this cluster are listed in Table 3. Several of these pathways, including the peroxisome proliferator-activator receptor (PPAR) signaling, PPARα/retinoid X receptor-α activation, and nuclear respiratory factor (Nrf)2-mediated oxidative stress response, represent metabolic processes of critical importance for adequate heart function (10). PPAR-α and -γ are both coactivated by the PPAR coactivator-1 alpha (PGC-1α). Both PGC-1α and PPAR-α are preferentially expressed in tissues with high oxidative capacity, such as the heart (10). Through coactivation of PPAR-α and estrogen-related receptor alpha (ERR-α), PGC-1α activates genes involved in the cellular uptake and mitochondrial oxidation of fatty acids. We have previously reported that RVF is characterized by a complex metabolic derangement where downregulation of PGC-1α seems to play a pivotal role (12). Because carvedilol treatment had a significant effect on the gene expression of the PPAR signaling pathways, we measured the gene and protein expression of PGC-1α, as well as different PGC-1α target genes, in RV tissue obtained from carvedilol-treated rats (Fig. 5). Indeed, carvedilol treatment significantly increased the suppressed transcript levels of PGC-1α, ERR-α, and PPAR-α and significantly increased the protein levels of PGC-1α measured by Western blot. Interestingly, treatment with metoprolol, a selective β1-blocker did not modify the expression of PGC-1α, which could suggest a specific effect of carvedilol not explained only by adrenergic blockade. Furthermore, carvedilol significantly affected the expression of numerous PGC-1α target genes involved in fatty acid metabolism (Fig. 5C).
Fig. 4.
Genes in subcluster 2 were classified by biological process by the PANTHER classification system (44, 45). The top 4 processes are cellular, metabolic, developmental, and cellular component organization.
Table 3.
Top five canonical pathways of subcluster 2
| Pathway Name | P Value |
|---|---|
| PPAR signaling | 2.59 × 10−5 |
| PPARα/RXRα activation | 5.75 × 10−4 |
| Agrin interactions at neuromuscular junction | 8.10 × 10−4 |
| NRF2-mediated oxidative stress response | 8.76 × 10−4 |
| Prostate cancer signaling | 1.56 × 10−3 |
PPAR, peroxisome proliferator-activator receptor; RXR, retinoid X receptor; NRF, nuclear respiratory factor.
Fig. 5.
A: quantitative real-time PCR analysis of PPAR coactivator (PGC)-1α, estrogen-related receptor (ERR)-α, and peroxisome proliferator-activator receptor (PPAR)-α in control, failing (SuHx), carvedilol-treated (carvedilol), and metoprolol-treated (metoprolol) RVs (*P < 0.01, #P < 0.001). B: Top: quantitative real-time PCR analysis of PGC-1α in control, failing (SuHx), and carvedilol-treated (carvedilol) RVs (*P < 0.01) Bottom: Western blot analysis of PGC-1α in failing (SuHx) and carvedilol-treated (carvedilol) RVs. C: quantitative real-time PCR analysis of the PGC-1α downstream genes CD36, ACSL1, CPT1α, CPT1β, CPT2, and ACADM in control, failing (SuHx), and carvedilol-treated (carvedilol) RVs (*P < 0.01, #P < 0.001). D: mitochondrial efficiency measured as ADP/O ratio in control, failing (SuHx), and carvedilol-treated (carvedilol) RVs (*P < 0.01).
Reactive oxygen species created during cardiac stress increase levels of nuclear factor kappa B (NF-κB), which subsequently increases levels of proinflammatory cytokines such as IL-1, IL-6, and TNF-α (38). Increased expression of NF-κB inhibitor alpha (NF-κBIA) suppresses the transcriptional activity of NF-κB. In our microarray data set, expression of NF-κBIA was increased 2.1-fold in the SuHx + Carv RV compared with the SuHx RV. This is perhaps one of several mechanisms whereby carvedilol affects inflammation.
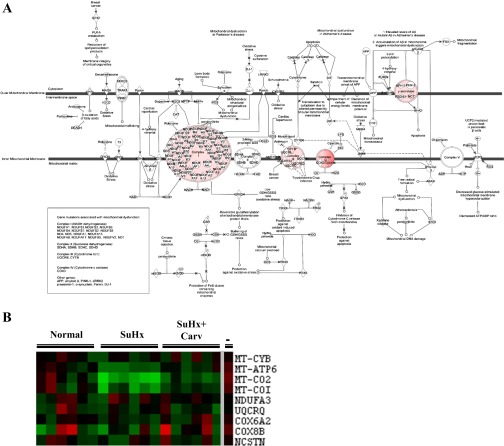
In addition to those pathways listed in Tables 2 and 3, carvedilol treatment significantly affected mitochondrial function (Fig. 6). Indeed, we found an increase in expression of genes encoding mitochondrial enzymes (Fig. 6) after carvedilol treatment. Cytochrome c oxidase (complex IV of the electron transport chain) is the final enzyme in the electron transport chain, which catalyzes the oxidation of cytochrome c and reduction of oxygen to water. We found four subunits of cytochrome c oxidase encoding genes increased in their expression when we examined the microarray data set, and their expression changes are listed in Table 4. Because it is likely that these expression changes could be beneficial to the heart and could reflect a trend toward normalization in RV mitochondrial function, we evaluated mitochondrial efficiency in isolated mitochondria from failing RV tissue obtained from rats treated with carvedilol. Figure 5D demonstrates that mitochondria obtained from SuHx RVs had a significantly reduced ADP/O ratio, which measures mitochondrial respiratory efficiency. A lower ADP/O ratio represents high oxygen consumption per unit of ADP provided. In addition, carvedilol treatment partially normalized the ADP/O ratio.
Fig. 6.
A: canonical pathway analysis identified the pathways from the Ingenuity Pathway Analysis library of canonical pathways that were most significant to the dataset. The P value for the mitochondrial dysfunction canonical pathway is 5.16 × 10−3. Genes are colored based on fold-change comparison between the SU5416/hypoxia + carvedilol (treated) RV and the SU5416/Hypoxia (failing) RV. Red indicates a relatively higher expression in the treated RV than the failing RV, and green indicates a relatively higher expression in the failing RV than the treated RV. B: microarray gene expression of genes encoding proteins involved in mitochondrial dysfunction in subcluster 2. The first 3 major columns on the left labeled “normal,” “SuHx,” and “SuHxCarv” denote expression in the normal, SU5416/hypoxia, and SU5416/hypoxia + carvedilol RVs. Red coloration indicates increased expression compared with the average normal RV expression level, while green indicates reduced expression. The small columns represent different animals treated with the same condition. The last major column to the right of the gray line labeled “-” denotes the difference in expression between the SU5416/hypoxia + carvedilol and SU5416/hypoxia RVs. Red coloration indicates increased expression in the SU5416/hypoxia RV with carvedilol treatment compared with an untreated SU5416/hypoxia RV, and green indicates reduced expression in the SU5416/hypoxia + carvedilol RV compared with the SU5416/hypoxia RV.
Table 4.
Genes differentially expressed in the mitochondrial dysfunction pathway
| Gene Name | Fold Change | q Value, % | Description |
|---|---|---|---|
| CytB | 1.638 | 0.000 | cytochrome b |
| Ndufa3 | 1.494 | 1.230 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 3 |
| Cox6a2 | 1.641 | 0.316 | cytochrome c oxidase subunit VIa polypeptide 2 |
| Atp6 | 2.174 | 0.000 | ATP synthase 6, mitochondrial |
| BQ782568 | 1.489 | 1.230 | BQ782568 UI-R-FF0-cpk-h-15-0-UI.s1 NCI_CGAP_FF0 Rattus norvegicus cDNA clone UI-R-FF0-cpk-h-15-0-UI 3¢, mRNA sequence |
| Nctsn | 1.675 | 0.422 | nicastrin |
| Cox2 | 2.562 | 0.000 | cytochrome c oxidase II, mitochondrial |
| Cox1 | 2.207 | 0.000 | cytochrome c oxidase I, mitochondrial |
| Cox8b | 2.173 | 0.180 | cytochrome c oxidase, subunit VIIIb |
As shown in Fig. 2, genes of the PPAR signaling and NRF2-mediated oxidative stress response pathways increase in expression, suggesting a return toward normal expression levels after carvedilol treatment. Furthermore, carvedilol affects a large number of genes in the mitochondrial function pathway (Fig. 6). In the aggregate, these results could suggest that a change in energy substrate utilization is associated with the reversal of RVF and that carvedilol might be able to reverse the metabolic remodeling in failing RVs.
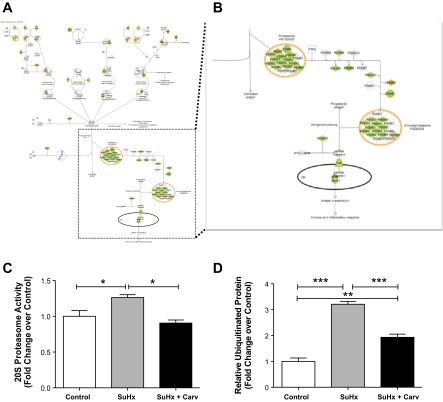
Protein ubiquitination.
We found a decrease in the expression in the SuHx + Carv RV of a large number of genes representing the protein ubiquitination pathway (Fig. 7). The protein ubiquitination system has been shown to be cardioprotective because of its ability to remove proapoptotic signaling molecules from the cell (1). A decreased expression of ubiquitination proteins leads to reduced proteasomal activities (28) and allows accumulation of proapoptotic proteins in the cell (1). A list of gene expression changes is provided in Table 5. We list genes representing several members of the DNAJ homolog family, several proteasome subunits, ubiquitin-conjugating enzymes, and ubiquitin-specific peptidases. Because this expression pattern suggested to us that the ubiquitin proteasome system is highly activated in RVF and that carvedilol treatment returns activity towards normal, we measured the levels of ubiquitinated proteins and the activity of the 20S proteasome. In support of our microarray data, we found a threefold increase in the levels of total ubiquitinated protein and evidence of heightened 20S proteasome activity in the SuHx RVs, while the SuHx + Carv RVs were characterized by normal or near normal activities.
Fig. 7.
A: canonical pathway analysis identified the pathways from the Ingenuity Pathway Analysis library of canonical pathways that were most significant to the dataset. The P value for the protein ubiquitination pathway is 6.59 × 10−11. Genes are colored based on fold-change comparison between the SU5416/hypoxia + carvedilol (treated) RV and the SU5416/Hypoxia (failing) RV. Red indicates a relatively higher expression in the treated RV than the failing RV, and green indicates a relatively higher expression in the failing RV than the treated RV. B: zoom-in of the same figure showing the 20S proteasome. C: 20S proteasome activity in control, failing (SuHx), and carvedilol-treated (SuHx + Carv) RV (*P < 0.05). D: total ubiquitinated protein in control, failing (SuHx), and carvedilol-treated (SuHx + Carv) RV (**P < 0.01, ***P < 0.001).
Table 5.
Genes differentially expressed in the protein ubiquitination pathway
| Gene ID | Fold Change | Entrez Gene Name |
|---|---|---|
| AMFR | 0.899 | autocrine motility factor receptor, E3 ubiquitin protein ligase |
| B2M | 0.546 | beta-2-microglobulin |
| CRYAB | 1.120 | crystallin, alpha B |
| DNAJA1 | 0.481 | DnaJ (Hsp40) homolog, subfamily A, member 1 |
| DNAJB1 | 0.534 | DnaJ (Hsp40) homolog, subfamily B, member 1 |
| DNAJB5 | 0.856 | DnaJ (Hsp40) homolog, subfamily B, member 5 |
| DNAJB6 | 1.083 | DnaJ (Hsp40) homolog, subfamily B, member 6 |
| DNAJC7 | 0.865 | DnaJ (Hsp40) homolog, subfamily C, member 7 |
| DNAJC9 | 0.550 | DnaJ (Hsp40) homolog, subfamily C, member 9 |
| DNAJC10 | 0.531 | DnaJ (Hsp40) homolog, subfamily C, member 10 |
| DNAJC11 | 1.119 | DnaJ (Hsp40) homolog, subfamily C, member 11 |
| DNAJC15 | 0.373 | DnaJ (Hsp40) homolog, subfamily C, member 15 |
| HLA-C | 0.541 | |
| HSCB | 0.537 | HscB iron-sulfur cluster co-chaperone homolog (E. coli) |
| HSP90AA1 | 1.075 | heat shock protein 90 kDa alpha (cytosolic), class A member 1 |
| HSP90AB1 | 0.506 | heat shock protein 90 kDa alpha (cytosolic), class B member 1 |
| HSPA4 | 0.856 | heat shock 70 kDa protein 4 |
| HSPA5 | 0.418 | heat shock 70 kDa protein 5 (glucose-regulated protein, 78 kDa) |
| HSPA8 | 0.436 | heat shock 70 kDa protein 8 |
| HSPA9 | 0.454 | heat shock 70 kDa protein 9 (mortalin) |
| HSPA14 | 0.431 | heat shock 70 kDa protein 14 |
| HSPA12B | 1.487 | heat shock 70kD protein 12B |
| HSPA1A/HSPA1B | 0.395 | heat shock 70 kDa protein 1A |
| HSPB2 | 0.871 | heat shock 27 kDa protein 2 |
| HSPB6 | 1.131 | heat shock protein, alpha-crystallin-related, B6 |
| HSPB7 | 1.460 | heat shock 27 kDa protein family, member 7 (cardiovascular) |
| HSPD1 | 0.453 | heat shock 60 kDa protein 1 (chaperonin) |
| HSPE1 | 0.402 | heat shock 10 kDa protein 1 (chaperonin 10) |
| NEDD4 | 0.503 | neural precursor cell expressed, developmentally down-regulated 4 |
| PSMA1 | 0.470 | proteasome (prosome, macropain) subunit, alpha type, 1 |
| PSMA2 | 0.565 | proteasome (prosome, macropain) subunit, alpha type, 2 |
| PSMA3 | 0.482 | proteasome (prosome, macropain) subunit, alpha type, 3 |
| PSMA4 | 0.452 | proteasome (prosome, macropain) subunit, alpha type, 4 |
| PSMA5 | 0.478 | proteasome (prosome, macropain) subunit, alpha type, 5 |
| PSMA6 (includes EG:246582) | 0.408 | proteasome (prosome, macropain) subunit, alpha type, 6 |
| PSMB2 | 0.480 | proteasome (prosome, macropain) subunit, beta type, 2 |
| PSMB3 | 0.507 | proteasome (prosome, macropain) subunit, beta type, 3 |
| PSMB4 | 0.494 | proteasome (prosome, macropain) subunit, beta type, 4 |
| PSMB5 | 0.516 | proteasome (prosome, macropain) subunit, beta type, 5 |
| PSMB6 | 0.420 | proteasome (prosome, macropain) subunit, beta type, 6 |
| PSMB9 | 0.528 | proteasome (prosome, macropain) subunit, beta type, 9 (large multifunctional peptidase 2) |
| PSMC1 | 0.571 | proteasome (prosome, macropain) 26S subunit, ATPase, 1 |
| PSMC2 | 0.524 | proteasome (prosome, macropain) 26S subunit, ATPase, 2 |
| PSMC4 | 0.492 | proteasome (prosome, macropain) 26S subunit, ATPase, 4 |
| PSMC6 | 0.398 | proteasome (prosome, macropain) 26S subunit, ATPase, 6 |
| PSMD1 | 0.468 | proteasome (prosome, macropain) 26S subunit, nonATPase, 1 |
| PSMD2 | 0.543 | proteasome (prosome, macropain) 26S subunit, nonATPase, 2 |
| PSMD7 | 0.566 | proteasome (prosome, macropain) 26S subunit, nonATPase, 7 |
| PSMD10 | 0.486 | proteasome (prosome, macropain) 26S subunit, nonATPase, 10 |
| PSMD14 | 0.398 | proteasome (prosome, macropain) 26S subunit, nonATPase, 14 |
| PSME2 | 0.520 | proteasome (prosome, macropain) activator subunit 2 (PA28 beta) |
| SMURF1 | 0.570 | SMAD specific E3 ubiquitin protein ligase 1 |
| SMURF2 | 1.137 | SMAD specific E3 ubiquitin protein ligase 2 |
| SUGT1 | 0.501 | SGT1, suppressor of G2 allele of SKP1 (S. cerevisiae) |
| TCEB1 | 0.478 | |
| TCEB2 | 0.421 | |
| THOP1 | 0.534 | thimet oligopeptidase 1 |
| UBA1 | 0.516 | ubiquitin-like modifier activating enzyme 1 |
| UBE2A | 0.453 | ubiquitin-conjugating enzyme E2A |
| UBE2D1 | 1.118 | ubiquitin-conjugating enzyme E2D 1 |
| UBE2D4 | 0.539 | |
| UBE2E2 | 0.499 | ubiquitin-conjugating enzyme E2E 2 |
| UBE2E3 | 0.382 | ubiquitin-conjugating enzyme E2E 3 |
| UBE2F | 0.468 | ubiquitin-conjugating enzyme E2F (putative) |
| UBE2G2 | 0.963 | ubiquitin-conjugating enzyme E2G 2 |
| UBE2I | 0.456 | ubiquitin-conjugating enzyme E2I |
| UBE2J2 | 0.835 | ubiquitin-conjugating enzyme E2, J2 |
| UBE2M | 0.492 | ubiquitin-conjugating enzyme E2M |
| UBE2N | 0.453 | ubiquitin-conjugating enzyme E2N |
| UBE2S | 0.496 | ubiquitin-conjugating enzyme E2S |
| UBE2V1 | 0.523 | ubiquitin-conjugating enzyme E2 variant 1 |
| UBE4A | 0.514 | ubiquitination factor E4A |
| UBE4B | 1.016 | ubiquitination factor E4B |
| UCHL3 | 0.464 | ubiquitin carboxyl-terminal esterase L3 (ubiquitin thiolesterase) |
| UCHL5 | 0.515 | ubiquitin carboxyl-terminal hydrolase L5 |
| USO1 | 0.524 | USO1 vesicle docking protein homolog (yeast) |
| USP7 | 1.198 | ubiquitin specific peptidase 7 (herpes virus-associated) |
| USP14 | 0.441 | ubiquitin specific peptidase 14 (tRNA-guanine transglycosylase) |
| USP15 | 0.863 | ubiquitin specific peptidase 15 |
| USP20 | 0.893 | ubiquitin specific peptidase 20 |
| USP32 | 1.284 | ubiquitin specific peptidase 32 |
| USP40 | 1.106 | ubiquitin specific peptidase 40 |
| USP42 | 1.309 | ubiquitin specific peptidase 42 |
| USP46 | 0.894 | ubiquitin specific peptidase 46 |
| USP47 | 0.937 | ubiquitin specific peptidase 47 |
| USP9X | 0.929 | ubiquitin specific peptidase 9, X-linked |
DISCUSSION
Right heart failure remains the main cause of death in patients with chronic severe PAH, and, although treatment of patients with vasodilator drugs may reduce the pulmonary vascular resistance, this effect is not necessarily accompanied by improvement of RV function (48, 49). We have proposed that the failing RV should be a treatment target in its own right (48), and we have begun to investigate the effects of drugs that target the heart and improve RV function in the setting of persistently elevated RV afterload (3, 47). In our SuHx model of chronic and progressive RVF (33), we could show that a 4 wk treatment with carvedilol was sufficient to reverse the established RVF and that this reversal was associated with an increased RV capillary density and improved hemodynamics (3). Here, we sought to examine whether carvedilol treatment affected the RVF gene expression “signature” (6). By applying the tool of a relatively small set of genes, the RVF prediction set, we found that carvedilol treatment of rats with RVF did not categorically alter the RVF gene expression signature (Fig. 1). Indeed, the overall structure of gene expression pattern of the carvedilol-treated RVs reflects the RVF gene expression pattern. However, additional data analysis reveals that carvedilol treatment altered the expression of subsets of genes that were not contained within the RVF prediction set (Fig. 2). This is of interest because this finding suggests that a limited number of genes and/or signaling pathways may be critical to RVF reversal. We concluded that a prediction set analysis is useful for distinguishing between failing and nonfailing RVs but that a more penetrating and expanded analysis is required to detect carvedilol treatment-associated gene expression alterations that may provide mechanistic clues that explain an improvement in RV function.
The major finding of our study is that carvedilol is associated with directional changes in the expression of a set of genes which are likely controlling the development of RV hypertrophy and RVF. Because carvedilol treatment brought about an improvement of RV performance, a decrease in RV hypertrophy, a reduction in myocardial fibrosis, and a normalization of the density of the myocardial capillaries (3), it appears to be justified to consider that the carvedilol treatment-associated gene expression changes reflect a reduction in cardiac stress and that carvedilol improves mechanisms of cardiac repair.
Our approach, using an RVF gene expression prediction set as a data filter, allowed us to uncover clusters of genes that encode proteins involved in angiogenesis and cell growth. For example, EGFR signaling is known to play a role in cardiac hypertrophy and in mediating β-arrestin-dependent cytoprotective effects via an interaction with β-adrenergic receptors (32). A decrease in the expression of eIF genes could suggest, at least on a transcriptional level, a decreased protein synthesis and partially explain the reduction in RV hypertrophy associated with carvedilol treatment (3). As shown in Table 2, genes encoding proteins in the eIF2-, eIF4-, and EGF pathways were increased in expression in the SuHx model when compared with the SuHx + Carv RV tissues. This suggests that these pathways have been affected by carvedilol to reduce RV hypertrophy.
That carvedilol affected the expression of genes encoding enzymes of sphingolipid pathways was unexpected. Karliner recently reviewed sphingosine kinase and spingosine-1 phosphate and their cardioprotective role in acute ischemia/reperfusion injury (17). Ceramide is considered injurious, causing heart dysfunction (34), and it has been acknowledged that carnitine palmitoyltransferase 1 (CPT1) deficiency (CPT1 oxidation) is associated with cardiac hypertrophy and increased heart tissue ceramide content (14). We also found that carvedilol affected the gene expression of members of the glucocorticoid receptor pathway. A study by Kuster et al. (23) implies that glucocorticoid receptors play an important part in post myocardial infarction heart remodeling.
Carvedilol treatment decreases the expression of many genes associated with cardiac hypertrophy. The higher than normal expression of FHL2 (Fig. 3) is reduced by carvedilol treatment. As FHL2 directly interacts with HIF-1α and represses HIF-1α-dependent transcription (16), overexpression of FHL2 represses pathological cardiac growth (15). We speculate that the high expression of FHL2 in the SuHx failing RVs could compromise HIF-1a-dependent angiogenesis, as would the overexpression of homeodomain-only protein X (Fig. 3) (18).
Finally, we detected that, in the failing RV, there was a highly significant overexpression of a very large number of genes encoding proteins of the ubiquitin proteasome pathways. Carvedilol treatment returned the expression of these genes and the heightened activity of these protein degrading systems toward normal (Fig. 7). These findings support the concept that, in RVF, the ubiquitin proteasome system is highly activated and, therefore, that RVF is associated with proteotoxicity. Ubiquitination is a multistep process involved in the regulation of a number of signal transduction pathways in the heart, among them p53, Fox0, calcineurin/NFAT, and estrogen receptor signaling (35). Indeed, treatment of mice with the proteasome inhibitor bortezomib causes cardiac hypertrophy, and proteasome inhibition of mice with experimentally induced transaortic constriction generates a maladaptive left ventricular dilation (5, 20). Based on such data, we suggest that the heart ubiquitin proteasome system is required for the adaptation of the RV to stress including: cell growth, apoptosis, and autophagy. The dampening effect of carvedilol on proteasome gene expression is consistent with the carvedilol-induced improved and more compensated RV function, which perhaps requires less ubiquitin proteasome activity. To reiterate, the activation of the ubiquitin proteasome system in the failing RV is also consistent with a response to a proteotoxic stress of the cardiomyocytes generated by accumulation of misfolded and aggregated proteins and defective cell organelles.
Conclusion
In conclusion, we show that microarray gene expression studies are useful to characterize RVF and to show that carvedilol alters the “RVF gene expression signature.” When the carvedilol-induced expression changes are viewed in the context of RVF reversal, then these pattern changes begin to explain molecular mechanisms of RVF and begin to characterize the components of the failure program that are reversible.
GRANTS
This work was supported by funds from the Victoria Johnson Center for Obstructive Lung Research. The chemiluminescence microplate reader is located in the VCU Nucleic Acids Research Facility core laboratory, which is supported, in part, by funding by NIH-CI Cancer Center Support Grant P30 CA-016059.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.I.D., R.N., H.J.B., P.F., and N.F.V. conception and design of research; J.I.D., D.K., and R.N. performed experiments; J.I.D., C.I.D., R.N., and H.J.B. analyzed data; J.I.D., J.G.-A., R.N., H.J.B., P.F., and N.F.V. interpreted results of experiments; J.I.D., J.G.-A., C.I.D., and P.F. prepared figures; J.I.D., J.G.-A., C.I.D., and N.F.V. drafted manuscript; J.I.D., J.G.-A., H.J.B., P.F., and N.F.V. edited and revised manuscript; J.I.D., J.G.-A., C.I.D., R.N., H.J.B., P.F., and N.F.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Daniela Farkas and Bernie J. Fisher for technical support. We acknowledge Karol Szczepanek and Edward J. Lesnefsky for the mitochondrial isolation and respirometry assay.
REFERENCES
- 1. Birks EJ, Latif N, Enesa K, Folkvang T, Luong le A, Sarathchandra P, Khan M, Ovaa H, Terracciano CM, Barton PJ, Yacoub MH, Evans PC. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res 79: 472–480, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 120: 1951–1960, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, Hoke NN, Kraskauskas D, Kasper M, Salloum FN, Voelkel NF. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med 182: 652–660, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bovill E, Westaby S, Crisp A, Jacobs S, Shaw T. Reduction of four-and-a-half LIM-protein 2 expression occurs in human left ventricular failure and leads to altered localization and reduced activity of metabolic enzymes. J Thorac Cardiovasc Surg 137: 853–861, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Chen B, Ma Y, Meng R, Xiong Z, Zhang C, Chen G, Zhang A, Dong Y. MG132, a proteasome inhibitor, attenuates pressure-overload-induced cardiac hypertrophy in rats by modulation of mitogen-activated protein kinase signals. Acta Biochim Biophys Sin (Shanghai) 42: 253–258, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol 45: 1239–1247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dudoit S, Fridyland J, Speed TP. Comparison of discrimination methods for the classification of tumors using gene expression data. J Am Stat Assoc 97: 77–87, 2002 [Google Scholar]
- 8. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucl Acids Res 30: 207–210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116: 615–622, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 37: 183–188, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassell B, Natarajan R, dos Remedios CG, Drake JI, Farkas L, Kraskauskas D, Wijesinghe DS, Chalfant CE, Bigbee J, Abbate A, Lesnefsky EJ, Bogaard HJ, Voelkel NF. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy due to pulmonary arterial hypertension. Circ Heart Fail 6: 136–144, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol 302: L363–L369, 2012 [DOI] [PubMed] [Google Scholar]
- 14. He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, Ding Y, Prasain J, Wood PA, Yang Q. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation 126: 1705–1716, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hojayev B, Rothermel BA, Gillette TG, Hill JA. FHL2 binds calcineurin and represses pathological cardiac growth. Mol Cell Biol 32: 4025–4034, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hubbi ME, Gilkes DM, Baek JH, Semenza GL. Four-and-a-half LIM domain proteins inhibit transactivation by hypoxia-inducible factor 1. J Biol Chem 287: 6139–6149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in the heart: z decade of progress. Biochim Biophys Acta 1831: 203–212, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katoh H, Yamashita K, Waraya M, Margalit O, Ooki A, Tamaki H, Sakagami H, Kokubo K, Sidransky D, Watanabe M. Epigenetic silencing of HOPX promotes cancer progression in colorectal cancer. Neoplasia 14: 559–571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Killion PJ, Sherlock G, Iyer VR. The Longhorn Array Database (LAD): an open-source, MIAME compliant implementation of the Stanford Microarray Database (SMD). BMC Bioinformatics 4: 32, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SY, Lee JH, Huh JW, Kim HJ, Park MK, Ro JY, Oh YM, Lee SD, Lee YS. Bortezomib alleviates experimental pulmonary arterial hypertension. Am J Respir Cell Mol Biol 47: 698–708, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Korn EL, Troendle JF, McShane LM, Simon R. Controlling the number of false discoveries: application to high-dimensional genomic data. J Stat Plan Infer 124: 379–398, 2004 [Google Scholar]
- 22. Kumpers P, Nickel N, Lukasz A, Golpon H, Westerkamp V, Olsson KM, Jonigk D, Maegel L, Bockmeyer CL, David S, Hoeper MM. Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J 31: 2291–2300, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Kuster DW, Merkus D, Kremer A, van Ijcken WF, de Beer VJ, Verhoeven AJ, Duncker DJ. Left ventricular remodeling in swine after myocardial infarction: a transcriptional genomics approach. Basic Res Cardiol 106: 1269–1281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol 44: 992–1001, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA 99: 11205–11210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lundgrin EL, Park MM, Sharp J, Tang WH, Thomas JD, Asosingh K, Comhair SA, Difilippo FP, Neumann DR, Davis L, Graham BB, Tuder RM, Dostanic I, Erzurum SC. Fasting 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Ann Am Thorac Soc 10: 1–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma XL, Yue TL, Lopez BL, Barone FC, Christopher TA, Ruffolo RR, Jr, Feuerstein GZ. Carvedilol, a new beta adrenoreceptor blocker and free radical scavenger, attenuates myocardial ischemia-reperfusion injury in hypercholesterolemic rabbits. J Pharmacol Exp Ther 277: 128–136, 1996 [PubMed] [Google Scholar]
- 28. Mearini G, Gedicke C, Schlossarek S, Witt CC, Kramer E, Cao P, Gomes MD, Lecker SH, Labeit S, Willis MS, Eschenhagen T, Carrier L. Atrogin-1 and MuRF1 regulate cardiac MyBP-C levels via different mechanisms. Cardiovasc Res 85: 357–366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, White M, Aban IB, Mujib M, Dell'Italia LJ, Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation 121: 252–258, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mi H, Guo N, Kejariwal A, Thomas PD. PANTHER version 6: protein sequence and function evolution data with expanded representation of biological pathways. Nucleic Acids Res 35: D247–D252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci 115: 175–183, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest 117: 2445–2458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Park TS, Goldberg IJ. Sphingolipids, lipotoxic cardiomyopathy, and cardiac failure. Heart Fail Clin 8: 633–641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Portbury AL, Ronnebaum SM, Zungu M, Patterson C, Willis MS. Back to your heart: ubiquitin proteasome system-regulated signal transduction. J Mol Cell Cardiol 52: 526–537, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. R Development Core Team R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2010 [Google Scholar]
- 37. Radmacher MD, McShane LM, Simon R. A paradigm for class prediction using gene expression profiles. J Comput Biol 9: 505–511, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Reiterer G, Toborek M, Hennig B. Quercetin protects against linoleic acid-induced porcine endothelial cell dysfunction. J Nutr 134: 771–775, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Saldanha AJ. Java Treeview–extensible visualization of microarray data. Bioinformatics 20: 3246–3248, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Simon R, Korn E, McShane L, Radmacher M, Wright G, Zhao Y. Design and Analysis of DNA Microarray Investigations. New York: Springer-Verlag, 2003 [Google Scholar]
- 41. Simon R, Lam A, Li MC, Ngan M, Menenzes S, Zhao Y. Analysis of gene expression data using BRB-ArrayTools. Cancer Inform 3: 11–17, 2007 [PMC free article] [PubMed] [Google Scholar]
- 42. Simon R, Radmacher MD, Dobbin K, McShane LM. Pitfalls in the use of DNA microarray data for diagnostic and prognostic classification. J Natl Cancer Inst 95: 14–18, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Sugden PH, Clerk A. Cellular mechanisms of cardiac hypertrophy. J Mol Med 76: 725–746, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13: 2129–2141, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thomas PD, Kejariwal A, Campbell MJ, Mi H, Diemer K, Guo N, Ladunga I, Ulitsky-Lazareva B, Muruganujan A, Rabkin S, Vandergriff JA, Doremieux O. PANTHER: a browsable database of gene products organized by biological function, using curated protein family and subfamily classification. Nucl Acids Res 31: 334–341, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J 40: 1555–1565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Voelkel NF, Natarajan R, Drake JI, Bogaard HJ. Right ventricle in pulmonary hypertension. Comp Physiol 1: 525–540, 2011 [DOI] [PubMed] [Google Scholar]
- 49. Vonk Noordegraaf A, Galie N. The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev 20: 243–253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yee HY, Paquet A, Dudoit S. Exploratory analysis for two-color spotted microarray data. R package version 1.20.0, 2007 [Google Scholar]
- 51. Yue TL, Cheng HY, Lysko PG, McKenna PJ, Feuerstein R, Gu JL, Lysko KA, Davis LL, Feuerstein G. Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J Pharmacol Exp Ther 263: 92–98, 1992 [PubMed] [Google Scholar]
- 52. Zhu Y, Lee C, Shen F, Du R, Young WL, Yang GY. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke 36: 1533–1537, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Zornoff LA, Skali H, Pfeffer MA, St. John Sutton M, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moye LA, Lewis SJ, Braunwald E, Solomon SD. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol 39: 1450–1455, 2002 [DOI] [PubMed] [Google Scholar]