Abstract
Emergent technologies of regenerative medicine have the potential to overcome the limitations of organ transplantation by supplying tissues and organs bioengineered in the laboratory. Pancreas bioengineering requires a scaffold that approximates the biochemical, spatial and vascular relationships of the native extracellular matrix (ECM). We describe the generation of a whole organ, three-dimensional pancreas scaffold using acellular porcine pancreas. Imaging studies confirm that our protocol effectively removes cellular material while preserving ECM proteins and the native vascular tree. The scaffold was seeded with human stem cells and porcine pancreatic islets, demonstrating that the decellularized pancreas can support cellular adhesion and maintenance of cell functions. These findings advance the field of regenerative medicine towards the development of a fully functional, bioengineered pancreas capable of establishing and sustaining euglycemia and may be used for transplantation to cure diabetes mellitus.
1. Introduction
The treatment of diabetes mellitus remains inadequate. Although exogenous insulin therapy is effective at preventing acute metabolic decompensation in type 1 diabetes, less than 40% of patients achieve and maintain therapeutic targets [1]. As a result, hyperglycemia-related organ damage remains a significant cause of morbidity and mortality among the diabetic population. Intensive glycemic control achieved through dietary modification, physical activity, oral hypoglycemics and exogenous insulin can significantly reduce, but not eliminate, the microvascular and macrovascular complications of diabetes mellitus.
Current best-practice guidelines for the management of diabetes are centered upon life-long lifestyle and pharmaceutical intervention. While these measures reduce the incidence of diabetic emergency and complication, they do not offer the possibility of remission or cure. β-cell replacement, through pancreas or islet cell transplantation, is the sole treatment capable of establishing long-term, stable euglycemia in type 1 diabetic patients.
Regenerative medicine promises to contribute to the advancement of islet transplantation through the development and implementation of microencapsulation technology and the exploitation of bioengineered microenvironments. Encapsulation is a means of immunoisolation, which serves to ‘camouflage’ the foreign antigens of the islet allo- or xeno-graft from host immune surveillance [2]. Encapsulation protocols involve packaging islets within semi-permeable, bio-inert membranes that selectively allow the passage of oxygen, glucose, nutrients, waste products and insulin while preventing penetration by immune cells [2, 3]. Theoretically, successful encapsulation eliminates the need for aggressive, life-long immunosuppression, with consequent improvements in β-cell viability and host morbidity. However, although promising results have been obtained in early animal studies, the clinical value of islet encapsulation has been limited by the following obstacles, recently reviewed by Vaithilingam and Tuch [3]: 1) poor biocompatibility of capsule materials; 2) inadequate immunoisolation due to the penetration of small immune mediators, like chemokines, cytokines and nitric oxide; 3) hypoxia secondary to failed revascularization.
Emerging, cutting edge technologies in regenerative medicine have recently allowed researchers to exploit and appreciate the advantages of preserving innate ECM for organ bioengineering investigations [4–7]. Indeed, innate ECM represents a biochemically, geometrically and spatially ideal platform for such investigations [8], it has both basic components (proteins and polysaccharides) and matrix-bound growth factors and cytokines preserved and at physiological levels [9], it retains an intact and patent vasculature which – when implanted in vivo – sustains the physiologic blood pressure [8], and it is able to drive differentiation of progenitor cells into an organ-specific phenotype [10, 11]. In other words, the natural innate ECM represents the requisite environment for cell welfare because it contains all indispensable information for growth and function [12]. Regenerative medicine is now exploring the possibility to use intact ECM from animal or human whole organs organ bioengineering purposes. ECM scaffolds from whole animal organs can be generated through detergent-based decellularization [5, 13]. Current decellularization protocols are capable of removing DNA, cellular material and cell surface antigens from the ECM scaffold while preserving attachment sites, structural integrity and vascular channels [14]. Successful recellularization of ECM scaffolds has been reported in several organ systems, including liver [15], respiratory tract [16], nerve [17], tendon [18], valve [19], bladder [20] and mammary gland [21]. Pancreas bioengineering lags behind other organs in the field, as only three studies to date report successful repopulation of decellularized pancreatic ECM [22–24]. However, these scaffolds described in these studies do not adequately reflect the size and structure of the adult human pancreas [23].
In this study, we sought to determine whether the porcine pancreas could serve as a suitable platform for the bioengineering of endocrine pancreas with emphasis on the insulin-producing compartment. We demonstrate that the porcine pancreas can be easily explanted and decellularized, retains native structural relationships and vascular channels, is amenable to repopulation via perfusion, and provides the cues necessary for cellular adhesion and proliferation.
2. Materials and methods
2.1. Porcine pancreas decellularization
Young adult pigs (20kg) were intravenously heparinized (400U/kg sodium heparin, NDC #25021-400-10, SAGENT Pharmaceuticals, Schaumburg, IL) then euthanized according to ACUC guidelines. Pancreata were excised en-bloc together with the spleen and upper duodenal segment then cannulated via the pancreatic duct and superior mesenteric vein with Kendall Curity™ 5Fr feeding tubes (155720, McKesson Healthcare, San Francisco, CA). Organs were flushed with 10U/mL sodium heparin in PBS (8 g/L sodium chloride [BP358, Fisher Scientific, Pittsburgh, PA], 0.2 g/L potassium chloride [BP366, Fisher Scientific], 1.15 g/L Sodium phosphate dibasic anhydrous [S5136, Sigma-Aldrich, St. Louis, MO], 0.2 g/L potassium phosphate monobasic anhydrous [BP362, Fisher Scientific] in deionized water) then perfused with 1% Triton® X-100 (X100, Sigma-Aldrich) / 0.1% ammonium hydroxide (A669, Fisher Scientific) in PBS for 24hrs at 0.75L/hr per cannula using Masterflex L/S® Digital Drive peristaltic pumps, Easy-Load II pump heads and silicone tubing (Cole-Palmer Instrument Co., Vernon Hills, IL). Pancreata were rinsed with PBS at the same flow rate for five days. To create scaffolds for culture, decellularized whole pancreata were blotted and sliced into 5mm sections. Sections were cut with a 7mm biopsy punch to create culture scaffold discs, which were placed in PBS and sterilized via 1MRad (10,000Gy) gamma irradiation. Scaffolds were stored at 4°C and used within 7 days, and prior tissue culture experiments were given two further PBS washes then soaked in Dulbecco’s Minimum Essential Medium-High Glucose (DMEM-HG, HyClone Laboratories, Logan, UT) overnight.
2.2. Confirmation of patent vasculature
Conray® (Iothalamate Meglumine, Mallinckrodt Inc, St Louis, MO) contrast agent was diluted in distilled water and perfused through the vasculature at a rate of 30mL/min. Fluoroscopy was captured via a Siemens SIREMOBIL Compact L C-arm. Fluorescein isothiocyanate (FITC)-conjugated dextran beads (FD70, Sigma-Aldrich) suspended in PBS were perfused into the superior mesenteric vein of the decellularized pancreas and visualized under ultraviolet light. Images were captured by a Fujifilm LAS-3000 charge-couple device camera.
2.3. Scanning electron microscopy (SEM) analysis
Scaffold pieces were fixed in 2.5% SEM-grade glutaraldehyde (G5882, Sigma-Aldrich) in PBS then dehydrated in successive alcohols prior to critical-point drying with carbon dioxide. Samples were sputter-coated and visualized via Hitachi S-2600N scanning electron microscope with accompanying manufacturer’s image-capture software.
2.4. Histological assessment of tissues
Scaffolds were fixed overnight in 10% neutral-buffered formalin then transferred to 60% ethanol prior to dehydration in successive alcohols, immersion in chloroform and finally embedding in paraffin wax. Samples were sectioned onto glass slides and stored at 4°C. Rehydrated sections were stained with histochemical dyes for either hematoxylin and eosin (black/pink, nuclei/tissue respectively), Alcian blue and Sirius red (blue/red, proteoglycans/collagens respectively) or with Masson’s Trichrome method (collagen/nuclei/cytoplasm, blue/black/red respectively). Sections were dehydrated again and mounted with Surgipath® MM24® Mounting Medium (3801122, Microsystems GmbH Wetzlar, Germany). All images were captured on a Leica Microsystems DM4000B upright microscope with ImagePro software, Ver. 6.3.
2.5. Collagen quantification
Decellularized pancreas samples were frozen and lyophilized and collagen levels were quantified using the Sircol Soluble Collagen Assay Kit (Biocolor Ltd., Newtownabbey, UK). Briefly, collagens were solubilized overnight in an acid-pepsin enzymatic solution with a pepsin concentration of 0.1 mg/mL in 0.5 M acetic acid. Purified samples were conjugated with Sircol reagent and measured at an absorbance of 555 nm.
2.6. Immunostaining procedures
Rehydrated sections were quenched of endogenous peroxidase activity with 3% hydrogen peroxide then blocked with serum-free protein block (X0909, Dako NA Inc., Carpinteria, CA). Primary antibodies or isotype controls were diluted in antibody diluent (S3022, Dako) and incubated on slides overnight at 4°C. Purchased primary antibodies were anti-laminin (L9393, Sigma-Aldrich), anti-human nuclear antigen (MA1-83365, Thermo Fisher Scientific Inc., Pittsburgh, PA), anti-type I collagen (1310-01, Southern Biotech, Birmingham, AL), anti-type III collagen (1320-01, Southern Biotech), anti-type IV collagen (1340-01, Southern Biotech), anti-elastin (ab9519, Abcam, Cambridge, MA), anti-entactin (ab77179, Abcam) and anti-fibronectin (sc-81767, Santa Cruz Biotechnology Inc., Dallas, TX). Anti-insulin primary antibody was a gift from Mercodia AB, Uppsala, Sweden. IgG fragment isotype controls (mouse, sc-2025; rabbit, sc-2027 and goat, sc-2028) were purchased from Santa Cruz Biotechnology Inc. Negative controls lacked primary antibody. Sections were blocked again prior to application secondary antibodies also diluted in antibody diluent, and incubated for 1 hr at room temperature. Secondary antibodies used were anti-rabbit IgG (BA-1000, Vector Laboratories, Burlingame, CA), anti-goat IgG (BA-5000, Vector Laboratories) and anti-mouse IgG (BA-9200, Vector Laboratories). Slides were washed with PBS, incubated with Vectastain® RTU ABC Reagent (PK-7100, Vector Laboratories) for 30 mins at room temperature then washed again with PBS. Once diaminobenzidine chromogen (ImmPACT™ DAB Peroxidase Substrate Kit, SK-4105, Vector Laboratories) was developed, sections were washed with distilled water and mounted with VectaMount™ AQ aqueous mounting medium (H-5501, Vector Laboratories).
2.7. Seeding of acellular pancreatic scaffolds
hAFSC were obtained as previously described [25] and cultured in Chang medium (Minimum Essential Medium alpha modification, HyClone Laboratories) supplemented with 1% Penicillin-Streptomycin (HyClone Laboratories), 1% L-glutamine (HyClone Laboratories), 15% embryonic stem fetal bovine serum (HyClone Laboratories), 18% Chang B and 2% Chang C supplements (Irvine Scientific, Santa Ana, CA). Select hAFSC lines were transfected with lentiviral particles for stable expression of green fluorescing protein (GFP) [26]. Following overnight soaking in serum-free medium, pancreatic scaffolds were seeded statically with hAFSC in complete Chang medium then maintained in a humidified environment at 37°C with 5% CO2. Proliferation of hAFSC on pancreatic scaffolds was assessed with CellTiter 96® AQueous One Solution MTS Cell Proliferation Assay (G3580, Promega, Madison, WI).
2.8. Islet isolation and culture
Porcine pancreata were obtained as detailed above then cannulated via the splenic artery and flushed with 1L of ice-cold sterile University of Wisconsin solution. Islets were isolated as previously described [27]. Briefly, pancreata were infused with a 1.5 mg/mL collagenase P (11213873001, Roche Applied Science, Indianapolis, IA) solution followed by 30 min cold ischemia. After digestion at 37°C, pancreata were subjected to mild mechanical disruption and islets were purified out via mesh strainers followed by OptiPrep™ (Cosmo Bio USA, Inc., Carlsbad, CA) density gradient separation. Islets were seeded onto acellular pancreatic matrix biopsy punches described above or cultured alone, both in a modified CMRL-based serum-free media (15–110-CV, Mediatech Inc., Manassas, VA).
2.9. Islet viability and functionality studies
Islets were placed in perifusion chambers (3DKUBES™, Kiyatec, Pendleton, SC) and pre-perifused at 37°C for 1 hr in Krebs-Ringer solution (K4002, Sigma-Aldrich) with 0.2% BSA (A9418, Sigma-Aldrich), pH 7.4, containing glucose at basal levels of 3.3 mM (equivalent to 60 mg/dL in the blood). After collection of basal effluent samples for 30 mins, glucose concentration in the perifusate was increased to synthesize hyperglycemic levels at 11.1 mM (200 mg/dL) with effluent samples then collected for 60 mins before perifusate was returned to basal levels and final samples collected. All samples were collected on ice then stored frozen until radioimmunoassay for presence of porcine insulin. For assessment of the metabolic activities of the islets, the MTS Cell Proliferation Assay was used again. Scaffolds seeded with 20 islets were placed into a 96-well plate with CMRL-based medium. After 3 and 7 days of culture, islet cell metabolic activity was assessed using the MTS assay as follows: The CMRL-based medium was removed and replaced with 100 µL of 10% MTS working solution in CMRL. After a 4-hour incubation period under standard culture conditions, the MTS solution was removed and placed in the 96-well plate. The colorimetric change was then measured by absorbance spectroscopy at 490 nm.
2.10. Statistics
All studies were performed with at least three biological replicates, with all assays performed in triplicate. Statistical analyses were performed where stated, using GraphPad Prism software, Ver. 5.0 (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Decellularization of the pancreas with detergent perfusion
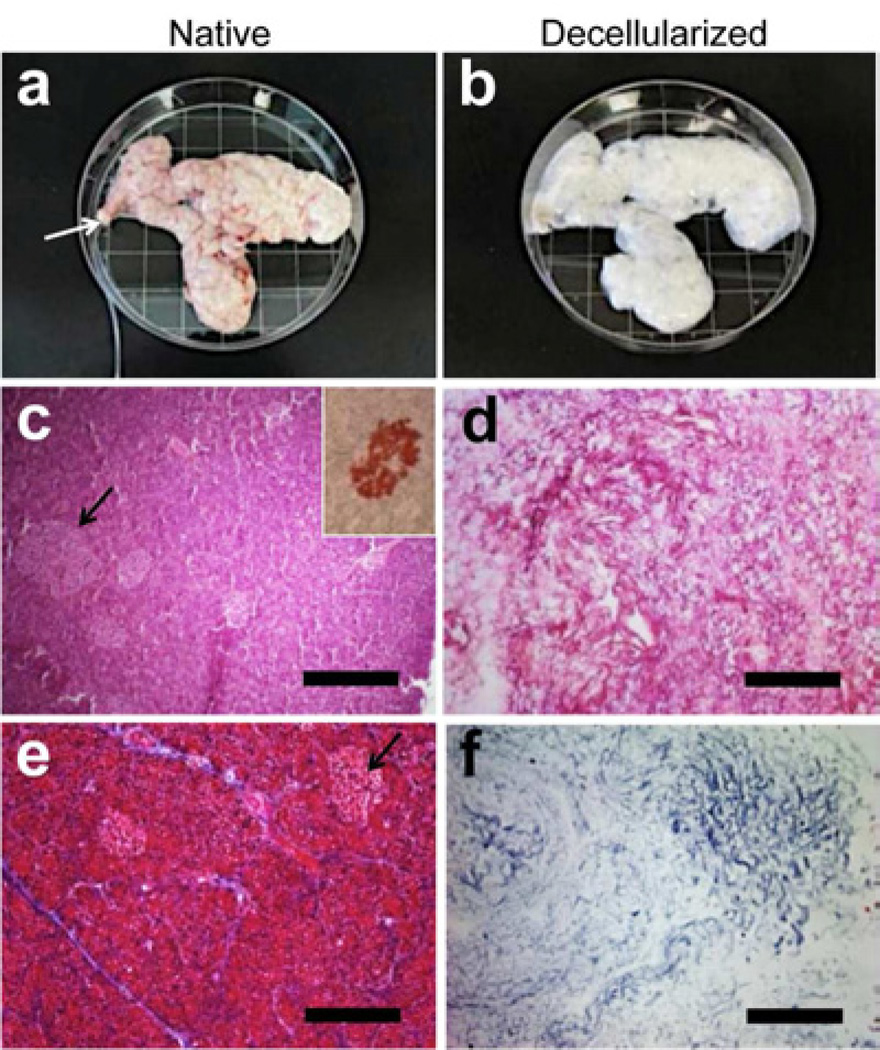
Following perfusion with of detergent solution, the porcine pancreata displayed a marked change from normal pink color to a mostly white, translucent appearance (compare Fig. 1a with 1b). The dense cellularity of the native pancreas, including islet structures (Fig. 1c and inset), was removed and the decellularized pancreatic matrix demonstrated a complete lack of nuclear staining (Fig. 1d). Masson’s trichrome staining confirmed the removal of cellular material (Fig 1e, red stain) and preservation of collagen fibers (Fig 1f, blue stain). To further confirm preservation of pancreatic tissue structure, while removing the cellular material, scanning electron microscopy (SEM) denoted the fibrous nature of the acellular tissue, distinct microvessel (≤ 200 µm) and islet structures (Supplemental Fig 1).
Figure 1. Decellularization of porcine pancreas.
(a) Gross image of porcine pancreas upon collection, approximately 15 cm in width, with cannulated pancreatic duct (arrow). (b) The pancreas after decellularization, showing opaque appearance. Hematoxylin and eosin staining of native (c) and acellular (d) pancreata, demonstrating the dense cellularity of the native pancreas and appearance of islets (arrows and inset; immunostaining for insulin), compared with acellular pancreas, which lacks cellularity. Masson’s trichrome staining of native (e) and acellular (f) pancreata, demonstrating cellularity of the native pancreas, compared with collagenous material and absence of cellular material in the acellular pancreas. All scale bars = 200µm.
3.2. Acellular pancreas retains patent vasculature
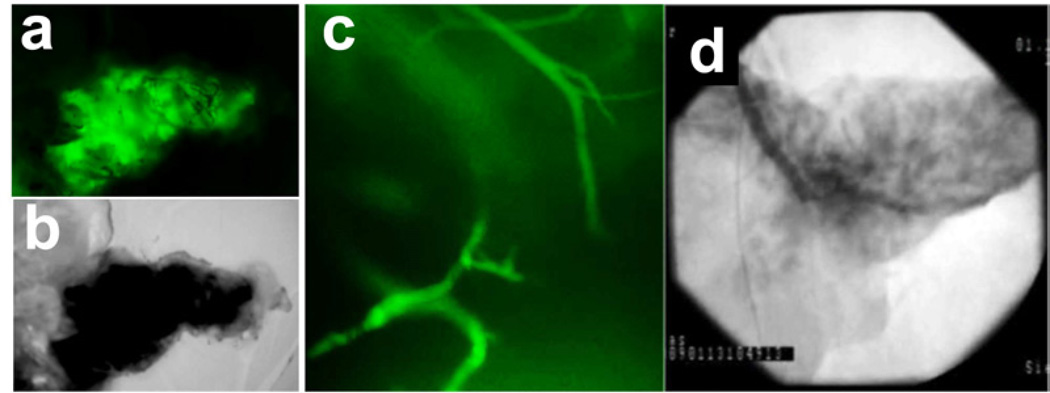
Maintaining an intact vascular network is not only important for the delivery of the decellularization detergent throughout the organ, but also as potential means for cell seeding of the scaffold. To confirm the integrity of the vascular tree and to demonstrate that fluid injected into the vasculature flowed through it rather than extravasate throughout the organ, a suspension of fluorescently-labeled dextran particles was perfused through the superior mesenteric and splenic arteries. The fluorescent suspension was distributed evenly across the pancreas parenchyma (Fig. 2a, b), with individual vessels identified under high-power magnification (Fig. 2c). To confirm the intact nature of the vasculature we infused an x-ray fluoroscopic radio-opaque dye through the superior mesenteric and splenic arteries. Time-dependent distribution of the dye revealed the major vessels of the acellular pancreas and subsequent distribution through the whole organ (Fig. 2d).
Figure 2. Preservation of intact vasculature in the decellularized porcine pancreas.
(a, b) Perfusion of fluorescein isothiocyanate (FITC)-labeled dextran particles inside the acellular porcine pancreas, shown under fluorescent and bright light microscopy, respectively. (c) High magnification of panel (a) shows intact vessels inside the acellular pancreas perfused with FITC-labeled dextran particles. (d) Fluoroangiograph of acellular porcine pancreas vasculature following perfusion of Conray® contrast agent.
3.3.The ECM composition of the acellular pancreas
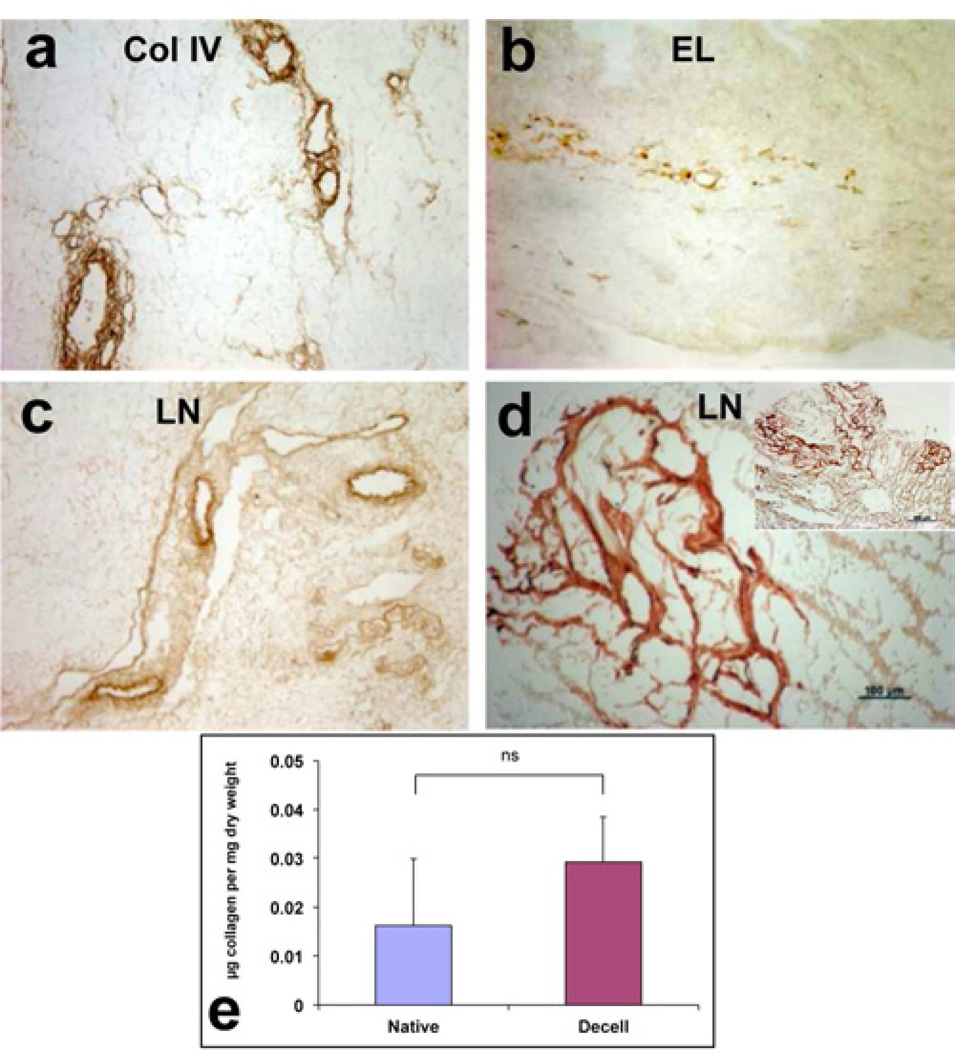
One major advantage of producing tissue-specific ECM is the preservation of the natural composition of such ECM, which provides authentic tissue micro-environment. Specifically, the pancreatic parenchyma is composed of specific ECM molecules that have a significant role in tissue development and regeneration. Immunostaining for specific ECM proteins showed specific distribution of collagen VI in the basement membrane of the pancreatic vessels and the acini (Fig 3a, arrows and arrowheads, respectively). Elastin is localized in the basement membrane of large and small vascular structures and the ducts (Fig 3b, arrows). Immunostaining for laminin revealed strong presence around the vascular structures (Fig 3c, arrows), as well as, in discrete structures in the acellular pancreas, probably the native sites of the pancreatic islets (Fig 3c, d, arrowheads). Comparison of these results with the report by Meyer et al [28] shows essentially the same pattern of expression and localization of the ECM proteins in the native porcine pancreas. Assessment of total collagen showed comparable amounts in native and acellular pancreas (Fig 3e), indicating that the decellularization process did not remove a significant amount of the major component of the ECM.
Figure 3. Characterization of ECM proteins in acellular porcine pancreas.
(b–j) Immunohistochemical staining for collagen type IV (Col IV, a), elastin (EL, b) and laminin (LN, c, d). High magnification image of laminin staining show discreet staining, presumably at the site of an islet (d). (k) Assessment of total collagen in native and acellular porcine pancreata. Values expressed as mean ± SEM, n=3, p=0.48 with Student’s t-test. Scale bars = 200µm (a–c), 500µm (d, inset) and 100µm (d).
3.4. Cell seeding on acellular pancreas ECM
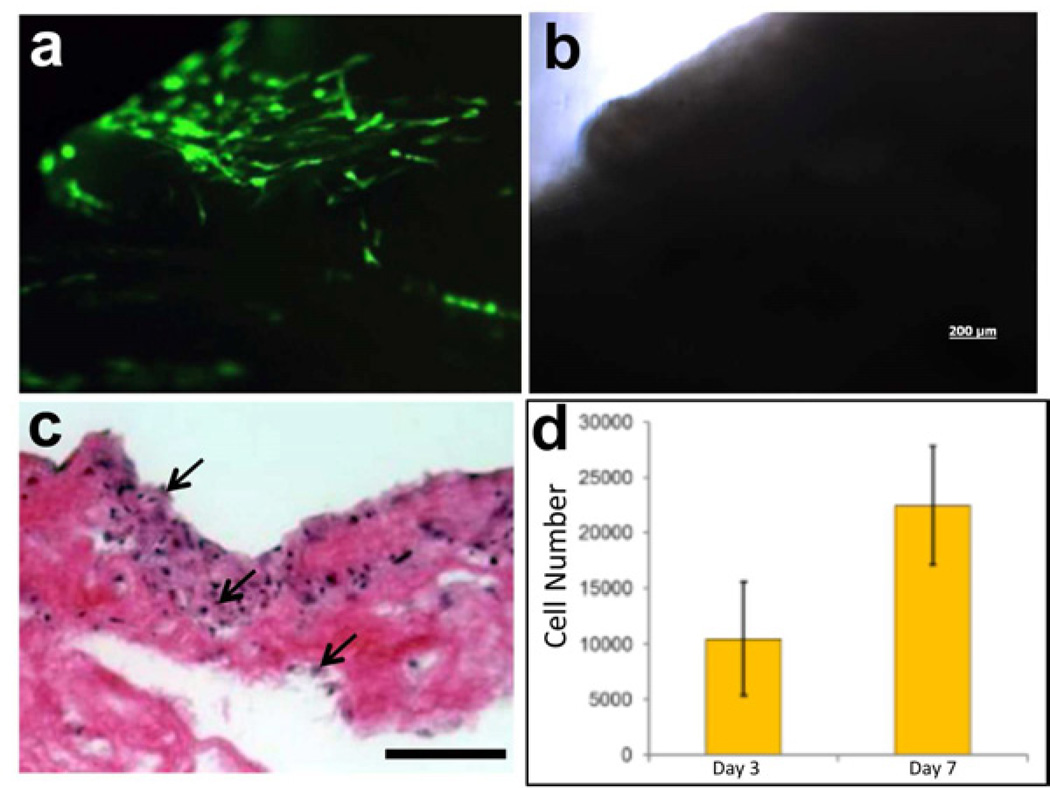
To assess the cellular compatibility of the acellular pancreas ECM, we seeded GFP-expressing human amniotic fluid-derived stem cells (hAFSC) and examined their growth. The human AFSC were proposed as a potential source of insulin-secreting cells [29, 30]. Forty eight hours post seeding the cells spread on the surface of the acellular pancreas ECM (Fig. 4a), while after 7 days the cells were found on the surface and inside the scaffold Fig 4c, arrows). Assessment of cell growth showed significant, two-fold increase, in cell numbers between day 3 and day 7 post seeding (Fig 4d).
Figure 4. Cell growth on acellular porcine pancreas.
(a) Fluorescent and bright field (b) images of GFP-labeled human amniotic fluid-derived stem cells (hAFSC) cultured on porcine acellular pancreatic matrix shown 48 hours after seeding. (c) H&E staining of sections of acellular porcine pancreas seeded with hAFSC 7 days after seeding (scale bar = 100µm). (d) Proliferation of hAFSC seeded onto acellular porcine pancreas, measured by MTS assay, shows increase in cell number from day 3 to day 7. Values expressed as mean ± stdev, n=4.
3.5. Maintaining islet functionality on acellular pancreas ECM
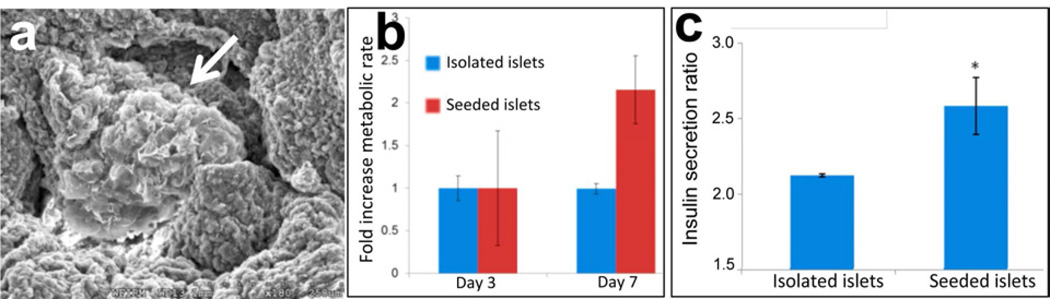
To demonstrate the potential of the acellular pancreas ECM to support pancreatic function, isolated porcine islets were seeded on the acelllular pancreas and insulin secretion was measured over time (Fig 5). SEM images show an individual islet settled inside the acellular pancreas (Fig 5a, arrow). Islet seeded on the acellular pancreas showed both an increase in their metabolic rate (Fig 5b), between days 3 and 7, and insulin secretion (Fig 5c and Supp Fig 2), compared with isolated islets. It is noteworthy in Supp Fig 2 that insulin secretion by islets after 3 days on either plastic or embedding in the pancreas ECM scaffold was pulsatile under basal and high glucose conditions, a phenomenon that we had previously described in freshly isolated islets [31].
Figure 5. Islet function on acellular porcine pancreas.
(a) SEM image of acellular porcine pancreas seeded with porcine islets after 3 days (arrow). (b) Islets seeded on porcine acellular pancreatic matrix showed a two-fold increase of metabolic activity, from day 3 to day 7, in comparison to islets maintained in culture media, as measured by MTS assay. (c) Pig islets in culture media or seeded onto porcine acellular pancreatic matrix for 3 days were subject to a glucose challenge test. Islets seeded onto scaffolds demonstrated a significantly increased insulin secretion ratio (highest peak to mean basal values) compared with islets in culture media. Values expressed as mean ± stdev, n=3, *p<0.05.
4. Discussion
The current study corroborates the idea that acellular porcine pancreas ECM matrices can serve a formidable platform for insulin-producing bioengineered tissue. The porcine pancreas can be easily explanted and decellularized, retains native structural and biochemical relationships and vascular channels, is amenable to cell repopulation, and provides the cues necessary for cellular adhesion and proliferation. Furthermore, the acellular porcine pancreas has the capacity to support the critical mass of islets required to meet insulin requirements for human patients. To our knowledge, the repopulation of whole, decellularized animal pancreata has not been fully reported in the bioengineering literature. These findings provide evidence that porcine pancreas ECM may represent a valuable platform for studies aimed at bioengineering a bioartificial pancreas to treat diabetes mellitus. Pancreas transplantation, first performed by Kelly et al. [32], yields higher rates of insulin independence than islet transplantation [33] and has been shown to improve secondary complications of diabetes compared to insulin treatment [34–36] and quality of life [37]. Despite these advantages, donor shortage, surgical morbidity and the need for lifelong immunosuppression significantly limit clinical application. Furthermore, comparative analyses have shown that transplantation improves overall survival rate only when performed as part of a simultaneous pancreas and kidney transplant [33, 38]. Alternatively, β-cell replacement has been attempted through the transplantation of isolated pancreatic islets, as described by the seminal so-called ‘Edmonton Protocol’ [39]. Although subsequent studies have demonstrated improvements in diabetic complications and quality of life following islet transplantation [40, 41], no studies to date have reported improved outcomes over insulin therapy [42]. Furthermore, islet transplantation requires 2–4 whole cadaveric pancreata per recipient [39], which would impoverish an already-limited organ pool.
In organ bioengineering and regeneration the seeding of cells on supporting scaffolding material seems to offer an attractive strategy to address clinical needs. Organ bioengineering requires a scaffold that approximates the biochemical, spatial and vascular relationships of the native tissue ECM. Although synthetic matrix analogues can satisfy some of these requirements, they may not express the complete complement of native ECM components, specifically required for in vivo islet attachment, survival and function [3]. Native ECM scaffolds has the potential to provide proper micro-environment for islets intended for transplantation [43]. Furthermore, the 3D structure of native ECM has been shown to determine the topographical arrangement of pancreatic endocrine cells [44], which influences islet secretory activity and survival [44]. Although successful recellularization of ECM scaffolds has been reported in several organ systems [15–20], pancreas bioengineering lags behind other organs in the field, as only a handful of studies to date report successful recellularization of native pancreatic ECM [22, 24]. A suitable scaffold for functional tissue engineering should 1) be easily explanted and decellularized, 2) retain native structural relationships and vascular channels, 3) be amenable to repopulation via immersion or perfusion, and 4) provide the cues necessary for cellular adhesion and proliferation. In the current study we demonstrated that acellular porcine pancreas satisfies these criteria, and thus serves as an ideal platform for further bioengineering investigation to address the clinical need of insulin supplementation. Although successful repopulation of acellular murine pancreata has been previously reported, these scaffolds do not adequately reflect the size and structure of the adult human pancreas [23]. De Carlo et al. [23] implanted diabetic rats with PVA/PEG tubular capsules containing slices of decellularized rat pancreas repopulated with differentiated murine islets. The pancreatic matrix significantly extended the duration of glucose-stimulated insulin release, suggesting that the pancreatic matrix favors long-term physiological response. Upon implantation, the islet capsules reduced blood sugar levels, although euglycemia was not achieved. Conrad et al. [22] have describe successful recellularization of rat pancreatic matrix with human islet cells and supportive mesenchymal stem cells. The islets showed preserved glucose-stimulated insulin response, cell viability, subcellular anatomy and attachments. Goh [24] also reported successful repopulation of decellularized mouse and cow cadaveric pancreata with mouse embryonic stem cells. However, the Conrad et al. and Goh studies have only been presented as abstracts, and the complete findings from these experiments remain to be published. The porcine pancreas is a more suitable platform, as it has the physiological and structural capacity to support the critical mass of β cells required to meet human insulin requirements.
Decellularization protocols involve the repeated irrigation of cadaveric tissues with detergents, acids or bases through the innate vasculature. We achieved successful decellularization of porcine pancreas by perfusing the pancreatic vasculature with detergents administered via cannulas in the pancreatic duct and superior mesenteric vein. Although organs with a higher fat content, like the pancreas, are thought to require additional lipid solvents [17], we were able to achieve optimal results with standard non-ionic cellular disruption mediums. As certain detergents have been shown to disrupt ECM components [45], we chose Triton X-100, a mild non-ionic detergent with proven efficacy in whole-organ decellularization [46]. The porcine pancreas has a sparse basement membrane with few cell-to-matrix adhesions compared to human or murine pancreata [47], which may facilitate decellularization. Decellularization was confirmed through nuclear staining and SEM analyses, which demonstrated the lysis and removal of cellular content. Imaging studies further demonstrated that our decellularization protocol preserves the vascular network intact, allowing for effective scaffold repopulation.
Previous studies have reported that pancreatic decellularization may disrupt the protein ultrastructure of the ECM [48]. Characterization studies of the acellular porcine pancreas showed widespread distribution of all essential structural proteins, including different types of collagen, elastin, fibronection and laminin (Fig. 3). These results are in good agreement with the expression and localization of these ECM proteins in the native porcine pancreas, as reported by Meyer et al [28]. The preservation of the native ECM proteins is essential, as laminins and proteoglycans appear necessary for the regionalization and differentiation of progenitor cells into pancreatic lineages [49]. Collagens, glycoproteins and glycosaminoglycans have also been shown to prevent β cell apoptosis triggered by the loss of cellular adhesion experienced during harvesting, termed anoikis [50]. Native ECM proteins also bind, store and regulate the activity of growth factors including TGF-β1 [22], which plays a role in development, function and regeneration [22] of pancreatic islets. Finally, laminin plays a key role in endocrine function, as they have been shown to independently enhance islet survival and insulin release in vitro [44]. The ratio of laminin isoforms varies between embryonic development and adulthood, suggesting that individual isoforms may selectively promote different aspects of pancreatic maturation, proliferation and secretion [51]. Future studies will determine whether the complement of ECM proteins expressed by the adult porcine pancreas is sufficient to promote β cell differentiation and function.
To the best of our knowledge, successful repopulation of a clinically-relevant size of acellular porcine pancreas has not been previously reported. We tested the cell compatibility of the acellular porcine pancreas by seeding hAFSC, as a potential stem cell source for generation of β cells [29, 30]. Islet tissue only comprises 1–2% of the adult human pancreas, and the non-islet support cells may be essential for islet viability and function [52]; as well, stromal cells seem to be essential for supporting growth and differentiation of progenitor cells [53]. In our study, partial recellularization was histologically confirmed, demonstrating that porcine pancreatic ECM is capable of supporting the growth of human amniotic progenitor cells. As anticipated earlier [5], our findings support the possibility of ‘semi-xenotrasnplantation’, the transplantation of animal-derived matrices populated with human cells. We also demonstrated that the porcine pancreatic ECM promotes glucose-mediated insulin release when repopulated with differentiated islets in culture. β cell replacement has been previously attempted through the transplantation of isolated pancreatic islets, as described by the seminal ‘Edmonton Protocol’ [39]. The transplantation of isolated islets has yielded poor outcomes, due in part to the disruption of the native pancreatic ECM during harvesting. The pancreatic ECM supports the native islet vasculature, and ECM disruption necessitates neo-angiogenesis prior to reperfusion [54]. Additionally, disruption of the native ECM appears to compromise the survival [55], engraftment [56], and immune-tolerance [57] of transplanted islets. Interestingly, the capability of ECM to support and protect pancreatic islets viability has been shown in previous studies. Survival and function of β cells, encapsulated in immunoprotective gels for transplantation in the treatment of insulin-dependent diabetes, was enhanced by including ECM components [50, 58]. Moreover, insulinoma cells encapsulated with polyethylene glycol added with collagen IV and laminin induced a significant increase in insulin secretion when compared with cells inserted in standard capsules [59]. Overall, our results suggest that the native ECM may prove enhance routine islet transplantation in the future.
5. Conclusions
This study demonstrated successful harvest and decellularization of porcine pancreas. The acellular pancreas retains its native structure and intact vascular channels. We further showed that the acellular porcine pancreas supports cell growth, including stem cells and pancreatic islets. These results suggest that acellular porcine pancreas ECM can serve as a platform for bioengineering a bioartificial pancreas to treat diabetes mellitus.
Supplementary Material
SEM image of (a) fibrous nature of the acellular porcine pancreas, (b) a pancreatic vessel showing an open lumen and a thicker wall (c) an islet in the native pancreatic parenchyma and (d) a putative site of an islet in the acellular pancreas. Scale bars = 20µm (a) and 200µm (b–d).
Time course data from perfusion of porcine islets in culture media (blue) and porcine islets seeded onto acellular porcine pancreas (green). Both groups were cultured for three days in low glucose and then subjected to a glucose challenge, as described in Materials and Methods. Low glucose levels (3.3mM) represented physiological euglycemia of 60 mg/dL, and the transition to high glucose (11.1mM) represented upper limit postprandial levels of 200mg/dL. Both groups displayed increased insulin secretion during high glucose perfusion, with islets seeded onto scaffolds demonstrating higher peak insulin secretion values, observed, specifically after 50, 55 and 75 minutes. Both groups showed a reduction of insulin secretion after a return to low (basal) glucose concentration
Acknowledgements
This work was supported by research grants R01EB8009 from NIH/NIBIB to AA and SS and R01DK080897 to ECO. The authors would like to thank Dr. James Jordan Department Cardiothoracic surgery, Wake Forest School of Medicine, Mr. Alexander Pirro and Ms. Natasha Lee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orlando G, Stratta RJ, Light J. Pancreas transplantation for type 2 diabetes mellitus. Curr Opin Organ Transplant. 2011;16:110–115. doi: 10.1097/MOT.0b013e3283424d1f. [DOI] [PubMed] [Google Scholar]
- 2.Opara EC, Mirmalek-Sani SH, Khanna O, Moya ML, Brey EM. Design of a bioartificial pancreas. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2010;58:831–837. doi: 10.231/JIM.0b013e3181ed3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaithilingam V, Tuch BE. Islet transplantation and encapsulation: an update on recent developments. Rev Diabet Stud. 2011;8:51–67. doi: 10.1900/RDS.2011.8.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Orlando G, Baptista P, Birchall M, De Coppi P, Farney A, Guimaraes-Souza NK, et al. Regenerative medicine as applied to solid organ transplantation: current status and future challenges. Transpl Int. 2011;24:223–232. doi: 10.1111/j.1432-2277.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33:7756–7764. doi: 10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Orlando G, Farney AC, Iskandar SS, Mirmalek-Sani SH, Sullivan DC, Moran E, et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg. 2012;256:363–370. doi: 10.1097/SLA.0b013e31825a02ab. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53:293–305. doi: 10.1002/hep.24012. [DOI] [PubMed] [Google Scholar]
- 10.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338–2347. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng SL, Narayanan K, Gao S, Wan AC. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials. 2011;32:7571–7580. doi: 10.1016/j.biomaterials.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 12.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 16.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998–1005. doi: 10.1016/j.athoracsur.2011.05.018. discussion -6. [DOI] [PubMed] [Google Scholar]
- 17.Crapo PM, Medberry CJ, Reing JE, Tottey S, van der Merwe Y, Jones KE, et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials. 2012;33:3539–3547. doi: 10.1016/j.biomaterials.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinello T, Bronzini I, Volpin A, Vindigni V, Maccatrozzo L, Caporale G, et al. Successful recellularization of human tendon scaffolds using adipose-derived mesenchymal stem cells and collagen gel. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1557. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Honge JL, Funder J, Hansen E, Dohmen PM, Konertz W, Hasenkam JM. Recellularization of aortic valves in pigs. Eur J Cardiothorac Surg. 2011;39:829–834. doi: 10.1016/j.ejcts.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 20.Loai Y, Yeger H, Coz C, Antoon R, Islam SS, Moore K, et al. Bladder tissue engineering: tissue regeneration and neovascularization of HA-VEGF-incorporated bladder acellular constructs in mouse and porcine animal models. J Biomed Mater Res A. 2010;94:1205–1215. doi: 10.1002/jbm.a.32777. [DOI] [PubMed] [Google Scholar]
- 21.Wicha MS, Lowrie G, Kohn E, Bagavandoss P, Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982;79:3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson JJ, Lutey BA, Suzuki Y, Toennies HM, Kelley DG, Kobayashi DK, et al. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med. 2011;183:876–884. doi: 10.1164/rccm.201005-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Carlo E, Baiguera S, Conconi MT, Vigolo S, Grandi C, Lora S, et al. Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: in vitro and in vivo studies. Int J Mol Med. 2010;25:195–202. [PubMed] [Google Scholar]
- 24.Goh SK. Perfusion-Decellularization of Pancreatic Matrix – A Scaffold for Bio-Engineered Pancreas. AIChE. Minneapolis Convention Center. 2011 [Google Scholar]
- 25.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nature biotechnology. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 26.Klages N, Zufferey R, Trono D. A stable system for the high-titer production of multiply attenuated lentiviral vectors. Molecular therapy : the journal of the American Society of Gene Therapy. 2000;2:170–176. doi: 10.1006/mthe.2000.0103. [DOI] [PubMed] [Google Scholar]
- 27.Ching CD, Harland RC, Collins BH, Kendall W, Hobbs H, Opara EC. A reliable method for isolation of viable porcine islet cells. Archives of surgery. 2001;136:276–279. doi: 10.1001/archsurg.136.3.276. [DOI] [PubMed] [Google Scholar]
- 28.Meyer T, Czub S, Chodnewska I, Beutner U, Hamelmann W, Klock G, et al. Expression pattern of extracellular matrix proteins in the pancreas of various domestic pig breeds, the Goettingen Minipig and the Wild Boar. Annals of transplantation : quarterly of the Polish. Transplantation Society. 1997;2:17–26. [PubMed] [Google Scholar]
- 29.Chun SY, Mack DL, Moorefield E, Oh SH, Kwon TG, Pettenati MJ, et al. Pdx1 and controlled culture conditions induced differentiation of human amniotic fluid-derived stem cells to insulin-producing clusters. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Mack DL, Williams JK, Mirmalek-Sani SH, Moorefield E, Chun SY, et al. Genetic Modification of Primate Amniotic Fluid-Derived Stem Cells Produces Pancreatic Progenitor Cells in vitro. Cells Tissues Organs. 2013 doi: 10.1159/000345816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opara EC, Atwater I, Go VL. Characterization and control of pulsatile secretion of insulin and glucagon. Pancreas. 1988;3:484–487. doi: 10.1097/00006676-198808000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61:827–837. [PubMed] [Google Scholar]
- 33.Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA. 2003;290:2817–2823. doi: 10.1001/jama.290.21.2817. [DOI] [PubMed] [Google Scholar]
- 34.Navarro X, Sutherland DE, Kennedy WR. Long-term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol. 1997;42:727–736. doi: 10.1002/ana.410420509. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med. 1990;322:1031–1037. doi: 10.1056/NEJM199004123221503. [DOI] [PubMed] [Google Scholar]
- 36.Fioretto P, Mauer SM, Bilous RW, Goetz FC, Sutherland DE, Steffes MW. Effects of pancreas transplantation on glomerular structure in insulin-dependent diabetic patients with their own kidneys. Lancet. 1993;342:1193–1196. doi: 10.1016/0140-6736(93)92183-t. [DOI] [PubMed] [Google Scholar]
- 37.Zehrer CL, Gross CR. Quality of life of pancreas transplant recipients. Diabetologia. 1991;34(Suppl 1):S145–S149. doi: 10.1007/BF00587643. [DOI] [PubMed] [Google Scholar]
- 38.Mohan P, Safi K, Little DM, Donohoe J, Conlon P, Walshe JJ, et al. Improved patient survival in recipients of simultaneous pancreas-kidney transplant compared with kidney transplant alone in patients with type 1 diabetes mellitus and end-stage renal disease. Br J Surg. 2003;90:1137–1141. doi: 10.1002/bjs.4208. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 40.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8:1990–1997. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 41.Tharavanij T, Betancourt A, Messinger S, Cure P, Leitao CB, Baidal DA, et al. Improved long-term health-related quality of life after islet transplantation. Transplantation. 2008;86:1161–1167. doi: 10.1097/TP.0b013e31818a7f45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cravedi P, Remuzzi A, Remuzzi G. Comment on: Robertson (2010) Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010;59:1285–1291. Diabetes. 59:e13. doi: 10.2337/db10-0848. author reply e4. [DOI] [PubMed] [Google Scholar]
- 43.Cheng JY, Raghunath M, Whitelock J, Poole-Warren L. Matrix components and scaffolds for sustained islet function. Tissue engineering Part B, Reviews. 2011;17:235–247. doi: 10.1089/ten.TEB.2011.0004. [DOI] [PubMed] [Google Scholar]
- 44.Lucas-Clerc C, Massart C, Campion JP, Launois B, Nicol M. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol. 1993;94:9–20. doi: 10.1016/0303-7207(93)90046-m. [DOI] [PubMed] [Google Scholar]
- 45.Samouillan V, Dandurand-Lods J, Lamure A, Maurel E, Lacabanne C, Gerosa G, et al. Thermal analysis characterization of aortic tissues for cardiac valve bioprostheses. J Biomed Mater Res. 1999;46:531–538. doi: 10.1002/(sici)1097-4636(19990915)46:4<531::aid-jbm11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Baptista PM, Orlando G, Mirmalek-Sani SH, Siddiqui M, Atala A, Soker S. Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6526–6529. doi: 10.1109/IEMBS.2009.5333145. [DOI] [PubMed] [Google Scholar]
- 47.van Deijnen JH, Hulstaert CE, Wolters GH, van Schilfgaarde R. Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man. Cell Tissue Res. 1992;267:139–146. doi: 10.1007/BF00318700. [DOI] [PubMed] [Google Scholar]
- 48.Tchen TTJ, Jiang H, Badylak S, Ogilvie JB. Decellularization Of Pancreatic Extracellular Matrix For A Tissue-Engineered Pancreas. Pittsburgh, PA: Abstract University of Pittsburgh, McGowan Institute for Regenerative Medicine; 2007. [Google Scholar]
- 49.Higuchi Y, Shiraki N, Kume S. In vitro models of pancreatic differentiation using embryonic stem or induced pluripotent stem cells. Congenit Anom (Kyoto) 2011;51:21–25. doi: 10.1111/j.1741-4520.2010.00307.x. [DOI] [PubMed] [Google Scholar]
- 50.Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14:1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes, obesity & metabolism. 2008;10(Suppl 4):119–127. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 52.Rorsman P, Braun M. Regulation of Insulin Secretion in Human Pancreatic Islets. Annu Rev Physiol. 2012 doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira MS, Jahnen-Dechent W, Labude N, Bovi M, Hieronymus T, Zenke M, et al. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials. 2012;33:6987–6997. doi: 10.1016/j.biomaterials.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 54.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749–763. doi: 10.1007/s00125-002-0827-4. [DOI] [PubMed] [Google Scholar]
- 55.Thomas F, Wu J, Contreras JL, Smyth C, Bilbao G, He J, et al. A tripartite anoikis-like mechanism causes early isolated islet apoptosis. Surgery. 2001;130:333–338. doi: 10.1067/msy.2001.116413. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu H, Ohashi K, Utoh R, Ise K, Gotoh M, Yamato M, et al. Bioengineering of a functional sheet of islet cells for the treatment of diabetes mellitus. Biomaterials. 2009;30:5943–5949. doi: 10.1016/j.biomaterials.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 57.Bennet W, Sundberg B, Song Z, Elgue G, Wennberg L, Richards A, et al. Porcine islets of langerhans isolated from normal and hDAF transgenic pigs elicit the same acute inflammatory reaction during exposure to human blood; inhibition of the response with soluble complement receptor 1 and heparin. Transplant Proc. 2000;32:1065. doi: 10.1016/s0041-1345(00)01123-4. [DOI] [PubMed] [Google Scholar]
- 58.Huang G, Greenspan DS. ECM roles in the function of metabolic tissues. Trends Endocrinol Metab. 2012;23:16–22. doi: 10.1016/j.tem.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber LM, Anseth KS. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27:667–673. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SEM image of (a) fibrous nature of the acellular porcine pancreas, (b) a pancreatic vessel showing an open lumen and a thicker wall (c) an islet in the native pancreatic parenchyma and (d) a putative site of an islet in the acellular pancreas. Scale bars = 20µm (a) and 200µm (b–d).
Time course data from perfusion of porcine islets in culture media (blue) and porcine islets seeded onto acellular porcine pancreas (green). Both groups were cultured for three days in low glucose and then subjected to a glucose challenge, as described in Materials and Methods. Low glucose levels (3.3mM) represented physiological euglycemia of 60 mg/dL, and the transition to high glucose (11.1mM) represented upper limit postprandial levels of 200mg/dL. Both groups displayed increased insulin secretion during high glucose perfusion, with islets seeded onto scaffolds demonstrating higher peak insulin secretion values, observed, specifically after 50, 55 and 75 minutes. Both groups showed a reduction of insulin secretion after a return to low (basal) glucose concentration