Abstract
Silent mating type information regulation 2 homolog 1 (SIRT1) is implicated in the control of skeletal muscle mitochondrial content and function through deacetylation of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and participation in the SIRT1/PGC-1α axis. The SIRT1/PGC-1α axis control of skeletal muscle mitochondrial biogenesis is an important therapeutic target for obesity and obesity-related metabolic dysfunction, as skeletal muscle mitochondrial dysfunction is implicated in the pathogenesis of multiple metabolic diseases. This review will establish the importance of the SIRT1/PGC-1α axis in the control of skeletal muscle mitochondrial biogenesis, and explore possible pharmacological and physiological interventions designed to activate SIRT1 and the SIRT1/PGC-1α axis in order to prevent and/or treat obesity and obesity-related metabolic disease. The current evidence supports a role for therapeutic activation of SIRT1 and the SIRT1/PGC-1α axis by both pharmaceuticals and exercise in the treatment and prevention of metabolic disease. Future research should be directed toward the feasibility of pharmaceutical activation of SIRT1 in humans and refining exercise prescriptions for optimal SIRT1 activation.
Keywords: SIRT1, PGC-1α, resveratrol, obesity, metabolic disease, exercise
Introduction
Decreases in mitochondrial content and function accompanying the development of overweight and obesity represent an underlying mechanism of several metabolic disorders including insulin resistance, dyslipidemia, type II diabetes, hypertension, and cardiovascular disease.1,2 In 2005, an estimated 23.2% of the worldwide adult population was overweight and 9.8% was obese.3 Current projections predict that 58.7% of the global adult population will be overweight or obese by 2030,4 an estimate that potentially represents a massive economic burden for healthcare systems worldwide. In the United States, these rates would increase obesity-related costs to 16% (USD860 billion) of total healthcare expenditure.4 Type II diabetes alone is already costing USD376 billion each year globally, a figure that is projected to grow 30% in the next 20 years.5,6 Given the economic and health costs associated with overweight and obesity, and their associated diseases, there is an urgent need for effective preventative and therapeutic interventions targeting weight gain and metabolic disease.
Skeletal muscle accounts for approximately 40% of body mass7 and plays a critical role in the maintenance of metabolic health. Healthy skeletal muscle has a highly oxidative phenotype, readily oxidizes lipids, is sensitive to insulin, and efficiently stores glucose.8,9 Consistent with the importance of mitochondria in healthy muscle, impairments in skeletal muscle function that contribute to obesity-related disease (eg, decreased fatty acid oxidation, impaired insulin sensitivity) are causally linked to decreases in mitochondrial content and function.10–15 In fact, decreases in mitochondrial content are apparent in early-stage overweight and obesity, and appear to contribute to both weight gain per se and to the metabolic dysfunction that underlies the development of metabolic disease.10,16
Genetic control of skeletal muscle mitochondrial content is regulated via a complex network of signaling pathways, transcriptional factors, and transcription cofactors. Peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) is a transcription factor coactivator that has been implicated as a key regulator of mitochondrial gene expression and mitochondrial biogenesis (Figure 1).17 The central role of PGC-1α in the regulation of mitochondrial content has positioned this protein as an important target in therapies aimed at preventing and/or treating disease.18,19 Silent mating type information regulation 2 homolog 1 (SIRT1) interacts with PGC-1α in skeletal muscle increasing PGC-1α’s transcriptional activity through deacetylation.20 Given its apparent ability to activate PGC-1α and increase mitochondrial content in skeletal muscle,20 SIRT1 has itself been implicated as a key player in metabolic health.21,22 It should be noted that SIRT1 is not solely responsible for the control of PGC-1α acetylation status, and evidence of normal mitochondrial biogenesis in mice lacking SIRT1 deacetylase activity suggests an inherent redundancy in the PGC-1α deacetylation pathway.23 Despite this redundancy, the bulk of evidence suggests an important role of SIRT1 in skeletal muscle adaptations; thus, there is considerable interest in interventions designed to target SIRT1 and the SIRT1/PGC-1α axis as a means of increasing mitochondrial content, skeletal muscle function, and metabolic health.
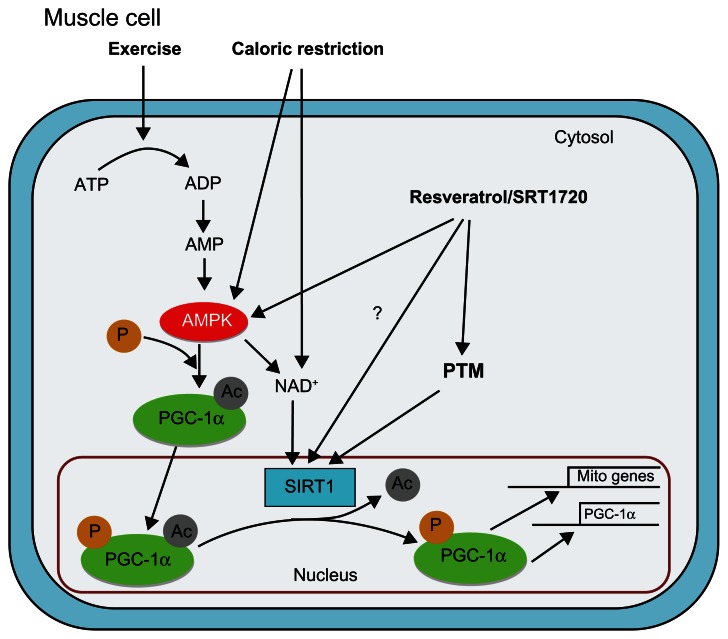
Figure 1.
Model of pathways leading to activation of the silent mating type information regulation 2 homolog 1 (SIRT1)/peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) axis in skeletal muscle: SIRT1 deacetylase activity is directly influenced through posttranslational modification and indirectly through exercise, caloric restriction, and pharmaceutical activation.
Notes: Exercise-induced activation of adenosine monophosphate-activated protein kinase increases nicotinamide adenine dinucleotide, a necessary substrate in the SIRT1 deacetylase reaction, and directly phosphorylates cytosolic PGC-1α prior to nuclear translocation. Caloric restriction activates adenosine monophosphate-activated protein kinase and increases nicotinamide adenine dinucleotide, while pharmaceuticals (resveratrol/SRT1720) activate adenosine monophosphate-activated protein kinase, induce SIRT1 posttranslational modification, and may augment SIRT1 deacetylase activity through direct interaction (dashed line). SIRT1 activates PGC-1α through deacetylation, increasing PGC-1α-mediated autoexpression and transcription of mitochondrial genes.
Abbreviations: Ac, acetyl; ADP, adenosine diphosphate; AMP, adenosine monophosphate; AMPK, adenosine monophosphate-activated protein kinase; ATP, adenosine triphosphate; NAD+, nicotinamide adenine dinucleotide; P, phosphate; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; PTM, posttranslational modification; SIRT1, silent mating type information regulation 2 homolog 1.
Within this context, this review will first examine the role of skeletal muscle mitochondria in the development of obesity and metabolic disorders and the importance of the SIRT1/PGC-1α axis in the determination of skeletal muscle mitochondrial content. Once the importance of the SIRT1/PGC-1α axis to metabolic health has been established, potential interventions – pharmacological and otherwise – designed to activate SIRT1 in an attempt to prevent and/or treat obesity and obesity-related metabolic disorder will be examined.
Skeletal muscle mitochondria: mechanisms of metabolic disease
Although not all individuals who are overweight or obese develop metabolic disorders, in general, there appears to be a progression from overweight, to obese, to metabolically diseased. Consistent with this idea, being overweight as a young adult is highly predictive of obesity later in life,24 and adult obesity is strongly associated with increased risk for many diseases, including insulin resistance, type II diabetes, and cardiovascular disease.1,2,11,25 Changes in mitochondrial content and function are implicated in the development of several metabolic diseases. While the importance of mitochondrial dysfunction is controversial,26 there is significant evidence that mitochondrial content is altered in metabolic dysfunction. For example, the decline in skeletal muscle mitochondria associated with altered metabolic health is eloquently demonstrated by observations that mitochondrial oxidative capacity is lower in obese than in lean adults, and further decreased in type II diabetics (ie, lean > obese > type II diabetes).10 Further, a positive correlation has been observed between mitochondrial oxidative capacity and insulin sensitivity in lean and obese young adults27,28 and in sedentary and exercise-trained older adults.29 While it is not clear whether mitochondrial dysfunction (defined as either decreases in individual mitochondrial oxidative capacity and/or decreased mitochondrial content) precedes initial weight gain, there is substantial evidence that mitochondrial function is impaired in both obesity11,28,30,31 and type II diabetes.14,32 At present, there is still debate regarding the exact mechanism(s) linking mitochondrial dysfunction to metabolic disease; however, the current literature suggests that either impaired fatty acid oxidation and/or increased oxidative stress resulting from altered mitochondrial function are central in the etiology of metabolic disease.
Fatty acid oxidative capacity is correlated with insulin sensitivity in obese individuals30 and impairments in lipid oxidation, accompanied by a concurrent upregulation of fatty acid transport,30 appear to underlie the accumulations of intramuscular fat associated with obesity and metabolic disease.11 Impaired lipid oxidation in obese skeletal muscle appears to be related to dysfunctional mitochondria lacking appropriate oxidative enzyme activity, decreased mitochondrial content within skeletal muscle, or a combination of both.10–12,16,26,33 As individuals progress from obesity to metabolic disease, there is a decrease in the content or quality of mitochondria, which results in an accumulation of intramuscular fat that is intimately related to decreases in metabolic health.
The accumulation of intramuscular fat is implicated in the development of a number of comorbidities, particularly insulin insensitivity and type II diabetes.8,34–37 Intramuscular lipid accumulation and the associated increases in lipid species, particularly diacylglycerol38 and ceramides,35,39 have been linked to the disruption of the insulin receptor cascade.26 Decreased insulin action (insulin insensitivity and the eventual development of insulin resistance and type II diabetes) is independently associated with the progression of hypertension and hyperlipidemia, and is known to be atherogenic.34,40
In addition to a decreased capacity to oxidize fat, impaired mitochondrial function also contributes to chronic increases in oxidative stress via increased lipid peroxidation. Decreased fatty acid usage results in slowed intramuscular lipid turnover and a resulting maladaptive peroxidation of intramuscular lipids and reactive lipid species accumulation.41,42 Lowered lipid turnover rates, coupled with dysfunctional mitochondria prone to reactive oxygen species production, facilitate the peroxidation of lipids and the production of lipotoxic lipid species in the intramuscular lipid pool.43 These lipotoxic lipid species further interfere with mitochondrial function and intracellular signaling through the disruption of protein and DNA structure, potentiating lipid peroxidation in a damaging cycle that contributes to the development of metabolic disease (see Schrauwen and Hesselink for a detailed review).43
The importance of skeletal muscle mitochondria for overall metabolic health is illustrated through the association of mitochondrial dysfunction and metabolic disease. The accumulation of damaging reactive lipid species within the muscle is hypothesized to result from impaired mitochondrial function, and the ensuing oxidative stress is implicated in the pathophysiology of a host of metabolic diseases. Specifically, oxidative stress is suggested to contribute to the development of obesity and several other diseases associated with metabolic syndrome, including coronary artery disease, hypertension, and type II diabetes. For more detailed information on the role of oxidative stress in obesity and metabolic disease, the reader is referred to Furukawa et al and Roberts and Sindhu.44,45 The activation of PGC-1α has been implicated in the control of antioxidant expression and the prevention of mitochondrial oxidative damage in mice,46 reinforcing the importance of targeting the SIRT1/PGC-1α axis to improve metabolic health. Improving skeletal muscle mitochondrial content may reverse this lipid accumulation and potentially ameliorate the associated metabolic dysfunction. Thus, skeletal muscle mitochondrial content, through its role in the determination of intramuscular fat content, is intimately related to systemic metabolic health.
Genetic control of skeletal muscle mitochondrial content: the SIRT1/PGC-1α axis
Plasticity is a defining characteristic of skeletal muscle; thus, there is great potential for the remodeling of metabolic function towards a healthy phenotype in all populations. For example, exercise training can significantly increase mitochondrial oxidative activity and fat oxidation, decrease intramuscular lipid accumulation and oxidative stress, and improve insulin sensitivity – all parameters of healthy skeletal muscle.36,47–49 These adaptations are largely due to increases in mitochondrial content and function resulting from the upregulation of a genetic program of mitochondrial protein controlled through the SIRT1/PGC-1α axis.
PGC-1α drives the transformation of skeletal muscle towards an oxidative phenotype via interaction with, and activation of, a plethora of transcription factors involved in the induction of mitochondrial genes encoded within both nuclear and mitochondrial DNA.17,18,50–52 While the regulation of the transcriptional activity of PGC-1α is complex,53,54 induction of PGC-1α-mediated transcription is accomplished through the acute activation of PGC-1α55,56 and chronic upregulation of PGC-1α protein content via an autoregulatory loop.57 Importantly, the acute activation of PGC-1α, accomplished via posttranslational modification, appears to be an essential first step in the induction of PGC-1α-mediated transcription of mitochondrial genes.21 PGC-1α is posttranslationally modified by phosphorylation via p38 mitogen-activated protein kinase and adenosine monophosphate-activated protein kinase (AMPK).58–60 In addition, PGC-1α acetylation status is also implicated in PGC-1α transcriptional activity and mitochondrial biogenesis.20,61 For example, acetylation levels of PGC-1α are inversely correlated with oxidative capacity in murine skeletal muscle,62 while genetic mutation of acetylation sites on PGC-1α (mimicking the deacetylated state) markedly increase PGC-1α transcriptional activity and mitochondrial biogenesis in skeletal muscle cells.62
Through the deacetylation of acetylated-lysine residues, the sirtuins (a family of evolutionarily conserved deacetylase) modify protein activity and have been implicated in a variety of cellular processes, including the stress response and cellular energy control in mammals.22,63,64 While seven sirtuins have been identified in humans, several of which are implicated in metabolic control, including the mitochondrial sirtuins SIRT3, SIRT4, and SIRT5,65 the current review will focus on SIRT1 in skeletal muscle and metabolic disease. SIRT1 directly interacts with PGC-1α in mouse hepatocytes,61 and is responsible for deacetylation of PGC-1α in 293T cells61 and PC12 neuronal cells.66 In addition, SIRT1 interacts with PGC-1α in C2C12 skeletal muscle cells,20 and SIRT1-mediated deacetylation of PGC-1α appears to play an important, but perhaps nonessential,23 role in the induction of PGC-1α-mediated transcription.62 In support of this evidence from cells, SIRT1 is implicated in caloric restriction, exercise, and aminoimidazole carboxamide ribonucleotide-induced upregulation of mitochondrial content and function in skeletal muscle of mice.20,62,67 Further, in transgenic animal models, overexpression of SIRT1 consistently improves insulin sensitivity68,69 and exerts a protective effect against metabolic disorders commonly associated with high-fat feeding.70 This evidence implicates the SIRT1/PGC-1α axis in skeletal muscle in the upregulation of genes implicated in mitochondrial content,71,72 as well as fatty acid oxidation and energy expenditure,20 and illustrates the importance of this axis for any intervention designed to reverse skeletal muscle dysfunction. As such, targeting the SIRT1/PGC-1α axis may be a practical treatment model for obesity and metabolic disorder.
Physiological control of SIRT1 activity
In vivo, SIRT1 activity is regulated by substrate availability, posttranslational modification, and the formation of both inhibiting and activating complexes.73 Activation of SIRT1 through one, or several, of these mechanisms is expected to upregulate mitochondrial gene expression via the SIRT1/PGC-1α axis, resulting in improved mitochondrial and metabolic function.
Substrate availability is implicated in SIRT1 activation, primarily through changes in cellular redox potential. Nicotinamide adenine dinucleotide (NAD+) is a substrate in SIRT1-mediated deacetylation, which suggests changes in NAD+ can affect SIRT1 activity.61,74 Consistent with this hypothesis, SIRT1 activity (measured as a decrease in PGC-1α acetylation status) and NAD+ are increased concomitantly during nutrient deprivation in hepatocytes61 and C2C12 skeletal muscle cells,20,61 and following nutrient deprivation67 and exercise62,67 in intact mouse muscle. Recent reports suggest that AMPK, an intracellular energy sensor,75 mediates increases in NAD+ during periods of nutrient restriction, and thus plays a key role in regulating SIRT1 activity and transcriptional programs controlled through the SIRT1/PGC-1α axis.62,67 Additionally, nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in the NAD+ salvage pathway,76,77 is implicated in the control of intramuscular NAD+ and thus SIRT1 activity. NAMPT is upregulated following interventions known to activate SIRT1 in skeletal muscle – specifically, exercise training in humans78 and acute exercise in mice.67 These results comprise the main evidence supporting the hypothesis that SIRT1 activity is increased in response to altered energy status through increased substrate availability via AMPK, NAMPT, or both. Interestingly, in both cell and animal models,49,79–81 SIRT1 activity does not always correspond to changes in NAD+. These results suggest that SIRT1 activity is under more complex control than can be explained through alterations in substrate availability alone.
Several recent lines of evidence have implicated posttranslational modifications of SIRT1 and the formation of inhibitory82,83 and activating complexes in the control of SIRT1 deacetylase activity.84,85 A number of phosphorylation sites have been identified on SIRT1,86,87 and phosphorylation of these sites consistently increases SIRT1 deacetylase activity.86–88 In addition to phosphorylation, SIRT1 activity is influenced by sumoylation,89 methylation,90 and nitrosylation.91 Sumoylation by the small ubiquitin-like modifier family of enzymes increases SIRT1 catalytic activity,89 while it is unclear whether methylation directly affects SIRT1 deacetylase activity.90 Transnitrosylation of SIRT1 has also been demonstrated, resulting in increased acetylation of SIRT1 targets, suggesting an inhibitory effect on SIRT1 deacetylase activity with nitrosylation.91 In addition to posttranslational modifications, SIRT1 deacetylase activity can be modified through the formation of regulatory complexes. Specifically, SIRT1 deacetylase activity is inhibited when forming a complex with deleted in breast cancer-185 and activated when forming a complex with active regulator of SIRT1.84 These types of SIRT1 activity regulations (both posttranslational modifications and regulatory complex formation) have not been thoroughly explored in skeletal muscle, making it an important direction for future research.
The physiological pathways controlling SIRT1 activity, outlined above, can be activated by both exercise and caloric restriction. The exact mechanism by which caloric restriction impacts SIRT1 activity remains unclear, although it is hypothesized that caloric restriction initiates a disturbance to metabolic homeostasis, activating AMPK and subsequently increasing cellular NAD+, resulting in an increased activation of SIRT1.20,21,61,62,67 Caloric restriction may also increase SIRT1 activity through activation of NAMPT, increasing flux through the NAD+ salvage pathway.92 Despite the uncertainty surrounding the mechanism of caloric restriction-mediated SIRT1 activation, it is apparent that caloric restriction increases SIRT1 deacetylation activity and can play a role in the activation of the SIRT1/PGC-1α axis, increasing fatty acid oxidation in skeletal muscle,62 and providing protective metabolic effects against an energy imbalance.93 For a more detailed review on the role of SIRT1 in caloric restriction, the reader is referred to Canto and Auwerx.21,93
Pharmacological activation of SIRT1
In addition to physiological activation, SIRT1 can also be pharmacologically activated through interactions with a number of natural and synthetic molecules. Resveratrol, a naturally occurring polyphenol found in grape skins, has been implicated in the activation of SIRT1.72,94–96 While resveratrol administration can increase SIRT1 activity by as much as eight-fold,96 the exact mechanism of activation remains elusive. Although it was initially believed that resveratrol interacts with and activates SIRT1 directly, more recent investigations have demonstrated that this interaction may be an artifact of the fluorophore detection method,97,98 and in vivo resveratrol-mediated activation of SIRT1 appears to result from activation of upstream targets.72,99,100 The synthetic production of SIRT1 activators has also been successful; in particular, SRT1720 increases SIRT1 deacetylase activity more potently than resveratrol in both cell lines and within skeletal muscle of mice.94,96,101 However, as with resveratrol, whether SRT1720 activates SIRT1 directly or indirectly remains unclear.98,101 Despite the uncertainty surrounding the mechanism of these molecules and their interaction with SIRT1, their ability to increase SIRT1 deacetylase activity is well established. The potential for activation of the SIRT1/PGC-1α axis through pharmacological means makes SIRT1 an intriguing target for future therapeutic intervention.
Targeting SIRT1 activation as therapeutic intervention
The remainder of this review will explore the potential benefits and limitations of targeting SIRT1, through pharmacological and physiological interventions, for the purpose of activating the SIRT1/PGC-1α axis to stimulate mitochondrial biogenesis and improve skeletal muscle function and metabolic health.
Health benefits associated with pharmaceutical activation of SIRT1
The pharmacological activation of SIRT1 exerts its therapeutic effects on skeletal muscle through the SIRT1/PGC-1α axis. By increasing mitochondrial content and function through PGC-1α-mediated transcription, improved skeletal muscle function may prevent or reverse obesity and metabolic disease. In skeletal muscle, resveratrol activates SIRT1 resulting in deacetylation of PGC-1α and the induction of a genetic profile associated with improved mitochondrial function and fatty acid metabolism.72 As mentioned previously, while SIRT1 is required for resveratrol-mediated deacetylation of PGC-1α in mouse embryonic fibroblasts,72 there is some debate regarding resveratrol’s direct interaction with SIRT1,98,102 with some evidence suggesting that resveratrol’s effects may occur via an AMPK-mediated mechanism.72,99,100 Regardless, AMPK and SIRT1 are believed to function in concert in skeletal muscle,67 and this uncertainty only questions whether resveratrol acts directly on SIRT1, not whether resveratrol activates the SIRT1/PGC-1α axis.
Consistent with the above, activation of the SIRT1/PGC-1α axis following resveratrol treatment in mice increases mitochondrial and fatty acid gene expression and increases mitochondrial content in skeletal muscle.72 Accompanying these improvements in skeletal muscle function are improved insulin sensitivity in mouse models72,99,103 and a resistance to the deleterious effects of aging and a high-fat diet on metabolic health.72,103,104 At present, there are relatively few studies examining the metabolic impact of resveratrol in humans. However, a recent study in obese males indicates that 30 days of dietary resveratrol supplementation increased skeletal muscle SIRT1, PGC-1α protein content, and intrinsic mitochondrial function and improved several markers of cardiovascular and metabolic health.105 Two other studies have also reported beneficial effects of dietary resveratrol in humans, including improved glucose tolerance in older adults106 and a reduction of the oxidative and inflammatory response to a high-fat meal.107 While these studies provide promising initial findings indicating that targeting SIRT1 via resveratrol supplementation may prove therapeutic for obesity and obesity-related disease, randomized controlled studies are still needed to confirm these beneficial effects in a larger population. In addition, there are little data regarding whether a prolonged intake of resveratrol has any negative implications either within skeletal muscle, within other organs (liver for example), or on overall metabolic health in humans.
The use of the synthetic SIRT1 activator SRT1720 has been proposed to selectively activate SIRT1 with greater potency, efficacy, and selectivity than resveratrol.94,96,101 The chronic administration of SRT1720 in mice decreases acetylation of PGC-1α and a number of other SIRT1 targets94 and increases oxidative capacity, lipid oxidation, insulin sensitivity, and provides a protective effect against diet-induced obesity.94,95,101 In addition to protective metabolic effects, mice on high-fat diets given SRT1720 ran twice the distance in endurance trials and improved muscle function in a variety of functional tests when compared to controls fed the same diet.94 These skeletal muscle adaptations make SRT1720 an appealing molecule for therapeutic intervention. Similar to resveratrol, controversy exists regarding whether SRT1720 activates SIRT1 through direct interaction, or acts indirectly via activation of AMPK through alterations in cellular energy status.98 Thus the metabolic adaptations attributed directly to SRT1720–SIRT1 interaction versus an AMPK-mediated SIRT1 activation are difficult to distinguish.94 Phase I and II clinical trials examining the safety and metabolic benefits of a drug closely related to SRT1720 (ie, SRT2104) are currently underway and represent an important look into the ability of SRT1720/2104 to increase mitochondrial content in skeletal muscle and improve metabolic function in obesity and metabolic disease.
At present, interventions with both resveratrol and SRT1720 in mice and in preliminary human studies (resveratrol only) appear to activate the SIRT1/PGC-1α axis, improve mitochondrial content in skeletal muscle, and improve metabolic health. These findings support the suggestion that these pharmaceuticals are promising potential agents for the prevention and treatment of obesity and its related diseases, and warrant further investigation.
Health benefits of the SIRT1/PGC-1α axis activation during exercise
Exercise, as a component of lifestyle intervention, is an alternative to pharmacological interventions aimed at improving skeletal muscle mitochondrial function and metabolic health.108 An emerging line of evidence suggests that exercise targets SIRT1, and the resulting activation of the SIRT1/PGC-1α axis underlies many of the beneficial effects associated with exercise.109,110
An acute bout of exercise in rat and mouse muscle increases SIRT1 activity109 and deacetylation of PGC-1α immediately, and for several hours following exercise.62,67 Exercise training is also implicated in the activation of SIRT1 in animal models, with both increases in SIRT1 activity49,80,81,111 and deacetylation of PGC-1α112 observed following chronic contractile activity and treadmill running. Activation of SIRT1 was also observed in human skeletal muscle after 2 weeks and 6 weeks of exercise training.71,109 While skeletal muscle SIRT1 protein content is also elevated following acute exercise and exercise training in both rats113,114 and humans,115,116 the implications of elevated SIRT1 content in muscle remain unclear.117 It should also be noted that although PGC-1α deacetylation was normal in mice expressing SIRT1 lacking deacetylase activity,23 the majority of observations in intact animal and human skeletal muscle support the involvement of SIRT1 in mediating PGC-1α acetylation status. Also consistent with the contention that SIRT1 and the SIRT1/PGC-1α axis play a critical role in the control of skeletal muscle mitochondrial content are numerous animal studies reporting that increased SIRT1 activity is accompanied by increases in PGC-1α transcriptional activity.80,81,113,114,118
The activation of the SIRT/PGC-1α axis by exercise is accompanied by increases in markers of oxidative capacity and mitochondrial content in animals71,80,81 and humans.71,116 Further, markers of improved fatty acid oxidation and insulin sensitivity are also found in animals and humans accompanying activation of the SIRT1/PGC-1α axis by exercise.71,114,116 In addition to mitochondrial adaptations, activation of the SIRT1/PGC-1α axis is linked to the attenuation of age-related decline in skeletal muscle health in exercised rodents49,111,113 via decreased oxidative stress and DNA damage, factors also implicated in the pathogenesis of many metabolic disorders associated with obesity.44 Consistent with these observations, exercise training improves oxidative capacity and fatty acid oxidation in skeletal muscle from obese adults,13,119,120 improves insulin sensitivity in obesity and type II diabetes,121,122 and decreases both risk factors for, and symptoms of, metabolic disease.123,124 In summary, exercise appears to activate the SIRT1/PGC-1α axis and improve skeletal muscle mitochondrial function and metabolic health. These results highlight the preventative and therapeutic potential of exercise for obesity and obesity-related disease.
As an alternative treatment in obesity and metabolic disease, exercise has several inherent advantages over pharmaceutical intervention. First, the improved metabolic function associated with exercise comes at minimal financial cost, while a pharmaceutical intervention carries a substantial financial commitment from both the individual and healthcare provider.4,6 Second, in addition to improved skeletal muscle mitochondrial function and metabolic/cardiovascular health, regular exercise is associated with a myriad of beneficial effects ranging from the prevention and treatment of mental disorders125 and cancer126 to alleviating symptoms and improving quality of life in many chronic diseases.127 Third, exercise is implicated in a systemic improvement of health with little to no risk of adverse side effects.108,128 Pharmaceuticals are often associated with undesirable side effects, and are inherently designed to be specific, eliminating the possibility of a systemic health improvement. Finally, there is evidence that exercise, as part of a lifestyle intervention, induces superior improvements compared to pharmaceutical intervention in subjects with metabolic disease.121 In light of these arguments, it makes both health and financial sense that exercise becomes a first-line tool in both the prevention and treatment of obesity and obesity-related disease.
Conclusion and future direction
The current review has focused on the contribution of SIRT1 to skeletal muscle function and metabolic health. Future studies should not only continue to investigate SIRT1 function, but should also focus on other members of the sirtuin family. The contribution of SIRT1 to overall metabolic health occurs, in part, through SIRT1’s influence on PGC-1α and skeletal muscle mitochondrial function. As skeletal muscle mitochondrial dysregulation is implicated in obesity and obesity-related metabolic disease, SIRT1 has become an attractive target for therapeutic intervention. The activation of SIRT1, and consequently the SIRT1/PGC-1α axis, results in upregulation of mitochondrial genes and improved skeletal muscle mitochondrial content and function. SIRT1 activity can be modified by pharmaceuticals and exercise, providing an array of options to pursue for implementing a therapeutic intervention. A clear need for further investigation of the feasibility of pharmaceutical intervention in humans is evident as, for the most part, human trials are in their infancy or are nonexistent. With exercise, exploration of the optimal dose and intensity will expand the possibility of tailored prescriptions targeting SIRT1 activity.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ. 2007;176(8):S1–S13. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16(10):2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(3):293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Kamei Y, Miura S, Suzuki M, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279(39):41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 8.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 9.Dyson PA. The therapeutics of lifestyle management on obesity. Diabetes Obes Metab. 2010;12(11):941–946. doi: 10.1111/j.1463-1326.2010.01256.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279(5):E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 12.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54(1):8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE. Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol. 2007;103(1):21–27. doi: 10.1152/japplphysiol.01228.2006. [DOI] [PubMed] [Google Scholar]
- 14.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 15.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holloway GP, Bonen A, Spriet LL. Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am J Clin Nutr. 2009;89(1):455S–462S. doi: 10.3945/ajcn.2008.26717B. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 19.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27(7):728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 20.Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1 alpha. EMBO J. 2007;26(7):1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canto C, Auwerx J. PGC-1 alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444(7121):868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 23.Philp A, Chen A, Lan D, et al. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) deacetylation following endurance exercise. J Biol Chem. 2011;286(35):30561–30570. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman KM, Craig CL, Gauvin L, Katzmarzyk PT. Tracking of obesity and physical activity from childhood to adulthood: the Physical Activity Longitudinal Study. Int J Pediatr Obes. 2009;4(4):281–288. doi: 10.3109/17477160802596171. [DOI] [PubMed] [Google Scholar]
- 25.Boyko EJ, de Courten M, Zimmet PZ, Chitson P, Tuomilehto J, Alberti KG. Features of the metabolic syndrome predict higher risk of diabetes and impaired glucose tolerance: a prospective study in Mauritius. Diabetes Care. 2000;23(9):1242–1248. doi: 10.2337/diacare.23.9.1242. [DOI] [PubMed] [Google Scholar]
- 26.Holloway GP. Mitochondrial function and dysfunction in exercise and insulin resistance. Appl Physiol Nutr Metab. 2009;34(3):440–446. doi: 10.1139/H09-028. [DOI] [PubMed] [Google Scholar]
- 27.Hickey MS, Weidner MD, Gavigan KE, Zheng D, Tyndall GL, Houmard JA. The insulin action-fiber type relationship in humans is muscle group-specific. Am J Physiol. 1995;269(1 Pt 1):E150–E154. doi: 10.1152/ajpendo.1995.269.1.E150. [DOI] [PubMed] [Google Scholar]
- 28.Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal-muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9(2):273–278. [PubMed] [Google Scholar]
- 29.Houmard JA, Egan PC, Neufer PD, et al. Elevated skeletal muscle glucose transporter levels in exercise-trained middle-aged men. Am J Physiol. 1991;261(4 Pt 1):E437–E443. doi: 10.1152/ajpendo.1991.261.4.E437. [DOI] [PubMed] [Google Scholar]
- 30.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13(14):2051–2060. doi: 10.1096/fasebj.13.14.2051. [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277(6 Pt 1):E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 32.Asmann YW, Stump CS, Short KR, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55(12):3309–3319. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- 33.Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL. Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab. 2004;287(6):E1076–E1081. doi: 10.1152/ajpendo.00177.2004. [DOI] [PubMed] [Google Scholar]
- 34.Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90(5A):11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- 35.Holland WL, Knotts TA, Chavez JA, Wang L, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65(6 Pt 2):S39–S46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 36.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49(4):467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 37.Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46(6):983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 38.Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav. 2008;94(2):242–251. doi: 10.1016/j.physbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Defronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 41.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 42.Samocha-Bonet D, Heilbronn LK, Lichtenberg D, Campbell LV. Does skeletal muscle oxidative stress initiate insulin resistance in genetically predisposed individuals? Trends Endocrinol Metab. 2010;21(2):83–88. doi: 10.1016/j.tem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes. 2004;53(6):1412–1417. doi: 10.2337/diabetes.53.6.1412. [DOI] [PubMed] [Google Scholar]
- 44.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84(21–22):705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1 alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106(48):20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Bruce CR, Thrush AB, Mertz VA, et al. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291(1):E99–E107. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- 48.Gibala MJ, Little JP, van Essen M, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575(Pt 3):901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrara N, Rinaldi B, Corbi G, et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11(1):139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 50.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 51.Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann N Y Acad Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lira VA, Benton CR, Yan Z, Bonen A. PGC-1 alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299(2):E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol. 2008;59(Suppl 7):5–18. [PubMed] [Google Scholar]
- 54.Gibala M. Molecular responses to high-intensity interval exercise. Appl Physiol Nutr Metab. 2009;34(3):428–432. doi: 10.1139/H09-046. [DOI] [PubMed] [Google Scholar]
- 55.Wright DC, Han D, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1 alpha expression. J Biol Chem. 2007;282(1):194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 56.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588(Pt 23):4795–4810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Handschin C, Rhee J, Lin JD, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1 alpha expression in muscle. Proc Natl Acad Sci U S A. 2003;100(12):7111–7116. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puigserver P, Rhee J, Lin J, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPAR gamma coactivator-1. Mol Cell. 2001;8(5):971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 59.Jaeger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1 alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1 alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282(26):18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- 61.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1 alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 62.Canto C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the “magnificent seven,” function, metabolism and longevity. Ann Med. 2007;39(5):335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 64.Yang T, Sauve AA. NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 2006;8(4):E632–E643. doi: 10.1208/aapsj080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flick F, Luscher B. Regulation of sirtuin function by posttranslational modifications. Front Pharmacol. 2012;3:29. doi: 10.3389/fphar.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1 alpha. J Biol Chem. 2005;280(16):16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 67.Canto C, Jiang LQ, Deshmukh AS, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11(3):213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8(4):333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bordone L, Cohen D, Robinson A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6(6):759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 70.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gurd BJ, Yoshida Y, McFarlan JT, et al. Nuclear SIRT1 activity, but not protein content, regulates mitochondrial biogenesis in rat and human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):R67–R75. doi: 10.1152/ajpregu.00417.2010. [DOI] [PubMed] [Google Scholar]
- 72.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 73.Zschoernig B, Mahlknecht U. SIRTUIN 1: regulating the regulator. Biochem Biophys Res Commun. 2008;376(2):251–255. doi: 10.1016/j.bbrc.2008.08.137. [DOI] [PubMed] [Google Scholar]
- 74.Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- 75.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8(10):774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 76.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279(49):50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 77.Yang H, Yang T, Baur JA, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130(6):1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costford SR, Bajpeyi S, Pasarica M, et al. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298(1):E117–E126. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson RM, Latorre-Esteves M, Neves AR, et al. Yeast life-span extension by calorie restriction is independent of NAD fluctuation. Science. 2003;302(5653):2124–2126. doi: 10.1126/science.1088697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chabi B, Adhihetty PJ, O’Leary MF, Menzies KJ, Hood DA. Relationship between Sirt1 expression and mitochondrial proteins during conditions of chronic muscle use and disuse. J Appl Physiol. 2009;107(6):1730–1735. doi: 10.1152/japplphysiol.91451.2008. [DOI] [PubMed] [Google Scholar]
- 81.Gurd BJ, Yoshida Y, Lally J, Holloway GP, Bonen A. The deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis. J Physiol. 2009;587(Pt 8):1817–1828. doi: 10.1113/jphysiol.2008.168096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451(7178):583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- 83.Escande C, Chini CC, Nin V, et al. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120(2):545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28(2):277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 85.Nin V, Escande C, Chini CC, et al. Role of deleted in breast cancer 1 (DBC1) in SIRT1 activation induced by protein kinase A and AMP activated protein kinase. J Biol Chem. 2012 May 3; doi: 10.1074/jbc.M112.365874. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gerhart-Hines Z, Dominy JE, Jr, Blattler SM, et al. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+ Mol Cell. 2011;44(6):851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sasaki T, Maier B, Koclega KD, et al. Phosphorylation regulates SIRT1 function. PLoS One. 2008;3(12):e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nasrin N, Kaushik VK, Fortier E, et al. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4(12):e8414. doi: 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Y, Fu W, Chen J, et al. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9(11):1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X, Wang D, Zhao Y, et al. Methyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1) Proc Natl Acad Sci U S A. 2011;108(5):1925–1930. doi: 10.1073/pnas.1019619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kornberg MD, Sen N, Hara MR, et al. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12(11):1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423(6936):181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Canto C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20(7):325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feige JN, Lagouge M, Canto C, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8(5):347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 95.Smith JJ, Kenney RD, Gagne DJ, et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 97.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280(17):17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 98.Pacholec M, Bleasdale JE, Chrunyk B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285(11):8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen S, Li J, Zhang Z, et al. Effects of resveratrol on the amelioration of insulin resistance in KKAy mice. Can J Physiol Pharmacol. 2012;90(2):237–242. doi: 10.1139/y11-123. [DOI] [PubMed] [Google Scholar]
- 100.Park SJ, Ahmad F, Philp A, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dai H, Kustigian L, Carney D, et al. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010;285(43):32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crandall JP, Oram V, Trandafirescu G, et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012 Jan 4; doi: 10.1093/gerona/glr235. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ghanim H, Sia CL, Korzeniewski K, et al. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J Clin Endocrinol Metab. 2011;96(5):1409–1414. doi: 10.1210/jc.2010-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 109.Gurd BJ. Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab. 2011;36(5):589–597. doi: 10.1139/h11-070. [DOI] [PubMed] [Google Scholar]
- 110.Benton CR, Wright DC, Bonen A. PGC-1 alpha-mediated regulation of gene expression and metabolism: implications for nutrition and exercise prescriptions. Appl Physiol Nutr Metab. 2008;33(5):843–862. doi: 10.1139/H08-074. [DOI] [PubMed] [Google Scholar]
- 111.Koltai E, Szabo Z, Atalay M, et al. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131(1):21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li L, Pan R, Li R, et al. Mitochondrial biogenesis and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation by physical activity: intact adipocytokine signaling is required. Diabetes. 2011;60(1):157–167. doi: 10.2337/db10-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ljubicic V, Joseph AM, Adhihetty PJ, et al. Molecular basis for an attenuated mitochondrial adaptive plasticity in aged skeletal muscle. Aging (Albany, NY) 2009;1(9):818–830. doi: 10.18632/aging.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1 alpha protein expressions in rat skeletal muscle. Metabolism. 2008;57(7):986–998. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 115.Guerra B, Guadalupe-Grau A, Fuentes T, et al. SIRT1, AMP-activated protein kinase phosphorylation and downstream kinases in response to a single bout of sprint exercise: influence of glucose ingestion. Eur J Appl Physiol. 2010;109(4):731–743. doi: 10.1007/s00421-010-1413-y. [DOI] [PubMed] [Google Scholar]
- 116.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588(Pt 6):1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gurd BJ, Little JP, Perry CG. Does SIRT1 determine exercise-induced skeletal muscle mitochondrial biogenesis: differences between in vitro and in vivo experiments? J Appl Physiol. 2012;112(5):926–928. doi: 10.1152/japplphysiol.01262.2011. [DOI] [PubMed] [Google Scholar]
- 118.Dumke CL, Davis JM, Murphy EA, et al. Successive bouts of cycling stimulates genes associated with mitochondrial biogenesis. Eur J Appl Physiol. 2009;107(4):419–427. doi: 10.1007/s00421-009-1143-1. [DOI] [PubMed] [Google Scholar]
- 119.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab. 2005;288(4):E818–E825. doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- 120.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52(9):2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 121.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laaksonen DE, Lindstrom J, Lakka TA, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54(1):158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 123.Hawley JA, Gibala MJ. Exercise intensity and insulin sensitivity: how low can you go? Diabetologia. 2009;52(9):1709–1713. doi: 10.1007/s00125-009-1425-5. [DOI] [PubMed] [Google Scholar]
- 124.Tjonna AE, Lee SJ, Rognmo O, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wolff E, Gaudlitz K, von Lindenberger BL, Plag J, Heinz A, Strohle A. Exercise and physical activity in mental disorders. Eur Arch Psychiatry Clin Neurosci. 2011;261(Suppl 2):S186–S191. doi: 10.1007/s00406-011-0254-y. [DOI] [PubMed] [Google Scholar]
- 126.Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50(2):167–178. doi: 10.3109/0284186X.2010.529822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(Suppl 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 128.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]