Abstract
1,2,3,4-diepoxybutane (DEB) is the key carcinogenic metabolite of 1,3-butadiene (BD), an important industrial and environmental chemical present in urban air and in cigarette smoke. DEB is a genotoxic bis-electrophile capable of cross-linking cellular biomolecules to form DNA-DNA and DNA-protein cross-links (DPCs). In the present work, mass spectrometry-based proteomics was employed to characterize DEB-mediated DNA-protein cross-linking in human fibrosarcoma (HT1080) cells. Over 150 proteins including histones, high mobility group proteins, transcription factors, splicing factors, and tubulins were found among those covalently cross-linked to chromosomal DNA in the presence of DEB. A large portion of the cross-linked proteins are known factors involved in DNA binding, transcriptional regulation, cell signaling, DNA repair, and DNA damage response. HPLC-ESI+-MS/MS analysis of total proteolytic digests revealed the presence of 1-(S-cysteinyl)-4-(guan-7-yl)-2,3-butanediol conjugates, confirming that DEB forms DPCs between cysteine thiols within proteins and the N-7 guanine positions within DNA. However, relatively high concentrations of DEB were required to achieve significant DPC formation, indicating that it is a poor cross-linking agent as compared to antitumor nitrogen mustards and platinum compounds.
Keywords: DNA-protein cross-links, diepoxybutane, mass spectrometry, proteomics, western blot
Introduction
DNA-Protein cross-links (DPCs) are super-bulky DNA lesions that are formed upon irreversible bonding of proteins to chromosomal DNA in the presence of cross-linking agents. Due to their enormous size, DPCs can distort DNA helix and interfere with normal DNA-protein interactions, blocking DNA replication, transcription, repair, recombination, and chromatin remodeling.1 DPCs can be induced by various chemical and physical agents including 1,2,3,4-diepoxybutane (DEB),2,3 endogenous aldehydes,4,5 ionizing radiation,6 UV light,1 transition metals,7 and therapeutic agents including nitrogen mustards,8-10 platinum drugs,11 and haloethylnitrosoureas.12 Despite their ubiquitous formation in living cells, structural features and protein composition of DPC lesions are not well established due to their inherent heterogeneity and the difficulty of analyzing macromolecular conjugates containing both proteins and DNA.
1,2,3,4-Diepoxybutane (DEB) is a carcinogenic, clastogenic, and genotoxic diepoxide produced upon metabolic activation of 1,3-butadiene (BD)(Scheme 1). BD is a known animal and human carcinogen present in automobile exhaust, urban air, and cigarette smoke.13,14 The potent biological activity of DEB is attributed to its ability to cross-link cellular biomolecules. Initial alkylation of adenine and guanine bases in DNA by DEB produces 2-hydroxy-3,4-epoxybut-1-yl (HEB) monoadducts, which can undergo further reactions, such as hydrolysis and alkylation of neighboring nucleobases to form DNA-DNA cross-links.15 Alternatively, the epoxide groups of 2-hydroxy-3,4-epoxybut-1-yl (HEB) lesions react with nucleophilic amino acid side chains of adjacent proteins, forming bulky DPC lesions (Scheme 1).3,15
Scheme 1.

Metabolic activation of 1,3-butadiene to 1,2,3,4-diepoxybutane (DEB) and the formation of DNA-protein cross-links by DEB.
DNA-protein cross-linking by DEB was first observed by Jelitto et al.15 These authors employed alkaline elution methodology to detect DPC formation in liver tissue of BD-exposed B6C3F1 mice.15 More recently, it has been shown that DEB forms DPC involving specific proteins such as O6-alkylguanine DNA-alkyltransferase (AGT), 3 GAPDH,16 and histones;17 and that overexpression of these proteins in bacteria can increase the toxicity and mutagenicity of DEB.16-19 Our laboratory used a mass spectrometry-based approach to demonstrate that in DEB-induced DPC lesions, the N-7 position of guanine in DNA is cross-linked to cysteine side chains within the proteins.3 We further investigated DPC formation by DEB through an affinity capture approach coupled with mass spectrometry.2 Biotinylated DNA duplexes were incubated with protein extracts from human cervical carcinoma (HeLa) cells in the presence of DEB, and the resulting DPCs were captured on streptavidine beads and identified by mass spectrometry based proteomics. A total of 39 proteins were identified, including those known to participate in transcriptional regulation (e.g., GAPDH), chromatin remodeling (e.g., actin), and DNA repair (e.g., AGT).2 However, these in vitro experiments were limited to synthetic DNA duplexes which cannot model normal DNA-protein interactions observed in cells.
The purpose of the present study was to characterize DNA-protein cross-linking in human fibrosarcoma (HT1080) cells treated with toxic concentrations of DEB. Proteins covalently trapped on DNA were isolated from cells by a modified phenol/chloroform extraction methodology recently developed by our group.8 Thermal hydrolysis was used to release DPCs from DNA backbone in the form of protein-guanine conjugates. Proteins participating in cross-linking were separated by SDS-PAGE and identified by mass spectrometry-based proteomics. A total of 152 cross-linked proteins were found, including those known to be involved in transcriptional regulation, apoptosis, DNA repair, DNA damage response, chromatin remodeling, cell motility, and cell signaling. HPLC-ESI+-MS/MS analysis of total proteolytic digests revealed that DEB cross-links cysteine thiols within proteins to the N-7 guanine positions within DNA. Comparison of protein lists to those previously generated for mechlorethamine and cisplatin-induced DNA-protein cross-linking in cells8 indicates that while some proteins are targeted by all three bis-electrophiles, these cross-linking agents show significant selectivity in regard to the proteins that they cross-link to DNA in human cells.
Experimental Section
Safety statement
DEB is a known carcinogen and must be handled with adequate safety precautions. Phenol and chloroform are toxic chemicals that should be handled in a well-ventilated fume hood with appropriate personal protective equipment.
Chemicals and Reagents
DEB, ammonium bicarbonate, ammonium acetate, phenylmethanesulfonyl fluoride (PMSF), leupeptin, pepstatin, aprotinin, dithiothreitol (DTT), iodoacetamide, chloroform, ribonuclease A, nuclease P1, and alkaline phosphatase were purchased from Sigma (St. Louis, MO). UltraPure™ Buffer-Saturated Phenol was obtained from Invitrogen (Carlsbad, CA). Mass spectrometry-grade trypsin was purchased from Promega (Madison, WI). Proteinase K was obtained from New England Biolabs (Beverly, MA). Primary polyclonal antibodies specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH, SC-25778), poly (ADP-ribose) polymerase 1 (PARP, SC-7150), DNA-(apurinic- or apyrimidinic-site) lyase (ref-1, SC-5577), histone-H4 (SC-10810), nucleophosmin (B23, SC-5564), and nucleolin (SC-13057) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Cys-N7G-BD and Cys-[15N]-N7G-BD were prepared as described previously.3
Cell Culture
Human fibrosarcoma (HT1080) cells20 were obtained from the American Type Culture Collection. The cells were maintained as exponentially growing monolayer cultures in Dulbecco’s modified Eagle’s medium supplemented with 9% fetal bovine serum (FBS), in a humidified incubator at 37 °C with 5% CO2.
Cytotoxicity experiments
Human fibrosarcoma (HT1080) cells were plated in Dulbecco’s modified Eagle’s medium containing 9% FBS at a density of 5 × 105 cells/dish and permitted to adhere overnight. On the following morning, cells (in duplicate) were treated with a range of DEB concentrations for 3 h at 37 °C. Trypan blue exclusion was used to confirm viability. Cells surviving treatment were counted in a hemocytometer, and cytotoxicity was expressed as the number of cells surviving DEB treatment relative to buffer-treated controls.
DPCs isolation from DEB-treated cells
HT1080 cells in culture were treated with DEB (0, 0.05, 0.10, 1.0, or 2.0 mM) for 3 h at 37 °C. Following exposure, the cells were washed with ice cold phosphate-buffered saline (PBS) and re-suspended in PBS to a final density of ~2 × 106 cells/mL. To isolate nuclei, cells were lysed by adding an equal volume of 2× cell lysis buffer (20 mM Tris-HCl/10 mM MgCl2/2%(v/v) Triton-X100/0.65 M sucrose), incubated on ice for 5 min, and centrifuged at 2000× g for 10 min at 4 °C. The nuclear pellets were re-suspended in a saline-EDTA solution (75 mM NaCl/24 mM EDTA/1% (w/v) SDS, pH 8.0) containing RNase A (10 μg/mL) and a protease inhibitor cocktail (1 mM PMSF; 1 μg/mL pepstatin; 0.5 μg/mL leupeptin; 1.5 μg/mL aprotinin) to a concentration of ~5 × 106 nuclei/mL and incubated for 2 h at 37 °C with gentle shaking. To remove free proteins, nuclear lysates were extracted with Tris-buffer saturated phenol and chloroform, and DPC-containing DNA was precipitated with cold ethanol. DNA amounts and its purity were estimated by UV and subsequently determined by quantitation of dG in enzymatic hydrolysates as described below.
Enzymatic Digestion of DNA and dG Quantitation
To quantify the DNA isolated from HT1080 cells and to detect any RNA contamination, approximately 5 μg aliquot of DNA from each sample was taken and subjected to neutral thermal hydrolysis (1 h at 70 °C) to release protein-guanine conjugates from the DNA backbone. Partially depurinated DNA was digested to 2′-deoxynucleosides in the presence of nuclease P1 (1 U), alkaline phosphatase (10 U), and 45 ng coformycin (to prevent deamination of dA) in 5 mM ZnCl2/50 mM ammonium acetate (pH 5.3) buffer for 20 h at 37 °C. Enzymatic digests were passed through Amicon Ultra-0.5 mL Centrifugal Filters (10K MWCO, Millipore, Temecula, CA) to remove proteins prior to HPLC-UV analysis.
Quantitative analysis of dG in enzymatic digests was conducted by HPLC-UV on an Agilent Technologies HPLC System (1100 model) equipped with a diode array UV detector and an autosampler. The sample was loaded on a Zorbax SB-C8 column (4.6 × 150 mm, 5 μm, from Agilent Technologies, Palo Alto, CA) was eluted with a gradient of 150 mM ammonium acetate (A) and acetonitrile (B). Solvent composition was held at 0% B for 2 min, followed by a linear increase to 3% B over 13 min, and further to 30% B over 3 min, where it was kept for the final 7 min of HPC run. UV absorbance was monitored at 260 nm. With this method, dG eluted as a sharp peak at ~13.5 min. dG amounts were determined by comparing HPLC peak areas to a calibration curve constructed by injecting known dG amounts.
Mass Spectrometric Identification of Cross-Linked Proteins
To identify cellular proteins that become covalently attached to chromosomal DNA in DEB -treated cells, HT1080 cells (~107 cells, in triplicate) were treated with 2 mM DEB or buffer control for 3 h at 37 °C, and chromosomal DNA containing any covalently cross-linked proteins was isolated by phenol/chloroform extraction and quantified as described above. DNA (26 μg) was subjected to neutral thermal hydrolysis to release protein-guanine conjugates, dried under vacuum, and reconstituted in 1 × NuPAGE Sample Buffer (Invitrogen, Carlsbad, CA). Proteins were separated using NuPAGE® Novex® 12% Bis-Tris Gels (Invitrogen, Carlsbad, CA) and stained with SimplyBlue Safe stain (Invitrogen, Carlsbad, CA). The gel lanes were excised and divided into five sections encompassing the entire molecular weight range, and each section was further diced into ~1 mm pieces. The proteins present within the gel pieces were subjected to in-gel tryptic digestion as described elsewhere.8,21 In brief, gel pieces were rinsed with 25 mM ammonium bicarbonate, and the protein thiols were subjected to reduction with DTT (300 mM) and alkylation with iodoacetamide. The gel pieces were then dehydrated by incubation with acetonitrile, dried under vacuum, and reconstituted in 25 mM ammonium bicarbonate buffer. Mass spectrometry-grade trypsin (2–3 μg) was added, and the samples were digested overnight at 37 °C. The resulting tryptic peptides were extracted with 60% acetonitrile containing 0.1% aqueous formic acid, evaporated to dryness, and desalted using ZipTip C18 (Millipore, Temecula, CA). Samples were reconstituted in 0.1% formic acid for HPLC–ESI+–MS/MS analysis.
HPLC–ESI+–MS/MS analyses of tryptic peptides were conducted on a ThermoScientific LTQ Orbitrap Velos mass spectrometer (Thermo Scientific Corp., Waltham, MA) in line with an Eksigent nanoLC 2D HPLC pump, a nanospray source, and an Xcalibur 2.1.0 software for instrument control. Peptide mixtures (8 μL) were loaded onto a Symmetry C18 trapping column (180 μm × 20 mm, Waters, Milford, MA) using 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) at a flow composition of 95% A and 5% B at 5 μL/min for 3 minutes. Following trapping, the HPLC flow was decreased to 0.3 μL/min and reversed to elute the peptides off the trap column. The mixtures were separated on a capillary column (75 μm ID, 10 cm packed bed, 15 μm orifice) created by hand packing a commercially purchased fused-silica emitter (New Objective, Woburn MA) with Zorbax SB-C18 5 μm separation media (Agilent, Santa Clara, CA). The gradient program started at 5% B, followed by a linear increase to 60% B over 60 min, and further to 95% B in 5 min. Liquid chromatography was carried out at an ambient temperature. Centroid MS-MS scans were acquired using an isolation width of 2.5 m/z, an activation time of 30 ms, an activation Q of 0.25, 35% normalized CID collision energy, and 1 microscan with a max ion time of 100 ms for each MS/MS scan. The mass spectrometer was calibrated prior to each analysis, and the spray voltage was adjusted to assure a stable spray. Typically, the tune parameters were as follows: spray voltage of 1.6 kV, a capillary temperature of 275 °C, and an S-lens RF Level of 50%. Peptide MS/MS spectra were collected using data-dependent scanning, in which one full scan mass spectrum was followed by eight MS/MS spectra. Dynamic exclusion was enabled for 60 s, and singly charged species were excluded from MS/MS.
Spectral data were analyzed using an in-house developed software pipeline “TINT” that linked raw data extraction, database searching, and probability scoring. Raw data were extracted and converted to the mzXML format using ReadW. Spectra that contained fewer than 6 peaks or had less than 20 measured total ion current (TIC) were excluded. Data were processed using the SEQUEST v.27 algorithm22 on a high speed, multiprocessor Linux cluster in the Minnesota Super Computing Institute at the University of Minnesota. Peptide spectra were searched against NCBI derived human protein database v200806 combined with its reversed counterpart, along with common protein contaminants totaling 70,711 entries. Search parameters included trypsin specificity and up to 2 missed cleavage sites. Cysteine carboxamidomethylation (+57.0215 Da) was set as a fixed modification, and methionine oxidation (+15.9949 Da) was set as a variable modification. Precursor mass tolerance was set to 1.25 m/z within the calculated average mass, and fragment ion mass tolerance was set to 0.5 m/z of their monoisotopic mass. The identified peptides were filtered using Scaffold 3 software (Proteome Software, INC., Portland, OR), to a target false discovery rate (FDR) of 5%. The FDR was calculated with the following expression FDR = (2R)/(R+F)*100, where R is the number of passing reversed peptide identifications and F is the number of passing forward (normal orientation) peptide identifications. The second round of filtering removed proteins supported by less than two distinct peptide identifications in the analyses. Indistinguishable proteins were recognized and grouped. Parsimony rules were applied to generate a minimal list of proteins that explained all of the peptides that passed our entry criteria.23 Any proteins also found in control samples were excluded from the final list.
Western blot analysis of identified proteins
HT1080 cells (~ 107) were treated with DEB (0, 0.05, 0.5, 1.0, or 2.0 mM) for 3 h at 37 °C. Chromosomal DNA, along with any covalently bound proteins, was extracted and quantified as described above. Approximately 100 μg of DNA from each sample was subjected to neutral thermal hydrolysis (1 h at 70 °C) to release protein-guanine conjugates from the DNA backbone. Proteins were separated by NuPAGE® Novex® 12% Bis-Tris Gels (Invitrogen, Carlsbad, CA) and transferred to Trans-blot nitrocellulose membranes (Bio-Rad, Hercules, CA). Following blocking in Tris-buffered saline (TBS) containing 5% (w/v) bovine serum albumin, the membranes were incubated with the primary antibody against the target protein (glyceraldehyde 3-phosphate dehydrogenase (GAPDH), poly(ADP-ribose) polymerase 1 (PARP), DNA-(apurinic- or apyrimidinic-site) lyase (ref-1), histone-H4, nucleophosmin (B23), and nucleolin) for 3 h at room temperature, rinsed with TBS buffer, and incubated overnight at 4 °C with the corresponding alkaline phosphatase-conjugated secondary antibody. The blots were washed and developed with SIGMA Fast BCIP/NBT (Sigma, St. Louis, MO) according to manufacturer’s instructions.
Isotope dilution HPLC–ESI+–MS/MS analysis of Cys-N7G-BD in DEB-exposed cells
HT1080 cells (~ 106) were treated with DEB (0, 0.05, 0.1, 1.0, or 2.0 mM for 3 h at 37 °C). Chromosomal DNA containing DPCs was isolated by the phenol/chloroform extraction procedure described above, and DNA samples (50 μg) were subjected to neutral thermal hydrolysis (1 h at 37 °C) to release protein-guanine conjugates from the DNA backbone. Proteins were cleaved with trypsin (10 μg protein in 25 mM ammonium bicarbonate, overnight at 37 °C), and the resulting peptides were further digested to amino acids in the presence of proteinase K (10 μg in 250 μL H2O, overnight at 37 °C). The digests were spiked with Cys-[15N]-N7G-BD internal standard (500 fmol), followed by off-line HPLC purification of Cys-N7G-BD. An Agilent Technologies HPLC system (1100 model) incorporating a diode array detector, an autosampler, and a fraction collector was fitted with a Supelcosil LC-18-DB (4.6 × 250 mm, 5 μm) column (Sigma-Aldrich, St. Louis, MO). The column was eluted at a flow rate of 1 mL/min using 15 mM ammonium acetate, pH 4.9 (A) and acetonitrile (B). The solvent composition was changed linearly from 0 to 24% B over 24 min and further to 60% B in 6 min. HPLC fractions containing Cys-N7G-BD (7.5-9 min) were collected, dried under vacuum, and reconstituted in water (25 μL) for HPLC–ESI+–MS/MS analysis.
Quantitative analyses of Cys-N7G-BD were conducted with a Thermo Scientific-Dionex-UltiMate® 3000 capillary HPLC system (Thermo Scientific Corp., Waltham, MA) interfaced to a Thermo-Finnigan TSQ Vantage mass spectrometer (Thermo Scientific Corp., Waltham, MA). Chromatographic separation was accomplished with a Phenomonex Synergi Hydro-RP C18 column (250 mm × 0.5 mm, 4 μm) eluted with a gradient of 15 mM ammonium acetate, pH 5.0 (A) and acetonitrile: isopropyl alcohol (1:1) (B) at a flow rate of 10 μL/min. Solvent composition was linearly changed from 4% to 20% in 8 min further and to 30% over 5 min, and brought back to 4% in 3 min. Under these conditions, Cys-N7G-BD and its internal standard (Cys-[15N]-N7G-BD) eluted at ~ 7 min. Electrospray ionization was achieved at a spray voltage of 3100 V and a capillary temperature of 270 °C. Collision induced dissociation was performed with Ar as a collision gas (1.5 mTorr) at a collision energy of 23 V. Instrument parameters were optimized for maximum response during infusion of a standard solution of Cys-N7G-BD. HPLC-ESI+-MS/MS analysis was performed in the selected reaction monitoring mode by following the neutral loss of guanine from protonated molecules of Cys-N7G-BD (m/z 359.0 [M + H]+ → 208.2 [M + H – Gua]+) and the corresponding mass transition of Cys-[15N5]-N7G-BD (m/z 364.0 [M + H]+ → 208.2 [M + H – 15N5 -Gua]+). Relative response ratios of the HPLC-ESI+-MS/MS peak areas in extracted ion chromatograms corresponding to the analyte and its internal standard were used to obtain concentration dependence curves for Cys-N7G-BD in cells treated with DEB.
Results
Concentration-Dependent Formation of DPCs in Human Cell Cultures Following DEB Treatment
To monitor DPC formation in vivo, human fibrosarcoma (HT1080) cells (~107) were treated with increasing concentrations of DEB (0, 0.05, 0.1, 0.5, 1.0 or 2.0 mM) for 3 h. Chromosomal DNA containing any covalent DPCs was isolated using modified phenol/chloroform extraction as described in our previous publication.8 Our earlier studies have established that DEB-mediated DNA-protein cross-linking takes place at the N7 position of guanine to produce hydrolytically labile N7-guanine adducts.3 Therefore, DEB-induced protein-guanine conjugates were released from DNA by thermal hydrolysis and resolved by 12% SDS-PAGE (Scheme 2, Figure 1). The proteins were visualized using SimplyBlue SafeStain (Figure 1A). We found that while some endogenous DPCs were present in untreated cells (lanes 2-3 in Figure 1), a significant increase of protein band intensities was observed in cells treated with 0.1-2 mM DEB (lanes 4–8 in Figure 1A, Figure 1B). In our previous studies, 80- and 20- fold lower concentrations of mechlorethamine and cisplatin, respectively, were required to achieve similar levels of cross-linking (reference8 and Ming et al., manuscript in preparation), suggesting that DEB is less efficient at inducing DNA-protein cross-links. Alternatively, DEB-induced DPCs may have shorter half-lives in cells due to spontaneous depurination and/or active repair. On the basis of these results, 2.0 mM DEB was selected for the proteomics experiments.
Scheme 2.
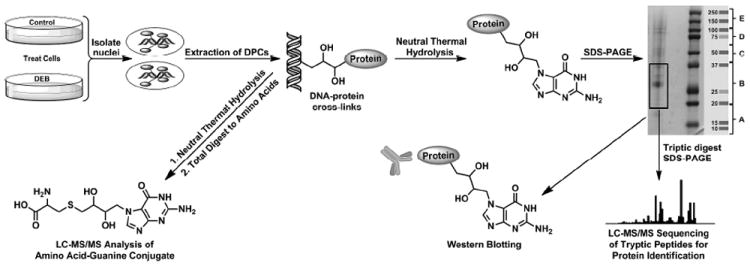
Strategy for the isolation and analysis of DPCs from DEB-treated mammalian cell cultures. Genomic DNA is isolated from control and treated cells using modified phenol-chloroform extraction in the presence of proteasome inhibitors. DPCs are released form the DNA backbone using thermal hydrolysis and are characterized by MS based proteomics, western blotting, and HPLC-ESI-MS/MS of amino acid-nucleobase conjugates in enzymatic digests.
Figure 1.

Concentration-dependent formation of DNA–protein cross-links in human fibrosarcoma (HT1080) cells treated with DEB. Cells were treated with 0–2 mM DEB for 3 h, and chromosomal DNA containing cross-linked proteins was isolated by modified phenol/chloroform extraction in the presence of proteasome inhibitors. Proteins (from 26 μg DNA) were released from DNA by thermal hydrolysisin the form of protein-guanine conjugates, separated by 12% SDS-PAGE, and visualized by staining with SimplyBlue SafeStain (A). Densitometric analysis of protein bands: normalized band intensity values were obtained by subtracting the values observed in controls. Error bars represent the standard error from two independent experiments (B).
Identification of Cross-linked Proteins by Mass Spectrometry-Based Proteomics
To identify the proteins participating in cross-linking, HT1080 cells (~107 cells, in triplicate) were treated with 2.0 mM DEB, while control cells were treated with DEB-free buffer. DPCs were extracted by the modified phenol/chloroform extraction method described above,8 and samples corresponding to 26 μg of DNA were subjected to neutral thermal hydrolysis to release protein-guanine conjugates from the DNA backbone (Scheme 2). We have previously shown that this step facilitates the separation and identification of proteins participating in DPC formation by mass spectrometry.24
SDS-PAGE analysis of protein-guanine conjugates in DEB-treated samples (Figure 2) has revealed distinct protein bands (lanes 6-8 in Figure 2), while the untreated samples exhibited only weak protein signals corresponding to endogenous DPCs (lanes 2- 4 in Figure 2). Protein-containing gel lanes were cut into five sections covering the entire molecular weight range 10- 250 kDa (A-E in Figure 2) and excised from the gel. Gel slices were individually subjected to in-gel tryptic digestion, and the resulting peptides were extracted from the gel and subjected to HPLC–ESI+–MS/MS analysis for protein identification. MS/MS analysis of tryptic peptides yielded characteristic b- and y-series fragment ions that were used to determine amino acid sequence and to identify the corresponding proteins (see spectra of two representative peptides in Figure 3). Database searching and parsimony analysis of the MS/MS spectral data resulted in identification of 152 proteins participating in cross-linking to the chromosomal DNA from DEB-treated cells (Table 1). Each protein was identified by a minimum of two unique peptides, and any proteins observed in control samples were excluded from the list.
Figure 2.

SDS-PAGE analysis of samples employed in the proteomics studies of DEB-induced DNA-protein cross-linking. HT1080 cells (~107) were treated with 0 (lanes 2, 3, and 4) or 2 mM DEB (lanes 6, 7, and 8) for 3 h. Following modified phenol/chloroform extraction of DNA in the presence of proteasome inhibitors and thermal hydrolysis to release proteins, the cross-linked proteins were separated by 12% SDS-PAGE and visualized by staining with SimplyBlue SafeStain. Proteins present in the 10–250 kDa molecular weight range were excised from the gel, subjected to in-gel tryptic digestion, and analyzed by HPLC–ESI+–MS/MS.
Figure 3.

Representative HPLC–ESI+–MS/MS spectra of tryptic peptides used in the identification of DPCs involving nucleolin (A) and histone-H4 (B).
Table 1.
| Swiss-Prot ID | Identified Protein | % coverage | No. of unique peptides | Total spectra | Primary cellular function | Protein MW (Da) |
|---|---|---|---|---|---|---|
| 1 Q13442 | 28 kDa heat- and acid-stable phosphoprotein | 14.9% | 2 | 2 | Cell Signalling/Motility/Architecture | 20630.8 |
| 2 P35613 | Basigin | 7.0% | 2 | 2 | 42199.7 | |
| 3 P25440 | Bromodomain-containing protein 2 | 2.1% | 2 | 2 | 88063.3 | |
| 4 P16070 | CD44 antigen | 6.9% | 4 | 6 | 81553.4 | |
| 5 Q07065 | Cytoskeleton-associated protein 4 | 4.7% | 2 | 2 | 66022.2 | |
| 6 Q08554 | Desmocollin-1 | 4.5% | 4 | 4 | 99988.1 | |
| 7 Q02413 | Desmoglein-1 | 3.1% | 2 | 2 | 113748.9 | |
| 8 P06753 | Isoform TM30nm of Tropomyosin alpha-3 chain | 10.9% | 2 | 2 | 29033.3 | |
| 9 Q03252 | Lamin-B2 | 3.5% | 2 | 2 | 67689.8 | |
| 10 P55081 | Microfibrillar-associated protein 1 | 6.2% | 2 | 2 | 51958.7 | |
| 11 P46821 | Microtubule-associated protein 1B | 1.2% | 2 | 2 | 270620.2 | |
| 12 P19105 | Myosin regulatory light chain 12A | 18.7% | 3 | 3 | 19795.3 | |
| 13 P35579 | Myosin-9 | 1.2% | 2 | 2 | 226537.5 | |
| 14 Q09666 | Neuroblast differentiation-associated protein AHNAK | 0.4% | 2 | 2 | 629104.4 | |
| 15 P41219 | Peripherin | 4.5% | 2 | 3 | 53652.0 | |
| 16 Q15149 | Plectin-1 | 0.7% | 2 | 3 | 531784.5 | |
| 17 O00592 | Podocalyxin | 3.9% | 2 | 2 | 58635.0 | |
| 18 P12273 | Prolactin-inducible protein | 13.7% | 2 | 2 | 16573.1 | |
| 19 Q13283 | Ras GTPase-activating protein-binding protein 1 | 4.3% | 2 | 2 | 52162.8 | |
| 20 P07996 | Thrombospondin-1 | 2.1% | 2 | 2 | 129381.7 | |
| 21 Q13428 | Treacle protein | 1.7% | 2 | 2 | 152102.5 | |
| 22 P09493 | Tropomyosin alpha-1 chain | 6.7% | 2 | 2 | 32710.0 | |
| 23 P67936 | Tropomyosin alpha-4 chain | 9.7% | 2 | 2 | 28522.4 | |
| 24 P07951 | Tropomyosin beta chain | 6.7% | 2 | 2 | 32851.7 | |
| 25 Q71U36 | Tubulin alpha-1A chain | 3.8% | 2 | 2 | 50135.7 | |
| 26 Q13885 | Tubulin beta-2A chain | 5.4% | 2 | 2 | 49907.1 | |
| 27 P05556 | Integrin beta-1 | 6.0% | 4 | 4 | 88415.1 | |
| 28 Q16643 | Drebrin | 5.6% | 2 | 2 | 71428.6 | |
| 29 Q01469 | Fatty acid-binding protein, epidermal | 13.3% | 2 | 2 | 15164.4 | |
| 30 P14923 | Junction plakoglobin | 2.8% | 2 | 2 | 81745.9 | |
| 31 P62263 | 40S ribosomal protein S14 | 15.9% | 2 | 2 | Cellular Homeostasis/Cell Cycle | 16272.9 |
| 32 P60866 | 40S ribosomal protein S20 | 19.3% | 2 | 2 | 13373.0 | |
| 33 P62851 | 40S ribosomal protein S25 | 24.0% | 4 | 5 | 13743.0 | |
| 34 P62753 | 40S ribosomal protein S6 | 14.1% | 3 | 3 | 28681.7 | |
| 35 P08865 | 40S ribosomal protein SA | 13.6% | 3 | 4 | 32854.1 | |
| 36 P14625 | Endoplasmin | 3.7% | 3 | 3 | 92471.7 | |
| 37 O60841 | Eukaryotic translation initiation factor 5B | 6.2% | 5 | 5 | 138831.5 | |
| 38 P14314 | Glucosidase 2 subunit beta | 8.0% | 4 | 4 | 59425.8 | |
| 39 P04406 | Glyceraldehyde-3-phosphate dehydrogenase | 14.9% | 3 | 3 | 36053.4 | |
| 40 Q9H1E3 | Nuclear ubiquitous casein and cyclin-dependent kinases substrate | 22.6% | 3 | 4 | 27296.7 | |
| 41 Q14978 | Nucleolar and coiled-body phosphoprotein 1 | 2.7% | 2 | 2 | 73604.2 | |
| 42 Q9NR30 | Nucleolar RNA helicase 2 | 3.3% | 2 | 2 | 87346.0 | |
| 43 P19338 | Nucleolin | 18.3% | 14 | 18 | 76615.9 | |
| 44 P06748 | Nucleophosmin | 13.9% | 3 | 5 | 32575.5 | |
| 45 P12270 | Nucleoprotein TPR | 1.4% | 2 | 2 | 267289.3 | |
| 46 Q06830 | Peroxiredoxin-1 | 15.1% | 3 | 4 | 22110.9 | |
| 47 Q15061 | WD repeat-containing protein 43 | 3.8% | 2 | 3 | 74890.8 | |
| 48 Q86VM9 | Zinc finger CCCH domain-containing protein 18 | 6.8% | 6 | 6 | 106379.8 | |
| 49 Q01105 | Protein SET | 7.2% | 2 | 2 | 33489.4 | |
| 50 Q8IZQ5 | Selenoprotein H | 17.2% | 2 | 2 | 13453.5 | |
| 51 Q8IYB3 | Serine/arginine repetitive matrix protein 1 | 3.0% | 2 | 2 | 102337.5 | |
| 52 Q9UQ35 | Serine/arginine repetitive matrix protein 2 | 6.3% | 12 | 13 | 299621.6 | |
| 53 P26583 | High mobility group protein B2 | 11.5% | 2 | 5 | DNA Damage Response/DNA Repair | 24034.6 |
| 54 O15347 | High mobility group protein B3 | 7.0% | 2 | 2 | 22980.7 | |
| 55 P07305 | Histone H1.0 | 11.9% | 2 | 2 | 20864.1 | |
| 56 Q02539 | Histone H1.1 | 5.6% | 2 | 4 | 21843.2 | |
| 57 P16403 | Histone H1.2 | 9.9% | 2 | 3 | 21365.8 | |
| 58 Q96QV6 | Histone H2A type 1-A | 12.2% | 2 | 2 | 14234.2 | |
| 59 P62807 | Histone H2B type 1-C/E/F/G/I | 7.9% | 2 | 3 | 13906.6 | |
| 60 Q16695 | Histone H3.1t | 11.8% | 2 | 2 | 15508.9 | |
| 61 P62805 | Histone H4 | 38.8% | 4 | 4 | 11367.7 | |
| 62 Q3SYE8 | Putative high mobility group protein B3-like-1 | 20.0% | 2 | 2 | 14606.9 | |
| 63 P23246 | Splicing factor, proline- and glutamine-rich | 4.2% | 2 | 2 | 76149.5 | |
| 64 P62988 | Ubiquitin | 32.9% | 2 | 2 | 8565.3 | |
| 65 Q14444 | Caprin-1 | 6.4% | 4 | 4 | RNA Processing/mRN A Splicing | 78364.4 |
| 66 P61978 | Heterogeneous nuclear ribonucleoprotein K | 5.0% | 2 | 2 | 50978.5 | |
| 67 P22626 | Heterogeneous nuclear ribonucleoproteins A2/B1 | 7.1% | 2 | 2 | 37430.3 | |
| 68 Q9UKD2 | mRNA turnover protein 4 homolog | 18.8% | 4 | 4 | 27561.3 | |
| 69 P67809 | Nuclease-sensitive element-binding protein 1 | 23.5% | 4 | 7 | 35923.8 | |
| 70 O00567 | Nucleolar protein 56 | 5.6% | 2 | 2 | 66052.0 | |
| 71 Q9Y2X3 | Nucleolar protein 58 | 4.5% | 3 | 3 | 59580.2 | |
| 72 Q9UMY1 | Nucleolar protein 7 | 16.7% | 3 | 3 | 29426.9 | |
| 73 Q86U42 | Polyadenylate-binding protein 2 | 9.5% | 3 | 3 | 32749.5 | |
| 74 Q7L014 | Probable ATP-dependent RNA helicase DDX46 | 1.0% | 2 | 2 | 117366.1 | |
| 75 Q99848 | Probable rRNA-processing protein EBP2 | 15.7% | 4 | 4 | 34852.9 | |
| 76 O75526 | RNA-binding motif protein, X-linked-like-2 | 5.6% | 2 | 2 | 42816.1 | |
| 77 P49756 | RNA-binding protein 25 | 4.7% | 3 | 3 | 100189.1 | |
| 78 Q9Y5S9 | RNA-binding protein 8A | 21.8% | 3 | 5 | 19889.4 | |
| 79 Q9UKM9 | RNA-binding protein Raly | 5.9% | 2 | 3 | 32463.9 | |
| 80 Q9Y3B9 | RRP15-like protein | 10.3% | 3 | 3 | 31484.5 | |
| 81 Q13435 | Splicing factor 3B subunit 2 | 3.0% | 2 | 2 | 100229.4 | |
| 82 Q07955 | Splicing factor, arginine/serine-rich 1 | 37.9% | 9 | 20 | 27745.1 | |
| 83 O75494 | Splicing factor, arginine/serine-rich 13A | 8.8% | 2 | 2 | 31301.7 | |
| 84 Q8TF01 | Splicing factor, arginine/serine-rich 18 | 3.4% | 2 | 2 | 92578.3 | |
| 85 Q01130 | Splicing factor, arginine/serine-rich 2 | 12.7% | 2 | 6 | 25477.1 | |
| 86 Q9BRL6 | Splicing factor, arginine/serine-rich 2B | 7.5% | 3 | 5 | 32288.5 | |
| 87 P84103 | Splicing factor, arginine/serine-rich 3 | 26.2% | 4 | 5 | 19330.0 | |
| 88 Q13243 | Splicing factor, arginine/serine-rich 5 | 13.6% | 3 | 3 | 31264.8 | |
| 89 Q16629 | Splicing factor, arginine/serine-rich 7 | 8.8% | 2 | 2 | 27367.5 | |
| 90 Q13242 | Splicing factor, arginine/serine-rich 9 | 17.2% | 4 | 4 | 25542.9 | |
| 91 O75683 | Surfeit locus protein 6 | 7.5% | 3 | 3 | 41451.7 | |
| 92 P62995 | Transformer-2 protein homolog beta | 17.0% | 5 | 9 | 33666.7 | |
| 93 P08621 | U1 small nuclear ribonucleoprotein 70 kDa | 12.1% | 5 | 6 | 51558.4 | |
| 94 O00566 | U3 small nucleolar ribonucleoprotein protein MPP10 | 7.1% | 4 | 4 | 78866.8 | |
| 95 O43290 | U4/U6.U5 tri-snRNP-associated protein 1 | 3.8% | 2 | 2 | 90257.1 | |
| 96 O95218 | Zinc finger Ran-binding domain-containing protein 2 | 10.3% | 3 | 3 | 37405.4 | |
| 97 P62241 | 40S ribosomal protein S8 | 11.5% | 2 | 2 | Transcriptional Regulation/Translation | 24206.4 |
| 98 P46781 | 40S ribosomal protein S9 | 9.8% | 2 | 2 | 22592.5 | |
| 99 P05387 | 60S acidic ribosomal protein P2 | 67.0% | 4 | 5 | 11665.5 | |
| 100 Q07020 | 60S ribosomal protein L18 | 12.2% | 2 | 2 | 21635.2 | |
| 101 P84098 | 60S ribosomal protein L19 | 17.9% | 3 | 3 | 23467.4 | |
| 102 P35268 | 60S ribosomal protein L22 | 18.8% | 2 | 2 | 14787.3 | |
| 103 P62829 | 60S ribosomal protein L23 | 17.9% | 2 | 2 | 14865.9 | |
| 104 P62750 | 60S ribosomal protein L23a | 32.1% | 5 | 9 | 17696.2 | |
| 105 P83731 | 60S ribosomal protein L24 | 13.4% | 2 | 3 | 17779.5 | |
| 106 P47914 | 60S ribosomal protein L29 | 14.5% | 2 | 3 | 17753.0 | |
| 107 P62899 | 60S ribosomal protein L31 | 18.4% | 2 | 2 | 14463.2 | |
| 108 P18124 | 60S ribosomal protein L7 | 14.1% | 4 | 5 | 29227.7 | |
| 109 P39687 | Acidic leucine-rich nuclear phosphoprotein 32 family member A | 19.7% | 6 | 8 | 28586.1 | |
| 110 Q92688 | Acidic leucine-rich nuclear phosphoprotein 32 family member B | 13.5% | 2 | 5 | 28788.7 | |
| 111 P53999 | Activated RNA polymerase II transcriptional coactivator p15 | 29.9% | 3 | 3 | 14395.9 | |
| 112 Q9NYF8 | Bcl-2-associated transcription factor 1 | 4.2% | 3 | 3 | 106125.3 | |
| 113 P83916 | Chromobox protein homolog 1 | 14.6% | 2 | 2 | 21418.1 | |
| 114 Q13185 | Chromobox protein homolog 3 | 13.1% | 2 | 2 | 20812.0 | |
| 115 P45973 | Chromobox protein homolog 5 | 10.0% | 2 | 2 | 22225.6 | |
| 116 Q5QJE6 | Deoxynucleotidyltransferase terminal-interacting protein 2 | 6.2% | 4 | 5 | 84471.1 | |
| 117 P16989 | DNA-binding protein A | 7.5% | 2 | 2 | 40089.4 | |
| 118 Q8WXX5 | DnaJ homolog subfamily C member 9 | 8.9% | 2 | 2 | 29910.3 | |
| 119 Q14919 | Dr1-associated corepressor | 10.2% | 2 | 2 | 22350.4 | |
| 120 P29692 | Elongation factor 1-delta | 8.9% | 2 | 2 | 31121.9 | |
| 121 P35269 | General transcription factor IIF subunit 1 | 6.4% | 2 | 2 | 58241.9 | |
| 122 Q7Z4V5 | Hepatoma-derived growth factor-related protein 2 | 6.7% | 3 | 3 | 74318.3 | |
| 123 Q99729 | Heterogeneous nuclear ribonucleoprotein A/B | 8.7% | 3 | 3 | 36225.2 | |
| 124 Q14103 | Heterogeneous nuclear ribonucleoprotein D0 | 7.3% | 2 | 2 | 38434.5 | |
| 125 P17096 | High mobility group protein HMG-I/HMG-Y | 37.4% | 5 | 14 | 11676.2 | |
| 126 P52926 | High mobility group protein HMGI-C | 56.9% | 4 | 7 | 11831.9 | |
| 127 P50502 | Hsc70-interacting protein | 6.5% | 2 | 2 | 41332.4 | |
| 128 P17096-2 | Isoform HMGA1b of High mobility group protein HMG-I/HMG-Y | 14.6% | 2 | 4 | 10679.2 | |
| 129 P43243 | Matrin-3 | 3.8% | 2 | 2 | 94626.7 | |
| 130 Q96DR8 | Mucin-like protein 1 | 17.8% | 2 | 2 | 9039.3 | |
| 131 Q13765 | Nascent polypeptide-associated complex subunit alpha | 13.5% | 2 | 3 | 23383.3 | |
| 132 P23497 | Nuclear autoantigen Sp-100 | 2.2% | 2 | 100418.8 | ||
| 133 Q9H930 | Nuclear body protein SP140-like protein | 4.6% | 2 | 2 | 50075.6 | |
| 134 P17480 | Nucleolar transcription factor 1 | 3.1% | 2 | 2 | 89409.6 | |
| 135 Q99733 | Nucleosome assembly protein 1-like 4 | 9.3% | 3 | 3 | 42823.9 | |
| 136 O75475 | PC4 and SFRS1-interacting protein | 10.0% | 4 | 5 | 60103.9 | |
| 137 Q8NC51 | Plasminogen activator inhibitor 1 RNA-binding protein | 7.6% | 3 | 3 | 44965.8 | |
| 138 Q6NZI2 | Polymerase I and transcript release factor | 15.4% | 4 | 4 | 43476.5 | |
| 139 P51531 | Probable global transcription activator SNF2L2 | 1.4% | 2 | 2 | 181283.0 | |
| 140 Q8IZL8 | Proline-, glutamic acid- and leucine-rich protein 1 | 4.1% | 2 | 2 | 119700.6 | |
| 141 Q9NY61 | Protein AATF | 6.6% | 3 | 3 | 63135.0 | |
| 142 P35659 | Protein DEK | 6.7% | 2 | 2 | 42675.9 | |
| 143 Q96ST2 | Protein IWS1 homolog | 3.1% | 2 | 2 | 91956.1 | |
| 144 P61244 | Protein max | 14.4% | 2 | 2 | 18275.3 | |
| 145 P05109 | Protein S100-A8 | 20.4% | 2 | 2 | 10835.0 | |
| 146 P06702 | Protein S100-A9 | 24.6% | 2 | 2 | 13242.3 | |
| 147 Q58FF3 | Putative endoplasmin-like protein | 4.0% | 2 | 2 | 45859.7 | |
| 148 Q8WVC0 | RNA polymerase-associated protein LEO1 | 3.0% | 2 | 2 | 75405.3 | |
| 149 Q9Y2W1 | Thyroid hormone receptor-associated protein 3 | 5.6% | 5 | 5 | 108668.9 | |
| 150 Q14011 | Cold-inducible RNA-binding protein | 14.5% | 2 | 2 | 18648.3 | |
| 151 P35637 | RNA-binding protein FUS | 7.2% | 2 | 2 | 53426.0 | |
| 152 Q15059 | Bromodomain-containing protein 3 | 2.8% | 2 | 2 | 79542.5 |
In an effort to better understand the biological significance of DEB-induced DNA-protein cross-linking, the identified proteins were compiled according to their cellular distribution, participation in biological processes, and molecular functions using the GO database available from the European Bioinformatics Institute (http://www.ebi.ac.uk/QuickGO, Figure 4). The majority of the observed proteins (103 total, 67.7%) are classified as known nuclear proteins (Figure 4A), including histones, high mobility group proteins, matrin-3, and tubulin. An additional 28 proteins (18.4%) are classified as cytoplasmic, and 11 (7.2%) are categorized as membrane-bound proteins (Figure 4A). Nuclear proteins are most likely to participate in DPC formation due to their proximity to DNA. However, it is important to note that some of the identified proteins may be present in more than one cellular compartment due to their participation in multiple biological processes (see below).
Figure 4.
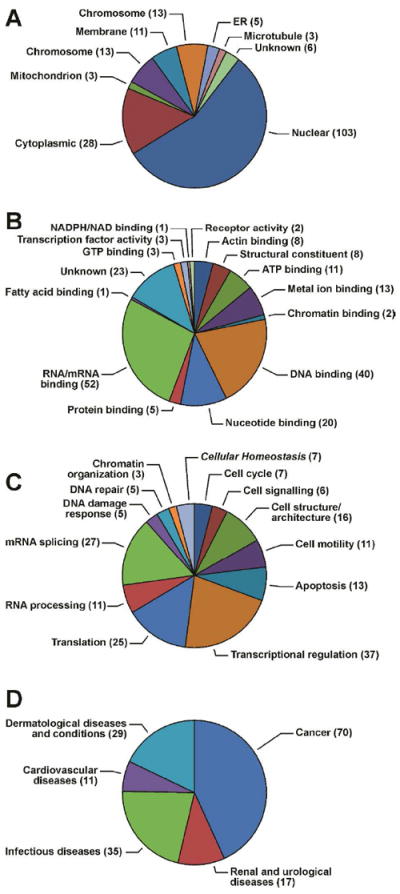
GO annotations for proteins involved in DEB-induced DPC formation in human HT1080 cells: (A) cellular distributions, (B) molecular functions, (C) biological processes, and (D) disease associations. The number of proteins falling into each category is labeled on the charts.
In regard to molecular functions, a large portion of the proteins participating in DPC formation by DEB (N = 92, > 60%) are known to participate in binding to DNA and RNA (Figure 4B). This is consistent with many of the identified proteins playing a role in transcriptional regulation (Figure 4C). This group includes high mobility group protein HMG-I (involved in base excision repair),25 Bcl-2 associated transcription factor 1 (plays a role in tumor suppression via the induction of apoptosis),26 and apoptosis-antagonizing transcription factor (functions as a general inhibitor of the histone deacetylase, HDAC1, anti-apoptosis, and response to DNA damage).27 We also observed proteins involved in cell motility/signaling/ architecture (e.g. lamin-B2, tubulin, ras GTPase activating protein, podocalyxin Plectin-1, and tropomyosin) (Table 1 and Figure 4C), and cellular homeostasis/cell cycle (GAPDH, nucleolin, nucleophosmin (B23)) (Table 1 and Figure 4C). An additional 11 proteins (6.4% of total) are involved in RNA processing, including zinc finger Ran-binding domain-containing protein-2, and transformer-2 protein homologue β (Table1 and Figure 4C).
Many of the proteins are counted in multiple GO categories due to their participation in diverse cellular processes. For example, B23 is involved in ribosome biogenesis,28 histone assembly,29 cell proliferation,30 and regulation of p53 tumor suppressor protein (TP53).31 As a result, B23 is listed under two GO annotation categories: Cell cycle and apoptosis (Figure 4C). It is also possible that the available GO annotations may not take into account protein’s secondary cellular localizations, biological processes, and molecular functions. However, the prevalence of proteins involved in transcriptional regulation among the cross-linked proteins is consistent with their proximity and direct binding interactions with DNA.
Western Blot Analysis of Cross-Linked Proteins
The identities of a subset of proteins detected by mass spectrometry-based proteomics were further confirmed by western blot analysis using commercial antibodies (Figure 5). HT1080 cells (~107) were treated with increasing concentrations of the DEB (0, 0.05, 0.1, 0.5, 1.0 or 2.0 mM) for 3 h. Chromosomal DNA containing any covalent DPCs was isolated by the modified phenol/chloroform extraction methodology described above. DNA from each sample (100 μg) was subjected to neutral thermal hydrolysis, and the released protein-guanine conjugates were separated by SDS-PAGE, transferred to nitrocellulose membranes, and subjected to western blot analysis using commercial antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH), poly(ADP-ribose) polymerase 1 (PARP), DNA-(apurinic- or apyrimidinic-site) lyase (ref-1), histone-H4, nucleophosmin (B23), and nucleolin. These proteins were selected based on their detection by mass spectrometry (Table 1) or their previously demonstrated ability to form DPCs in the presence of other bis-electrophiles.3,8,24 A concentration-dependent formation of DPCs to PARP, nucleolin, and GAPDH was observed (Figure 5). Since these proteins are also targeted by nitrogen mustards and cisplatin, our results suggest that they are particularly susceptible to DPC formation by bis-electrophiles. In contrast, we did not detect Ref1, histone H4, or nucleolin by western blotting (not shown).
Figure 5.
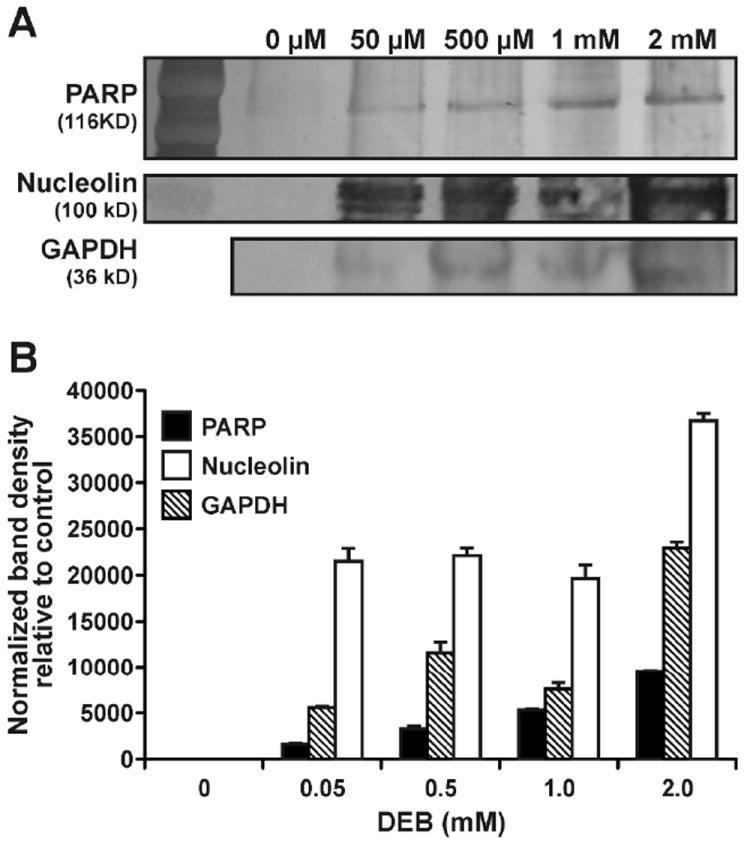
Western blot analysis of DEB-induced DPCs in HT1080 cells. Following treatment with 0, 0.05, 0.5, 1.0, or 2.0 mM DEB, DNA and covalently cross-linked proteins were isolated by phenol/chloroform extraction. Proteins (from 100 μg DNA) were released by thermal hydrolysis, separated by SDS-PAGE, and transferred to nitrocellulose membranes. Western blotting was performed using primary antibodies specific for PARP, nucleolin, and GAPDH. (A) Densitometric analysis of protein bands: normalized band intensity values were obtained by subtracting the values observed in controls. Error bars represent the standard error from two independent experiments (B).
HPLC-ESI+-MS/MS Detection of Cys-N7G-BD Conjugates as Evidence for DPC Formation
In order to quantify the formation of covalent DPCs in cells treated with increasing concentrations of DEB, HT1080 cells (~106) were incubated with 0, 0.1, 0.5, 1.0, or 2.0 mM DEB, and the chromosomal DNA was extracted as described above (Scheme 2). Our previous studies conducted with recombinant proteins and cell free extracts2,3 have revealed that DEB induced cross-linking takes place mainly between the cysteine sulfhydryl side chain within proteins and the N7-position to form 1-(S-cysteinyl)-4-(guan-7-yl)-2,3-butanediol (Cys-N7G- BD) conjugates (Scheme 2), therefore the quantitative HPLC-ESI+-MS/MS analyses have focused on Cys-N7G- BD. Equal DNA amounts (50 μg) were taken from each sample and subjected to neutral thermal hydrolysis to release protein-guanine conjugates from the DNA backbone. Proteins were enzymatically digested to amino acids, and the resulting digests were spiked with known amounts of Cys-[15N]-N7G-BD and subjected to off-line HPLC purification. Conjugate amounts in cells were determined by isotope dilution HPLC-ESI+-MS/MS in the selected reaction monitoring mode as described previously.2,8,24,32 Cys-N7G-BD conjugates were detected in proteolytic hydrolysates of samples from DEB-treated HT1080 cells, but not in untreated cells (Figure 6A). These data confirm that DEB induced DNA-protein cross-linking takes place between the side chain sulfhydryls of cysteine residues in proteins and the N7-position of guanine bases in chromosomal DNA. Furthermore, Cys-N7G-BD amounts in cells were dependent on DEB concentration, reaching the levels of 6 lesions per million normal nucleotides in cells treated with 2 mM DEB (Figure 6B).
Figure 6.

HPLC-ESI+-MS/MS analysis of 1-(S-cysteinyl)-4-(guan-7-yl)-2,3-butanediol (Cys-N7G-BD) conjugates in total proteolytic digests. HT1080 cells were exposed to 0, 0.1, 0.5, 1.0, or 2.0 mM DEB for 3 h. Following extraction of DPC-containing chromosomal DNA, equal DNA amounts from each sample were subjected to thermal and enzymatic hydrolysis to release amino acid-nucleobase conjugates. The samples were subjected to offline HPLC to enrich for Cys-N7G-BD prior to HPLC-ESI+-MS/MS analysis. Quantification of Cys-N7G-BD was accomplished using isotope dilution with Cys-15N5-N7G-BD. Shown are extracted ion chromatograms corresponding to HT1080 cells incubated in the absence of DEB (negative control) and samples treated with 2 mM DEB (A) and concentration dependent formation of Cys-N7G-BD in DEB-treated HT1080 cells (B).
Discussion
Reversible DNA-protein interactions are essential for normal cell function. Chromosomal DNA is packaged in the nucleus by wrapping around histone octamers, and reversible binding of regulatory proteins to their recognition motifs controls DNA replication and transcription. Protein binding also mediates DNA repair and cellular responses to DNA damage. Any interruption of these dynamic interactions is likely to have serious consequences for cell viability and genetic stability.
DNA-protein cross-links are bulky macromolecular conjugates that form when proteins become covalently trapped in the DNA strand. These ubiquitous lesions that can be induced by a variety of chemical and physical drugs, environmental toxins, ionizing radiation, endogenous aldehydes, and free radical generating systems.33 Covalent DPCs have been shown to accumulate in an age-dependent fashion in brain and heart tissues, probably a result of exposure to endogenous reactive oxygen species, lipid peroxidation products, and transition metals. Due to their considerable size and their pronounced effects on DNA structure and DNA-protein interactions, DPCs are hypothesized to interfere with replication, transcription, and repair, potentially leading to mutations and cell death.1
Experimental evidence is available in support of a role of DPCs in the biological activity of bis-electrophiles.33 For example, the cytotoxicity and mutagenicity of several bifunctional alkylation agents including 1,2-dibromoethane, dibromomethane, and DEB, are enhanced in bacteria which over-express human O6-alkylguanine DNA alkyltransferase (AGT) protein, presumably due to the formation of toxic AGT-DNA cross-links.34 In our recent study, AGT proteins containing DNA-reactive 2-hydroxy-3,4-epoxybutyl groups were introduced into mammalian cell lines, generating covalent AGT-DNA cross-links and inducing statistically significant levels of cell death and mutations at the hypoxanthine-guanine phosphoribosyltransferase gene (HPRT) (Tretyakova et al., submitted for publication). However, the extent of DPC formation in cells and the identities of the participating proteins have not been established, limiting our understanding of the role of DPCs in the biological activity of common antitumor drugs and in the development of cardiovascular disease, cancer, and age-related neurodegeneration.
Recently, our laboratory developed a practical and effective approach for isolating DPC lesions from cultured cells using a modified phenol/chloroform extraction method.8 The approach has been successfully used to characterize DPCs induced by antitumor nistrogen mustards and cisplatin in human cells and in blood of cancer patients undergoing chemotherapy (Ming et al., manuscript in preparation and Gherezghiher et al., unpublished observations). In the present work, the same methodology was applied to characterize DNA-protein cross-linking in human HT1080 cells treated with cytotoxic concentrations of DEB, the genotoxic metabolite of 1,3-butadiene (Scheme 1). Following DPC isolation from treated cells, neutral thermal hydrolysis was employed to selectively release DPC from the DNA backbone in the form of protein-guanine conjugates (Scheme 2).8 Proteins participating in cross-linking to DNA were identified by mass spectrometry-based proteomics and immunoblotting. Each protein was identified based on sequence of at least two unique peptides, and any proteins present in control samples were excluded.
A total of 152 proteins were found to form cross-links to chromosomal DNA in the presence of DEB (Table 1). These proteins are involved in a variety of cellular functions, including transcriptional regulation (e.g. matrin-3, high mobility group protein B3, and 40S ribosomal protein S20), cell signaling and architecture (e.g. lamin-B2, basigin, and myosin regulatory light chain 12A), regulation of cell cycle (e.g. GAPDH, tubulin beta-2A chain, and nucleolar protein 58), DNA damage response and repair (e.g. histone proteins), and RNA processing (e.g. splicing factor, arginine/serine-rich 3, ATP-dependent RNA helicase, and nucleolar RNA helicase). The identified proteins have been associated with variety of diseases including cancer, renal and urological diseases, cardiovascular diseases, and dermatological disease and conditions as revealed by Ingenuity Pathway Analysis (IPA) (Figure 4D).
Previously, it has been reported that DEB forms DPCs with recombinant AGT,3 GAPDH,16 and histones. 17 More recently, our laboratory employed an affinity capture approach to identify 39 proteins which participate in DEB-mediated cross-linking to DNA in cell free protein extracts from HeLa cells.2 Only two of the previously identified proteins (GAPDH, and heterogeneous nuclear ribonucleoprotein K)2 were also observed in the present study (Table 1), while the remaining proteins were not detected from in vitro cross-linking experiments. This may be partially explained by differences in protein expression profiles between human ovarian carcinoma (HeLa)2 and fibrosarcoma (HT1080) cells.35 However, the most dramatic difference between the two studies is the nature of the DNA employed. Our in vitro studies2 utilized short synthetic DNA duplexes representing codons 10-15 of K-ras protooncogene (5′-GGA GCT GGT GGC GTAGGC-3′). These duplexes lacked nucleosomal structure and were unlikely to participate in sequence-specific interactions with DNA-binding proteins. In contrast, experiments described in the present report were conducted in intact human cells, where DNA is organized in chromatin and involves specific interactions between DNA and proteins, potentially facilitating DPC formation. Indeed, ~ 10-fold lower concentrations of DEB were required to achieve the same extent of crosslinking in intact cells as compared to in vitro experiments.2 Both experiments required millimolar levels of DEB to achieve sufficient numbers of cross-links to enable their MS analysis. Although these concentrations are unlikely to be achieved in vivo, it is expected that the same proteins are targeted following exposure to lower concentratons of DEB.
When the list of proteins identified in the present study of DEB-mediated DNA-protein cross-linking in HT1080 cells (Table 1) is compared to a similar list of mechlorethamine-cross linked proteins,8 eighteen proteins are found in common, including, Bcl-2-associated transcription factor 1, B23, matrin-3, high mobility group protein HMG-I/HMG-Y, heterogeneous nuclear ribonucleoprotein A/B, and zinc finger CCCH domain-containing protein 18 (Figure 7A). Similarly, sixty seven proteins identified in the present work, including lamin-B2, nucleolin, myosin-9, matrin-3, histone H4, high mobility group protein HMG-I, AATF, B23, GAPDH, and peroxiredoxin-1 have also been found to form DPCs in HT1080 cells treated with cisplatin (Figure 7B) (Ming et al., manuscript in preparation). The remaining proteins were not common between the three lists, which is not surprising given the distinct mechanisms of cross-link formation by the three bis-electrophiles. In particular, while DEB and nitrogen mustards preferentially target cysteinyl residues within the proteins (Figure 7), cisplatin-mediated DPCs involve lysine and arginine residues (Ming et al., manuscript in preparation).8
Figure 7.
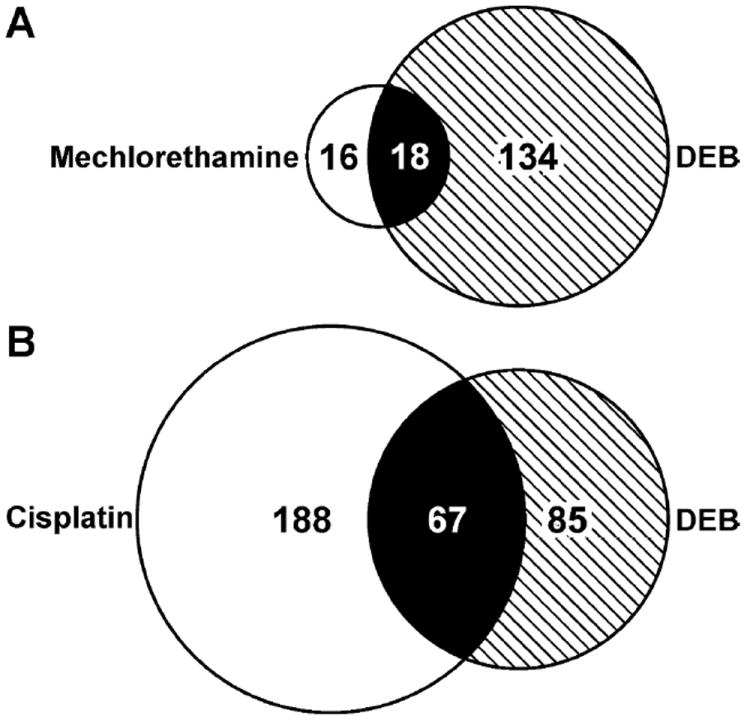
Venn diagrams showing the overlaps between proteins that form cross-links to chromosomal DNA in DEB-treated HT1080 cells (2 mM) with proteins that form DPCs in the presence of mechlorethamine (25μM) (A) and proteins that form DPCs in the presence of cisplatin (100 μM) (B).
Due to their unusually bulky nature as compared to the conventional DNA lesions, DPCs are expected to interrupt many critical cellular processes such as DNA replication, DNA repair, recombination, transcription, and chromatin remodeling. Previously, several possible mechanisms for DPCs repair have been proposed including proteolytic degradation, nucleotide excision repair (NER), and homologous recombination (HR). For example, Nakano et al. studied DPC repair in bacteria and mammalian cells and found that NER is involved in the repair of DPCs involving proteins less than12-14 kDa in size in bacteria and less than 8-10 kDa in mammalian cells, whereas HR is responsible for the repair of oversized DPCs.36 This would suggest that the majority of DPCs induced by DEB are repaired by HR, due to their significant size (Table 1).
In conclusion, our study demonstrates that the treatment of human fibrosarcoma cells with DEB induces DNA-protein cross-links to a variety of cellular proteins, including those participating in chromatin remodeling, translation, DNA replication, DNA repair, RNA metabolism, transcriptional regulation, and apoptosis. Although DEB-mediated DNA-protein cross-linking is relatively inefficient as compared to that induced by nitrogen mustards and platinum compounds, the resulting bulky, helix distorting DPC lesions have a considerable potential to interfere with critical cellular processes such as replication and transcription, potentially triggering programmed cell death and/or genotoxic outcomes.
Supplementary Material
Acknowledgments
We thank Pratik Jagtap (Minnesota Supercomputing Institute, University of Minnesota) for his help with proteomic data analyses and Bob Carlson (University of Minnesota Masonic Cancer Center) for preparing figures for this manuscript. Funding for this research was provided by the National Cancer Institute (CA-100670).
Abbreviations
- AGT
O6-alkylguanine DNA alkyltansferase
- Cys-N7G-BD
1-(S-cysteinyl)-4-(guan-7-yl)-2,3-butanediol
- DEB
1,2,3,4-diepoxybutane
- DPC
DNA-protein cross-link
- DTT
dithiothreitol
- FBS
fetal bovine serum
- FDR
false discovery rate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
glutathione
- HPLC-ESI+-MS/MS
high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry
- PARP
poly(ADP-ribose) polymerase I
- PBS
phosphate-buffered saline
- PMSF
phenylmethanesulfonyl fluoride
- Ref-1
DNA-(apurinic- or apyrimidinic-site) lyase
- TBS
Tris-buffered saline
- XRCC-1
x-ray cross-complementing protein I
Reference List
- 1.Barker S, Weinfeld M, Murray D. DNA-protein crosslinks: their induction, repair, and biological consequences. Mutat Res. 2005;589:111–135. doi: 10.1016/j.mrrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Michaelson-Richie ED, Loeber RL, Codreanu SG, Ming X, Liebler DC, Campbell C, Tretyakova NY. DNA-protein cross-linking by 1,2,3,4-diepoxybutane. J Proteome Res. 2010;9:4356–4367. doi: 10.1021/pr1000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeber R, Rajesh M, Fang Q, Pegg AE, Tretyakova N. Cross-linking of the human DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane. Chem Res Toxicol. 2006;19:645–654. doi: 10.1021/tx0600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murata-Kamiya N, Kamiya H. Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA. Nucleic Acids Res. 2001;29:3433–3438. doi: 10.1093/nar/29.16.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merk O, Speit G. Significance of formaldehyde-induced DNA-protein crosslinks for mutagenesis. Environ Mol Mutagen. 1998;32:260–268. doi: 10.1002/(sici)1098-2280(1998)32:3<260::aid-em9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Barker S, Weinfeld M, Zheng J, Li L, Murray D. Identification of mammalian proteins cross-linked to DNA by ionizing radiation. J Biol Chem. 2005;280:33826–33838. doi: 10.1074/jbc.M502477200. [DOI] [PubMed] [Google Scholar]
- 7.Zhitkovich A, Voitkun V, Kluz T, Costa M. Utilization of DNA-protein cross-links as a biomarker of chromium exposure. Environ Health Perspect. 1998;106(Suppl 4):969–974. doi: 10.1289/ehp.98106s4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michaelson-Richie ED, Ming X, Codreanu SG, Loeber RL, Liebler DC, Campbell C, Tretyakova NY. Mechlorethamine-induced DNA-protein cross-linking in human fibrosarcoma (HT1080) cells. J Proteome Res. 2011;10:2785–2796. doi: 10.1021/pr200042u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker JM, Parish JH, Curtis JP. DNA-DNA and DNA-protein crosslinking and repair in Neurospora crassa following exposure to nitrogen mustard. Mutat Res. 1984;132:171–179. doi: 10.1016/0167-8817(84)90035-x. [DOI] [PubMed] [Google Scholar]
- 10.Ewig RA, Kohn KW. DNA damage and repair in mouse leukemia L1210 cells treated with nitrogen mustard, 1,3-bis(2-chloroethyl)-1-nitrosourea, and other nitrosoureas. Cancer Res. 1977;37:2114–2122. [PubMed] [Google Scholar]
- 11.Kloster M, Kostrhunova H, Zaludova R, Malina J, Kasparkova J, Brabec V, Farrell N. Trifunctional dinuclear platinum complexes as DNA-protein cross-linking agent. Biochemistry. 2004;43:7776–7786. doi: 10.1021/bi030243e. [DOI] [PubMed] [Google Scholar]
- 12.Ewig RA, Kohn KW. DNA-protein cross-linking and DNA interstrand cross-linking by haloethylnitrosoureas in L1210 cells. Cancer Res. 1978;38:3197–3203. [PubMed] [Google Scholar]
- 13.Morrow NL. The industrial production and use of 1,3-butadiene. Environ Health Perspect. 1990;86:7–8. doi: 10.1289/ehp.90867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 15.Jelitto B, Vangala RR, Laib RJ. Species differences in DNA damage by butadiene: role of diepoxybutane. Arch Toxicol Suppl. 1989;13:246–249. doi: 10.1007/978-3-642-74117-3_42. [DOI] [PubMed] [Google Scholar]
- 16.Loecken EM, Guengerich FP. Reactions of glyceraldehyde 3-phosphate dehydrogenase sulfhydryl groups with bis-electrophiles produce DNA-protein cross-links but not mutations. Chem Res Toxicol. 2008;21:453–458. doi: 10.1021/tx7003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loecken EM, Dasari S, Hill S, Tabb DL, Guengerich FP. The bis-electrophile diepoxybutane cross-links DNA to human histones but does not result in enhanced mutagenesis in recombinant systems. Chem Res Toxicol. 2009;22:1069–1076. doi: 10.1021/tx900037u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thier R, Pemble SE, Kramer H, Taylor JB, Guengerich FP, Ketterer B. Human glutathione S-transferase T1-1 enhances mutagenicity of 1,2-dibromoethane, dibromomethane and 1,2,3,4-diepoxybutane in Salmonella typhimurium. Carcinogenesis. 1996;17:163–166. doi: 10.1093/carcin/17.1.163. [DOI] [PubMed] [Google Scholar]
- 19.Thier R, Muller M, Taylor JB, Pemble SE, Ketterer B, Guengerich FP. Enhancement of bacterial mutagenicity of bifunctional alkylating agents by expression of mammalian glutathione S-transferase. Chem Res Toxicol. 1995;8:465–472. doi: 10.1021/tx00045a019. [DOI] [PubMed] [Google Scholar]
- 20.Rasheed S, Nelson-Rees WA, Toth EM, Arnstein P, Gardner MB. Characterization of a newly derived human sarcoma cell line (HT-1080) Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Shin NY, Liu Q, Stamer SL, Liebler DC. Protein targets of reactive electrophiles in human liver microsomes. Chem Res Toxicol. 2007;20:859–867. doi: 10.1021/tx700031r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates JR, III, Eng JK, McCormack AL. Mining genomes: correlating tandem mass spectra of modified and unmodified peptides to sequences in nucleotide databases. Anal Chem. 1995;67:3202–3210. doi: 10.1021/ac00114a016. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Chambers MC, Tabb DL. Proteomic parsimony through bipartite graph analysis improves accuracy and transparency. J Proteome Res. 2007;6:3549–3557. doi: 10.1021/pr070230d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeber RL, Michaelson-Richie ED, Codreanu SG, Liebler DC, Campbell CR, Tretyakova NY. Proteomic analysis of DNA-protein cross-linking by antitumor nitrogen mustards. Chem Res Toxicol. 2009;22:1151–1162. doi: 10.1021/tx900078y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Summer H, Li O, Bao Q, Zhan L, Peter S, Sathiyanathan P, Henderson D, Klonisch T, Goodman SD, Droge P. HMGA2 exhibits dRP/AP site cleavage activity and protects cancer cells from DNA-damage-induced cytotoxicity during chemotherapy. Nucleic Acids Res. 2009;37:4371–4384. doi: 10.1093/nar/gkp375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Lu ZG, Miki Y, Yoshida K. Protein kinase C delta induces transcription of the TP53 tumor suppressor gene by controlling death-promoting factor Btf in the apoptotic response to DNA damage. Mol Cell Biol. 2007;27:8480–8491. doi: 10.1128/MCB.01126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie J, Guo Q. Apoptosis antagonizing transcription factor protects renal tubule cells against oxidative damage and apoptosis induced by ischemia-reperfusion. J Am Soc Nephrol. 2006;17:3336–3346. doi: 10.1681/ASN.2006040311. [DOI] [PubMed] [Google Scholar]
- 28.Maggi LB, Jr, Kuchenruether M, Dadey DY, Schwope RM, Grisendi S, Townsend RR, Pandolfi PP, Weber JD. Nucleophosmin serves as a rate-limiting nuclear export chaperone for the Mammalian ribosome. Mol Cell Biol. 2008;28:7050–7065. doi: 10.1128/MCB.01548-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuwaki M, Matsumoto K, Tsujimoto M, Nagata K. Function of nucleophosmin/B23, a nucleolar acidic protein, as a histone chaperone. FEBS Lett. 2001;506:272–276. doi: 10.1016/s0014-5793(01)02939-8. [DOI] [PubMed] [Google Scholar]
- 30.Grisendi S, Bernardi R, Rossi M, Cheng K, Khandker L, Manova K, Pandolfi PP. Role of nucleophosmin in embryonic development and tumorigenesis. Nature. 2005;437:147–153. doi: 10.1038/nature03915. [DOI] [PubMed] [Google Scholar]
- 31.Bai RY, Ouyang T, Miething C, Morris SW, Peschel C, Duyster J. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood. 2000;96:4319–4327. [PubMed] [Google Scholar]
- 32.Loeber R, Michaelson E, Fang Q, Campbell C, Pegg AE, Tretyakova N. Cross-linking of the DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of antitumor nitrogen mustards. Chem Res Toxicol. 2008;21:787–795. doi: 10.1021/tx7004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ide H, Shoulkamy MI, Nakano T, Miyamoto-Matsubara M, Salem AM. Repair and biochemical effects of DNA-protein crosslinks. Mutat Res. 2011;711:113–122. doi: 10.1016/j.mrfmmm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Valadez JG, Liu L, Loktionova NA, Pegg AE, Guengerich FP. Activation of bis-electrophiles to mutagenic conjugates by human O6-alkylguanine-DNA alkyltransferase. Chem Res Toxicol. 2004;17:972–982. doi: 10.1021/tx049897u. [DOI] [PubMed] [Google Scholar]
- 35.Fountoulakis M, Tsangaris G, Oh J, Maris A, Lubec G. Protein profile of the HeLa cell line. J Chromatogr A. 2004;1038:247–265. doi: 10.1016/j.chroma.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Nakano T, Morishita S, Katafuchi A, Matsubara M, Horikawa Y, Terato H, Salem AM, Izumi S, Pack SP, Makino K, Ide H. Nucleotide excision repair and homologous recombination systems commit differentially to the repair of DNA-protein crosslinks. Mol Cell. 2007;28:147–158. doi: 10.1016/j.molcel.2007.07.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


