Abstract
Non-obese diabetic (NOD) mice develop Sjögren’s-like syndrome (Ss) and a gradual loss of saliva secretory function. Our previous study showed that injections of matched normal spleen cells with Complete Freund’s Adjuvant (CFA) reversed salivary gland dysfunction in 14-week-old NOD mice, which had established Ss. The spleen and bone marrow are closely related organs, and both are among the first sites of hematopoiesis during gestation. Noticing a rapidly increasing number of clinical trials using bone marrow (BM) cells treatments for autoimmune diseases, we tested if BM cells can prevent Ss and restore salivary glands’ function. We injected CFA and MHC class I-matched normal BM cells in 7-week-old NOD mice, which had not yet developed Ss. We found at week 52 post-treatment that all NOD mice receiving BM cells and CFA had a recovery of salivary flow and were protected from Ss and diabetes. BM cells-treated mice had their salivary function restored quantitatively and qualitatively. Saliva flow was higher (p < 0.05) in BM cells-transplanted mice when compared to control mice, which continued to deteriorate over time. Total proteins, epidermal growth factor, amylase, and electrolytes concentrations in saliva of BM cells-treated mice were not significantly changed at week 44 and 52 post-therapy when compared to pre-therapy (when the mice did not have Ss). Restoration of salivary flow could have resulted from a combination of rescue and paracrine effects from BM cells. This study suggests that a combined immuno- and cell-based therapy can permanently prevent Ss and restored salivary function in NOD mice.
Keywords: Sjögren’s syndrome, Bone marrow cells, Cell therapy, Xerostomia, Salivary gland, Saliva
1. Introduction
Sjögren’s syndrome, which affects people with a frequency of 1 in 100, is characterized by an autoimmune destruction of the salivary and lacrimal glands. As a result, patients with Sjögren’s syndrome suffer from dry mouth and dry eyes (Delaleu et al., 2005; Lee et al., 2009). Salivary glands have various cell types: acinar cells which are responsible for water and proteins secretion, ductal cells that principally regulate the composition of saliva, and myoepithelial cells surrounding the acini and ducts (Lombaert et al., 2008). In Sjögren’s syndrome the immune system attacks the salivary glands, particularly the acinar cells. This leads to a loss of saliva secretion and the patients’ quality of life is severely compromised due to xerostomia (dry mouth), severe dental caries, and oral infections (Fox and Speight, 1996; Delaleu et al., 2005; Lombaert et al., 2008; Lee et al., 2009; Nikolov and Illei, 2009). Unfortunately, there is no suitable treatment for Sjögren’s syndrome, because the current pharmacological therapy that depends on the stimulation of residual acinar cells frequently fails, since in many cases all the salivary secretory tissue has already been lost (Tran et al., 2006). Regeneration of destroyed salivary glands and restoration of their function would greatly improve the quality of life for these patients.
Non-obese diabetic (NOD) mice, a frequently used animal model of Sjögren’s syndrome and type 1 diabetes mellitus (T1DM), both exhibit infiltrates of lymphocytes in the salivary glands (sialadenitis) with a gradual loss of salivary function and in the pancreas (insulitis) (Jonsson et al., 2007; Kodama et al., 2003; Lee et al., 2009; Soyfoo et al., 2007a,b; Tran et al., 2007). The reduced saliva output is similar to what is observed in patients (Tran et al., 2007). Previous studies from our group and collaborators have shown that a two-limb intervention can permanently restore lost function in Sjögren’s syndrome and T1DM in NOD mice (Kodama et al., 2003; Tran et al., 2007). This two-limb intervention consists of using complete Freund’s adjuvant (CFA) as the first limb, and then combining this with matched Major Histocompatibility Complex (MHC) class I spleen cells, as the second limb. The rationale of this two-limb intervention is that cellular immunity (T lymphocytes) plays a major role in the pathophysiology of Sjögren’s syndrome (Katsifis et al., 2007). NOD mice have a defect in the production of the low-molecular-weight protein 2, LMP2, leading to a problem in T cell selection. This results in the presence of autoreactive T cells and development of autoimmune diabetes and Sjögren’s-like syndrome (Hayashi and Faustman, 1999). LMP2 is a catalytic subunit of the proteosomes, which are very large protein complexes inside all eukaryotes and their role is to degrade all proteins that the cell has no more use for. This proteasome defect also impairs the processing of nuclear factor κB (NF-κB), a transcription factor that stimulates the expression of genes involved in a wide variety of biological functions, including the protection from TNF-α induced apoptosis. The disruption of its function in antigen presenting cells results in the escape of autoreactive T cells from proper immune selection. NF-κB defect also increases the apoptosis of misselected T-cells by TNF-α-induced apoptosis (Ryu et al., 2001). Treatment of NOD mice with a TNF-α inducer such as CFA, promotes the apoptosis of autoreactive T-cells and eventually removes the autoimmunity (Kodama et al., 2003; Tran et al., 2007). Once the autoimmunity is removed, restoration of salivary glands’ function was achieved.
Our previous report used spleen cells (Kodama et al., 2003). However, this population of cells is not easily obtained from patients (except from trauma cases). Bone marrow cells (BM cells) are clinically easier to harvest (either by needle aspiration or by mobilization to the blood). The spleen and bone marrow are closely related organs, and both are among the first sites of hematopoiesis during gestation. There are reports that BM cells have been used to treat autoimmune diseases (Bocelli-Tyndall et al., 2007; Burt et al., 2009; Lowenthal et al., 1993; Miniati et al., 2009; Oyama et al., 2005, 2007; Saccardi et al., 2006; Tran et al., 2003; Van Laar and Tyndall, 2006). BM cells have been suggested as a source of multipotent stem cells; particularly the marrow derived stromal cells, also known as mesenchymal stem cells (MSCs) with their ability to repair non-hematopoietic organs, including the salivary gland and pancreas (Couzin, 2006; Lombaert et al., 2006, 2008; Orlic et al., 2001; Urban et al., 2008). MSCs that can be isolated from a variety of tissues were shown to interact with all cells of the innate and adaptive immune system to modulate their function. Following systemic administration they home to injured tissues and can suppress the pro-inflammatory cytokines to help survival. In addition to immuno-modulation they can also regenerate bone, fat, cartilage and cells of other lineages (Uccelli and Pistoia, 2008; Zhao et al., 2009). The plasticity and immunosuppressive capability of MSCs have made them a novel therapeutic choice in autoimmune diseases (Aguayo-Mazzucato and Bonner-Weir, 2010; Zhao et al., 2009). MSCs can assist in the regeneration of the pancreas and salivary glands in mice (Lombaert et al., 2008; Urban et al., 2008). Transplantation of BM cells boosted levels of serum insulin and decreased blood sugar levels in diabetic mice by mechanisms such as the re-construction of β-cell islets (the insulin-secreting cells), secretion of growth factors to endothelial progenitor cells, or by direct cell differentiation (Aguayo-Mazzucato and Bonner-Weir, 2010; Uccelli and Pistoia, 2008). In summary, BM cells are capable to differentiate into other cell types, as well as to provide a beneficial effect by secreting cytokines and/or growth factors (Burt et al., 2009; Coppes et al., 2009; Uccelli and Pistoia, 2008). From all these reasons, the objective of this study was to assess whether the use of BM cells, instead of spleen cells, in our two-limb intervention (described above) could prevent Sjögren’s syndrome and restore salivary glands’ function in NOD mice. In addition, this study improved on our previous one (Tran et al., 2007) by: (a) providing therapy at an early time point (7-week-old NOD mice, as compared to our previous study using 14-week-old NOD mice with established Sjogren’s syndrome), (b) following the mice daily for 52 weeks, (c) by including a group of NOD mice treated with CFA only (to allow comparison to the group of mice treated with CFA + BM cells), and (d) by assessing the composition/quality of saliva (as compared to its quantity only). These improvements all add new information to the current literature.
2. Materials and methods
2.1. Animals
Seven-week old female NOD mice (which had not yet developed Sjögren’s syndrome; Taconic Farms, Germantown, NY) and aged-matched CByF1B6F1/J (CByB6F1) mice (Jackson Laboratory) were maintained under pathogen-free conditions in the Animal facility at McGill University. Female NOD mice were divided into three different groups (10 mice per group) and were followed for 52 weeks after treatments with either: (a) bone marrow cells transplant plus CFA (BM cells group), (b) only CFA injected (CFA group), or (c) no cell injection, no CFA, but daily injections of insulin to control blood sugar levels (Control group).
2.2. Cell transplantation
Bone marrow cells of male CByB6F1 mice (1 × 107 cells) were harvested and freshly injected into female NOD recipients (of the BM cells group) through the tail vein, twice a week for six consecutive weeks (Kodama et al., 2003; Tran et al., 2007; Urban et al., 2008; Zhao et al., 2009). No bone marrow cells were injected into NOD mice in the CFA group or Control groups. Complete Freund’s adjuvant (CFA, Difco, Detroit, MI) was freshly mixed with an equal volume of physiological saline and then injected (50 μl) into each hind footpad simultaneously with the first bone marrow cells injection. CFA was also injected once in NOD mice of the CFA group. No CFA was injected in mice of the Control group.
2.3. Salivary flow rate
Secretory function of the salivary glands (salivary flow rate) was obtained by inducing mild gas anesthesia to NOD mice with 5% Isoflurane, and 1.5–1 L/min Oxygen (as per animal facility protocols at McGill University). Whole saliva was collected after stimulation of secretion using 0.5 mg Pilocarpine/kg body weight administered subcutaneously. Saliva was obtained from the oral cavity by micropipette, placed into pre-weighed 0.5-ml microcentrifuge tubes. Saliva was collected for a 10-min period and its volume determined gravimetrically. Salivary flow rate was determined at baseline (week 0, when NOD mice were 7-week old; before transplantation started) and at weeks 2, 12, 34, 38, and 52 post-transplantation.
2.4. Blood sugar
Blood glucose levels from NOD mice were monitored once a week (Accu-Check, Roche Diagnostics, Laval, QC, Canada). The mice were diagnosed with diabetes after observing two consecutive daily blood glucose concentrations of >200 mg/dl. These diabetic mice were injected with insulin on a daily basis (Humulin N, Lilly, ON, Canada).
2.5. Saliva compositions
2.5.1. Total proteins concentration
The concentration of proteins in saliva was measured by Bicinchoninic Acid Assay (BCA) method. It was measured at the beginning of experiment in week 0 and at the end in week 52 (Thermo Fisher Scientific Cat no # 23225).
2.5.2. Epidermal growth factor (EGF)
Concentration of EGF in saliva was measured by ELISA method at baseline (week 0) and at the end of the experiment (week 52 post-transplantation) (R&D System, Minneapolis, MN, USA, Cat no # MGE00).
2.5.3. Amylase activity
Amylase activity in saliva was measured by colorimetric method at the beginning (week 0) and at the end of study (week 52) (Salimeterics, PA, USA, Cat no # 1-1902).
2.5.4. Electrolytes (Na+, K+, Cl−, and Ca2+) concentration
Salivary sodium, potassium, chloride, and calcium were analyzed on a Beckman Coulter DXc 800 automated chemistry analyzer using the urine chemistry mode. The sodium assay was an indirect potentiometric method based on a sodium sensitive electrode. Potassium was measured with a potassium sensitive electrode consisting of a valinomycin PVC membrane cast. An indirect potentiometry utilizing a solid-state chloride electrode was used to measure chloride. Calcium was also measured by an ion selective electrode consisting of a calcium ionophore membrane cast (Beckman Coulter Synchron systems, CA, USA).
2.6. Histology analysis
2.6.1. Immunostaining and fluorescence in situ hybridization (FISH)
Salivary glands were collected, immersed in Optimal Cutting Temperature (OCT) compound, and then frozen at −80°C. Serial frozen sections were cut in a cryostat at 4–8 μm thickness, mounted on poly l lysine coated microscope slides and immersion fixed in 2% paraformaldehyde fixative for 10 min (Tran et al., 2007; Toth et al., 2008). Immunostaining was performed by primary antibody that was detected by the Sternberger peroxidase antiperoxidase (PAP) method followed by a Fluorescein Isothiocyanate-Conjugated (FITC)-tyramide signal amplification (TSA System, Invitrogen, Carlsbad, USA). The primary antibody used was against Na+/K+/2Cl− co-transporter (NKCC1) (graciously donated by Dr. James Turner, NIH) to detect salivary acinar cells. A digoxigenin-labeled riboprobe was then added to recognize a repeat sequence (pY353B) on the mouse Y chromosome. Then the samples were blocked with endogenous peroxidase (DAKO, CA, USA#S2001). The riboprobe was then detected using an antibody to digoxigenin conjugated to peroxidase (Roche, Indianapolis, USA). This peroxidase was visualized by tyramide signal amplification with a Alexa Fluor 594 fluorochrome-tyramide reagent (TSA System, Invitrogen, Carlsbad, USA). All sections were stained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Invitrogen#D3571), a chromosomal marker to label all nuclei and then mounted with buffer Tris. Finally the slides were visualized using a Leica DM6000 fluorescent microscope equipped with Volocity software (Tran et al., 2007).
2.6.2. Focus score
Focus score is defined as the number of foci, infiltrated lymphocytes (at least 50 inflammatory cells), per 4 mm2 of tissue. After NOD mice were sacrificed, their submandibular glands were removed immediately. Half of a gland per mouse was fixed in 10% formalin and embedded in paraffin. Sections were cut at 5–8 μm thicknesses and subsequently stained with hematoxylin and eosin. This method is an accepted way to determine the severity of the sialadenitis (the inflammatory cells infiltrate found in the salivary glands).
2.7. Statistical analysis
To determine statistical significant differences (p < 0.05), Linear Mixed Models and ANOVA analysis (Tukey’s Post Hoc test) were used. Subjects between and within the groups were compared at different time points using SPSS version 17 (IBM, USA).
3. Results
3.1. Salivary flow rate
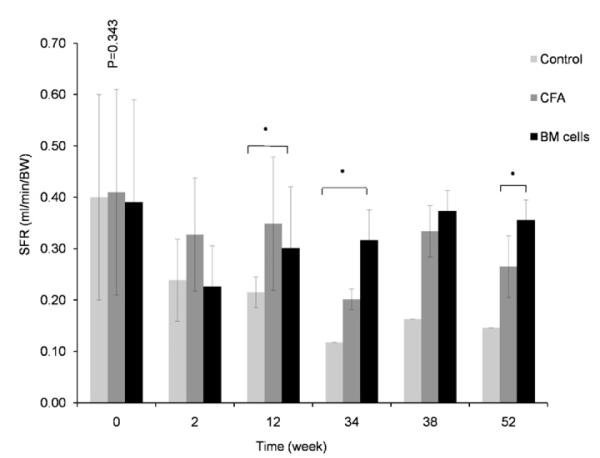
Overall salivary flow rates (SFR) gradually increased in the BM cells-treated and CFA injected groups for up to 1-year post transplantation. SFR of non-treated NOD mice, on the other hand, continued to decrease until the end of experiment (Figs. 1 and 2). Mice in BM cells-treated group had a decline in their SFR for 2 weeks post-treatment. After that, SFR started to recover and continued to improve until the end of the experiment, reaching comparable levels of SFR in baseline. The SFR was significantly higher in BM cells-transplanted group than in the non-treated group (p < 0.05).
Figs. 1.
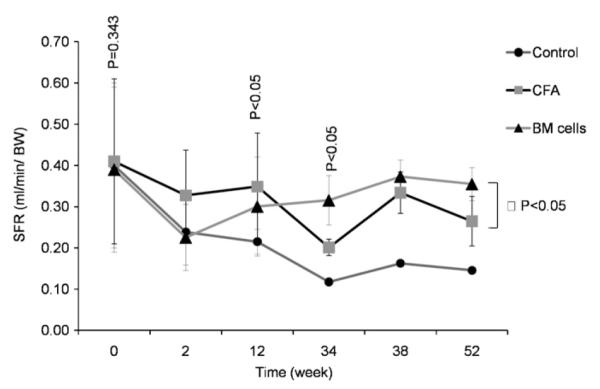
Effects of treatment on the restoration of salivary flow rates (SFR). Stimulated salivary flow rate of BM cells-treated (triangle), CFA-treated (square), and untreated NOD mice (circle) during the 52-week time course following therapy. SFR in BM cells-treated mice has recovered. Mice in BM cells-treated group (triangle) had a decline in their SFR for 2 weeks post-treatment. After that period, SFR started to recover and continued to improve until the end of the experiment, reaching comparable levels to before Sjögren’s-like syndrome. SFR was significantly different in BM cells-transplanted group when compare to SFR in control-group (p < 0.05).
Figs. 2.
Effects of treatment on the restoration of salivary flow rates (SFR). Stimulated salivary flow rate of BM cells-treated (triangle), CFA-treated (square), and untreated NOD mice (circle) during the 52-week time course following therapy. SFR in BM cells-treated mice has recovered. Mice in BM cells-treated group (triangle) had a decline in their SFR for 2 weeks post-treatment. After that period, SFR started to recover and continued to improve until the end of the experiment, reaching comparable levels to before Sjögren’s-like syndrome. SFR was significantly different in BM cells-transplanted group when compare to SFR in control-group (p < 0.05).
3.2. Blood glucose
All mice were normoglycaemic at the start of the experiment, at 7 weeks of age (week 0). Non-treated NOD mice (i.e. only receiving injections of insulin; no CFA or BM cells) developed diabetes during the course of the experiment and 90% of them died by 30 weeks post-transplantation (Fig. 3) (p < 0.05). The first mouse in the control group was diagnosed with diabetes within 2 weeks of the follow-up period. Animals in the 2 treated groups (CFA + BM cells; or CFA alone), however, showed a relatively stable blood sugar level and survived during the observation period of 52 weeks post-transplantation.
Fig. 3.
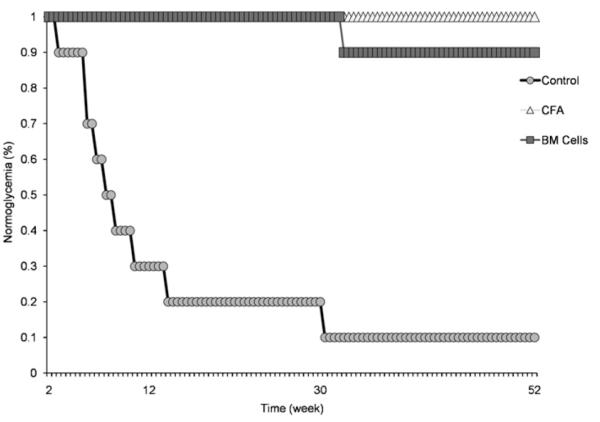
Kaplan–Meier plot for normoglycaemia in BM cells-treated (square), CFA-treated (triangle), and untreated (circle) NOD mice monitored for 66 weeks (for 52 weeks post-transplantation). All mice were normoglycaemic at the start of the experiment, at 7 weeks of age (week 0). The first diabetic mouse was diagnosed in the control group (circle) at 2 weeks post-therapy and 90% of mice in this group died within 30 weeks post-therapy (p < 0.05). Mice in the treated groups (square and triangle) on the other hand, had a stable level of blood sugar during the course of the experiment. Only one mouse in the BM cells-treated group showed high level of blood sugar at 30 weeks post-transplantation.
3.3. Total proteins concentration
Total proteins concentration in saliva has not changed significantly from week 52 (end of experiment) when compared to week 0 (when mice were healthy) among the treated groups (p > 0.05) (Fig. 4). Total proteins concentration in saliva of control mice could not be measure at week 52 as 90% of these mice had died due to their diabetes.
Fig. 4.
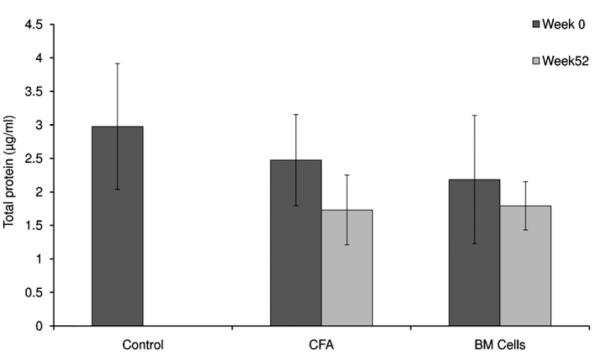
Total proteins concentration in saliva. The concentration of total proteins did not significantly change in NOD mice of treated groups (CFA and BM cells) between week 0 (when the mice were healthy) and week 52 following therapy (p > 0.05). Total proteins concentration in saliva of control mice could not be measured at week 52 as 90% of these mice had died due to their diabetes.
3.4. Epidermal growth factor (EGF) and amylase activity
Epidermal growth factor levels were higher in the CFA and CFA + BM group at week 52 when compared to week 0. However these differences were not statistically significant between and within the groups (Fig. 5; p = 0.083). Similarly, the levels of amylase activity also exhibited lower levels in the CFA group when comparing week 0 and week 52, although this appears contra-indicative of the role of CFA in reversing this common symptom of Sjögren’s syndrome (Fig. 6; p = 0.170).
Fig. 5.
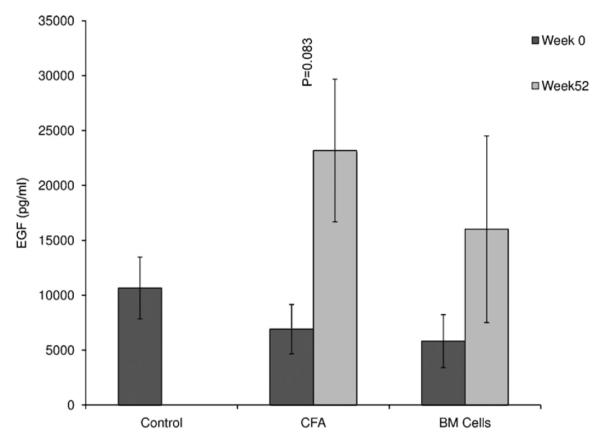
Concentration of EGF in saliva. EGF concentration was not significantly different among the experimental groups in week 0 (baseline) and week 52, 1 year after treatment, although EGF concentration was higher in week 52 when compared to week 0 (p = 0.083).
Fig. 6.
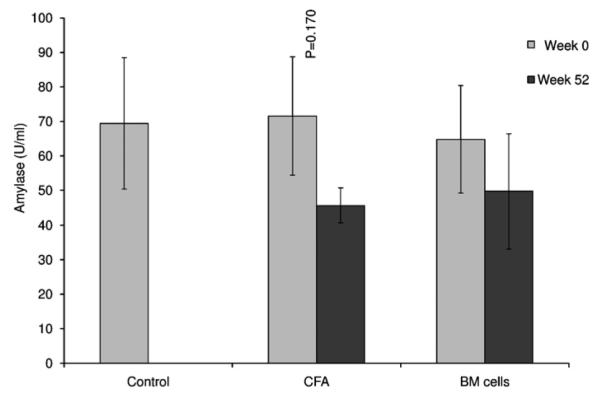
Amylase activity in saliva. Although the activity of amylase in saliva was higher in treated groups (CFA and BM cells) at week 0 (baseline) when compared to week 52 post-therapy, this was not statistically significant (p = 0.170).
3.5. Electrolytes in saliva
In general, the electrolyte concentrations decreased in the BM cells-transplanted group. The levels of Na+ and Cl− decreased in week 44 compared to their levels in week 4 post-transplantation (Fig. 1 Supplemental). This may be due to the increase of SFR in the BM cells-treated mice during the 52-week follow-up period. The level of K+, on the other hand, had a slight increase. The level of Na+, Cl− and Ca2+ did not change in the CFA-treated group during the same interval of observation (Fig. 1 Supplemental). The concentration of salivary electrolytes (Na+, K+, Cl− and Ca2+) in the control-group mice increased during the course of the study. This can be explained by the decrease of saliva secretion measured from these mice.
3.6. Immunocytochemistry (ICC) combined with fluorescence in situ hybridization (FISH)
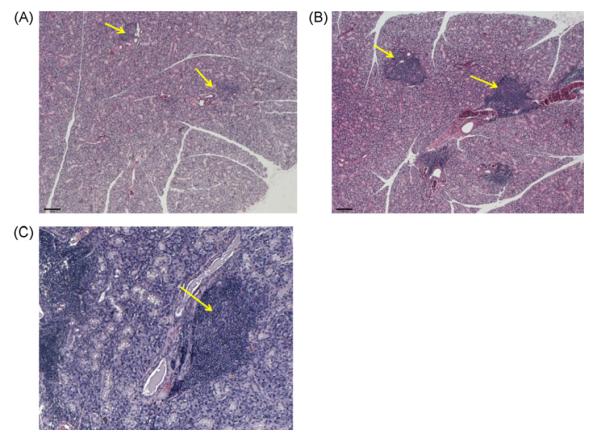
To investigate the mechanism of the return of SFR in BM cells-treated NOD mice, we examined their salivary tissues for cell chimerism and inflammatory signs. Restoration of SFR could be the result of rescue of the salivary gland by removing autoimmunity, or by a combination of rescue and tissue regeneration that overcomes the destruction caused by autoimmunity. Regeneration could mainly be a de novo process, in which the glands are being regenerated by their endogenous stem cells (internal source). Another possibility is if the male donor BM cells are able to differentiate into salivary epithelial cells. We observed a very small number of male donor cells which had differentiated into salivary epithelial cells (<0.1%), including acinar cells (detected by the NKCC1 antibody) (Fig. 7).
Fig. 7.
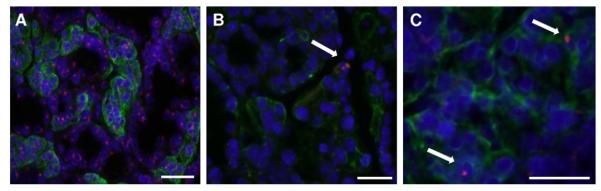
Double immunostaining in mouse salivary glands. (A) Male mouse salivary gland tissue used here as a positive control for the Y chromosomal probe. The Y chromosome signal is a pink dot, and the co-transporter for Na-K-2Cl type 1 is a green signal that surrounds the cell membrane (NKCC1 is a marker of salivary acinar cells). Nuclei are stained in blue with DAPI. (B and C) We observed very few (<0.1%) Y chromosome positive cells in NKCC1-positive cells (shown by the arrows) in several BM cells-treated female NOD mice. The scale bar represents 34 μm in all three panels. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.7. Focus score
Focus score in the salivary glands was not significantly different between non-treated and treated groups (p > 0.05). The average focus scores were 3.13 in the control group, 2.4 in the CFA-treated group, and 2.3 in the BM cells-treated group. The size of the average lymphocytic infiltrate, however, was slightly larger in the non-treated mice when compared to the treated groups (Fig. 8).
Fig. 8.
Focus score between treated and non-treated mice. Light micrographs of mouse salivary glands stained with hematoxylin and eosin. Focus score was not significantly different between non-tread and treated groups. The size of the focus score, however, was slightly bigger in the non-treated mice (B) when compared to the BM cells and CFA-treated mice (A and C) (p > 0.05).
4. Discussion
This study demonstrates that a two-limb therapy, simultaneous administration of CFA and BM cells can prevent the development of Sjögren’s syndrome and type I diabetes mellitus in female NOD mice. We transplanted 7-week-old female NOD mice (which had normal glucose and saliva production levels) with CFA and BM cells. We measured effectiveness of this treatment using these criteria: (1) monitoring the progression to Sjögren’s syndrome and diabetes, (2) measuring salivary flow rate (SFR), and (3) examining saliva composition. Our results showed that the treatment administered in young (7-week-old) NOD mice completely protected mice (100%) from progression to Sjögren’s syndrome and diabetes. This percentage is higher than the 85% reported by Kodama et al. (2003) for end-stage diabetic NOD mice treated with this two-limb therapy at 22–40 weeks of age using splenocytes. Our data points at the importance of an early intervention using this two-limb therapy. We have demonstrated SFR improvements from our previous study when treatment started for 14-week-old NOD mice (with already established Sjögren’s syndrome). However, our previous study did not evaluate saliva compositions. In the current study, we assessed the salivary glands’ functions both quantitatively (SFR), and qualitatively (electrolytes, amylase activity, etc).
In general, 60–80% of female NOD mice develop Sjögren’s syndrome and type 1 diabetes mellitus around 8 weeks of age, even though almost all mice exhibit sialadenitis and insulitis (Jonsson et al., 2007; Lee et al., 2009; Yamano et al., 1999). Symptoms from this disorder become more severe with time, and the majority of these animals die due to complications caused by their diabetes and Sjögren’s syndrome (Jonsson et al., 2007; Yamano et al., 1999; Hu et al., 1992). We, nevertheless, were able to keep the BM cells + CFA treated animals alive for more than 1-year post-transplantation. Mice in CFA treated group also survived until the end of study. The therapeutic effects of CFA on diabetes have been shown in many studies (Ryu et al., 2001; Kodama et al., 2003) including this one. However for Sjögren’s syndrome, our results showed that SFR of CFA-treated mice decreased after 38 weeks post-transplantation. In other words, we found that CFA had a transient effect on salivary glands. Our study did not test alternative adjuvants but only CFA. We selected CFA as it is the most applicable and efficient available adjuvant that can be used when subcutaneously injected (Hayashi and Faustman, 1999; Qin et al., 1993). CFA can increase the level of TNF-α better, higher, and faster and eventually remove the autoimmunity (Qin et al., 1993; Ryu et al., 2001).
Salivary gland dysfunction occurs in NOD mice with increasing age and this trend was reported by Jonsson et al. (2006) andSoyfoo et al. (2007a,b) (Jonsson et al., 2006; Soyfoo et al., 2007a,b). Our finding showed that salivary glands’ function was quantitatively and qualitatively restored in the BM cells + CFA treated mice. Saliva compositions including total proteins, epidermal growth factor, amylase activity, and electrolyte concentrations in treated mice were restored to their initial levels as those at baseline (week 0) of the experiment, when the mice were still healthy. Small number of male donor BM cells (less than 0.1%) settled in the salivary gland and differentiated into salivary epithelial cells. Our method of detection for donor Y-chromosome BM cells is as robust (if not better) than for GFP cells (Faustman et al., 2006). A clear mechanism for our findings has not been proposed so far. Since there is no suitable available treatment for Sjögren’s syndrome, our study is a significant step in support of a combined immuno- and cell-based therapy. The restoration mechanism of salivary function and its composition are probably driven by gland rescue and paracrine stimulation done by BM cells (Tran SD, Submitted) (Coppes et al., 2009; Tran et al., 2007). Transplanted BM cells, including mesenchymal and epithelial cells secrete cytokines and growth factors, which might stimulate the remaining progenitor cells in the glands to proliferate and generate new cells as well as inhibit the secretion of pro-inflammatory cytokines by the infiltrating lymphocytes. Therefore regeneration of the salivary is a result of de novo regeneration (Coppes et al., 2009). A paracrine mechanism has also been reported for pancreatic regeneration in a murine model by Aguayo-Mazzucato and Bonner-Weir (2010). Restoration of the glands’ function could have resulted from mediators secreted by the donor cells when they were systemically transplanted, as explained by Horwitz and Dominici (Aguayo-Mazzucato and Bonner-Weir, 2010; Horwitz and Dominici, 2008). BM cells can also increase angiogenesis by regenerating endothelial cells, as reported by Lombaert et al. (2008). We, however, could not find any endothelial cell of donor origin in the NOD mice. In contrast to the pancreas, as reported by Kodama et al. (2003), we did not find a significant change in focus score (lymphoid infiltrates within the glands) among the experimental groups (BM cells, CFA, or control group). This data is similar to our previous study (Tran et al., 2007). The size of the salivary lymphocytic infiltrates (focus score) in our study was relatively bigger in the control group as compare to the BM cells-transplanted group; although this did not achieve a statistical significant difference. There seems to be no obvious association between focus score appearance and salivary glands dysfunction (Haga, 2002; Jonsson et al., 2006).
5. Conclusion
In conclusion, we demonstrated that a two-limb, CFA and BM cells, intervention if applied at an early age can permanently prevent diabetes and Sjögren’s-like syndrome, two forms of autoimmune diseases in the NOD mice. With recent findings that human patients with Sjögren’s syndrome have the same proteasome defects in their LMP2 subunit as the NOD mice, they might likely benefit from this treatment. However, further investigation is needed to find the mechanism of how and which population of cells from transplanted bone marrow leads to resolution of the autoimmune disease.
Supplementary Material
Acknowledgments
We wish to thank Drs. R James Turner (NIDCR/ NIH, for donating the NaKCl-cotransporter antibody), Mari Kaartinen, Dieter Reinhardt, Noreen Jane Bider, Jose Correa, Yonit Rebibo, and Neena Hanif. We also wish to thank the staff at the McGill University animal facility and the Centre for Bone and Periodontal Research. This study was supported in part by research funding from the Canadian Institutes of Health Research (CIHR), Canada Research Chair, and the Intramural Research Program of the National Institute of Dental and Craniofacial Research (NIDCR), NIH.
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.biocel.2010.08.008.
References
- Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:139–48. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- Bocelli-Tyndall C, Bracci L, Spagnoli G, Braccini A, Bouchenaki M, Ceredig R, et al. Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatology (Oxford) 2007;46:403–8. doi: 10.1093/rheumatology/kel267. [DOI] [PubMed] [Google Scholar]
- Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8:244–53. doi: 10.1016/S1474-4422(09)70017-1. [DOI] [PubMed] [Google Scholar]
- Coppes RP, vander Goot A, Lombaert IM. Stem cell therapy to reduce radiation-induced normal tissue damage. Semin Radiat Oncol. 2009;19:112–21. doi: 10.1016/j.semradonc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Couzin J. Clinical trials. A shot of bone marrow can help the heart. Science. 2006;313:1715–6. doi: 10.1126/science.313.5794.1715a. [DOI] [PubMed] [Google Scholar]
- Delaleu N, Jonsson R, Koller MM. Sjogren’s syndrome. Eur J Oral Sci. 2005;113:101–13. doi: 10.1111/j.1600-0722.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- Faustman DL, Tran S, Kodama S, Lodde BM, Szalayova I, Key S, Toth ZE, Mezey E, Chong, et al. Nishio, et al. Suri, et al. on diabetes reversal in NOD mice. Science. 2006:314. doi: 10.1126/science.1129918. Comment on papers by. [DOI] [PubMed] [Google Scholar]
- Fox PC, Speight PM. Current concepts of autoimmune exocrinopathy: immunologic mechanisms in the salivary pathology of Sjogren’s syndrome. Crit Rev Oral Biol Med. 1996;7:144–58. doi: 10.1177/10454411960070020301. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Faustman D. NOD mice are defective in proteasome production and activation of NF-kappaB. Mol Cell Biol. 1999;19:8646–59. doi: 10.1128/mcb.19.12.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga HJ. Clinical and immunological factors associated with low lacrimal and salivary flow rate in patients with primary Sjögren’s syndrome. J Rheumatol. 2002;29:305–8. [PubMed] [Google Scholar]
- Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10:771–4. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- Hu Y, Nakagawa Y, Purushotham KR, Humphreys-Beher MG. Functional changes in salivary glands of autoimmune disease-prone NOD mice. Am J Physiol. 1992;263:E607–14. doi: 10.1152/ajpendo.1992.263.4.E607. [DOI] [PubMed] [Google Scholar]
- Jonsson MV, Delaleu N, Brokstad KA, Berggreen E, Skarstein K. Impaired salivary gland function in NOD mice: association with changes in cytokine profile but not with histopathologic changes in the salivary gland. Arthritis Rheum. 2006;54:2300–5. doi: 10.1002/art.21945. [DOI] [PubMed] [Google Scholar]
- Jonsson MV, Delaleu N, Jonsson R. Animal models of Sjogren’s syndrome. Clin Rev Allergy Immunol. 2007;32:215–24. doi: 10.1007/s12016-007-8012-7. [DOI] [PubMed] [Google Scholar]
- Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjogren’s syndrome: contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol. 2007;32:252–64. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- Kodama S, et al. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223–7. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- Lee BH, Tudares MA, Nguyen CQ. Sjogren’s syndrome: an old tale with a new twist. Arch Immunol Ther Exp (Warsz) 2009;57:57–66. doi: 10.1007/s00005-009-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Wierenga PK, Kok T, Kampinga H, de Haan G, Coppes RP. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res. 2006;12:1804–12. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]
- Lowenthal RM, Cohen ML, Atkinson K, Biggs JC. Apparent cure of rheumatoid arthritis by bone marrow transplantation. J Rheumatol. 1993;20:137–40. [PubMed] [Google Scholar]
- Miniati I, Guiducci S, Conforti ML, Rogai V, Fiori G, Cinelli M, et al. Autologous stem cell transplantation improves microcirculation in systemic sclerosis. Ann Rheum Dis. 2009;68:94–8. doi: 10.1136/ard.2007.082495. [DOI] [PubMed] [Google Scholar]
- Nikolov NP, Illei GG. Pathogenesis of Sjogren’s syndrome. Curr Opin Rheumatol. 2009;21:465–70. doi: 10.1097/BOR.0b013e32832eba21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Barr WG, Statkute L, Corbridge T, Gonda EA, Jovanovic B, et al. Autologous non-myeloablative hematopoietic stem cell transplantation in patients with systemic sclerosis. Bone Marrow Transplant. 2007;40:549–55. doi: 10.1038/sj.bmt.1705782. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005;128:552–63. doi: 10.1053/j.gastro.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Qin HY, Sadelain MW, Hitchon C, Lauzon J, Singh B. Complete Freund’s adjuvant-induced T cells prevent the development and adoptive transfer of diabetes in nonobese diabetic mice. J Immunol. 1993;150:2072–80. [PubMed] [Google Scholar]
- Ryu S, Kodama S, Ryu K, Schoenfeld D, Faustman D. Reversal of established autoimmune diabetes by restoration of endogenous beta cell function. J Clin Invest. 2001;108:63–72. doi: 10.1172/JCI12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardi R, Kozak T, Bocelli-Tyndall C, Fassas A, Kazis A, Havrdova E, et al. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler. 2006;12:814–23. doi: 10.1177/1352458506071301. [DOI] [PubMed] [Google Scholar]
- Soyfoo MS, DeVriese C, Debaix H, Martin-Martinez MD, Mathieu C, Devuyst O, et al. Modified aquaporin 5 expression and distribution in submandibular glands from NOD mice displaying autoimmune exocrinopathy. Arthritis Rheum. 2007a;56:2566–74. doi: 10.1002/art.22826. [DOI] [PubMed] [Google Scholar]
- Soyfoo MS, Steinfeld S, Delporte C. Usefulness of mouse models to study the pathogenesis of Sjogren’s syndrome. Oral Dis. 2007b;13:366–75. doi: 10.1111/j.1601-0825.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- Toth ZE, Leker RR, Shahar T, Pastorino S, Szalayova I, Asemenew B, et al. The combination of granulocyte colony-stimulating factor and stem cell factor significantly increases the number of bone marrow-derived endothelial cells in brains of mice following cerebral ischemia. Blood. 2008;111:5544–52. doi: 10.1182/blood-2007-10-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran SD, Kodama S, Lodde BM, Szalayova I, Key S, Khalili S, et al. Reversal of Sjogren’s-like syndrome in non-obese diabetic mice. Ann Rheum Dis. 2007;66:812–4. doi: 10.1136/ard.2006.064030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran SD, Pillemer SR, Dutra A, Barrett AJ, Brownstein MJ, Key S, et al. Differentiation of human bone marrow-derived cells into buccal epithelial cells in vivo: a molecular analytical study. Lancet. 2003;361:1084–8. doi: 10.1016/S0140-6736(03)12894-2. [DOI] [PubMed] [Google Scholar]
- Tran SD, Dipasquale ST, Cotrim G, Bandyopadhyay AP, Riddle BC, Mooney K, et al. Re-engineering primary epithelial cells from rhesus monkey parotid glands for use in developing an artificial salivary gland. Tissue Eng. 2006;12:2939–48. doi: 10.1089/ten.2006.12.2939. [DOI] [PubMed] [Google Scholar]
- Uccelli AML, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Urban VS, Kiss J, Kovacs J, Gocza E, Vas V, Monostori E, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–53. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- Van Laar JM, Tyndall A. Adult stem cells in the treatment of autoimmune diseases. Rheumatology (Oxford) 2006;45:1187–93. doi: 10.1093/rheumatology/kel158. [DOI] [PubMed] [Google Scholar]
- Yamano S, Atkinson JC, Baum BJ, Fox PC. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol. 1999;92:265–75. doi: 10.1006/clim.1999.4759. [DOI] [PubMed] [Google Scholar]
- Zhao S, Wehner R, Bornhauser M, Wassmuth R, Bachmann M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2009;2:130–8. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.